Abstract
Rhizotrons provide valuable information about plant root production, but measurements are usually made in units of root length per unit surface area of observation window surface. These measurement units are not easily comparable to above-ground plant growth. To address this deficiency, several techniques have been developed to convert rhizotron measurement units into root mass production per unit ground area. In this study, four different conversion methods were applied to the same dataset of rhizotron measurements. This data was used to reveal the effect of conversion method upon estimates of the temporal variation in, and annual magnitude of, gross root mass production. Application of four different conversion methods resulted in gross root production estimates ranging between 2.1 and 11.4 t ha−1 year−1. Temporal variation in gross root mass production also varied between methods. All current methods for quantifying root production are likely to cause some disturbance and bias. Based upon a comparison of the sources of error present in each conversion method, we assess which methods are likely to produce the most reliable estimates of root biomass production per unit ground area, and propose additional measurements which could further improve accuracy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A significant fraction of carbon (C) assimilated by plants is allocated below-ground to sustain growth and maintenance of root tissue (Jackson et al. 1997; Högberg et al. 2001). As a store of C and other nutrients, roots are relatively dynamic (Eissenstat et al. 2000), responding quickly to environmental changes with potentially large consequences for ecosystem biogeochemical cycling. Several methodologies have been developed to record root production and turnover (see reviews by Vogt et al. 1998, Smit et al. 2000, Hendricks et al. 2006), but most face significant problems from inferring root activity based upon occasional measurements. In this context, rhizotrons or minirhizotrons are increasingly popular because they can record in situ root production and turnover at high temporal frequency. However, a key limitation of such methods is that the measurement unit (root length per unit surface area of observation window surface) is not directly comparable with above-ground plant production, which is usually quantified in units of biomass per unit ground area. Several methods have been presented in the literature to convert rhizotron length measurements into units of biomass per unit area (see references and further details in the “Materials and methods” section). However, to the authors’ knowledge, there has been no review of these different conversion methods.
The purpose of this study, therefore, was to compare four different conversion methods by applying them to the same dataset of rhizotron measurements to estimate gross fine root (< 2 mm diameter) biomass production per unit ground area. This data was used to reveal differences in temporal variation in, and annual magnitude of, root biomass production estimates (t ha−1) derived from the conversion methods. There were no means to calculate a completely unbiased estimate of root growth at the site, against which the different conversion methods could have been compared to derive absolute error values. Instead, we assess potential sources of bias inherent in each conversion method, calculate uncertainties in key parameters required for each conversion method and then outline potential approaches to minimizing methodological biases.
Materials and methods
Study site
The study site is a one-hectare plot (see Table 1 for further plot details) located in the Caxiuanã National Forest, Pará State, north-eastern Brazil (1°43'3.5''S, 51°27'36''W). The forest is a lowland terra firme rainforest with a high annual rainfall (∼ 2500 mm) but a pronounced dry season between July and December. The soil type is a highly weathered yellow Oxisol (U.S Department of Agriculture classification system). For further details of soil texture and chemistry in the region, see Ruivo and Cunha (2003).
Supplementary measurements
Standing crop fine root (< 2 mm diameter) mass was recorded at nine replicate points in the plot, at the beginning of November 2004. Soil cores (14 cm diameter, 30 cm depth) were extracted using opposable semi-circular cutting blades, and the roots were removed by dry manual sorting following the approach outlined by Metcalfe et al. (2007).
