Abstract
Rice seedlings were grown in hydroponic culture to determine the effects of external Zn and P supply on plant uptake of Cd in the presence or absence of iron plaque on the root surfaces. Iron plaque was induced by supplying 50 mg l−1 Fe2+ in the nutrient solution for 2 day. Then 43-day-old seedlings were exposed to 10 μmol l−1 Cd together with 10 μmol l−1 Zn or without Zn (Zn–Cd experiment), or to 10 μmol l−1 Cd with 1.0 mmol l−1 P or without P (P–Cd experiment) for another 2 day. The seedlings were then harvested and the concentrations of Fe, Zn, P and Cd in dithionite–citrate–bicarbonate (DCB) extracts and in roots and shoots were determined. The dry weights of roots and shoots of seedlings treated with 50 mg l−1 Fe were significantly lower than when no Fe was supplied. Adsorption of Cd, Zn and P on the iron plaque increased when Fe was supplied but Cd concentrations in DCB extracts were unaffected by external Zn or P supply levels. Cd concentrations in shoots and roots were lower when Fe was supplied. Zn additions decreased Cd concentrations in roots but increased Cd concentrations in shoots, whereas P additions significantly increased shoot and root Cd concentrations and this effect diminished when Fe was supplied. The percentage of Cd in DCB extracts was significantly lower than in roots or shoots, accounting for up to 1.8–3.8% of the plant total Cd, while root and shoot Cd were within the ranges 57–76% and 21–40% respectively in the two experiments. Thus, the main barrier to Cd uptake seemed to be the root tissue and the contribution of iron plaque on root surfaces to plant Cd uptake was minor. The changes in plant Cd uptake were not due to Zn or P additions altering Cd adsorption on iron plaque, but more likely because Zn or P interfered with Cd uptake by the roots and translocation to the shoots.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium is of great concern due to its threat to the food chain and human health. General interest in Cd in rice arose with the occurrence of Itai–Itai disease in Japan caused by Cd intake from rice (Obata and Umebayashi 1997). Tsukahara et al. (2003) showed that rice was the most important source of Cd in the Japanese diet, accounting for 30–40% of total Cd intake (Watanabe et al. 2000). Cd pollution of paddy soils originates mainly from industrial processes and phosphatic fertilizers or manures, together with irrigation with contaminated wastewater (Junta et al. 1998). Once discharged, Cd is highly persistent in the environment. It is readily absorbed by and translocated within rice plants (Shah et al. 2001), and Cd accumulation in rice grain can pose a high potential risk to human health despite the occurrence of lower Cd concentrations in polished than in unpolished rice grain (Kim et al. 1980). A recent literature survey (J.L. Zhang, unpublished) has indicated that the transfer coefficient of Cd between rice shoots and grain ranges from about 11 to 40% and depends on the soil type, plant genotype and environmental conditions. Lowering shoot Cd uptake is therefore of prime importance for reducing Cd concentrations in rice grain.
There are many factors affecting Cd uptake by rice (Chaney and Hornick 1978). The ubiquity of iron plaque observed on rice root surfaces has stimulated investigations on the effects of iron plaque on plant elemental uptake. The formation of iron plaque on the root surfaces of rice and other wetland plants is due to the release of oxygen and oxidants in the rhizosphere and the subsequent oxidation of ferrous to ferric iron and the precipitation of iron oxide or hydroxide on the root surface (Armstrong 1967; Chen et al. 1980b; Taylor et al. 1984). Mineralogical investigations in wetland fields have shown that the composition of iron plaque in Phalaris arundinacea is mainly ferrihydrite (∼63%) with smaller amounts of goethite (32%) and minor amounts of siderite (5%; Hansel et al. 2001), or predominately goethite in Juncus bulbosus (Chabbi 1999) and rice (Chen et al. 1980a). Iron plaque therefore has chemical properties similar to those of iron oxides in the soil and can thus sequester both cations and anions and alter the uptake and accumulation of elements by plants. Iron plaque on rice root surfaces has been shown to sequester As (Liu et al. 2004a, b and Liu et al. 2005), Al (Chen et al. 2006) and Se (Zhou et al. 2007), alleviate toxicities of Cu, Ni and Zn to plants (Greipsson 1994, 1995; Greipsson and Crowder 1992) and enhance Zn uptake when the amount of iron plaque was up to 12.1 g kg−1 root dry weight (Zhang et al. 1998) and P uptake up to 24.5 g kg−1 root dry weight when Fe(OH)3 was supplied to induce the formation of iron plaque (Zhang et al. 1999). However, studies have also suggested that iron plaque is not the main barrier to Cd uptake by rice (Liu et al. 2007).
