Abstract
Experiments to assess the capability of different combinations of iron (Fe) compounds and adjuvants to provide Fe via foliar application to Fe-deficient plants have been carried out. A total of 80 formulations containing (1) one of five Fe-compounds [FeSO4·7H2O, Fe(III)-citrate, Fe(III)-Ethylenediaminetetraacetic acid (EDTA), Fe(III)-Diethylenetriamine pentaacetic acid (DTPA), Fe(III)-Iminodisuccinic acid (IDHA)], (2) a surfactant (Mistol, alkyl-polyglucoside1 or alkyl-polyglucoside2), and (3) an adjuvant (glycerol, methanol or glycine–betaine) were studied with respect to leaf wetting ability and surface tension. From the initial formulations only 26 resulted in adequate leaf wetting, 20 with alkyl-polyglucoside2 and 3 each with Mistol and alkyl-polyglucoside1, and some of them (four with alkyl-polyglucoside2, one with Mistol, and three with alkyl-polyglucoside1) were found to have inadequate surface tension values for use as foliar fertilizers. In a second experiment, 20 formulations containing alkyl-polyglucoside2 and one each of the five Fe-compounds and adjuvants listed above, were used for a foliar experiment with Fe-deficient peach trees [Prunus persica (L.) Batsch] grown under field conditions. Iron-deficient shoots were sprayed only once and leaf re-greening was assessed over 6 weeks for leaf chlorophyll content (via SPAD measurements) and percentage of green leaf area (via image analysis). Foliar Fe application always resulted in leaf Chl increases, although different degrees of re-greening were observed for the various Fe-compounds tested. Best results were obtained after treatment with formulations containing (in a decreasing order): Fe(II)-sulfate, Fe(III)-citrate, Fe(III)-EDTA, Fe(III)-IDHA, and Fe(III)-DTPA. A positive effect of adding glycerol, methanol or glycine–betaine was often observed, although the effect depended on each Fe-containing compound, indicating the existence of significant interactions between spray components. Results are of importance while trying to critically evaluate the potential of Fe sprays as a viable strategy to remedy plant Fe deficiency under field conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Iron (Fe) deficiency chlorosis is a common nutritional disorder affecting plants, and a limiting factor for fruit agricultural production in many areas of the world. Iron deficiency impairs fruit quality and yield, and can ultimately lead to tree death (Álvarez-Fernández et al. 2003, 2006). Fruit production under soil conditions leading to Fe deficiency requires continuous (every year) treatment with Fe-containing compounds. The most efficient practice to control Fe deficiency in fruit trees is currently the supply of synthetic Fe(III)-chelates to the root system, although these treatments are very costly. For instance, in the northeast of Spain ∼45,000 ha of fruit crops with high-economic value (peach and pear) require treatment with Fe(III)-chelates, increasing growers’ costs by 25 million € every year (Álvarez-Fernández et al. 2004). The possibility to deliver small amounts of Fe to fruit trees via foliar sprays could be a target-oriented, cheaper strategy to overcome Fe deficiency in fruit crops, although variable responses to Fe sprays have been often reported (see Abadía et al. 2002a; Fernández and Ebert 2005, and references therein). Development of suitable Fe spray formulations is currently hindered by the limited understanding of the mechanisms involved in the penetration, translocation, and bioavailability of the Fe-containing solutions applied to the foliage (Fernández et al. 2005).
The cuticle that covers all aerial plant parts is the limiting barrier for the exchange of water and ions between the plant and the surrounding environment (Schönherr and Schreiber 2004). The cuticle is mainly composed of cutin, cuticular waxes (intra- and epi-cuticular) and polysaccharides, and therefore shows both hydrophobic and hydrophilic properties (Popp et al. 2005). Moreover, there is wide micro-structural diversity among leaf surfaces and wax regeneration processes in living leaf surfaces also occur (Barthlott and Neinhuis 1997; Koch et al. 2004). In the last four decades, research concerning foliar uptake of agrochemicals has chiefly focused on investigating penetration trough the cuticle (Schönherr 2002). Non-charged molecules are thought to cross cuticles by dissolving and diffusing in lipophilic domains made of cutin and cuticular waxes (Schönherr et al. 2005), whereas ionic species, which are not lipid-soluble and would be subsequently excluded from the lipophilic pathway, would be capable of crossing lipid membranes only through aqueous pores (Schönherr 2000). These pores are still poorly known, although they are thought to be very small in size, with a radius of 0.45 nm found in Citrus aurantium L. polymer matrix membranes (Schönherr 1976; Schönherr and Schreiber 2004). Recently, it has been observed that the size of molecular Ca species was less limiting for ionic species (entering through aqueous pores) than for neutral species (entering through cutin and waxes) (Schönherr and Schreiber 2004). The process of cuticular water transport has been recently investigated using an experimental design based on the formation of AgCl precipitates in the cuticle polar pores (Schreiber et al. 2006). Cuticular water sorption has also been attributed to a polysaccharide fraction with a high-hydration capacity (Domínguez and Heredia 1999), which may consist of a reticulum of microfibrils ramifying and stretching through the cuticular membrane (Jeffree 1996). On the other hand, the significance of the stomatal pathway regarding the penetration of leaf-applied chemicals remains unclear, although there is evidence that it may constitute an alternative route for the uptake of foliar sprays (Currier and Dybing 1959; Eichert et al. 2002).
