Abstract
Accurate estimation of soil nitrogen (N) supply in the field is required to optimize fertilizer N management and to minimize environmental N losses in humid environments. Laboratory-based measures of N availability were evaluated as predictors of field-based indices of soil N supply within potato production systems. Pre-plant soil samples (0–15 cm) were collected from a series of forty treatments in established potato trials located in Atlantic Canada and Maine, USA. Total plant N uptake at topkill with no fertilizer N applied (PNU0N), PNU0N plus soil mineral N to 30 cm depth at harvest and relative yield were considered as field-based indices of soil N supply. The potentially mineralizable N (N0) was determined by aerobic incubation at 25°C and periodic leaching for 24 weeks. A series of laboratory-based measures of soil N supply were measured in soil samples. Pre-plant soil nitrate or total mineral N at 0–30 cm depth was the best single predictor of PNU0N (r = 0.67 and 0.71, respectively) and relative yield (r = 0.58 and 0.61). The ultraviolet absorbance of 0.01 M NaHCO3 extract at 205 nm (NaHCO3-205) was suitable as a predictor of PNU0N and relative yield in each growing season, however, the relationship between this parameter and PNU0N or relative yield varied somewhat among years. A combination of pre-plant mineral N plus N mineralized in the first 2 weeks period of incubation after re-wetting is proposed as a more robust measure of N availability compared with use of mineral N alone.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There is increasing concern over environmental N losses through nitrate leaching or nitrous oxide emissions within potato (Solanum tuberosum L.) production (Zebarth and Rosen 2007). One of the most practical approaches to reduce environmental losses and improve profitability is to tailor fertilizer N applications to meet crop fertilizer N requirements. The supply of N from the soil, which consists of N mineralized from soil organic matter and crop residues and residual mineral N from the previous growing season, is an important but variable contributor of N to crop production (Zebarth et al. 2005). In arid and sub-humid environments, residual mineral N from one season to the next represents a significant component of soil N supply. In such environments, pre-plant soil mineral N tests have been developed to estimate the magnitude of residual mineral N and thereby improve fertilizer N recommendations (Hergert 1987). These tests are successfully used in western Canada and USA to predict fertilizer N requirements for barley (Hordeum vulgare L.), wheat (Triticum aestivum L.) and corn (Zea mays L.) (Soper and Huang 1963; Dahnke and Johnson 1990). In humid environments, the loss of residual mineral N over winter results in soil N supply being dominated by in-season soil N mineralization; however, there is a lack of practical methods for routine estimation of soil N supply. Consequently, variation in soil N supply is commonly not considered in making crop fertilizer N recommendations in humid environments. Development of a reliable index of soil N supply for use in humid environments could improve fertilizer N recommendations, thereby optimizing economics of crop production and minimizing environmental N losses.
The most common approaches for estimation of soil N supply include measurement of crop N uptake in unfertilized plots (PNU0N) and accumulation of soil mineral N in vegetation-free plots (Schepers and Meisinger 1994). The former approach has the advantage of being conducted under field conditions where temperature, moisture, and aeration are representative of that experienced by the crop. It also incorporates the root interactions of the specific crop species, and is therefore expected to provide a better estimate of the crop specific soil N supply (Zebarth et al. 2005). Soil N supply also can be estimated as the sum of PNU0N plus residual soil mineral N in the root zone after crop harvest (Redman et al. 1989; Delphin 2000; Zebarth et al. 2005), hereafter referred to as PNU0N + SMNh. These approaches have been used for a variety of crop species including corn (Fox and Piekielek 1978), orchardgrass (Dactylis glomerata L.) (Bittman et al. 2004), broccoli (Brassica olearacea L. Italica) (Zebarth et al. 1995) and potato (Zebarth et al. 2005). While both PNU0N and PNU0N + SMNh provide measures of soil N supply to the crop, both parameters require information measured at crop harvest and therefore cannot be used as a predictor of soil N supply in making fertilizer N recommendations.
In some cases, increased N supply may not result in increased crop yield (Griffin and Hesterman 1991). Crop yield response to fertilizer N addition is therefore a practical alternative means of evaluating soil N supply. Some of the variation in absolute yield among sites which are not related to soil N supply can be eliminated by using relative yield i.e. yield from a zero fertilizer N rate plot divided by yield from a fully treated plot expressed as percentage (Evans 1987).