Zn and P are commonly associated with Cd in natural or anthropogenic sources. The chemical similarity of Cd and Zn leads to the co-existence of the two elements, and both antagonistic and synergistic interactions in plant uptake have been reported in hydroponic and field experiments (Hart et al. 2005; Kukier and Chaney 2002). P fertilizers are regarded as an important source of Cd pollution in cultivated soils, and Cd and P may have synergistic interactions (Lagriffoul et al. 1998; Moral et al. 1994).
Most published studies on rice have focused on the role of iron plaque in the uptake of one single element. However, a study by Geng et al. (2005) showed that As uptake by rice may involve a three-way interaction among P supply, the amount of iron plaque and uptake of As but the underlying mechanisms remain unclear. Taking together the significant role of Zn and P supply in rice Cd uptake and the contribution of iron plaque to elemental uptake by plants, the present study was conducted to investigate the effects of external Zn (Zn–Cd experiment) or P supply levels (P–Cd experiment) on Cd uptake by rice seedlings with root surface iron plaque. Our hypothesis was that the presence of Zn or P may lower the adsorption of Cd on root surface iron plaque and thus depress the uptake and accumulation of Cd by rice seedlings.
Materials and methods
Pre-culture of rice seedlings
Seeds of rice (Oryza stativa L. cv. II You 718) were surface sterilized in 30% v/v H2O2 for 30 min and then washed thoroughly with deionized water. Afterwards the seeds were dipped in saturated CaSO4 solution for 6 h to promote germination and then germinated in acid-washed quartz sand for 10 day. Each seedling was transferred to a 1.2-l ceramic container and grown in 1/2-strength nutrient solution for 3 day and then for a further 26 days in full strength solution with the following nutrient composition (μmol l−1): NH4NO3 500, NaH2PO4 · 2H2O 60, K2SO4 230, CaCl2 210, MgSO4 · 7H2O 160, Fe–EDTA 10, ZnSO4 · 7H2O 0.5, MnCl2 · 4H2O 0.5, (NH4)6Mo7O24 · 4H2O 0.05, H3BO3 0.2, CuSO4 · 5H2O 0.01. The nutrient solution was adjusted to pH 5.0 with NaOH or HCl and changed every 3 d.
Experimental treatments
Prior to plaque deposition on the root surfaces, seedlings were firstly incubated in deionized water (pH 5.5) for 12 h to minimize interference of other elements (especially phosphorus) with iron. Seedlings were then transplanted into nutrition solution containing 50 mg l−1 ferrous ion (Fe2+ as FeSO4 · 7H2O instead of the Fe–EDTA used in the pre-cultivation stage of the seedlings) but P-free nutrient solution (Fe50) for 2 days to induce the formation of iron plaque. Control seedlings were supplied with Fe–EDTA but P-free full nutrient solution to avoid Fe deficiency (Fe0). Afterwards all the seedlings were grown in normal nutrient solution for 1 d and then the Fe pre-treated seedlings (Fe50 and Fe0) were further split into two groups so that half were used for the Zn–Cd study (Zn–Cd experiment) and the other half for the P–Cd study (P–Cd experiment). In the Zn–Cd experiment, Fe50 and Fe0 seedlings were exposed to 10 μmol l−1 Cd (3CdSO4·8H2O) in solution, either with (Zn10) or without (Zn0) 10 μmol l−1 Zn. In the P–Cd experiment, seedlings were subjected to 10 μmol l−1 Cd either with (P1) or without (P0) 1.0 mmol l−1 P. Speciation calculated using Visual MINTEQ 2.3 showed that 97.3% of Cd was in the form of Cd2+ and 2.7% was present as CdHPO4 (aqueous form), indicating that no precipitation of Cd–P had occurred in the solution. The Cd concentration was chosen to represent heavily polluted conditions. The zinc concentration was based on published data on the interactions of Zn and Cd which showed that a molar ratio of Zn/Cd of 1:1 was a threshold value above which an antagonistic effect of Zn and Cd might occur (Girling and Peterson 1981; Green et al. 2001). The P concentration was based on Liu et al. (2004b). The duration of exposure to Zn or P was 2 days and the seedlings were then harvested. The total combination of the elements gave four treatments in each experiment: Fe0Zn0, Fe0Zn10, Fe50Zn0, Fe50Zn10 in the Zn–Cd experiment and Fe0P0, Fe0P1, Fe50P0 and Fe50P1 in the P–Cd experiment. Each treatment was set up in triplicate.