In addition to rhizotrons (see below), ingrowth cores (14 cm diameter, 30 cm depth) were used to estimate root production. In November 2004, soil cores were extracted from 16 points in each plot using opposable semi-circular cutting blades, and the roots were removed by dry manual sorting following the approach outlined by Metcalfe et al. (2007) and the remaining soil was reinserted into the same holes that it had been extracted from, surrounded by plastic mesh bags (1 cm mesh aperture diameter). Care was taken to pack the reinserted soil to a similar bulk density as surrounding undisturbed soil (∼ 1.5 Mg m−3). This was repeated four times (every 3 months) between November 2004 and November 2005. The amount of root material which grew into the mesh bags was used to calculate new root production for each 3-month interval. Roots removed from soil samples were immediately placed in plastic bags to minimize desiccation. Within 48 h of removal from the soil, root samples were cleaned of residual soil and detritus with a soft brush and scanned at high resolution (600 dpi). From the scans, root length (divided into 0.1 mm diameter categories) and volume was calculated using image analysis software (WinRHIZO Pro version 2003b, Regent Instruments, Canada). Root samples from both the standing crop and ingrowth cores were dried at 70°C to constant mass and weighed.
Rhizotron methodology and unit conversion
In this study, rhizotrons were constructed from wooden frames, supporting vertically orientated transparent plastic screens (21 cm width, 30 cm depth). In August 2004, nine rhizotrons were inserted into the soil along a regular grid within the study plot. Incremental root length extension was recorded every 15 days by tracing over roots visible at the rhizotron screen with a permanent marker pen. Measurements commenced in November 2004, after a 3 month equilibration period, and continued for 1 year. Over this period, only 18 roots were observed to completely disappear from the rhizotron screens (out of a total number of 588 observed roots). To assess the reliability of disappearance observations at the rhizotron screens, two rhizotrons, installed adjacent to the study plot, were trenched to sever roots visible at the screens. Root disappearance was clearly observed subsequent to trenching (Fig. 1), which indicated that the low root disappearance rate recorded at the un-trenched rhizotrons reflected a real biological pattern, rather than a deficiency of the experimental observer and/or equipment. Given the low level of observed root disappearance, and the difficulty associated with discerning whether visible roots were dead or not, root production values calculated in this study are gross production estimates. Root tracings were scanned, and root length in each diameter category was recorded for each measurement session using image analysis software (WinRHIZO Tron, Regent Instruments, Canada). Rhizotron root length was converted to root mass per unit ground area using the following different methods:
Method 1 (Taylor et al. 1970; Itoh 1985; Tingey et al. 2000): The area of the two-dimensional plane of soil sampled by the rhizotrons is known (rhizotron width×length), but without some assumption of how far this plane extends into the bulk soil, root length observed at the rhizotron screen cannot be converted into root length per unit soil volume and ground area. However, direct measurements of ingrowth core root length per unit soil volume were made (see Supplementary measurements, above), and so the soil volume that was assumed to be ‘sampled’ by the rhizotron screen was altered until the mean value of rhizotron root length production per unit soil volume was equal to that derived from ingrowth cores. Rhizotron root length per unit soil volume/ground area was then converted to mass using mean root mass per unit length, calculated from ingrowth core sample root length and mass measurements.
Method 2 (this study): Adjacent to the study plot, 22 pairs of rhizotrons and ingrowth cores were installed in November 2004. Each pair was situated 1 m apart from each other. Measurements from the rhizotron – ingrowth core pairs over a single 3-month measurement interval were used to establish a linear relationship between rhizotron root length extension and ingrowth core root mass production per unit ground area. This linear relationship was then applied to root length data from the rhizotrons installed within the plot (which were not paired with ingrowth cores) to estimate gross root production per unit area.
Method 3 (Hendricks et al. 2006): Percentage root production rate was calculated from the rhizotrons by dividing the length of new roots which appeared each new sample interval by the length of roots visible at the rhizotron screen in the previous sample interval, and multiplying by 100. Absolute root production per unit ground area was then estimated by multiplying percentage growth values by mean plot standing crop fine root mass recorded at the initiation of rhizotron length measurement (November 2004, see Supplementary measurements, above).
