Abstracts
Wheat varietal autotoxicity and varietal allelopathy were assessed based on plant extract and root exudate bioassays under laboratory conditions. Aqueous extract of wheat differed in varietal autotoxicity and varietal allelopathy, inhibiting wheat germination by 2–21%, radicle growth by 15–30%, and coleoptile growth by 5–20%, depending on the combination of the receiver and donor. Extracts of cv Triller or cv Currawong were more allelopathic to other wheat varieties than cv Batavia and cv Federation. Triller extract was more autotoxic than Federation. Assessment of root exudates by the equal-compartment-agar-method further identified the significant differences in varietal autotoxicity and varietal allelopathy of root exudates between wheat varieties, with root exudates of Triller or Batavia showing stronger autotoxic or allelopathic effects than Currawong or Federation. The varietal autotoxicity and allelopathy of root exudates also showed a characteristic radial inhibitory pattern in the agar growth medium. These results suggest that careful selection of suitable wheat varieties is necessary in a continuous cropping system in order to minimize the negative impacts of varietal allelopathy and varietal autotoxicity. Factors affecting autotoxicity in the field and strategies in autotoxicity management are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Allelopathy refers to the beneficial or harmful effects of one plant on another by the release of inhibitory substances from plants into the environment through root exudation, leaching and volatilization, and through the decomposition of plant residues (Rice 1984). Autotoxicity is an intraspecific allelopathy occur when a plant species releases chemical substances that inhibit or delay germination and growth of the same plant species (Putnam 1985). Application of the concepts of allelopathy and autotoxicity in depicting the chemical interactions between varieties within the same crop species results in “varietal allelopathy” and “varietal autotoxicity”. Varietal allelopathy occurs when plants of a given variety release chemical substances that inhibit or delay germination and growth of other varieties of the same crop species. On the other hand, varietal autotoxicity occurs when plants of a given variety release chemical substances that inhibit or delay germination and growth of the same variety.
Allelopathy of wheat (Triticum aestivum L.) has been extensively examined for its potential in weed management (Bertholdsson 2005; Wu et al. 2000b). Research on wheat allelopathy has progressed rapidly from the initial phase of evaluation of wheat allelopathy (Wu et al. 2000b, 2003a) to the identification of wheat allelochemicals (Villagrasa et al. 2006; Wu et al. 2002), the degradation of these compounds (Fomsgaard et al. 2004) and, further, to the identification of genetic markers associated with wheat allelopathy (Wu et al. 2003b). The genetic enhancement of allelopathic traits has provided a promising area for improved weed management (Wu et al. 1999). However, improved wheat allelopathic activity via biotechnological means might increase the negative effects on growth of other crops and on wheat itself.
Wheat has caused allelopathic inhibition to the growth and yield of crops such as rice (Oryza sativa L.), barley (Hordeum vulgare L.), rye (Secale cereale L.) (Hozumi et al. 1974), cotton [Gossypium hirsutum L. Merr.] (Hicks et al. 1989) and soybean (Glycine max L. Merr) (Protic 1977). Wheat straw was also allelopathic to a number of forage crops including sorghum (Sorghum bicolor L. Moench), pearl millet (Pennisetum glaucum L.), clusterbean (Cyamopsis tetragonoloba L.) and cowpeas (Vigna unguiculata L. Walp.) (Narwal et al. 1997).
Evidence of autotoxicity first appeared in an early report (Schreiner and Reed 1907), who claimed that the roots of wheat, oats, and certain other crop plants exude chemicals inhibitory to their own seedlings. Since then, autotoxicity has been identified in many field crops, including alfalfa (Medicago sativa L.) (Hedge and Miller 1990), rice (Chou and Chiou 1979), barley (Ben-Hammouda et al. 2002), wheat (Waller 1987; Young et al. 1989), asparagus (Asparagus officinalis L.) (Young 1984) and cucurbit crops (Yu et al. 2000). The ecological significance of autotoxicity has involved geographical distribution, adaptation to induced dormancy and prevention of seeds and propagules from decay (Friedman and Waller 1983).