The experiments were carried out in a controlled growth chamber in the Department of Plant Nutrition, China Agricultural University, Beijing. The day/night regime was 14/10 h, the average temperature regime was 25/20°C and the average photon flux density was 120–150 μmol m−2 s−1.
Extraction and determination of Fe, Cd, P and Zn on root surfaces and in roots and shoots
At harvest seedlings were separated into shoots and roots and then rinsed three times with deionized water. Iron plaque on fresh root surfaces was extracted using a modified dithionite–citrate–bicarbonate (DCB) method (Taylor and Crowder 1983; Otte et al. 1989). Briefly, the entire root system of each seedling was incubated for 60 min at 25°C in 60 ml 0.03 mol l−1 sodium citrate (Na3C6H5O7 · 2H2O) and 0.125 mol l−1 sodium bicarbonate (NaHCO3) with the addition of 1.2 g sodium dithionite (Na2S2O4). The extracts were transferred to 100-ml glass flasks and filtered into plastic containers for analysis. Roots appeared completely white after extraction and no visible damage to the roots was observed. After DCB extraction of the roots, roots and shoots were oven dried at 70°C for 3 days and weighed.
Oven dried root and shoot samples were ground. Sub-samples (approximate 0.16 g roots and 0.30 g shoots) were weighed into digestion tubes and moistened overnight with 5 ml mixed concentrated acid (87/13 HNO3/HClO4 by volume). On the following day the samples were heated on a digestion block at 90°C for 3 h, then at 140°C for 5 h, and 180°C for a further 2 h until little solution remained but without complete drying out. After cooling, the digests were transferred to 25-ml flasks with deionized water and filtered into plastic bottles. A reagent blank and a standard reference material (tomato, GSB 07-1264-2000, Chinese National Certified Reference Material) were included to verify the accuracy and precision of the digestion procedure and subsequent analysis.
Elemental concentrations in the DCB extracts and in the acid digests were measured by inductively coupled plasma-optical emission spectrometry (ICP-OES, Optima 3300 DV, Perkin Elmer, USA). An internal standard was included and negligible matrix effects were observed. Total uptake of the elements and the distributions of the elements in DCB extracts and in roots and shoots were calculated according to Liu et al. (2004b).
Statistical analysis
Data from each of the two experiments were subjected to two-way analysis of variance using SAS for Windows (Version 8.2, SAS Institute, Cary, NC). Data presented are means ± SD (n = 3) and were compared by least significant difference (LSD) at the 5% level.
Results
Appearance of iron plaque on root surfaces
The visual appearance of root surfaces differed greatly between the control and Fe2+ treated plants. Roots of plants subjected to 50 mg l−1 Fe2+ formed a deep orange color indicating the presence of iron plaque. The iron plaque was unevenly distributed and the color of the plaque became gradually darker towards the basal parts from 1 cm behind the root tips along the entire roots. Some root zones were heavily coated with iron plaque while others were lightly smeared. Root surfaces of the control seedlings had a relatively evenly distributed orange color and the root tips remained white. The control seedlings formed a weak iron plaque on the root surfaces due to the supply of Fe–EDTA during the pre-cultivation seedling stage.
Dry weights of shoots and roots
Seedling shoot and root dry weights were significantly affected by Fe additions and Fe×Zn or Fe×P (P < 0.05) interactions, but not by Zn or P additions in the nutrient solution (Table 1). The shoot and root dry weights of seedlings treated with Fe were significantly lower than of seedlings without Fe2+ in both experiments (Table 1). Zn supply significantly increased shoot and root dry weights at Fe50. At Fe50, shoot dry weights at P1 were significantly lower than at P0, while root dry weights at P1 were significantly higher than at P0 when no Fe was supplied (Table 1). A general trend was that shoot and root dry weights appeared to be depressed by Zn supply at Fe0, but increased at Fe50, but P supply showed the opposite trend.
Fe concentrations in DCB extracts, roots and shoots
Iron concentrations in DCB extracts and in roots and shoots of rice seedlings were significantly increased by Fe supply (P < 0.01) and the effect was significantly higher at Fe50 than at Fe0 (Table 2). Zn supply had no significant effect on Fe concentrations except that the mean root Fe concentration at Fe50 was lower at Zn10 than at Zn0. At Fe0, Fe concentrations in shoots and roots at P1 were significantly higher than at P0, and at Fe50, P supply had no significant effect on shoot or root Fe concentrations.