Method 4 (Bernier and Robitaille 2004): The number of roots contacting the rhizotron screen each time-step, together with root diameter, was used to calculate the total cross-sectional surface area of intersecting roots (XSr, mm2), using the following equation:
Where r is root radius (mm). Roots which branched after contact with the rhizotron observation screen were not counted. Using the product of equation 1, root production (Pr, t ha−1) for each rhizotron measurement session was calculated as:
Where Dr is root tissue density (g mm−3), Fc is the soil coarse fraction, α is the angle of the rhizotron observation screen relative to the ground, γ is the ground angle relative to the horizontal, and W is the width of the rhizotron observation screen (mm). Root density was calculated by dividing ingrowth core sample root volume by mass (see Bernier et al. 2005 for further details about calculating root density). The 104 value converts mm2 ground area into one hectare, and grams into tonnes. The additional multiplication factor of 2 is used because roots can only intersect with the rhizotron screen from the front. It was assumed that if there was not an empty space behind the rhizotron screen to allow for measurement and observation then an equal amount of roots would intersect from behind as well as from the front.
Data analysis and uncertainty calculation
Spatial variation in production recorded between the nine rhizotrons was quantified as standard errors (SE). Key sources of uncertainty for all conversion methods were quantified as 95% confidence intervals (CI), and were used to calculate mean ± 95% CI annual root production estimates derived from each conversion method. Differences in mean annual gross fine root production estimates derived from the different conversion methods were assessed with a univariate analysis of variance (ANOVA), using SPSS 13.0 for Windows (SPSS Inc., Chicago, U.S.A). Comparison between methods was carried out with the Tamhane T2 test, which does not assume equal variances. Data were subjected to a natural logarithmic transformation to achieve a normal distribution.
Results
Method and uncertainty calculation
For Method 1, the assumed soil thickness sampled was selected such that mean rhizotron root length per unit soil volume matched that of the ingrowth cores (0.6 cm cm−3, ± 95% CI of 0.2 cm cm−3). Based upon this approach, observations made at the rhizotron screens were assumed to be representative of a soil thickness of 0.19 cm (± upper and lower 95% CI of 0.11 and 0.05 cm respectively, Fig. 2) adjacent to the screen. This was equivalent to a sampled soil volume of 122 cm−3 (± upper and lower 95% CI of 70 and 33 cm−3 respectively). Rhizotron root length per unit ground area was then converted to mean ± 95% CI mass (Table 2 and Fig. 6) using a linear relationship (R 2 = 0.75) and 95% CI fitted between ingrowth core root length and mass (Fig. 3).
An example of the relationship between the thickness of soil layer that is assumed to be sampled by the rhizotron screen and the subsequent root production estimate, for a fixed visible root length. Lines indicate the assumed soil thickness by different studies. Error bars around the mean assumed thickness in this study indicate variation – 95% confidence intervals – derived from methodological uncertainty
For Method 2, a linear regression (R 2 = 0.43) and 95% CI were fitted between rhizotron root length density and ingrowth core root mass produced per unit ground area over a single 3-month interval (Fig. 4). The equations describing the best-fit line and 95% CI were then applied to root length data from the rhizotrons established within the plot, to estimate mean ± 95% CI root mass production per unit area (Table 2 and Fig. 6).
The relationship between ingrowth core root mass and rhizotron screen root density, over a single 3 month period. Each circle represents data from an ingrowth core-rhizotron pair located 1 m apart. The solid and dotted lines indicate the best-fit regression line and 95% confidence intervals, respectively
For Method 3, mean annual percentage root extension across all rhizotrons was 171% (± SE of 9%). Mean standing crop fine root mass, recorded at the beginning of the rhizotron measurement campaign, was 3.3 t ha−1 (± 95% CI of 1.1 t ha−1). Thus, fine root production was estimated by multiplying annual percentage root extension per rhizotron by mean ± 95% CI plot standing crop fine root mass (Table 2 and Fig. 6).
For Method 4, the mean number of roots intersecting each rhizotron screen over the year of study was 61 (± SE of 4). The majority of these intersecting roots (93%) were below 1 mm diameter. Calculated mean root tissue density was 0.29 g cm−3 (± 95% CI of 0.04 g cm−3), based upon measured ingrowth core root mass and volume from each 3 month measurement interval (16 ingrowth cores per interval, 4 intervals, 16 × 4 = 64 samples). Using equations 1 and 2, these key data allowed calculation of mean ± 95% CI fine root production per unit area (Table 2 and Fig. 6).