The pathways for Fe uptake in leaves are still poorly known (Fernández and Ebert 2005). Using different Fe-compounds and Populus x canescens cuticular membranes, Schönherr et al. (2005) observed that there was no correlation between molecular mass and penetration rates, and also that temperature (from 15 to 35°C) did not affect the penetration process. Furthermore, the permeability of cuticular membranes decreased with increasing concentrations of Fe-chelates, leading to the suggestion that Fe-chelates themselves may somehow reduce the size of aqueous pores. A reduction of the water conductance through fruit cuticles after Fe-treatments was also reported by Beyer et al. (2002) and Weichert et al. (2004). It has been suggested that 100% relative humidity (RH) would be required for a significant cuticular penetration of Fe-chelates, and that addition of hygroscopic humectants would favor foliar uptake (Schönherr et al. 2005). Evidence for the leaf penetration of several Fe-containing compounds was also obtained in attached leaves of Vicia faba L., Citrus madurensis Lour., and Nicotiana tabacum L. (Fernández 2004; Fernández et al. 2005). Working with field grown pear trees (Pyrus communis L.), Álvarez-Fernández et al. (2004) tested the re-greening effect of various foliar treatments including Fe(II)-sulfate, Fe(III)-Diethylenetriamine pentaacetic acid (DTPA), ascorbic, and citric acid. Whilst substantial chlorophyll (Chl) increments were always associated with Fe supply, the authors concluded that with the current state of knowledge, treatment with Fe sprays is still not an efficient alternative to the use of soil applied Fe chelates.
Many factors involved in the processes of Fe uptake by leaves, transport, and its distribution within the plant remain unclear, and in particular the significance of the leaf apoplast is not fully understood (Kosegarten et al. 2001; Larbi et al. 2001; Nikolic and Römheld 2003). Iron is thought to be taken up from the apoplast by leaf cells through a plasma membrane-bound Fe(III) reductase (Brüggemann et al. 1993), which is light dependent and does not increase with Fe-deficiency (Larbi et al. 2001), and may be regulated by changes in leaf apoplastic pH (Kosegarten et al. 2001; López-Millán et al. 2001). It has been hypothesized that apoplastic pH increases may depress the activity of the leaf plasma membrane-bound reductase, thereby hindering symplastic Fe uptake (Kosegarten et al. 2001; see Abadía et al. 2002b, for a review).
Given the complex scene determining the efficiency of Fe sprays and aware of the existing constraints and opportunities, the aim of this investigation was to assess the re-greening effect of optimized Fe-containing solutions applied to Fe chlorotic peach leaves under field conditions as a means to understand the mechanisms involved on the penetration and uptake of leaf-applied Fe, taking into account different Fe compounds, surfactants, and other adjuvants.
Materials and methods
Iron containing formulations for foliar sprays: iron sources, surface-active agents and adjuvants
All treatment solutions contained Fe at concentrations of 2 mM, supplied as five different Fe-compounds: Fe(II)SO4·7H2O (Panreac, Barcelona, Spain), Fe(III)-Ethylenediaminetetraacetic acid (EDTA), Fe(III)-DTPA, Fe(III)-Iminodisuccinic acid (IDHA) or Fe(III)-citrate, all of them dissolved in water type II analytical grade (obtained with an Elix-apparatus, Millipore, Bedford, MA, USA). The latter four Fe(III)-compounds were synthesized in the laboratory by complexing Fe(III) (FeCl3, acidic AAS standard, Merck, Darmstadt, Germany) with the corresponding ligand at 1:1 (Fe:ligand) ratios, excepting for Fe(III)-citrate, where the ratio was 1:20. The chelating agents employed were: K2EDTA·2H2O (Panreac), DTPA free acid (Merck), IDHA Na salt (Baypure CX 100 Solid, supplied by Lanxess, Leverkusen, Germany), and Na3-citrate·2H2O (Sigma, St. Louis, MO, USA). All solutions were adjusted to pH 5.0 to avoid altering the ion exchange properties of the cuticle (Fernández et al. 2005). excepting FeSO4·7H2O formulations, which were kept at pH 4.0 to keep Fe soluble and retarding the process of atmospheric oxidation (Fernández and Ebert 2005).