Cd concentrations in DCB extracts, roots and shoots
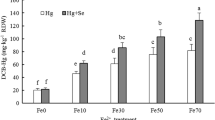
Irrespective of Zn or P supply levels, Cd concentrations in DCB extracts were significantly higher at Fe50 than at Fe0, and in contrast, root and shoot Cd concentrations at Fe50 were lower than at Fe0 and the effect was more pronounced in the P–Cd experiment. Zn supply in the nutrient solution significantly decreased root Cd concentrations and increased shoot Cd concentrations at both Fe supply levels. In comparison, P supply significantly increased root and shoot Cd concentrations at Fe0, and at Fe50 the increments of shoot and root Cd concentrations between P1 and P0 were smaller as shown by the significant interaction between Fe and P supply (Fig. 1, Table 3).
Cadmium concentrations in dithionite–citrate–bicarbonate (DCB) extracts and in roots and shoots of rice seedlings with (Fe50) or without (Fe0) iron plaque on root surfaces in the Zn–Cd and P–Cd experiments. Error bars: ±SD. Lower case a and b indicate significant differences between the two Zn or P supply levels at Fe50 or Fe0
Zn and P concentrations in DCB extracts, roots and shoots
Zinc concentrations in DCB extracts and roots were significantly higher at Fe50 than at Fe0, while no significant difference was observed in shoot Zn concentration between the two Fe treatments (Fig. 2, Table 4). P concentrations in DCB extracts and shoots at Fe50 were also higher than at Fe0, while root P concentrations showed the reverse trend. Zn concentrations in DCB extracts and in roots and shoots were significantly higher at Zn10 than at Zn0. External P supply had no significant effect on DCB–P or shoot P concentrations, while root P concentrations were higher at P1 than at P0. The difference in root P concentrations between the two P levels was more pronounced at Fe0 than at Fe50 as indicated by the Fe and P interactions on root P concentrations (P < 0.001).
Zinc and P concentrations in dithionite–citrate–bicarbonate (DCB) extracts and in roots and shoots of rice seedlings with (Fe50) or without (Fe0) iron plaque on root surfaces in the Zn–Cd and P–Cd experiments. Error bars: ±SD. Lower case a and b indicate significant differences between the two Zn or P supply levels at Fe50 or Fe0
Distribution of Cd, P and Zn in DCB extracts, roots and shoots
In both experiments the percentages of Cd in DCB extracts were significantly lower than in roots or shoots, accounting for up to approximately 1.8–3.8% of plant total Cd (Table 5). In contrast, the percentages of Cd in roots and shoots were up to around 57–73% and 23–40% respectively in the Zn–Cd experiment. The corresponding values in the P–Cd experiment were 73–76% and 21–24%. In both experiments DCB–Cd percentage was significantly higher at Fe50 than at Fe0. The percentages of Cd in roots and shoots were significantly affected by Zn supply in the Zn–Cd experiment (Table 5). At Fe0 shoot and root percentages were lower when Zn was supplied and the reverse was observed at Fe50. However, P supply had no significant effect on root Cd or shoot Cd percentage.
The percentages of Zn and P in shoots were much higher than in roots and DCB extracts, accounting for about 46–60% and 72–76% respectively of plant Zn and P uptake (Table 6). Fe supply had no significant effect on Zn distribution in different plant parts in the Zn–Cd experiment, but in the P–Cd experiment Fe supply increased DCB–P, decreased root P but showed no significant effect on shoot P percentage.