Method comparison
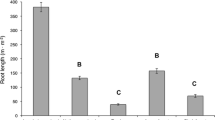
There was considerable variation in estimates of annual gross root production derived from the different conversion methods (Table 2). The estimate of annual root production provided by Method 4 was significantly lower (P < 0.004) than all other methods (Table 2 and Fig. 6). In addition, annual root production calculated by Method 1 was also significantly greater than the value estimated by Method 3 (P = 0.028). Methods 1, 2 and 3 all estimated an identical temporal pattern of root production (though the absolute magnitude differed slightly): peaking in July 2005, and declining markedly thereafter (Fig. 6). Method 4, however, estimated a different temporal pattern of root production: with no clear peak in growth towards the end of the wet season, but a possible rise in production in December 2004 (Fig. 6).
The degree of spatial variation (quantified as SE) surrounding estimates of root production was substantially higher for Method 4, compared to the other methods (Table 2 and Fig. 5). Uncertainties inherent in the conversion methodologies (quantified as 95% CI) were greatest for Methods 1 and 3 (Table 2).
Daily and monthly rainfall, and mean root length extension and intersection at the rhizotron screens, over the study period. Error bars indicate SE, n is 9. Root length extension is the raw data necessary for calculation of Methods 1, 2, and 3. Root intersection is required for calculation of Method 4
Discussion
Absolute magnitude of root production estimates
Using different rhizotron root length-mass conversion methods, on the same rhizotron data, caused changes in estimates of both the temporal pattern and overall magnitude of gross root mass production per unit ground area (Table 2 and Fig. 6). Root production estimates from Methods 1 and, to a lesser extent, 2 and 3 appear unusually high, in comparison to results from other studies in the region and an ingrowth core estimate from the study site (Table 2). In contrast, the estimate from Method 4 agrees more closely with ingrowth core data from the site, and in general with previous results from across the Amazon (Table 2). Though the other methods used to quantify root production in the region are also subject to biases and flaws (Vogt et al. 1998; Hendricks et al. 2006).
Mean root mass production for each conversion method, over the study period. Error bars indicate SE, n is 9.The temporal pattern of growth for Method 4 is not identical to root intersection rate (Fig. 5) because of the additional influence of the diameter of roots intersecting the rhizotron screen. Method 1 converted root length extension (Fig. 5) to length extension per unit ground area by making an assumption about the depth of soil “sampled” by the rhizotron screen (Fig. 2) and then converting this length to mass using the relationship in Fig. 3. Method 2 used paired measurements of ingrowth cores and rhizotrons to establish a linear relationship between root length visible at the rhizotron screen and root mass per unit ground area within the ingrowth cores (Fig. 4), which was then applied to root length extension observed at the study rhizotrons (Fig. 5). Method 3 used root length extension (Fig. 5) to calculate percentage root length increases each measurement interval, and applied them to fine root standing stock mass. Method 4 estimated the cross-sectional area of roots contacting the rhizotron screen, based upon root intersection rate (Fig. 5) and diameter of intersecting roots. From root area, root volume was calculated and converted to mass using measured root tissue density, following Eq. 2 (in the “Material and methods” section)
Methods 1 and 2 are calibrated with data from ingrowth cores. They are not, therefore, independent rhizotron estimates of root production. Instead, they rely upon the, perhaps questionable, accuracy of production estimates from ingrowth cores (Vogt et al. 1998, Steingrobe et al. 2000). For Method 1, there is substantial variation around measured mean ingrowth core root length per unit soil volume, which leads to considerable uncertainty in assumed soil thickness sampled by the rhizotron screen (Fig. 2) and consequently, estimates of root mass production (Table 2). A potential improvement to Method 1 could be to more rigorously define the depth to which the rhizotron field of view extends into the soil with direct measurements of objects placed at known distances from the observation screen. Method 2 is mainly limited by the lack of a strong correlation between root length density and root mass (R 2 = 0.43) measured in paired rhizotrons and ingrowth cores respectively, located 1 m apart (Fig. 4). If paired ingrowth cores and rhizotrons were located closer together this could improve the correlation but would increase disturbance and potentially introduce additional bias into the measurements.