Four different surface-active agents (surfactants) were tested at a concentration of 1 g l−1: Mistol (a mixture of ionic and non-ionic surfactants, Henkel, Barcelona, Spain), Silwet L-7607 (an organo-silicon surfactant, Witco Corporation, Tarrytown, NY, USA) and two non-ionic alkyl-polyglucoside surfactants, capryl-glucoside and alkyl (8–16) glucoside, herein referred to as alkyl-glucoside1 and alkyl-glucoside2, respectively (supplied by Cognis, Düsseldorf, Germany).
Three different adjuvants were also tested: 1% glycerol (Sigma), 5% methanol (Panreac), and 5 g l−1 glycine–betaine (Fluka, Madrid, Spain). Such compounds may act as synergists or humectants and facilitate the process of leaf penetration (Hazen 2000; Schönherr et al. 2005).
Methodologies to assess the physico-chemical characteristics of potential spray formulations: wetting ability and surface tension
We tested under laboratory conditions two physico-chemical characteristics, wetting ability and surface tension, in 80 different formulations containing Fe. These formulations contained: (1) 2 mM Fe solutions, obtained with five different Fe-compounds, (2) 1 g l−1 surfactant (alkyl-glucoside1, alkyl-glucoside2, Mistol or no surfactant), and (3) an adjuvant (glycerol, methanol, glycine–betaine or no adjuvant). All 80 possible combinations, including the Fe-compounds alone, were freshly prepared prior to measurement.
Estimates of the rate of wetting (integrating wetting, spreading, and retention) were obtained by dipping in the solutions fully expanded leaves, with a similar maturity stage, of the shrub Evonymus japonicus L. Leaves were harvested in the garden of the Aula Dei Experimental Station, CSIC (Zaragoza, Spain). These leaves are coriaceous and non-wettable, constituting therefore a good model for a worst-case scenario. Peach leaves are more wettable, but leaves of other fruit tree species affected by Fe deficiency such as citrus and pear would perform similarly to leaves of E. japonicus. Once the leaf was dipped in the solution, the average degree of wetting was recorded according to a visual rating scale. The wetting performance of formulations was classified in four grades as follows: (0) leaf completely dry, (1) ∼two-thirds of the leaf surface wet, (2) leaf wet, but with some dry areas, and (3) leaf completely wet. Six homogeneous E. japonicus leaves were used per treatment.
Also, the surface tension of the formulations was determined with a torsion balance apparatus for surface and interfacial tension measurement (Model OS, Worcs, UK). Formulations tested included the 80 previously screened in the wetting experiment, plus 20 more prepared with the surfactant Silwet L-7607, used as a low-surface tension check. Four surface tension measurements were taken per formulation.
Field plant material
Twenty-year-old peach trees [Prunus persica (L.) Batsch, cv. Babygold 10, grafted on seedling] grown on a flood-irrigated calcareous soil (Typical xerofluvent, clay-loamy texture, with 31% total CaCO3, 9.9% active CaCO3, 7 mg kg−1 DTPA-extractable Fe, 2.86% organic matter, and pH 8.0 in water) were used. The orchard was located in the Aula Dei Experimental Station, CSIC, had a frame of 3 × 4 m2, and was appropriately maintained in terms of nutrition, pruning, and pest and disease control. However, trees did not receive any exogenous Fe input for 2 years prior to the beginning of the foliar fertilization trial, and therefore developed Fe deficiency symptoms in springtime. The experiment was designed as a completely randomized block. Six trees with a similar leaf chlorosis level (average SPAD value of 14, corresponding to ∼100 μmol Chl m−2) were selected at the beginning of the trial.
Spray treatments with Fe formulations in peach trees growing in the field
From the analysis of the physico-chemical properties it was decided to use in field foliar spray trials only formulations containing alkyl-glucoside2, since they had a good wetting ability, low-surface tension values, and minimal interactions with Fe-compounds. All these selected formulations had wetting rates of three and relatively low-surface tension values.