Discussion
The amounts of iron plaque on the root surfaces of rice were approximately 8–10 g kg−1 at Fe0 and 45–48 g kg−1 at Fe50, which fell within the range (the lowest ∼0.15 g kg−1 and the highest ∼59 g kg−1) reported for other wetland plants (St-Cyr and Crowder 1989; Hansel et al. 2002) and rice (∼25–102 g kg−1, Chen et al. 1980b) in the field. Uptake of nutrient elements and/or metal(loids) can be mediated by the formation of ion plaque on root surfaces of wetland and some terrestrial plants. However, the effects of iron plaque on plant elemental uptake are controversial. Some workers have suggested that iron plaque can sequester metal(loid)s such as Al and As on root surfaces of rice and thus depress their translocation to the shoots (Batty et al. 2000; Chen et al. 2006; Greipsson 1994, 1995; Liu et al. 2004b). Others have shown that iron plaque has little effect on Cd uptake (Liu et al. 2007), and some have demonstrated that iron plaque can act as a P reservoir to enhance plant P uptake (Liang et al. 2006; Zhang et al. 1999). In the present study the distribution of Cd in DCB extracts (about 1.8–3.8%) was minor when compared to the proportions of Cd in the roots (∼57–76%) and shoots (∼21–40%, Table 5), and this was in marked contrast to the distribution of other anions (Liu et al. 2004a, b; Zhou et al. 2007) where DCB% accounted for the major proportions of the elements. Although the Cd concentrations in DCB extracts increased in Fe-treated plants, Cd concentrations in roots and shoots decreased significantly (except shoot Cd concentrations in the Zn–Cd experiment, Fig. 1), and the present study indicates that the contribution of iron plaque to plant Cd uptake was minor and the roots may have been the main tissue acting as a barrier to plant Cd uptake. These results differ from others (Chen et al. 2006; Batty et al. 2000; Greipsson 1994, 1995; Liu et al. 2004b) but agree with our conclusions (Liu et al. 2007) and those of Liu et al. (2005) who also found that root tissue was the main barrier to As uptake by rice plants when arsenite was supplied. Similar results were also reported for metal uptake by other wetland plants, for example Pb and Cd uptake by Typha latifolia (Ye et al. 1998), Cu, Zn and Pb uptake by water hyacinth (Vesk et al. 1999), and Fe, Mn, Cu and Zn uptake by Juncus bulbosus (Chabbi 1999). The increase in Fe nutritional level in plants may partially alleviate the potential toxic effects of Cd to plant growth, as shown by the decreased Cd concentrations in roots and shoots at Fe50 than at Fe0 in both experiments (Fig. 1). Fe has been shown to exert strong competition over other heavy metals for sensitive metabolic sites within the leaves (Kuo 1986; Taylor and Crowder 1983) or in the root tips (Chen et al. 2006). Mean shoot Fe concentrations in the present experiment at Fe50 (except at P0) were higher than the 448 mg kg−1 reported for normally grown rice plants (Ottow et al. 1982). However, no visible Fe toxicity symptoms were observed even though root dry weight decreased at Fe50 (Table 1), indicating that roots were more sensitive to the external Fe supply. In the literature, decreases (Ye et al. 2001; Liu et al. 2007) or increases (Greipsson and Crowder 1992; Greipsson 1994, 1995) or no changes in root dry weight have been reported in response to pre-treatment to induce the formation of iron plaque on root surfaces.
The distributions of P and Zn in different plant parts were in contrast with that of Cd (Fig. 2, Table 6). The proportions of Zn and P in shoots were much higher than in roots and DCB extracts, accounting for approximately 46–60% (Zn–Cd experiment) and 72–76% (P–Cd experiment) respectively of plant Zn and P uptake. Since P and Zn are essential elements for plant growth under our experimental conditions in which P and Zn concentrations were not high but shoot Cd concentrations were high, rice may have evolved mechanisms to increase plant uptake and translocation of Zn and P. For example, Zhang et al. (1998) found that phytosiderophores released by Fe-deficient plants enhanced Zn uptake by rice plants with iron plaque up to a particular amount of Fe, and enhanced shoot P uptake was assumed to be related to substances other than siderophores released by plants (Zhang et al. 1999). The overall role of iron plaque in elemental uptake by rice may vary among studies according to differences in culture methodology, duration of the treatments, the element or contaminant studied, or the amount of iron plaque on the root surfaces (Zhang et al. 1998; Otte et al. 1989), plant genotypes (Liu et al. 2004a) and to the status of nutrients such as P (Liu et al. 2004b) and Fe (Zhang et al. 1998). The present study and others highlight the complexity of transport of nutrient elements and/or metal(loids) from soil to rice shoots via the soil–rhizosphere–iron plaque–root–shoot circuit.