Methods 3 and 4 represent significant advances upon Methods 1 and 2, because they are not inter-calibrated with any other equipment or methodology. There is, therefore, no a priori reason to expect that production estimates from Methods 3 and 4 will agree with estimates from other methods (as is the case with Methods 1 and 2). However, in this study, there is substantial variation around our estimate of mean standing crop root mass, which causes considerable uncertainty in the estimate of annual root production derived from Method 3 (Table 2). In addition, the magnitude of gross root production estimates from Method 3 depend largely upon the rather subjective decision of what portion of the standing stock root crop percentage growth rates should be applied to. Hendricks et al. (2006) applied percentage increases only to standing crop roots finer than 0.5 mm in diameter, whereas, in this study, roots finer than 2 mm diameter were assumed to grow at the rates defined by the rhizotron measurements. In both cases, it is clearly a simplification to project uniform growth rates for standing crop roots beneath a certain diameter, and zero growth of thicker roots. This method could be significantly improved by recording growth rates for separate root diameter categories (measured with the rhizotrons) and applying these rates to the corresponding diameter fractions of standing crop root mass.
A key advantage of Method 4 is that it only requires parameters (date of root appearance, and diameter) which are relatively easily observed at the rhizotron screen (Bernier and Robitaille 2004), whereas Methods 1, 2, and 3 require tracing of roots growing at the rhizotron screen which is usually extremely laborious and time consuming, and often requires expensive image analysis software. However, Method 4 makes several assumptions – that roots are orientated randomly and that the root segments are independent of each other – which are likely to be violated under natural conditions. Though, problems arising from violation of these assumptions may be minimized by orientating the observation screens at approximately 45° to the vertical (Buckland et al. 1993; Horgan et al. 1993; Bernier and Robitaille 2004). A more readily quantifiable source of methodological uncertainty in Method 4 is variation around the calculated plot mean value of root tissue density (Bernier et al. 2005). This uncertainty is relatively minor compared to, for example, uncertainty introduced by variation in standing crop root mass for Method 3, or ingrowth core root length per unit soil volume for Method 1 (Table 2). It is likely that root density changes with root diameter (Bernier et al. 2005). Thus, one potential improvement to the current methodology would be to calculate density for roots of different diameters, and then apply the root diameter-specific density value to convert the estimated volumes of corresponding root diameter classes into mass per unit area. In this study, an additional apparent disadvantage of Method 4 is the higher level of spatial variation surrounding estimates of root production, compared to Methods 1, 2 and 3 (Table 2 and Fig. 6). This may be partly an artefact of the type of rhizotron used in this study, which is likely to underestimate the total number of, particularly very fine, roots intersecting with the rhizotron screen. The low number of root intersections (Fig. 5) results in a relatively high level of variation, whereas root length extension at the rhizotron screen after 1 year is invariably a larger number (Fig. 5), with a relatively lower level of variation between rhizotrons. Further work with Method 4 is required to assess whether high spatial heterogeneity in root production estimates is a consistent generic problem associated with the method, or instead is specific to the equipment and field site in this study.