Considerable effort has been made to develop laboratory-based measures of soil N mineralization which could be used as predictors of soil N supply in humid environments. A standard laboratory-based method for measuring potentially mineralizable N was first proposed by Stanford and Smith (1972). This method requires an incubation of soil for more than 20 weeks under optimum temperature and moisture conditions to assess the potentially mineralizable N (N0) and its mineralization rate coefficient (k). However, this method is time consuming, and not practical for routine use. Consequently, recent research has focused on the development of more rapid chemical extraction methods. Among several proposed laboratory-based methods, encouraging results have been reported for hot (100°C) KCl (Gianello and Bremner 1986a; Jalil et al. 1996), phosphate–borate buffer at pH = 11.2 (Gianello and Bremner 1986b), direct-distillation with NaOH (Stanford 1978; Sharifi et al. 2007), ultraviolet absorbance of 0.01 M NaHCO3 extracts at 200, 205 and 260 nm (Fox and Piekielek 1978; Hong et al. 1990), Illinois Soil N Test for amino sugar-N (Khan et al. 2001; Williams et al. 2007), microbial biomass-C or -N (Carter and MacLeod 1987; Deng et al. 2000) and particulate organic matter-C or -N (Willson et al. 2001). The advantage of a laboratory-based approach is that an estimate of the contribution of soil N mineralization to soil N supply can be obtained. However, such laboratory tests estimate soil N mineralization potential, do not account for the effects of environmental conditions on N mineralization under field conditions, or take into account losses of soil mineral N during the growing season due to leaching and denitrification.
The development and validation of laboratory-based predictors of field soil N supply generally rely on the statistical relationship between test results and standard field-based indices of soil N supply (e.g. PNU0N or PNU0N + SMNh). Few studies have assessed the relationship between N0 (as a standard laboratory-based index) and field-based methods of estimating soil N supply. Stanford et al. (1977) found that a simple statistical model that includes both pre-plant NO3–N and N0 could be used to predict sugar beet N uptake (r = 0.80, combining 2 years field data in Idaho). Carpenter-Boggs et al. (2000) reported that corn N uptake (without N fertilizer) was strongly correlated with total available N (mineralized N during 189d incubation plus pre-plant mineral N; r = 0.89). Kuo et al. (1996) reported that N mineralization from cover crops during a 98 d aerobic incubation had no relationship with corn N uptake or yield; however, combining mineralized N and pre-plant soil mineral N increased correlation coefficients (r = 0.75 and 0.86, for N uptake and yield, respectively). Net N mineralization during a 275 day aerobic incubation and corn field N mineralization estimated by the mass balance approach were correlated (r = 0.72); however, the laboratory incubation over-predicted field N mineralization (Delphin 2000).
Other studies mostly focused on assessment of the relationship between laboratory-based N availability tests and PNU0N or PNU0N + SMNh. Fox and Piekielek (1984) found ultraviolet absorbance of a boiling 0.01 M CaCl2 extract (121°C, 16 h) was not well correlated to N uptake in corn; however, when poorly drained soils and fields planted to legumes within the preceding 2 years were omitted, the correlation coefficient was improved (r = 0.65). Hong et al. (1990) evaluated the ability of several chemical soil N availability indices to predict the N supply of soil to corn in 49 field experiments over 3 years in Pennsylvania. They found significant correlations between soil N supply (PNU0N minus 75% of starter N fertilizer applied) and both pre-plant NO3–N concentration (r = 0.75) and NaHCO3-200 (r = 0.73) for 0–20 cm soil depth. McTaggart and Smith (1993) evaluated some indices of N availability across ten soils and three growing seasons in Scotland, and found that soil mineral N at planting and hot KCl (100°C) were reasonable predictors of barley N uptake with exclusion of a few sites (r = 0.88 and 0.81, respectively).
In the present study, laboratory-based measures of soil mineral N and soil N mineralization potential were evaluated as predictors of field-based indices of soil N supply within potato production systems in eastern Canada and the north-eastern USA, a region characterized by a cool Maritime climate and humid soil moisture regimes. These laboratory-based measures were previously evaluated as predictors of soil N mineralization potential (i.e. N0) (Sharifi et al. 2007).
Materials and methods
Field experiments
Soil samples were collected from a series of forty treatments in established potato trials located in New Brunswick and Prince Edward Island, Canada and Maine, USA, between 2000 and 2005. In Maine, samples were taken from the potato year of two different rotation trials in each of 2004 and 2005. In each plot, an area was established which received no fertilizer N; the remainder of the plot received an optimal fertilizer N rate. In New Brunswick and Prince Edward Island, samples were collected during 2000–2004 from the zero N rate plots within established N fertility experiments. All trials had at least four replicates. Soils in this region were mostly developed on medium-textured glacial till deposits, and were classified as Spodosols (Typic Haplorthods and Typic Haplohumods). Selected sites represented a range of soil characteristics, rotations and management history and practices of potato production in the region.
Trials were planted to Russet Burbank, Shepody or Atlantic, three primary processing potato cultivars in the region, in early to mid-May in rows 91 cm apart. The P and K fertilizers were applied at recommended rates. Standard commercial practices were used for tillage and weed, insect and disease control. No irrigation was applied.