Neither Zn nor P addition had any significant effect on Cd concentrations in DCB extracts while Cd concentrations in roots and shoots were significantly affected. This indicates that iron plaque on the root surfaces may not be the site where Zn and P affect Cd adsorption and uptake by and translocation within rice seedlings. This leads us to deviate partly from our initial hypothesis but may be explained by the minor role of iron plaque in plant Cd uptake. Studies on the deposition and distribution patterns of elements on iron plaque by means of in situ techniques such as scanning electron microscopy (SEM) analysis may help to provide further evidence to support our conclusions. Providing that small amounts of Cd are present in iron plaque, it is understandable that Zn or P may have little effect on Cd adsorption since the co-depositon of large amounts of other elements such as P and Zn and/or precipitates (in the case of P) may mask any effects of Cd. Few experiments reported in the literature have studied the effects of accompanying ions on elemental uptake by plants with iron plaque. Batty et al. (2000) combined solution culture with SEM to study the effects of iron plaque on root surfaces of Phragmites australis on plant Mn and Cu uptake. The solution culture work showed that adsorption of Cu and Mn on the plaque was affected by the pH value of the solution. At pH 6.0 shoot Cu concentrations were lower in the presence than in the absence of plaque while at pH 3.5 the potential effect of iron plaque was masked by activity of hydrogen ions. However, based on SEM analysis of both field and laboratory specimens, they suggested that iron plaque may have an extremely limited capacity to adsorb metals such as Mn or Cu. Kuo (1986) found that the adsorption of P by a synthetic hydrous Fe oxide did not affect Cd, Ca or Zn sorption. In contrast, Geng et al. (2005) provided evidence that external P concentrations affect As uptake and translocation in rice seedlings. The effect was attributed to an interaction among plant P nutrition, the formation of iron plaque on root surfaces, and As sequestration in iron plaque, indicating a possible indirect effect of external P supply on As uptake by plants although the direct competition of P on As uptake could not be fully excluded.
The decreased Cd concentrations in roots and increased Cd concentrations in shoots of plants with or without iron plaque (Fig. 1, Table 3) indicate that Zn may have inhibited root Cd uptake but not the transfer of Cd from roots to shoots. An antagonistic effect of Zn on Cd uptake has been reported from numerous studies and the effect may be due to strong competition between Zn and Cd uptake because of the chemical similarity of the two elements (Hart et al. 2005; White and Chaney 1980). Recently Hassan et al. (2005) showed that at high Cd supply (5 μmol l−1 Cd compared to 1 μmol l−1 Cd or without Cd), Zn (1.0 μmol l−1) significantly increased shoot Cd concentrations but reduced root Cd concentrations. Kukier and Chaney (2002) found that at high external Cd levels (2.56 and 4.55 μmol l−1 Cd), Zn (3.89 μmol l−1) significantly stimulated Cd translocation from roots to shoots in rice plants at later growth stages.
Our results also indicate that there is an interaction between P supply and Cd uptake by rice plants (Fig. 1, Table 3). P additions in the solution substantially enhanced the Cd concentrations in roots and shoots when no Fe was supplied and P and Cd showed a synergistic interaction. Moral et al. (1994) reported that P concentrations in roots, stems, leaves and fruits of tomato plants supplied with 30 mg l−1 Cd were higher than when supplied with 10 or 0 mg l−1 Cd. Lagriffoul et al. (1998) also found that P concentrations in maize leaves increased significantly when the Cd supply was over 5 μmol l−1 (0.56 mg l−1). In the present study the synergistic effect of P on Cd uptake was diminished when Fe was supplied (50 mg l−1), suggesting a possible three−factor interaction among Fe supply, P supply and Cd uptake. The mechanism by which Fe supply mediates the effect of P supply on Cd uptake requires further investigation.
In conclusion, our results suggest that rather than iron plaque, root tissue may be the main barrier to Cd uptake by rice plants. The adsorption of Cd on iron plaque is not affected by the external Zn or P supply. The uptake by and translocation of Cd in plants may be mediated by external P and Zn supply levels. Zn and P additions increased shoot Cd concentrations. Conventional agricultural practices to increase rice yields such as P fertilizer application may pose a potential risk of higher Cd uptake by rice plants from contaminated soils. However, high Fe concentrations in plants may help, to some extent, to minimize additional Cd uptake by plants.