Temporal pattern of root production estimates
Root production estimated by Methods 1, 2 and 3 peaked towards the end of the wet season (March–May) and declined steadily over the course of the dry season (August–October) and the beginning of the wet season (Fig. 6). These results are corroborated by other studies of root dynamics in tropical forests (e.g.: Sanford 1989, Cavelier 1989, Sánchez-Gallén and Alvarez-Sánchez 1996, Yavitt and Wright 2001, Green et al. 2005). It is difficult to identify any clear seasonal pattern in root production estimated with Method 4 (Fig. 6), because of substantial spatial heterogeneity in the rate of root intersection with the rhizotron screens (Fig. 5). While root intersection rate increases towards the end of the wet season (Fig. 5), the average diameter of roots intersecting declines, and therefore the total estimate of production shows little consistent seasonal change (Fig. 6). Methods 1, 2 and 3 all depend upon the same raw data, root length extension at the rhizotron screen (Fig. 5), to estimate production. However, both total root length and length extension rate at the rhizotron screen are likely to be influenced by the presence of the screen (Withington et al. 2003), and therefore may not be representative of root production in the surrounding soil. In contrast, Method 4 calculates production based upon a different, but related parameter: the number of roots intersecting with the rhizotron screen at each measurement interval (Fig. 5). Any growth subsequent to intersection with the screen is potentially biased and therefore is not considered. However, there potentially remains some element of bias in the rate of root intersection with the rhizotron screen, since this is likely to be affected by the soil environment immediately adjacent to the rhizotron which may be different to the bulk soil. Clearly, longer time-series of root production to capture inter-annual variation, together with measurements of other potential environmental drivers (e.g.: soil temperature, litter fall) in different environments are required reinforce these interpretations.
Conclusions
The different methods available for converting rhizotron measurement units result in different estimates of gross root biomass production. All of the methods assessed are potentially biased and so additional measurements are proposed which could improve the accuracy of estimates. A key advantage of Methods 3 and 4, compared to methods 1 and 2, is that they do not involve inter-calibration with other data and therefore provide independent evaluation of root production. Method 3 is limited by uncertainty surrounding standing crop root mass, while Method 4 appears to have a lower degree of methodological uncertainty but makes several assumptions which may often be violated in natural environments. Therefore, researchers should carefully consider the relative merits of each conversion technique before choosing one.
References
Bernier PY, Robitaille G (2004) A plane intersect method for estimating fine root productivity of trees from minirhizotron images. Plant Soil 265:165–173
Bernier PY, Robitaille G, Rioux D (2005) Estimating the mass density of fine roots of trees for minirhizotron-based estimates of productivity. Can J For Res 35:1708–1713
Buckland ST, Campbell CD, Mackie-Dawson LA, Horgan GW, Duff EI (1993) A method for counting roots observed in minirhizotrons and theoretical conversion to root length density. Plant Soil 153:1–9
Cavelier J (1989) Root biomass, production and the effect of fertilisation in two tropical rain forests. PhD Thesis University of Cambridge, U.K
Cavelier J, Wright SJ, Santamaria J (1999) Effects of irrigation on litterfall, fine root biomass and production in a deciduous lowland forest in Panama. Plant Soil 211:207–213
Cuevas E, Medina E (1988) Nutrient dynamics within Amazonian forests II: fine root growth, nutrient availability and leaf litter decomposition. Oecologia 76:222–235
Eissenstat DM, Wells CE, Yanai RD, Whitbeck JL (2000) Building roots in a changing environment: implications for root longevity. New Phytol 147:33–42
Fisher RA, Williams M, Lola da Costa A, Malhi Y, da Costa R F, Almeida S, Meir PW (2007) The response of an eastern Amazonian rain forest to drought stress: results and modelling analyses from a through-fall exclusion experiment. Glob. Change Biol in press