Soil samples were taken from 0 to 7.5 cm depth in 2000 to 2003 and from 0 to 15 cm depth in 2004 and 2005. Samples were taken before planting and any fertilizer application. Soils were air dried and sieved (<2 mm) before analysis. Soil pH was determined in a 1:1 soil:water suspension. Particle-size distribution was determined by the pipette method after organic matter removal by hydrogen peroxide (Gee and Bauder 1986). Soil total organic-C and -N were measured by dry combustion method using a LECO CNS-1000 analyzer (LECO Corporation, St. Joseph, MI, USA.) after carbonate removal. The physicochemical properties of the tested soils are summarized in Table 1.
Separate soil samples were collected from 0 to 30 cm depth in each plot just prior to planting and after tuber harvest for determination of soil mineral N concentration. Samples were frozen until analysis. These soils were extracted with 1.7 M KCl (20 g field moist soil/ 100 ml extractent; 30 min shaking time) and concentrations of NO3–N and NH4–N (KCl–NO3 and KCl–NH4, respectively) in the extract determined spectrophotometrically using a Technicon TRAACS 800 autoanalyzer (Zebarth and Milburn 2003). Soil KCl–NO3 and KCl–NH4 concentrations were converted to units of kg N ha−1 using soil bulk density measured by the soil core method at each site (Zebarth and Milburn 2003). The sum of pre-plant KCl–NO3 and KCl–NH4 contents at 0–30 cm is hereafter referred to as soil mineral N.
Field-based indices of soil N supply
Four adjacent whole potato plants in one row were harvested from each plot just prior to topkill, and partitioned into tubers, vines and readily recoverable roots, and dry matter and N accumulation of each plant component was determined as in Zebarth and Milburn (2003). In addition, tuber fresh weight yield was determined in the zero fertilizer N rate plot, and in either an adjacent area of the same plot or in another N rate treatment in the same trial, which received the recommended fertilizer N rate.
Total plant N uptake in zero fertilizer N rate plots including vines, tubers and readily recoverable roots measured at topkill was considered as PNU0N (Meisinger 1984; Zebarth et al. 2005). The PNU0N + SMNh was estimated by adding 0–30 cm depth mineral N content measured at tuber harvest to PNU0N (Meisinger 1984; Zebarth et al. 2005). Relative yield was calculated as the total tuber yield for the zero N rate plots divided by the yield for the recommended fertilizer N rate and expressed as a percentage.
Laboratory-based measures of potentially mineralizable N or N availability
The long term aerobic incubation procedure described by Campbell et al. (1993), a modification of the method of Stanford and Smith (1972), was used to measure potentially mineralizable N. Soil samples were rewetted to field capacity during the first leaching and incubated at 25°C for 24 weeks as described by Sharifi et al. (2007). The soils were leached periodically (every 2 weeks for the first 12 weeks and every 4 weeks thereafter) with 0.01 M CaCl2 followed by a zero-N nutrient solution (Campbell et al. 1993). Leachates were analyzed for NH4–N and NO3–N using a Technicon TRAACS 800 auto-analyzer (Zebarth and Milburn 2003). The N mineralized in the first 2 weeks period was not used for the curve fitting procedure because this represents the initial flush of mineralization upon rewetting. The following first-order kinetic model was fitted to data using the Marquardt iteration method:
where Nmin is the cumulative amount of N mineralized at time, t, N0 is potentially mineralizable N, and k is the mineralization rate coefficient (Campbell et al. 1993). Three different pools of mineralizable N based on long term aerobic incubation were calculated (Sharifi et al. 2007): [Pool-I]. The flush in soil mineral N which occurs in the first 2 weeks period following rewetting. This pool represents the mineralization of a labile organic N pool. [Pool-II] The cumulative amount of N which mineralized between 2 and 24 weeks and is representative of the release of an intermediate pool of organic N [Pool-III]. The amount of N which was predicted to be potentially mineralizable based on curve fitting but did not mineralize during the incubation period.
Hot KCl-extractable NH4–N (HKCl–NH4) was determined by heating 9.0 g soil with 60 ml of 2 M KCl on a digestion block set at 100°C for 4 h (Gianello and Bremner 1986a) followed by filtration and analysis for NH4–N as described above. The ultraviolet absorbance of the NaHCO3 0.01 M extract at 205 and 260 nm (NaHCO3-205 and NaHCO3-260), Illinois Soil N Test (ISNT), phosphate–borate buffer extractable N (PBN), and NaOH direct-distillation -NH4 (NaOH-DD) were determined in soil samples as described by Sharifi et al. (2007). The chloroform fumigation extractable C was determined as an index of soil microbial biomass C (MBC) (Voroney et al. 1993). Particulate organic matter C and N (POM-C and POM-N) were determined by passing a 25-g dispersed air-dried soil sample through a 53-μm sieve (Gregorich and Ellert 1993). Retained sand and macroorganic matter were dried and weighed. Carbon and N concentrations were then determined by dry combustion using a LECO CNS-1000 and the masses of C and N per gram of air-dry soil were calculated as POM-C and POM-N, respectively. The NaOH-DD, POM-C and POM-N were only measured for the 2004–2005 samples. Measurement units were converted to units of kg N ha−1 using soil bulk density measured by the soil core method at each site in each sampling year (Blake and Hartge 1986).