Conservation farming including stubble retention has been widely adopted by farmers worldwide because of its advantages in soil and moisture conservation and fuel savings, but poor early growth of wheat and yield reduction have sometimes been observed in the reduced or no-tillage farming systems where wheat residues are retained in the field (McCalla and Army 1961). The cause of the yield reduction could be due to reduced soil temperature, physical barriers, and nitrogen immobilization (Kimber 1973; Hairston et al. 1987), as well as the phytotoxic chemicals released from decomposing wheat residues (Kimber 1967; McCalla and Haskins 1964). In the field, the inclusion of wheat in a farming system, either with monocropping or in rotation with other crops, may cause higher autotoxic effects in comparison with those cropping systems without wheat (Waller 1987; Young et al. 1989).
Confirmation under controlled environments showed that wheat residue water extracts were autotoxic to the germination and seedling growth of wheat (Alam 1990; Guenzi et al. 1967; Kimber 1967). In solution culture, Protic et al. (1980) found that wheat seedling root volume, total root area and active root area were decreased with an increase in the quantity of the wheat residue amended into the nutrient solution.
Much of the knowledge on autotoxicity in crops has resulted from the study of plant residues. Investigation of the autotoxic effects of root exudates is scarce. Young (1984) demonstrated that root exudates of asparagus inhibited the growth of the radicle and shoot of asparagus seedlings, suggesting asparagus autotoxicity is a possible mechanism for the yield decline and replanting failure. Yu et al. (2000) found that root exudates of many cucurbit crops possess strongly autotoxic potential. No information is available on the autotoxic effects of wheat root exudates, although such exudates contained phytotoxic chemicals (Nakano et al. 2006; Wu et al. 2001, 2002) and were inhibitory to annual ryegrass (Lolium rigidum Gaud.) (Wu et al. 2000b). This paper evaluates varietal allelopathy and varietal autotoxicity of root exudates and residue extracts in wheat and describes the differential sensitivity (tolerance) of wheat varieties to root exudates of wheat seedlings.
Materials and methods
Collection of wheat material and preparation of plant extract
Based on a previous research of wheat phytotoxic activity of aqueous extract on the growth of L. rigidum (Wu et al. 2003a), four wheat varieties (Triller, Batavia, Currawong and Federation) were chosen and used in this study. A previous plant extract bioassay was adopted with slight modification (Wu et al. 2003a). Shoots of the four varieties were collected from the field just prior to harvest. After the removal of grain spike, leaves and stems were combined and oven-dried at 40°C for 72 h, and ground to pass through a sieve of 0.25 mm. Ten grams of residue powder from each wheat variety were extracted with 100 ml of distilled water in a glass jar for 48 h at 20°C. The mixture was filtered through four layers of cheese cloth and the resulting filtrate was centrifuged at 10,000 rpm for 15 min at 10°C. The supernatant was then vacuum-filtered through one layer of microfilter paper (Whatman, 0.25 μm). The sterilized filtrate was designated as full strength (100%).
Autotoxicity assessment of wheat residue extract
Wheat varieties Triller and Batavia were used as the receivers. Twenty-five seeds (non surface-sterilized) of each of the receivers were sown onto 9 cm petri dishes lined with one layer of Whatman No.1 filter paper. Six millitre of each extract (1/3 of the full strength) from four donor varieties (Triller, Batavia, Currawong and Federation) were delivered to each petri dish, and distilled water (6 ml) was used as control. Each petri dish with its cover was sealed with a piece of parafilm to reduce evaporation. All dishes were maintained in a tissue culture room at 23°C with fluorescent lights for 24 h as described previously (Wu et al. 2003a). The fluorescent light intensity was 4.17 ± 0.18 × 103 lux. Germinated seeds with >1 mm radicle were recorded and root lengths measured after 5 days of incubation. Treatments included a full factorial of four donor varieties by two receivers.