Green JJ, Dawson LA, Proctor J, Duff EI, Elston DA (2005) Fine root dynamics in a tropical rainforest is influenced by rainfall. Plant Soil 276:23–32
Högberg P, Nordgren A, Buchmann N, Taylor AFS, Ekblad A, Högberg MN, Nyberg G, Ottosson-Löfvenius M, Read DJ (2001) Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature 411:789–792
Hendricks JJ, Hendrick RL, Wilson CA, Mitchell RJ, Pecot SD, Guo D (2006) Assessing the patterns and controls of fine root dynamics: an empirical test and methodological review. J Ecol 94:40–57
Horgan GW, Buckland ST, Mackie-Dawson LA (1993) Estimating three-dimensional line process densities from tube counts. Biometrics 49:899–906
Itoh S (1985) In situ measurement of rooting density by micro-rhizotron. Soil Sci Plant Nutrit 31:653–656
Jackson RB, Mooney HA, Schulze E-D (1997) A global budget for fine root biomass, surface area, and nutrient contents. Proc Nat Acad Sci USA 94:7362–7366
Jordan CF, Escalante G (1980) Root productivity in an Amazonian rain forest. Ecol 61:14–18
Metcalfe DB, Williams M, Aragão LEOC, da Costa ACL, de Almeida SS, Braga AP, Gonçalves PHL, de Athaydes Silva Junior J, Malhi Y, Meir P (2007) A method for extracting plant roots from soil which facilitates rapid sample processing without compromising measurement accuracy. New Phytol 174:697–703
Ruivo MLP, Cunha ES (2003) Mineral and organic components in archaeological black earth and yellow latosol in Caxiuanã, Amazon, Brazil. In: Tiezzi E, Brebbia CA, Uso JL (eds) Ecosystems and sustainable development. WIT, London, pp 319–329
Sánchez-Gallén I, Alvarez-Sánchez J (1996) Root productivity in a lowland tropical forest in Mexico. Vegetatio 123:109–115
Sanford RL (1989) Fine root biomass under a tropical forest light gap opening in Costa Rica. J Trop Ecol 5:251–256
Sanford RL (1990) Fine root biomass under light gap openings in an Amazon rain forest. Oecologia 83:541–545
Silver WL, Thompson AW, McGroddy ME, Varner RK, Robertson JR, Dias JD, Silva H, Crill P, Keller M (2005) Fine root dynamics and trace gas fluxes in two lowland tropical forest soils. Glob Change Biol 11:290–306
Smit A, Bengough A, Engels C, van Noordwijk M, Pellerin S, van de Geijn SC (2000) Root methods: a handbook. Springer, Berlin, Heidelberg, New York
Steingrobe B, Schmid H, Claassen N (2000) The use of the ingrowth core method for measuring root production of arable crops – influence of soil conditions inside the ingrowth core on root growth. J Plant Nutrit Soil Sci 163:617–622
Taylor HM, Huck MG, Klepper B, Lund ZF (1970) Measurement of soil-grown roots in a rhizotron. Agron J 62:807–809
Tingey DT, Phillips DL, Johnson MG (2000) Elevated CO2 and conifer roots: effects on growth, life span and turnover. New Phytol 147:87–103
Vogt KA, Vogt DJ, Bloomfield J (1998) Analysis of some direct and indirect methods of estimating root biomass and production of forests at an ecosystem level. Plant Soil 200:71–89
Withington JM, Elkin AD, Bulaj B, Olesinski J, Tracy KN, Bouma TJ, Oleksyn J, Anderson LJ, Modrzyński J, Reich PB, Eissenstat DM (2003) The impact of material used for minirhizotron tubes for root research. New Phytol 160:533–544
Yavitt JB, Wright JS (2001) Drought and irrigation effect on fine root dynamics in a tropical moist forest, Panama. Biotropica 33:421–434
Acknowledgements
This work was supported by a Natural Environment Research Council (UK) PhD studentship and a Standard Research Grant (NER/A/S/2003/1609), a Royal Society (UK) Dudley Stamp Memorial Fund award, and an Elizabeth Sinclair Fund award (School of Geosciences, University of Edinburgh, UK). The manuscript benefited from valuable comments by P. Y. Bernier. The authors would like to thank Leonardo Sá, Ima Vieira, and Samuel Almeida for their scientific support and collaboration, the Museu Paraense Emilio Goeldi for the use of its field station and laboratory facilities, and Bene and Joca for committed field work assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Philippe Hinsinger
Rights and permissions
About this article
Cite this article
Metcalfe, D.B., Meir, P. & Williams, M. A comparison of methods for converting rhizotron root length measurements into estimates of root mass production per unit ground area. Plant Soil 301, 279–288 (2007). https://doi.org/10.1007/s11104-007-9447-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-007-9447-6