Statistical analyses
Correlation analyses and regression analyses using linear and segmented models were used to assess the relationships between the laboratory-based measures of N availability and field-based indices of soil N supply in each group of soils and overall (SAS Institute Inc., Cary, NC, Version 8.2).
Results and discussion
Field-based indices of soil N supply
The PNU0N ranged from 39 to 156 kg N ha−1 with an average of 79 kg N ha−1 (Table 2). Likewise, PNU0N + SMNh ranged from 47 to 185 kg N ha−1 with an average of 106 kg N ha−1. Soil mineral N prior to planting was the equivalent of 25–96% (average of 48%) of PNU0N and 14–107% (average of 36%) of PNU0N + SMNh within individual treatments. The combination of pre-plant soil mineral N plus pool-I was equivalent to 48–158% (average of 95%) of PNU0N and 36–125% (average of 71%) of PNU0N + SMNh within individual sites. Relative yield ranged from 39 to 100% with an average of 72%.
Laboratory-based measures of soil N supply
The parameter N0, estimated from the periodically-leached incubation, was considered the standard measure of soil N mineralization potential, representing pools II and III of mineralizable N. The N0 ranged from 64 to 187 kg N ha−l (Table 2) and represented an average of 6% of total organic-N. The measured values for N0 were at the low end range of N0 values reported by Carter and MacLeod (1987; 79–445 kg N ha−1 if a soil bulk density of 1.2 Mg m−3 is assumed) for Prince Edward Island, Canada and by Milburn et al. (1990; 177–281 kg N ha−1 if a soil bulk density of 1.1 Mg m−3 is assumed) for five potato trials in New Brunswick, Canada. The slightly higher N0 values in these reports compared with the values in our study can be attributed to the higher incubation temperatures, longer periods of incubation and use of sites with a history of manure application. Pool-I values ranged from 17 to 58 kg N ha−1. Pool-II and -III values ranged from 49 to 140 and 2 to 74 kg N ha−1, respectively, and represented an average of 80 and 20% of N0, respectively. Pool-III had the highest variability among potentially mineralizable N components (CV = 92% compared with CVs of 24, 27, and 18% for N0, pool-I and pool-II, respectively).
The calculated values of k ranged from 0.037 to 0.124 week−1 (average of 0.080 week−1) (Table 2). This range is similar to the range of values reported by Jalil et al. (1996; 0.031–0.138 week−1) and Curtin and Wen (1999; 0.025–0.178 week−1) but slightly higher than the values reported by Stanford and Smith (1972) for 39 soils collected throughout the USA (average of 0.054 week−1) and by Milburn et al. (1990) for 5 potato trials in New Brunswick, Canada (average of 0.051 week−1).
The KCl–NO3 ranged from 7 to 113 kg N ha−l (average of 26 kg N ha−l) (Table 2). The KCl–NH4 ranged from 4 to 30 kg N ha−l (average of 12 kg N ha−l). The soil mineral N ranged from 17 to 131 kg N ha−l (average 39 kg N ha−l), which on average consisted of 68% NO3–N and 32% NH4–N. The amount of NH4–N extracted by HKCl–NH4 ranged from 16 to 60 kg N ha−l, representing 0.7–2.5% of total organic-N. The ultraviolet absorbance of 0.01 M NaHCO3 extracts ranged from 0.50 to 2.67 at 205 nm and from 0.24 to 0.50 at 260 nm. The ISNT ranged from 91 to 413 kg N ha−l, representing 5–17% of total organic-N. The NaOH-DD extractable N ranged from 212 to 478 kg N ha−l for 2004 and 2005 samples, which represented 16–26% of total organic-N. The MBC ranged from 39 to 509 kg C ha−l which accounted for 0.2–17% (average 6%) of soil total organic-C. The POM-N ranged from 86 to 1118 kg N ha−l for 2004 and 2005 samples, and was equivalent to 4–61% (average 33%) of total organic-N. The POM-N was the largest measured organic-N fraction among tested methods. The POM-C ranged from 5.4 to 10.5 Mg C ha−1 and was equivalent to 21 to 52% (average 20%) of soil total organic-C. The KCl–NO3, POM-N, KCl–NH4 and MBC had the highest CVs among the tested laboratory-based measures of soil N supply (CV = 86, 62, 61, and 55%, respectively).