Autotoxicity assessment of root exudates of wheat seedlings
The four wheat varieties were used as the donors and receivers, respectively. The equal-compartment-agar-method (ECAM) was employed to study the autotoxicity of wheat root exudates, because this method successfully separates competition from allelopathy (Wu et al. 2000b). Briefly, wheat seeds were surface-sterilized by soaking the seeds in 70% ethanol for 2.5 min, followed by 4 rinses in sterilized distilled water. They were then soaked in 2.5% sodium hypochlorite solution for 15 min followed by 5 rinses in sterilized distilled water. The surface-sterilized seeds of the wheat genotypes were soaked in sterilized water for the imbibition of water in light at 25°C for 24 h and then rinsed with fresh sterilized water. The wheat seeds were then germinated in light at 25°C for another 24 h.
Twelve pre-germinated seeds of each donor wheat varieties were uniformly selected and aseptically sown on the agar surface with the embryo up, in three rows on one half of a glass beaker (500 ml) pre-filled with 30 ml of 0.3% water agar. The beaker was wrapped with a piece of parafilm and placed in a controlled growth cabinet with a daily light/dark cycle of 13 h/11 h and a temperature cycle at 25°C/13°C. The fluorescent light intensity in the cabinet was 3.56 ± 0.16 × 103 lux. After the growth of wheat seedlings for 7 days, 12 pre-germinated seeds of each receiver wheat variety were aseptically sown on the agar surface in three rows on the other side of the beaker. A piece of pre-autoclaved white paperboard was inserted across the centre and down the middle of the beaker with the lower edge of the paperboard kept 1 cm above the agar surface. The entire beaker was thereby divided into two equal compartments that were occupied separately by donor and receiver wheat seedlings. Any autotoxins produced and released by wheat seedlings were able to diffuse throughout the entire agar medium to affect the growth of receiver wheat. After the sowing of receiver wheat, the beaker was again wrapped with parafilm and placed back in the growth cabinet for continuous growth of 10 days. The growth of each receiver wheat without the donor wheat plants was treated as controls. The radicle and shoot lengths of both donor and receiver wheat seedlings were measured after 10 days of growth of the receiver wheat in the growth cabinet. Experimental design was a factorial of four donor varieties by four receivers.
Autotoxic radial zone of root exudates of wheat seedlings
The above ECAM bioassay was used. Triller and Federation were used as the donor as well as the receiver varieties. Twelve pre-germinated wheat seeds were uniformly selected and aseptically sown in a semi-circle pattern on the agar surface to one side of glass beaker (2000 ml) as illustrated in Fig. 1. The donor wheat seedlings were carefully cut from near the agar surface and removed after 7 days of growth. Thirty-six pre-germinated seeds of the receiver varieties Triller or Federation were sown on the agar surface in three concentric lines around the donor wheat seedlings site (Fig. 1), with each concentric line having 12 pre-germinated wheat seeds. The three concentric lines were distanced from the edge of the donor wheat seedlings at 2, 5, 8 cm, respectively. The growth of the receivers without the donor wheat plants was treated as controls. The root and shoot lengths of receiver wheat seedlings were measured after 10 days of growth of the receivers.
Experimental design and statistical analysis
A randomized complete block design with four replicates was used for each of the experiments described above. These experiments were not repeated over time due to the consistent, reproducible results achieved previously under the controlled conditions (Wu et al. 2000a, 2000b). All experimental data were subjected to analysis of variance using Genstat 5 (Release 3.2) and the treatment means were tested separately with least significant difference (l.s.d.) at a 5% level of probability. Percentage of the inhibition on the growth of receiver wheat was calculated as (control – data with donor)/control × 100.