The re-greening effects of different Fe-compounds and adjuvants, using always alkyl-glucoside2 as a surfactant, were evaluated for a 6-week period after a single foliar spray of 20 different formulations containing 1 g l−1 alkyl-glucoside2. Iron was supplied at 2 mM concentrations as Fe(II)-sulfate, Fe(III)-citrate, Fe(III)-EDTA, Fe(III)-DTPA or Fe(III)-IDHA. Also, three adjuvants (1% glycerol, 5% methanol, and 5 g l−1 glycine–betaine) were evaluated, as compared to pure Fe-compound sprays. Solutions containing only adjuvants and alkyl-glucoside2 as well as pure water were also applied as controls.
Leaf sprays were applied with a commercial hand sprayer, both on the adaxial and abaxial leaf surface, until full wetting (i.e., until solution run-off). Peach leaves are known to have stomata only in the abaxial surface, and this was confirmed by our own microscopic observations. However, both sides were treated to mimic usual field spraying techniques. Treatments were applied from 6:00 to 8:00 solar time on June 20th, 2005 (a sunny day, with 70% RH and 20°C at the time of treatment). Each of the five Fe-compounds was applied to one specific tree, which was separated from other Fe-treatments by at least one non-treated tree to avoid cross-contamination between Fe-compounds. In each tree, four different southwest oriented, sun exposed vegetative shoots (not bearing fruits) were treated, each with a different adjuvant (glycerol, methanol, glycine-betaine or no adjuvant). A separate tree was used to perform adjuvant-only (no Fe) treatments in different branches as controls.
Methodologies to estimate leaf re-greening: SPAD and image analysis
Leaf re-greening after treatment was assessed by two different methods: (1) measuring the degree of intensity of leaf re-greening (using a SPAD apparatus) and (2) estimating the percentage of green area having experienced significant re-greening (using a photo scanner and image analysis software). Each methodology gives a different assessment of the re-greening process.
In the first methodology, leaf Chl was monitored non-destructively with a SPAD apparatus (Minolta 502, Osaka, Japan), 1 day before spray application and then on a weekly basis during 6 weeks. This technique estimates the Chl concentration from red light absorbance measurements in a column-shaped cross-section of the leaf, with a 6 mm2 base surface. To account for leaf heterogeneity, averaged SPAD measurements from four different locations were taken per leaf. Seven different leaves across the same shoot (excluding non-fully developed leaves) were monitored per treatment. The SPAD method was calibrated by extracting pigments from leaf disks (previously measured with the SPAD apparatus) with pure acetone and then measuring Chl spectrophotometrically in the extracts (Abadía and Abadía 1993). The calibration curve correlating Chl concentration in peach leaves with SPAD values was: Y = −0.0002X 2 + 0.1942X-4.865 (R 2 = 0.992; Y and X were in SPAD units and Chl in μmol m−2, respectively).
A second methodology to assess leaf re-greening was based on the measurement of green areas as a percentage of the total leaf surface at the end of the experiment, by scanning (with an Epson Perfection 4870 Photo Scanner), once detached, the same leaves used for SPAD readings in each treatment, and analyzing the digital images obtained. The color distribution of the obtained images was analyzed with the image analysis application Carnoy Version 2.1 for Mac OS X (Schols et al. 2002). In each scanned leaf photograph, total surface was determined by calibrating the image size by standard image measurement techniques. Then, green areas were measured in each leaf following a similar procedure, by selecting an appropriate gray-scale threshold value. The resulting image was compared with the initial color image to ensure that the discrimination of colors was carried out appropriately. The green and total leaf area of seven leaves per shoot was measured, and the image analysis was performed twice per leaf image.
Statistical analysis
Data were statistically evaluated by two-way analysis of variance (ANOVA) with the program SPSS 11.0 to assess the significance of the main factors and the significance of interactions. Means were also compared using Duncan’s test at P < 0.05 in order to find significant differences between treatments.