Relationships between laboratory-based measures and field-based indices of soil N supply
Linear correlation coefficients were calculated between lab-based measures of soil N supply and field-based indices of soil N supply for 2000–2003, 2004–2005 and overall data (Table 3). All potentially mineralizable N parameters in the 2004–2005 data, except N0, had a significant (P < 0.05) correlation with at least one of the field-based indices of soil N supply (Table 3). The k and N0 k were significantly correlated with PNU0N and PNU0N + SMNh (r = 0.54–0.67). Pool-I was significantly correlated with PNU0N and relative yield (r = 0.47 and 0.69, respectively) and pool-II had a significant positive correlation with relative yield (r = 0.54). In contrast, pool-III had a significant negative correlation with PNU0N (r = −0.50). There were no significant correlations between mineralizable N parameters and field-based indices of soil N supply in the 2000–2003 data alone. In many cases significant correlations present in the 2004–2005 data were also reflected in the overall data.
Organic-C, organic-N and clay were poorly correlated with the different measures of soil N supply in this study. This reflects the narrow range of values resulting from the generally similar soil types from which the soil samples were collected. Consequently, the differences in mineralizable N among sites likely reflect variation in recent management practices and/or variation in environmental conditions.
Among lab-based measures of soil N supply in the 2004–2005 data, KCl–NO3 and soil mineral N at 0–30 cm depth were significantly correlated with all three field-based indices of soil N supply (Table 3; r = 0.51 to 0.68). The NaHCO3-205 was significantly correlated with PNU0N and PNU0N + SMNh (r = 0.73 and 0.61, respectively). There was also a significant correlation between POM-N and relative yield (r = 0.55).
The pattern of correlation coefficients between lab-based measures of soil N supply and field-based indices of soil N supply in the 2000–2003 data was similar to that for the 2004–2005 data. The KCl–NO3, soil mineral N at 0–30 cm depth and NaHCO3-205 were the only lab-based measures of soil N supply which were significantly correlated with at least two of the field-based indices of soil N supply in the 2000–2003 data (Table 3). The correlation coefficients between these measures and PNU0N + SMNh were decreased in the 2000–2003 data compared with the 2004–2005 data whereas the correlations between these measures and relative yield increased in the 2000–2003 data compared with the 2004–2005 data. This may reflect greater variation in the 2000–2003 data, which consisted of individual sites, compared with the 2004–2005 data, where 14 of 19 treatment means were from two rotation experiments in Maine.
The PNU0N was highly correlated with PNU0N + SMNh and relative yield in both the 2000–2003 and the 2004–2005 data (Table 3; overall r = 0.90 and 0.79, respectively). The correlation coefficients between lab-based measures of soil N supply and PNU0N + SMNh were generally lower compared with correlation coefficients between these measures and PNU0N or relative yield. This may reflect additional variation in PNU0N + SMNh which is the sum of parameters, i.e. soil mineral N in 0–30 cm depth at harvest plus PNU0N, or may reflect additional variation in availability in soil N late in the growing season which may not be reflected in plant uptake or yield (Griffin and Hesterman 1991).
Soils which had a preceding legume crop commonly had higher average values of PNU0N, PNU0N + SMNh and relative yield (104 kg N ha−1, 134 kg N ha−1 and 85%, respectively) compared with other soils (71 kg N ha−1, 91 kg N ha−1 and 67%, respectively). However, categorizing soils by preceding legume or non-legume crop did not increase the correlation coefficients between lab-based measures of soil N supply and field-based indices of soil N supply.
Prediction of soil N supply
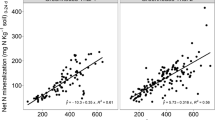
The plot of relative yield against the most successful lab-based measures of soil N supply were used to identify soils for which their PNU0N appeared to be limited by factors (soil or environmental) other than soil N supply. Only one site under potato-pea (Pisum sativum L.) – white clover (Trifolium repens L.) rotation in Maine in 2004 was eliminated from subsequent analyses using this approach (Fig. 1d).
The pool-I and N0 k had the strongest correlations with PNU0N (Table 3). However, plots of these parameters against PNU0N indicate that these parameters used alone would not be suitable for use as a predictor of soil N supply, particularly when the 2000–2003 data are considered (Figs. 2a,b). Similarly, these two parameters used alone did not appear to be suitable predictors of relative yield (Fig. 1a,b). The NaHCO3-205 appeared to be more suitable as a predictor of PNU0N and relative yield, however, the relationship between this parameter and PNU0N or relative yield varied somewhat among years (Figs. 1c, 2c). The pre-plant KCl–NO3 or soil mineral N at 0–30 cm depth, was the best single predictor of PNU0N (r = 0.65 and 0.68, respectively) (Fig. 2d) and also of relative yield (Fig. 1d).