Results
Varietal allelopathy and autotoxicity of wheat residue extracts
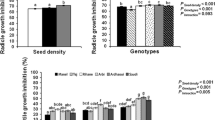
Responses of wheat varieties to wheat residue extract measured in germination and seedling growth differed among donor varieties (Table 1). Aqueous extract of Federation had the least inhibitory effect on the germination of Triller (2%), while the Currawong caused the strongest inhibition on the germination of Federation (21%). The four wheat varieties inhibited radicle growth by 15–30% and coleoptile by 5–20%. On average Currawong or Triller was more allelopathic than Batavia or Federation. As well, the two receiver varieties responded differentially to the donor extracts, with Federation being more sensitive than Triller when exposed to each of the donor extract.
The two varieties Triller and Federation differed in varietal autotoxicity (Triller on Triller and Federation on Federation) on germination, root and shoot growth, with Triller generally being more autotoxic than Federation when exposed to its own aqueous extract.
Varietal autotoxicity of wheat root exudates
The four wheat varieties Triller, Batavia, Currawong and Federation exhibited differential varietal autotoxic effects (Fig. 2). Autotoxic effect of root exudates on root growth was ranked in a decreasing order as Triller (38%) > Batavia (31%) > Federation (29%) > Currawong (18%). Root exudates of each of the donor varieties Triller, Batavia, Currawong and Federation were also autotoxic to the shoot growth of the respective variety by 21%, 12%, 6% and 2%. These results showed that the varietal autotoxicity of Triller or Batavia was more pronounced than Currawong or Federation.
Differential responses of wheat varieties to varietal allelopathy
Wheat varieties responded differentially to wheat varietal allelopathy (Table 2). Root growth of Batavia was the most sensitive to the varietal allelopathic effects of root exudates from donor Triller, resulting in 46% root growth inhibition of the receiver Batavia when compared to the control (absence of donor wheat), while Federation was the most sensitive variety to the root exudates from Batavia and Currawong, inhibiting root growth of Federation seedlings by 45% and 42%, respectively. Root exudates of donor Federation had similar allelopathic effects on the three receivers, Triller, Batavia and Currawong. On average, the varietal allelopathic effects on the root growth of other varieties were the highest in donor variety Triller (35%) and Batavia (37%), followed by Currawong (28%) and Federation (15%). The varietal allelopathic effects on shoot growth were ordered in a similar pattern as Triller (17%), Batavia (14%), Federation (11%) and Currawong (9%).
Radial effects of varietal autotoxicity and allelopathy of root exudates
The varietal autotoxic effect depended on the radial distance of the receivers to the donor. Root exudates of both donor wheat varieties had significant varietal autotoxic effects on the root growth of Triller on Triller and Federation on Federation, causing significant 9–47% autotoxic inhibition on root growth and 9–21% inhibition on shoot when compared to the control in absence of the donor wheat (Fig. 3A). The nearer of the receiver to the donor root exudates, the greater the autotoxic effects. On the average of the two receivers by two donor varieties, root growth of the receivers sown at a radial distance of 2, 5 and 8 cm to the donor wheat zone was inhibited by 35%, 26%, and 19%, respectively. The receiver plants of Triller in the absence of donor wheat (as a control) did not show radial growth pattern, having an average length of root and shoot of 141 and 134 cm, respectively. Similarly, the receiver plants of Federation in the absence of donor wheat had an average length of root and shoot of 129 and 140 cm, respectively. The radial varietal autotoxic effects of root exudates were also evidenced on shoot growth of the receivers (Fig. 3B). In addition, the varietal allelopathy (Triller on Federation and Federation on Triller) also followed a similar radial trend. Root exudates with Triller showed stronger varietal allelopathy and varietal autotoxicity than those of Federation (Fig. 3)
Radial effects of wheat root exudates on growth inhibition of root (A) and shoot (B) of receiver plants. The distances to the donor wheat were 2, 5, and 8 cm. Bars representing standard error of the mean. Percentage of the inhibition on the growth of receiver wheat was calculated as (control – data with donor)/control × 100
Discussion
The differences in varietal allelopathy and varietal autotoxicity of wheat residue extract identified in the present study are in agreement with Guenzi et al. (1967) and Kimber (1967). Guenzi et al. (1967) found that shoot aqueous extracts of nine wheat varieties differed in varietal allelopathy on the germination and seedling growth of wheat. Similar findings were obtained by Kimber (1967), showing that the variety, ‘Gabo’, exhibited a stronger inhibitory effect than cv Insignia. Straw extracts of Gabo and Insignia inhibited root growth of Gabo wheat seedlings by 27% and 4% respectively, and shoot growth by 43 and 12%, respectively.