Results
Iron formulations differ in leaf wetting ability and surface tension
A total of 80 Fe-containing formulations were prepared, based on all potential combinations, including: (1) different Fe-compounds at an Fe concentration of 2 mM (FeSO4·7H2O, Fe(III)-citrate, Fe(III)-EDTA, Fe(III)-DTPA, and Fe(III)-IDHA); (2) different surfactants (alkyl-glucoside1, alkyl-glucoside2, Mistol, and no surfactant), and (3) different adjuvants (glycerol, methanol, glycine–betaine, and no adjuvant). The degree of wetting was visually assessed as four different grades: full wetting (3), some dry areas (2), one-third of the leaf dry (1), and no wetting at all (0). Optimal leaf wetting was observed with all 20 alkyl-glucoside2-solutions, whereas only three alkyl-glucoside1- and three Mistol-containing formulations led to full leaf surface wetting (Table 1). The three formulations giving optimal wetting with Mistol were Fe sulfate/betaine, Fe(III)-EDTA/methanol, and Fe(III)-DTPA/methanol. The three formulations giving optimal wetting with alkyl-glucoside1 were Fe(III)-DTPA/methanol, Fe(III)-DTPA/no adjuvant, and Fe(III)-IDHA/glycerol. In all cases, solutions containing no surface-active agents had a wetting rate of zero.
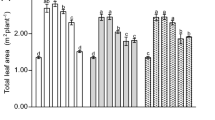
Surface tension measurements were carried out immediately after solution preparation (Fig. 1). Formulations tested were the 80 used in the wetting experiment plus 20 more built with the surfactant Silwet L-7607, used as a low-surface tension check. In the absence of surface-active agents, Fe-containing solutions had surface tension values similar to that of distilled water (i.e., above 60 mN m−1), significantly higher than those measured for all surfactant-containing formulations. According to the hypothesis of Schönherr and Bukovac (1972), a threshold value of 30 mN m−1 should be considered limiting for wetting purposes. Addition of both Mistol and alkyl-glucoside1 at rates of 1 g l−1 caused significant decreases in surface tension, which were markedly affected by the nature of the Fe-compound. When using Mistol, Fe(III)-EDTA, and Fe(III)-IDHA solutions still had a surface tension higher than 34 mN m−1, indicating the occurrence of interactions between the ionic surfactant and the negatively-charged Fe(III)-chelate molecules, whereas solutions including the other three Fe-compounds led to surface tension values of ∼30 mN m−1. The non-ionic surface active agent alkyl-glucoside1 also provided low-surface tension values (∼30 mN m−1) with three Fe compounds, whereas tensions of ∼36 mN m−1 were measured for Fe(III)-IDHA, and Fe(III)-DTPA formulations, indicating the occurrence of interactions with the two latter Fe-compounds. Alkyl-glucoside2 gave low-surface tensions with most Fe-containing formulations (∼30 mN m−1), excepting for Fe(III)-DTPA solutions, which had a tension of 34 mN m−1. Finally, significantly lower surface tension values (∼24 mN m−1) were measured for all Fe-compounds when using the organo-silicon surfactant Silwet L-7607. These surface tension values are similar to those reported by Knoche et al. (1991) and Neumann and Prinz (1975). Addition of glycerol, methanol or glycine-betaine did not alter significantly surface tension of solutions including Fe-compounds and surfactants (data not shown).
Surface tension of Fe-containing solutions (Fe(II)-sulfate, Fe(III)-citrate, Fe(III)-EDTA, Fe(III)-DTPA, and Fe(III)-IDHA) plus 1 g l−1 surfactant (Mistol, alkyl-glucoside1, alkyl-glucoside2, Silwet L-7607 or no surfactant, respectively). Solutions were freshly prepared and surface tension was measured immediately. Results are means ± SD (n = 4)
In conclusion, alkyl-glucoside2 was selected as the most suitable surface-active agent for foliar spray formulations among those tested, since in most cases it provided both optimal wetting and low-surface tension. Silwet L-7607 was not chosen since it has been reported to degrade at pH values below five (Knoche et al. 1991) and is also known to markedly interact with Fe compounds such as Fe(III)-citrate (Neumann and Prinz 1975), in both cases leading to a rapid increase in surface tension over time after solution preparation.
The effect of different concentrations of alkyl-glucoside2 on surface tension was also assessed (Fig. 2). Surface tension decreased linearly from concentrations of 0.001 to ∼0.02% alkyl-glucoside2. Concentrations higher than 0.02% and up to 5% led to a surface tension of ∼30 mN m−1. Therefore, an alkyl-glucoside2 concentration of 0.1% (1 g l−1) was used in further experiments; this concentration is in agreement with the values suggested elsewhere for other surfactants (Jansen et al. 1961; Schönherr 2001).