A stepwise regression was performed using each of the field-based indices of soil N supply as the dependent variable and the lab-based measures of soil N supply as independent variables. The relationships between PNU0N or relative yield and soil mineral N were not significantly improved by entering other lab-based measures of soil N supply to the model (results not presented). The relationship between PNU0N + SMNh and soil mineral N improved by entering MBC and pool-I to the model (partial r = 0.48, 0.35 and 0.22 for soil mineral N, MBC and pool-I, respectively).
A series of linear regression models were fit to determine the potential to predict PNU0N and relative yield using sequential addition of terms representing N pools of decreasing N availability (Table 4). For both PNU0N and relative yield, adding additional terms for pool-I, -II, and -III resulted in only minor increases in the proportion of variability over use of soil mineral N only. This suggests that in the absence of additional information on environmental conditions, consideration of N pools other than soil mineral N provided little additional predictive power.
While pre-plant soil mineral N is frequently used as a measure of soil N supply in arid and sub-humid environments (Hergert 1987), it may be less reliable in humid environments where soil N supply is dominated by soil N mineralization (Zebarth and Rosen 2007). Bélanger et al. (2001) found that pre-plant soil NO3–N concentration was positively correlated with marketable tuber relative yield (r = 0.50–0.55), and negatively correlated with optimal fertilizer N rate (r = −0.51 to −0.72) in Atlantic Canada, however they did not recommend it as a sole predictor of the optimal fertilizer N rate for potatoes. McTaggart and Smith (1993) reported a strong positive correlation between soil N supply and pre-plant soil mineral N content (r = 0.85), with some sites eliminated, which was attributed to the contribution of early N mineralization to soil mineral N prior to sampling. However, for the small number of fields in which significant carry-over of soil nitrate from the previous growing season occurs (Zebarth et al. 2003), such a relationship may not be applicable. Practical use of pre-plant soil mineral N concentration as a predictive test may be somewhat problematic because soil nitrate-N concentrations can change rapidly over time in spring, making the values sensitive to sampling date (McTaggart and Smith 1993; Zebarth and Paul 1997).
Two additional regression models were fit which represent the best practical options for representing the various pools of N (Table 4). This model considers the sum of pre-plant soil mineral N plus pool-I as a single term. Using the sum of these two parameters reduces variability (CV of 46% for soil mineral N vs 31% for soil mineral N plus pool-I) and makes the relationship less susceptible to errors associated with residual N from the previous growing season. Consequently, while this model does not improve predictive capacity, it should result in a more robust measure of N availability compared with use of soil mineral N alone (Fig. 3).
In the present study, pre-plant soil mineral N in the root zone (0–30 cm) alone or used in combination with a pool of readily mineralizable N (i.e. pool-I as a potentially mineralizable N component) represents the best predictor of soil N supply. The potentially mineralizable N components and lab-based measures of soil N supply, used alone and in the absence of additional environmental information, have limited potential as predictors of field-based indices of soil N supply. Previous studies have identified ultraviolet absorbance of boiling 0.01 M CaCl2 extract at 260 nm (Fox and Piekielek 1984), ultraviolet absorbance of 0.01 M NaHCO3 extract at 200 nm (Hong et al. 1990), HKCl–NH4 (McTaggart and Smith 1993) and ISNT (Williams et al. 2007) as suitable predictors of soil N supply. The limited variation in soil texture in comparison to previous studies, and use of non-manured sites, may have contributed to the limited predictive potential of these measures. We hypothesize that further improvements in predictive potential will require consideration of environmental conditions. While pools of soil mineral N and of readily mineralizable N (i.e. pool-I) can be quantified, a large proportion of soil N supply is likely contributed by the larger and less readily mineralizable pool of N (i.e. pool-II). The proportion of pool-II which mineralizes in any given growing season is likely sensitive to the environmental (e.g. soil moisture and temperature) conditions present.