Varietal autotoxicity of wheat root exudates was also demonstrated in this study. In addition to the inhibitory chemicals leached out from wheat residues, accumulation of organic substances exuded from root systems is another contributor to wheat autotoxicity. The present research showed that wheat root exudates were autotoxic to the growth of its own variety as well as allelopathic to other varieties. The wheat root exudate released into the agar medium showed a radial effect on varietal autotoxicity and varietal allelopathy. Root exudates autotoxicity has also been reported in asparagus (Young 1984) and cucurbit crops (Yu et al. 2000). It is therefore postulated that the autotoxins both exuded from living wheat plants and leached from wheat residues may accumulate in the field and determine the success of next wheat crop.
Research has shown that phytotoxic compounds are actively exuded by living wheat plants, passively leached into the soil, and convertibly produced in decaying wheat straw under certain field conditions (Guenzi et al. 1967; Lynch 1978). Phenolic acids, cyclic hydroxamic acids, short-chain fatty acids as well as many other phytotoxic chemicals have been identified from the residues and root exudates of wheat (Gaspar and Neves 1995; Nakano et al. 2006; Wu et al. 2001, 2002). The wheat varietal differences in the concentrations of allelochemicals in the shoots, roots and root exudates previously reported by Wu et al. (2002) might account for the variations of varietal autotoxicity and varietal allelopathy in wheat. In addition, biodegradation of wheat phytotoxic chemicals might also play an important role in wheat autotoxicity. Macías et al. (2003) reported that wheat coleoptiles were highly sensitive to the microbially-degraded products, i.e. 2-benzoxazolinone (BOA) and 3-aminophenoxazin-2-one (APO) derived from the hydroxamic acid DIBOA (2, 4-dihydroxy- 1,4-benzoxazin-3-one) of wheat (T. aestivum). Autotoxins such as phenolic acids have been reported to affect ion uptake, membrane permeability, photosynthesis and phytohormone balance (Yu 2001).
It is not clear if allelochemicals for the allelopathic effects are also acting as autotoxins for the autotoxic effects. The water-soluble saponins that are reported to exhibit allelopathic effects of alfalfa on other plants were not responsible for the autotoxic effects (Miller 1983). Water-soluble medicarpin, 4-methoxy medicarpin, sativa, 5-methoxy sativan, and chlorogenic acid are the possible agents to the autotoxicity of alfalfa (Dornbos et al. 1990; Miller et al. 1988). In wheat, phenolic acids seem to be involved in both allelopathic effects on weeds (Wu et al. 2002, 2003a) and autotoxic effects on wheat (Guenzi et al. 1967; Lodhi et al. 1987). The present research showed that both varietal allelopathy and varietal autotoxicity of Triller or Batavia root exudates were consistently stronger than Currawong or Federation.
This study provides laboratory-based evidence of the potential of varietal autotoxicity and varietal allelopathy in wheat, which could partly explain the frequently-reported autotoxic phenomenon of wheat in the field as a result of continuous cropping (Cast et al. 1990; Waller 1987; Young et al. 1989). For examples, Shodiev and Kaspari (1985) claimed that the presence of autotoxins exuded by wheat roots resulted in yield reduction of wheat in monoculture, compared with other rotations. Cast et al. (1990) found a trend towards greater autotoxicity in the soil of wheat by wheat under no-till, concluding that accumulation of phytotoxic chemicals occurred. Similarly, Oueslati et al. (2005) claimed that potential autotoxicity of barley detected by laboratory bioassays may be valuable in predicting whether a particular variety will affect the growth of subsequent barley crop in the field. We are currently validating laboratory results in the field.