References
Armstrong W (1967) The oxidizing activity of roots in water-logged soils. Physiol Plant 20:920–926
Batty LC, Baker AJM, Wheeler BD, Curtis CD (2000) The effect of pH and plaque on the uptake of Cu and Mn in Phragmites australis (Cav.) Trin ex. Steudel. Ann Bot 86:647–653
Chabbi A (1999) Juncus bulbosus as a pioneer species in acidic lignite mining lakes: Interactions, mechanism and survival strategies. New Phytol 144:133–142
Chaney RL, Hornick SB (1978) Accumulation and effects of cadmium on crops. In Cadmium 77: Proc 1st Int. Cadmium Conf. San Francisco, Metal Bulletin Ltd, London, pp 125–140
Chen CC, Dixon JB, Turner FT (1980a) Iron coatings on rice roots: Mineralogy and quantity influencing factors. Soil Sci Soc Am J 44:635–639
Chen CC, Dixon JB, Turner FT (1980b) Iron coatings on rice roots: Morphology and models of development. Soil Sci Soc Am J 44, 1113–1119
Chen RF, Shen RF, Gu P, Dong XY, Du CW, Ma JF (2006) Response of rice (Oryza sativa) with root surface iron plaque under aluminium stress. Ann Bot 98:389–395
Geng CN, Zhu YG, Liu WJ, Smith SE (2005) Arsenate uptake and translocation in seedlings of two genotypes of rice is affected by external phosphate concentrations. Aquat Bot 83:321–331
Girling CA, Peterson PJ (1981) The significance of the cadmium species in uptake and metabolism of cadmium in crop plants. J Plant Nutr 3:707–720
Green CE, Chaney RL, Bouwkamp J (2001) Interactions between cadmium uptake and phytotoxic zinc levels in rice (Oryza sativa L.) using chelator-buffered nutrient solution. Plant Soil 9:20–30
Greipsson S (1994) Effects of iron plaque on roots of rice on growth and metal concentration of seeds and plant tissues when cultivated in excess copper. Commun Soil Sci Plant Anal 25:2761–2769
Greipsson S (1995) Effects of iron plaque on roots of rice on growth of plants in excess zinc and accumulation of phosphorus in plants in excess copper or nickel. J Plant Nutr 18:1659–1665
Greipsson S, Crowder AA (1992) Amelioration of copper and nickel toxicity by iron plaque on roots of rice (Oryza sativa L.). Can J Bot 70:824–830
Hansel CM, Fendorf S, Sutton S, Newville M (2001) Characterization of Fe plaque and associated metals on the roots of mine-waste impacted aquatic plants. Environ Sci Technol 35:3863–3868
Hansel CM, La Force MJ, Fendorf S, Sutton S (2002) Spatial and temporal association of As and Fe species on aquatic plant roots. Environ Sci Tech 36(9):1988–1994
Hart JJ, Welch RM, Norvell WA, Clarke JM, Kochian LV (2005) Zinc effects on cadmium accumulation and partitioning in near-isogenic lines of durum wheat that differ in grain cadmium concentration. New Phytol 167:391–401
Hassan MJ, Zhang GP, Wu FB, Wei K, Chen ZH (2005) Zinc alleviates growth inhibition and oxidative stress caused by cadmium in rice. J Plant Nutr Soil Sci 168:255–261
Junta Y, Misdutaka Y, Kang YM, Huang B, Luo GB, Takashi K (1998) Heavy metal pollution of agricultural soils and sediments in Liaoning Province, China. Soil Sci Plant Nutr 44 (3):367–375
Kim MC, Shim KH, Chung DH, Cho KT (1980) Heavy metal contents in different bran layers of rice. J Korean Agric Chem Soc 23:141–149
Kukier U, Chaney RL (2002) Growing rice grain with controlled cadmium concentrations. J Plant Nutr 25:1793–1820
Kuo S (1986) Concurrent sorption of phosphate and zinc, cadmium, or calcium by a hydrous ferric oxide. Soil Sci Soc Am J 50:1412–1419
Lagriffoul A, Mocquot B, Mench M, Vangronsveld J (1998) Cadmium toxicity effects on growth, mineral and chlorophyll contents, and activities of stress related enzymes in young maize plants (Zea mays L.). Plant Soil 200:241–250
Liang Y, Zhu YG, Xia Y, Li Z, Ma Y (2006) Iron plaque enhances phosphorus uptake by rice (Oryza sativa) growing under varying phosphorus and iron concentrations. Ann Appl Biol 149:305–312
Liu HJ, Zhang JL, Zhang FS (2007) Role of iron plaque in Cd uptake by and translocation within rice (Oryza sativa L.) seedlings grown in solution culture. Environ Exp Bot 59:314–320
Liu WJ, Zhu YG, Smith FA, Smith SE (2004a) Do iron plaque and genotypes affect arsenate uptake and translocation by rice seedlings (Oryza sativa L.) grown in solution culture? J Exp Bot 55:1707–1713