Abbreviations
- HKCl–NH4 :
-
Extractable NH4–N with 2 M KCl at 100°C
- ISNT:
-
Illinois Soil N Test for amino sugar-N
- k :
-
Mineralization rate coefficient
- KCl–NO3 :
-
Extractable NO3–N with 1.7 M KCl at 0–30 cm soil depth prior to planting
- KCl–NH4 :
-
Extractable NH4–N with 1.7 M KCl at 0–30 cm soil depth prior to planting
- MBC:
-
Microbial biomass C by chloroform fumigation extraction method
- NaHCO3–200:
-
Ultraviolet absorbance of 0.01 M NaHCO3 extract at 200 nm
- NaHCO3-205:
-
Ultraviolet absorbance of 0.01 M NaHCO3 extract at 205 nm
- NaHCO3-260:
-
Ultraviolet absorbance of 0.01 M NaHCO3 extract at 260 nm
- NaOH-DD:
-
Direct-distillation with NaOH (12.5 M)
- N0 :
-
Potentially mineralizable N
- PNU0N :
-
Total plant N uptake measured at topkill with no fertilizer N application as a field-based index of soil N supply
- PNU0N+SMNh :
-
Total plant N uptake measured at topkill with no fertilizer N application plus soil mineral N at 0–30 cm soil depth measured at harvest as a field-based index of soil N supply
- POM-C:
-
Particulate organic matter C
- POM-N:
-
Particulate organic matter N
- Pool-I:
-
The flush in mineral N which occurs in the first 2 week period of incubation at 25°C following rewetting
- Pool-II:
-
Cumulative amount of N mineralized between 2 and 24 weeks of incubation at 25°C
- Pool-III:
-
N0 minus pool-II
References
Bélanger G, Walsh JR, Richards JE, Milburn PH, Ziadi N (2001) Predicting nitrogen fertilizer requirements of potatoes in Atlantic Canada with soil nitrate determinations. Can J Soil Sci 81:535–544
Bittman S, Zebarth BJ, Kowalenko CG, Hunt DE (2004) Season of year effect on response of orchardgrass to N fertilizer in a maritime climate. Can J Plant Sci 84:129–142
Blake GR, Hartge KH (1986) Bulk density. In: Klute A (ed) Methods of soil analysis. Part 1. 2nd edn. Agron. Monogr. 9. ASA and SSSA, Madison, WI, pp 363–375
Campbell CA, Ellert BH, Jame YW (1993) Nitrogen mineralization potential in soils. In: Carter MR (ed) Soil sampling and methods of analysis. Lewis Publishers, Boca Raton, FL, pp 341–349
Carpenter-Boggs L, Pikul JL Jr, Vigil MF, Riedell WE (2000) Soil nitrogen mineralization influenced by crop rotation and nitrogen fertilization. Soil Sci Soc Am J 64:2038–2045
Carter MR, MacLeod JA (1987) Biological properties of some Prince Edward Island soils: relationship between microbial biomass nitrogen and mineralizable nitrogen. Can J Soil Sci 67:333–340
Curtin D, Wen G (1999) Organic matter fractions contributing to soil nitrogen mineralization potential. Soil Sci Soc Am J 63:410–415
Dahnke WC, Johnson GV (1990) Testing soil for available nitrogen. In: Westerman RL (ed) Soil testing and plant analysis. SSSA Inc, Madison, WI, pp 127–139
Delphin JE (2000) Estimation of nitrogen mineralization in the field from an incubation test and from soil analysis. Agronomie 20:349–361
Deng SP, Moore JM, Tabatabai MA (2000) Characterization of active nitrogen pools in soils under different cropping systems. Biol Fertil Soils 32:302–309
Evans CE (1987) Soil test calibration. In: Brown JR (ed) Soil testing: sampling, correlation, calibration, and interpretation. SSSA Inc., Madison, WI, pp 23–29
Fox RH, Piekielek WP (1978) Field testing of several nitrogen availability indexes. Soil Sci Soc Am J 42:747–750
Fox RH, Piekielek WP (1984) Relationships among anaerobically mineralized nitrogen, chemical indexes, and nitrogen availability to corn. Soil Sci Soc Am J 48:1087–1090
Gee GW, Bauder JW (1986) Particle-size analysis. In: Klute A (ed) Methods of soil analysis. Part 1. 2nd edn. Agron. Monogr. 9. ASA and SSSA, Madison, WI, pp 383–411
Gianello C, Bremner JM (1986a) A simple chemical method of assessing potentially available organic nitrogen in soil. Commun Soil Sci Plant Anal 17:195–214
Gianello C, Bremner JM (1986b) Comparison of chemical methods of assessing potentially mineralizable organic nitrogen in soil. Commun Soil Sci Plant Anal 17:215–236
Gregorich EG, Ellert BH (1993) Light fraction and macroorganic matter in mineral soils. In: Carter MR (ed) Soil sampling and methods of analysis. Lewis Publication, Boca Raton, FL, pp 397–407
Griffin TS, Hesterman OB (1991) Potato response to legume and fertilizer nitrogen sources. Agron J 83:1004–1012
Hergert GW (1987) Status of residual nitrate–nitrogen soil tests in the United States of America. In: Brown JR (ed) Soil testing: sampling, correlation, calibration, and interpretation. SSSA Inc, Madison, WI, pp 73–88