Peach leaf re-greening effects of a single spray application with different Fe-compounds and adjuvants
The re-greening effects of a single foliar spray with different Fe-compounds and adjuvants, using always alkyl-glucoside2 as a surfactant, are shown in Tables 2 and 3. The two-way ANOVA (Table 2) showed that the effects of Fe compounds on three parameters used to assess leaf re-greening, i.e., final leaf Chl concentration and Chl increase (both measured with a SPAD meter) and final percentage of green leaf area (estimated via image analysis) were highly significant (P ≤ 0.001). The best Fe compound used was Fe(II)-sulfate, with other compounds being less effective, whereas no significant re-greening was obtained in the absence of Fe in the spray formulations (Table 3). The highest Chl increases 6 weeks after treatment were found with Fe(II)-sulfate, followed by Fe(III)-citrate, Fe(III)-IDHA, Fe(III)-EDTA, Fe(III)-DTPA, and alkyl-glucoside2-only (Table 3). When comparing the extent of the re-greened surface the ranking was quite similar, with the highest values being found with Fe(II)-sulfate, followed by Fe(III)-citrate, Fe(III)-EDTA, Fe(III)-DTPA, Fe(III)-IDHA, and alkyl-glucoside2-only (Table 3). In leaves treated with surfactant and adjuvants but without Fe, a significant part of the surface could be still considered as green (21% of the total surface, corresponding to the major leaf veins), a percentage similar to that found in Fe-deficient, untreated leaves (not shown).
The effect of the different adjuvants, on the other hand, was only significant at P ≤ 0.05 when considering the final Chl increase and the percentage of green leaf area (Table 3). A strong interaction (P ≤ 0.001) between the Fe-compounds and adjuvants was also found (see below for a detailed description) (Table 2).
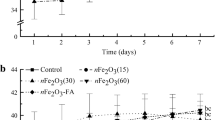
The time-course of re-greening after the foliar treatment with different Fe-containing compounds, expressed as a percentage increase in relation to the initial Chl values, is shown in Fig. 3. With all Fe-containing compounds, leaf Chl concentrations increased gradually in the first 2 weeks after the treatment, and in the case of Fe(II)-sulfate the increase continued for two more weeks (Fig. 3). In contrast, in leaves sprayed with Fe-free solutions Chl increased only slightly (less than 10%) in the first week and showed a decrease from week three on, to show at the end of the experimental period a 5% increase as compared to the initial values.
Evolution of the leaf Chl increases in Fe-deficient peach leaves after treatment with Fe sprays (Fe(II)-sulfate, Fe(III)-citrate, Fe(III)-EDTA, Fe(III)-DTPA, and Fe(III)-IDHA) plus 0.1% alkyl-glucoside2. Data represent mean Chl increases (%) in relation to the initial Chl concentration for each Fe treatment. Results are the average of the four adjuvant treatments for a given Fe-carrier ± SD (n = 28)
Interactions between Fe compounds and adjuvants in the re-greening of peach leaves
The interactions between the different Fe-compounds (all of the applied with alkyl-glucoside2) and adjuvants (glycerol, methanol, glycine–betaine or none) on both the leaf Chl level and the percentage of green leaf area are shown in detail in Fig. 4. Data indicate that the interactions between Fe-compounds and adjuvants were very marked.
Final Chl increase (measured via SPAD measurements) and green area per leaf (estimated via image analysis), 6 weeks after treatment with several Fe-compounds (from top to bottom, Fe(II)-sulfate, Fe(III)-citrate, Fe(III)-EDTA, Fe(III)-DTPA, and Fe(III)-IDHA) and different adjuvants (none, glycerol, methanol or glycine-betaine). All treatment solutions contained the surfactant alkyl-glucoside2 at a concentration of 0.1%. It should be kept in mind that in Fe-deficient, untreated leaves the percentage of green area was ∼21%. Values are means ± SD (n = 28)
The best re-greening results were obtained with Fe(II)-sulfate. When using this compound, the best re-greening effect (a 95 μmol m−2 increase in Chl with 78% of the leaf green at the end of the experiment) was obtained using the adjuvant methanol. However, glycerol and glycine–betaine also led to green leaf surface values of ∼80%, a value higher than that obtained without adjuvants (56%), although the intensity of the re-greening obtained did not reach that obtained with methanol. Even when using no adjuvants, Fe sulfate led to major increases in both Chl content and percentage of green area over the values found in the Fe-untreated controls. It should be kept in mind that in Fe-deficient, untreated leaves the percentage of green area was ∼21%.
Iron(III)-citrate was quite effective in increasing the percentage of green surface, with all formulations leading to values in the range 62–74%. However, this Fe-source did not always lead to large Chl concentration increases. When considering the Chl increase the best formulation was that containing glycerol, followed by the formulation not containing adjuvants. Both methanol and glycine-betaine led to smaller Chl increases.