The potential of varietal autotoxicity and varietal allelopathy in wheat was identified in this study. However, the expression of such potential in the field is a function of a number of biotic and abiotic factors such as wheat varieties used, quantity of residue, residue placement, level of decomposition, soil type, and climatic conditions (Guenzi et al. 1967; Kimber 1967; Wu et al. 2001). In general, straw genotype and straw quantity will determine the allelopathic potential in the field. The expression of this potential is highly dependent on soil types (Hairston et al. 1987; Putnam and Duke 1978; Rice 1984), soil cultivation (Lynch et al. 1981; Opoku et al. 1997), nutrient status, microbial activity, residue placement, degree of decomposition, and climatic conditions such as rainfall and temperature (Purvis 1990). In a similar study, Oueslati et al. (2005) reported that barley autotoxicity was affected by the variety used and the climatic conditions during the growing seasons. These biotic and abiotic factors interactively regulate the release, accumulation, transformation and dissipation of allelochemicals.
Wheat autotoxicity can be largely ameliorated by an effective management package, including a proper crop rotation to reduce the accumulation of autotoxins (Opoku et al. 1997; Putnam and Duke 1978), selection of crops and varieties tolerant to autotoxins due to the considerable variation in sensitivity among different crops and varieties (Hicks et al. 1989; Protic et al. 1980), and avoidance of the inhibitory period of decomposing residues by the delay in sowing (Tesar 1993). It appears that autotoxicity can be overcome by reducing the quantity of straw retained, increasing sowing rates (Hicks et al. 1989; Purvis and Jones 1990) and supplementing with nitrogen fertilizer (Rice 1984; Hairston et al. 1987). The applied N may play a dual role in increasing productivity because: (1) it compensates for the short-term depletion of N by immobilization, and (2) it also enhances the activity of soil microbes to break down potential toxins (Rice 1984). Ammonium ions may also detoxify phytotoxic chemicals (Chou and Chiou 1979). It has been suggested that a breeding program could be initiated to develop new varieties that do not produce autotoxins or are tolerant to autotoxins in order to overcome the autotoxicity in alfalfa (Chung and Miller 1995). A similar approach could be applied to overcome the autotoxicity in wheat and other crops.
References
Alam SM (1990) Effect of wheat straw extract on the germination and seedling growth of wheat (cv. Pavon). Wheat Information Service 71:16–18
Ben-Hammouda M, Ghorbal MH, Kremer RJ, Oueslati O (2002) Autotoxicity of barley. J Plant Nutri 25:1155–1161
Bertholdsson NO (2005) Early vigour and allelopathy - two useful traits for enhanced barley and wheat competitiveness with weeds. Weed Res 45:94–102
Cast KB, Mcpherson JK, Pollard AJ, Krenzer EG Jr, Waller GR (1990) Allelochemicals in soil from no tillage versus conventional-tillage wheat (Triticum aestivum) field. J Chem Ecol 16:2277–2289
Chou CH, Chiou SJ (1979) Autointoxication mechanism of Oryza sativa. II. Effects of culture treatments on the chemical nature of paddy soil and on rice productivity. J Chem Ecol 5:839–859
Chung IM, Miller DA (1995) Effect of alfalfa plant and soil extracts on germination and growth of alfalfa. Agron J 87:762–767
Dornbos DL Jr, Spencer GF, Miller RW (1990) Medicarpin delays alfalfa seed germination and seedling growth. Crop Sci 30:162–166