Hong SD, Fox RH, Piekielek WP (1990) Field evaluation of several chemical indexes of soil nitrogen availability. Plant Soil 123:83–88
Jalil A, Campbell CA, Schoenau J, Henry IL, Jame YW, Lafond GP (1996) Assessment of two chemical extraction methods as indices of available nitrogen. Soil Sci Soc Am J 60:1954–1960
Khan SA, Mulvaney RL, Hoeft RG (2001) A simple soil test for detecting sites that are nonresponsive to nitrogen fertilization. Soil Sci Soc Am J 65:1751–1760
Kuo S, Sainju UM, Jellum E (1996) Winter cover cropping influence on nitrogen mineralization, presidedress soil nitrate test, and corn yields. Biol Fertil Soils 22:310–317
McTaggart IP, Smith KA (1993) Estimation of potentially mineralisable nitrogen in soil by KCl extraction. Plant Soil 157:175–184
Meisinger JJ (1984) Evaluating plant-available nitrogen in soil-crop systems. In: Hauck RD (ed) Nitrogen in crop production. ASA, Madison, WI, pp 391–416
Milburn P, Richards JE, Gartley C, Pollock T, O’Neill H, Bailey H (1990) Nitrate leaching from systematically tiled potato fields in New Brunswick, Canada. J Environ Qual 19:448–454
Redman MH, Wigglesworth SA, Vinten AJA (1989) Nitrogen dynamics of a leguminous green manure. In: Hansen JA, Henriksen K (eds) Nitrogen in organic wastes applied to soils. Academic, London, pp 11–24
Schepers JS, Meisinger JJ (1994) Field indicators of nitrogen mineralization. In: Havlin JL, Jacobsen JS (eds) Soil testing: prospects for improving nutrient recommendations. Vol. Special Publ. 40. SSSA Inc, Madison, WI, pp 31–47
Sharifi M, Zebarth BJ, Burton DL, Grant CA, Cooper JM (2007) Evaluation of some indices of potentially mineralizable nitrogen in soil. Soil Sci Soc Am J 71:1233–1239
Soper RJ, Huang PM (1963) The effect of nitrate nitrogen in the soil profile on the response of barley to fertilizer nitrogen. Can J Soil Sci 43:350–358
Stanford G (1978) Evaluation of ammonium release by alkaline permanganate extraction as an index of soil nitrogen availability. Soil Sci 126:244–253
Stanford G, Smith SJ (1972) Nitrogen mineralization potentials of soils. Soil Sci Soc Am J 36:465–472
Stanford G, Carter JN, Westermann DT, Meisinger JJ (1977) Residual nitrate and mineralizable soil nitrogen in relation to nitrogen uptake by irrigated sugar beets. Agron J 69:303–308
Voroney RP, Winter JP, Beyaert RP (1993) Soil microbial biomass C and N. In: Carter MR (ed) Soil sampling and methods of analysis. Lewis Publication, Boca Raton, FL, pp 277–287
Williams JD, Crozier CR, White JG, Heiniger RW, Sripada RP, Crouse DA (2007) Illinois soil nitrogen test predicts southern U.S. corn economic optimum nitrogen rate. Soil Sci Soc Am J 71:735–744
Willson TC, Paul EA, Harwood RR (2001) Biologically active soil organic matter fractions in sustainable cropping systems. Appl Soil Ecol 16:63–76
Zebarth BJ, Paul JW (1997) Growing season nitrogen dynamics in manured soils in south coastal British Columbia: implications for a soil nitrate test for silage corn. Can J Soil Sci 77:67–76
Zebarth BJ, Milburn PH (2003) Spatial and temporal distribution of soil inorganic nitrogen concentration in potato hills. Can J Soil Sci 83:183–195
Zebarth BJ, Rosen CJ (2007) Research perspective on nitrogen BMP development for potato. Am J Potato Res 84:3–18
Zebarth BJ, Bowen PA, Toivonen PMA (1995) Influence of nitrogen fertilization on broccoli yield, nitrogen accumulation and apparent fertilizer-nitrogen recovery. Can J Plant Sci 75:717–725
Zebarth BJ, Leclerc Y, Moreau G, Gareau R, Milburn PH (2003) Soil inorganic nitrogen content in commercial potato fields in New Brunswick. Can J Soil Sci 83:425–429
Zebarth BJ, Leclerc Y, Moreau G, Sanderson JB, Arsenault WJ, Botha E, Wang-Pruski G (2005) Estimation of soil nitrogen supply in potato fields using a plant bioassay approach. Can J Soil Sci 85:377–386
Acknowledgments
Funding was provided by the GAPS program of Agriculture and Agri-Food Canada. Technical assistance was provided by H. Blizzard, G. Decker, D. Janes, M. Levesque, K. Pickering, and K. Terry.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Elizabeth (Liz) A. Stockdale.
Rights and permissions
About this article
Cite this article
Sharifi, M., Zebarth, B.J., Burton, D.L. et al. Evaluation of laboratory-based measures of soil mineral nitrogen and potentially mineralizable nitrogen as predictors of field-based indices of soil nitrogen supply in potato production. Plant Soil 301, 203–214 (2007). https://doi.org/10.1007/s11104-007-9438-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-007-9438-7