Iron(III) synthetic chelates were generally less effective than Fe(II)-sulfate. Using Fe(III)-EDTA led to the best results, either without adjuvants or with glycerol and glycine–betaine, when considering the percentage of green leaf area (56–68%), whereas methanol had a detrimental effect when compared to the treatment not including adjuvants. However, using glycerol and glycine-betaine led to higher increases in Chl content as compared to the no-adjuvant control. The fact that the treatment with no adjuvants produced an increase in the green area with almost no increase in total Chl content suggests that the re-greening was only superficial. In this case, methanol did slightly increase the Chl content.
The chelates Fe(III)-DTPA and Fe(III)-IDHA were even less effective than Fe(III)-EDTA. Using Fe(III)-DTPA, all formulations led to similar percentages of green area (41–40%). However, only the addition of methanol increased significantly the leaf Chl content as compared to the rest of treatments. With regard to Fe(III)-IDHA, the application of glycerol led to the highest green area rates (57%), followed by glycine–betaine and methanol (48 and 43%, respectively). Glycerol also induced the highest Chl increases with Fe(III)-IDHA, followed by the formulation containing glycine–betaine. In contrast, application of Fe(III)-IDHA alone or in combination with methanol led to very small Chl increases.
Discussion
Clear evidence for the re-greening of Fe-deficient peach leaves was gained after a single treatment with different foliar Fe treatments. Despite the variable plant responses to foliar Fe fertilization reported in the literature (see review by Fernández and Ebert 2005), several investigations have described the beneficial effects of foliar Fe sprays application to Fe-deficient fruit crops such as citrus, pear, peach, apple, mango, plum, and almond, in terms of increasing leaf Chl concentration and improving fruit yield and quality (Kadman and Gazit 1984; Sanz et al. 1992; Abadía et al. 2002a; Álvarez-Fernández et al. 2004, 2006). Results obtained in the present study support the idea that maximizing the chances for leaf penetration via optimizing spray formulations and application practices may improve significantly the efficiency of foliar Fe fertilization.
One of the conclusions of this investigation is that the physico-chemical characteristics must be taken into account when carrying out foliar spray studies. Out of the initial 80 possible Fe-compound/surfactant/adjuvant combinations, only 26 can be considered appropriate for foliar sprays with respect to optimal leaf wetting, and eight of them (four with alkylglucoside2, three with alkylglucoside1, and one more with Mistol) had values higher than 30 mN m−1 (Fig. 1). A low-surface tension will enable an intimate contact between the leaf surface and the solution, as well as facilitate the spontaneous infiltration of stomatal cavities (Schönherr and Bukovac 1972) and other possible hydrophilic domains of the cuticular layer. To our knowledge, this is the first study in which of the physico-chemical properties of Fe sprays have been considered a priori as key factors determining the success of foliar Fe fertilization.
Among the surfactants tested, alkyl-glucoside2, a non-ionic, alkyl (8–16) glucoside, was found to provide the best physico-chemical properties for use in foliar Fe fertilizer formulations, with optimal characteristics for leaf wetting and good surface tension values (excepting for formulations including Fe(III)-DTPA), as well as providing a relatively low degree of deleterious interactions with Fe-compounds. This non-phytotoxic, biodegradable surfactant has not been used before, to our knowledge, in Fe foliar fertilization studies. Results indicate that this surfactant and other similar compounds could improve markedly the effectiveness of Fe foliar fertilization.
With regard to the Fe-containing compounds applied in this study, the best results were recorded for Fe(II)-sulfate supplemented with alkyl-glucoside2 and methanol, with other Fe(II)-sulfate formulations giving also good results. The mechanism of leaf Fe(II) penetration is still unknown, although the Fe(II)-ion has been recently shown to penetrate V. faba leaves at a higher rate than most of the Fe-compounds tested (Fernández et al. 2005). Iron sulfate was also the best Fe-compound in field foliar fertilization experiments carried out with pear trees (Álvarez-Fernández et al. 2004). The low-molecular mass of the Fe(II) ion (56 g mol−1) may facilitate foliar penetration via stomata or hydrophilic cuticular pathways, although in theory it may readily precipitate and/or interact with the negative charges of cuticular and apoplastic components. Iron(II) may be oxidized and then chelated in the apoplast by endogenous ligands such as citrate or other organic acids and nicotianamine. Subsequently, it may follow reduction by the plasma membrane Fe(III)-chelate reductase, or may directly enter the cell via a Fe(II)-transporters as suggested by Álvarez-Fernández et al. (2004).