Fomsgaard IS, Mortensen AG, Carlsen SCK (2004) Microbial transformation products of benzoxazolinone and benzoxazinone allelochemicals - a review. Chemosphere 54:1025–1038
Friedman J, Waller GR (1983) Caffein hazards and their prevention in germinating seeds of coffee Coffea arabica L. J Chem Ecol 9:1099–1106
Gaspar EM, Neves HC (1995) Chemical constituents in allelopathic straw of wheat (Triticum aestivum L.). Allelopathy J 2:79–87
Guenzi WD, McCalla TM, Norstadt FA (1967) Presence and persistence of phytotoxic substances in wheat, oat, corn and sorghum residues. Agron J 59:163–165
Hairston JE, Sanford JO, Pope DF, Horneck DA (1987) Soybean-wheat doublecropping: implications from straw management and supplemental nitrogen. Agron J 79:281–286
Hedge RS, Miller DA (1990) Allelopathy and autotoxicity in alfalfa: characterization and effects of preceding crops and residue incorporation. Crop Sci 30:1255–1259
Hicks SK, Wendt CW, Gannaway JR, Baker RB (1989) Allelopathic effects of wheat straw on cotton germination, emergence, and yield. Crop Sci 29:1057–1061
Hozumi Y, Nakayama K, Yoshida K (1974) Allelopathy of wheat, barley and rye on the growth of rice plant. J Central Agric Exp Station 20:87–102
Kimber RWL (1967) Phytotoxicity from plant residues. I. The influence of rotted wheat straw on seedling growth. Aust J Agric Res 18:361–374
Kimber RWL (1973) Phytotoxicity from plant residues. III. The relative effect of toxins and nitrogen immobilization on the germination and growth of wheat. Plant Soil 38:543–555
Lodhi MAK, Bilal R, Malik KA (1987) Allelopathy in agroecosystems: wheat phytotoxicity and its possible roles in crop rotation. J Chem Ecol 13:1881–1891
Lynch JM (1978) Production and phytotoxicity of acetic acid in anaerobic soils containing plant residues. Soil Biol Biochem 10:131–135
Lynch JM, Ellis FB, Harper SHT, Christian DG (1981) The effect of straw on the establishment and growth of winter cereals. Agric Environ 5:321–328
Macías FA, Marín D, Oliveros-Bastidas A, Varela RM, Simonet AM, Carrera C, Molinillo JM (2003) Allelopathy as a new strategy for sustainable ecosystems development. Biol Sci Space 17:18–23
McCalla TM, Army TJ (1961) Stubble mulch farming. Adv Agron 13:125–196
McCalla TM, Haskins F (1964) Phytotoxic substances from soil microorganisms and crop residues. Bacteriol Rev 28:181–207
Miller DA (1983) Allelopathic effects of alfalfa. J Chem Ecol 9:1059–1072
Miller RW, Kleiman R, Powell RG, Putnam AR (1988) Germination and growth inhibitors of alfalfa. J Nat Prod 51:328–330
Nakano H, Morita S, Shigemori H, Hasegawa K (2006) Plant growth inhibitory compounds from aqueous leachate of wheat straw. Plant Growth Reg 48:215–219
Narwal SS, Sarmah MK, Nandal DP (1997) Allelopathic effects of wheat residues on growth and yield of fodder crops. Allelopathy J 4:111–120
Opoku G, Vyn TJ, Voroney RP (1997) Wheat straw placement effects on total phenolic compounds in soil and corn seedling growth. Can J Plant Sci 77:301–305
Oueslati O, Ben- Hammouda M, Ghorbal MH, Guezzah M, Kremer RJ (2005) Barley autotoxicity as influenced by varietal and seasonal variation. J Agron Crop Sci 191:249–254
Protic R (1977) Allelopathic activity of harvest remains of wheat and sugar beet on soyabean. Bioloski Vestnik 25:192
Protic R, Andelic M, Vasiljevic L (1980) Anatomical structure and function of the root system of wheat as dependent on allelopathic effects. Savremena-Poljoprivreda 28:243–256
Purvis CE (1990) Differential response of wheat to retained crop stubbles. I. Effect of stubble type and degree of composition. Aust J Agric Res 41:225–242