Liu WJ, Zhu YG, Smith FA, Smith SE (2004b) Do phosphorus nutrition and iron plaque alter arsenate (As) uptake by rice seedlings in hydroponic culture? New Phytol 162:481–488
Liu WJ, Zhu YG, Smith FA (2005) Effects of iron and manganese plaques on arsenic uptake by rice seedlings (Oryza sativa L.) grown in solution culture supplied with arsenate and arsenite. Plant Soil 277:127–138
Moral R, Gomez I, Pedreno JN, Mataix J (1994) Effects of cadmium on nutrient distribution, yield, and growth of tomato grown in soilless culture. J Plant Nutr 17:953–962
Obata H, Umebayashi M (1997) Effects of cadmium on mineral nutrient concentrations in plants differing in tolerance for cadmium. J Plant Nutr 20:97–105
Otte ML, Rozema J, Koster L, Haarsma MS, Broekman RA (1989) Iron plaque on roots of Aster tripolium L.: Interaction with zinc uptake. New Phytol 111:309–317
Ottow JGG, Benckiser G, Watanabe I (1982) Iron toxicity of rice as multiple nutritional stress. Trop Agr Res Series 15:167–179
Shah K, Kumar RG, Verma S, Dubey RS (2001) Effect of cadmium on lipid peroxidation, superoxide anion generation and activities of antioxidant enzymes in growing rice seedlings. Plant Sci 161:1135–1144
St-Cyr L, Crowder AA (1989) Factors affecting iron plaque on the roots of Phragmites australis (Cav.) Trin. ex Steudel. Plant Soil 116:85–93
Taylor GJ, Crowder AA (1983) Use of the DCB technique for extraction of hydrous iron oxides from roots of wetland plants. Am J Bot 70:1254–1257
Taylor GJ, Crowder AA, Rodden R (1984) Formation and morphology of an iron plaque on the roots of Typha latifolia L. grown in solution culture. Am J Bot 71:666–675
Tsukahara T, Ezaki T, Moriguchi J, Furuki K, Shimbo S, Matsuda-Inoguchi N, Ikeda M (2003) Rice as the most influential source of cadmium intake among general Japanese population. Sci Tot Environ 305:41–51
Vesk PA, Nockolds CE, Allaway WG (1999) Metal localization in water hyacinth roots from an urban wetland. Plant Cell Environ 22:149–158
Watanabe T, Zhang ZW, Moon CS, Shimbo S, Nakatsuka H, Matsuda-Inoguchi N, Higashikawa K, Ikeda M (2000) Cadmium exposure of women in general populations in Japan during 1991–1997 compared with 1977–1981. Int Arch Occup Environ Health 73:26–34
White MC, Chaney RL (1980) Zinc, cadmium and manganese uptake by soybean from two zinc-amended and cadmium-amended coastal plain soils. Soil Sci Soc Am J 44:308–313
Ye ZH, Baker AJM, Wong MH, Willis AJ (1998) Zinc, lead and cadmium accumulation and tolerance in Typha latifolia as affected by iron plaque on the root surface. Aquat Bot 61:55–67
Ye ZH, Cheung KC, Wong MH (2001) Copper uptake in Typha latifolia as affected by iron and manganese plaque on the root surface. Can J Bot 79:314–320
Zhang XK, Zhang FS, Mao DR (1998) Effect of iron plaque outside roots on nutrient uptake by rice (Oryza sativa L.). Zinc uptake by Fe-deficient rice. Plant Soil 202:33–39
Zhang XK, Zhang FS, Mao DR (1999) Effect of iron plaque outside roots on nutrient uptake by rice (Oryza sativa L.): Phosphorus uptake. Plant Soil 209:187–192
Zhou XB, Shi WM, Zhang LH (2007) Iron plaque outside roots affects selenite uptake by rice seedlings (Oryza sativa L.) grown in solution culture. Plant Soil 290:17–28.
Acknowledgements
The study was supported by the Program for Innovative Research Teams in Universities of the Chinese Ministry of Education (Grant IRT0511) and the British Council with the UK Department for International Development through their Development Partnerships in Higher Education program (Project DelPHE 64). We thank Dr Shihua Lu, Institute of Soils and Fertilizers, Sichuan Academy of Agriculture Sciences, for supplying the rice seeds and two anonymous reviewers for helpful suggestions that greatly improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Fangjie J. Zhao.
Rights and permissions
About this article
Cite this article
Liu, H.J., Zhang, J.L., Christie, P. et al. Influence of external zinc and phosphorus supply on Cd uptake by rice (Oryza sativa L.) seedlings with root surface iron plaque. Plant Soil 300, 105–115 (2007). https://doi.org/10.1007/s11104-007-9393-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-007-9393-3