Purvis CE, Jones GPD (1990) Differential response of wheat to retained crop stubbles. I. Other factors influencing allelopathic potential; intraspecific variation, soil type and stubble quantity. Aust J Agric Res 41:243–251
Putnam AR (1985) Allelopathic research in agriculture: past highlights and potential. In: Thompson AC (ed). The Chemistry of allelopathy: Biochemical interactions among plants, American Chemical Society, Washington, D.C. pp 1–8
Putnam AR, Duke WB (1978) Allelopathy in agroecosystems. Annual Rev Phytopath 16:431–451
Rice EL (1984) Allelopathy. 2nd edn, Academic Press, Orlando, Florida
Schreiner O, Reed HS (1907) The production of deleterious excretions by roots. Bull Torr Bot Club 34:279–303
Shodiev P, Kaspari VM (1985) On the role of allelopathy in agriculture. Uzbekskii Biologicheskii Zhurnal 2:22–24
Tesar MB (1993) Delayed seeding of alfalfa avoids autotoxicity after ploughing or glyphosate treatment of established stands. Agron J 85:256–263
Villagrasa M, Guillamon M, Labandeira A, Taberner A, Eljarrat E, Barcelo D (2006) Benzoxazinoid allelochemicals in wheat: distribution among foliage, roots, and seeds. J Agric Food Chem 54:1009–1015
Waller GR (1987) Allelopathic compounds in soil from no tillage vs conventional tillage in wheat production. Plant Soil 98:5–15
Wu H, Pratley J, Lemerle D, Haig T (1999) Crop cultivars with allelopathic capability. Weed Res 39:171–180
Wu H, Pratley J, Lemerle D, Haig T (2000a) Laboratory screening for allelopathic potential of wheat (Triticum aestivum) accessions against annual ryegrass (Lolium rigidum). Aust J Agric Res 51:259–266
Wu H, Pratley J, Lemerle D, Haig T (2000b) Evaluation of seedling allelopathy in 453 wheat (Triticum aestivum) accessions by Equal-Compartment-Agar-Method. Aust J Agric Res 51:937–944
Wu H, Pratley J, Lemerle D, Haig T (2001) Allelopathy in wheat (Triticum aestivum). Ann Appl Biol 139:1–9
Wu H, Haig T, Pratley J, Lemerle D, An M (2002) Biochemical basis for wheat seedling allelopathy on the suppression of annual ryegrass (Lolium rigidum). J Agric Food Chem 50:4567–4571
Wu H, Pratley J, Haig T (2003a) Phytotoxic effects of wheat extracts on a herbicide-resistant biotype of annual ryegrass (Lolium rigidum). J Agric Food Chem 51:4610–4616
Wu H, Pratley J, Ma W, Haig T (2003b) Quantitative trait loci and molecular markers associated with wheat allelopathy. Theor Appl Genet 107:1477–1481
Young CC (1984) Autointoxication in root exudates of Asparagus officinalis L. Plant Soil 82:247–253
Young CC, Thorne RLZ, Waller GR (1989) Phytotoxic potential of soil and wheat straw in rice rotation cropping systems of subtropical Taiwan. Plant Soil 120:95–101
Yu JQ, Sen S, Ya Q, Zhu Z, Wen H (2000) Autotoxic potential of cucurbit crops. Plant Soil 223:147–151
Yu JQ (2001) Autotoxic potential of cucurbit crops: phenomenon, chemicals, mechanisms and means to overcome. J Crop Prod 4:335–348
Acknowledgements
This research is jointly funded by both Charles Sturt University (CSU) and the Australian Cooperative Research Centre for Weed Management Systems.
Author information
Authors and Affiliations
Corresponding author
Additional information
Resposible Editor: Philippe Hinsinger
Rights and permissions
About this article
Cite this article
Wu, H., Pratley, J., Lemerle, D. et al. Autotoxicity of wheat (Triticum aestivum L.) as determined by laboratory bioassays. Plant Soil 296, 85–93 (2007). https://doi.org/10.1007/s11104-007-9292-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-007-9292-7