The second best re-greening Fe-compound was Fe(III)-citrate (245 g mol−1 molecular mass) alone or in combination with glycerol, followed by Fe(III)-EDTA (428 g mol−1) also in the presence of glycerol. Both Fe-compounds have been reported to penetrate bean leaves, but at a lower rate than Fe(II)-sulfate (Fernández et al. 2005). Iron(III)-citrate foliar application has been found to induce positive effects in pear (Álvarez-Fernández et al. 2004) and bean (Fernández et al. 2005). Iron(III)-DTPA and Fe(III)-IDHA, with molecular masses of 449 and 392 g mol−1, respectively, also induced leaf re-greening but to a lower extent than the rest of Fe-compounds tested. The poor effect of Fe(III)-DTPA formulations is likely associated with their relatively high-surface tension values. The lack of correlation found between the molecular mass of leaf-applied Fe-chelates and the re-greening efficiency is in agreement with recent findings (Fernández 2004; Schönherr et al. 2005), and would be in line with some mechanisms proposed for foliar uptake, including leaf penetration via stomata or through cuticular hydrophilic pathways. Molecular mass has also been shown to be less limiting for Ca-containing compounds when diffusion takes place through hydrophilic than through lipophilic domains (Schönherr and Schreiber 2004).
Interactions between Fe-compounds, surfactants, and adjuvants are extremely important, and in many cases they could inactivate the possible effect of foliar fertilizer preparations. First, significant interactions were seen when measuring surface tensions. For instance, formulations including Fe(III)-IDHA/Mistol, Fe(III)-IDHA/alkyl-glucoside1, Fe(III)-DTPA/alkyl-glucoside1, and DTPA/alkyl-glucoside1 had surface tension values higher than 33 mN m−1. A loss in surface tension and leaf wetting in the presence of Fe-containing compounds has also been described for the surfactant Silwet L-77 (Neumann and Prinz 1975; Horesh and Levy 1981; Knoche et al. 1991). Whereas interactions between the ionic Mistol molecules and the negatively charged Fe-chelates or Fe-salts can be expected, the nature of the interactions between the non-ionic akyl-polyglucoside surfactants and some of the Fe-compounds used remains unclear and deserves further study. Second, major interactions between the various Fe-compounds and adjuvants in the presence of alkyl-glucoside2 on peach leaf re-greening have also been observed, as summarized in Table 4. Using all three adjuvants in addition to alkyl-glucoside2 induced significant beneficial effects, depending on every particular Fe-carrier, whereas treatment with Fe-free adjuvant solutions did neither increase the percentage of leaf green area nor induce Chl content increases. In association with Fe(II)-sulfate, methanol promoted a more intense Chl increase than that found with glycerol and glycine–betaine, without causing any supplementary effect on the extent of leaf surface undergoing re-greening, suggesting that the addition of methanol facilitates Fe availability to cells located deep inside the leaf. A beneficial effect of applying diluted methanol solutions in combination with foliar Fe sprays has been described by Nonomura et al. (1995).
Mechanisms acting during the process of plant cuticular penetration of Fe-compounds include interactions of a variable nature (e.g., electro-chemical or osmotic), which may induce the clogging of hydrophilic pathways (Beyer et al. 2002; Weichert et al. 2004), as supported by the relatively lower penetration rate of more concentrated versus more diluted Fe-containing solutions (Fernández 2004; Schönherr et al. 2005). Consequently, all Fe-compounds were applied in this study at a relatively low concentration (i.e., 2 mM Fe), in contrast to most published Fe spray trials (Fernández and Ebert 2005). Also, and whereas it has been concluded that 100% RH would be required for the penetration of Fe-chelates (Schönherr et al. 2005), a physiological response has been observed in this study for all Fe-chelates applied to chlorotic peach leaves under field conditions, confirming previous reports with other crops (Rombolà et al. 2000; Abadía et al. 2002 a, b; Álvarez-Fernández et al. 2004). These results may imply that a successful leaf penetration of Fe could have taken place during droplet drying. The adjuvants tested in this trial caused significant improvements on the performance of Fe-containing solutions, probably by facilitating the leaf penetration process in terms of “plasticizing” (solubilizing) the cuticle (methanol) or maintaining Fe sprays in a liquid form for a longer period due to their hydroscopicity (glycerol and glycine–betaine).
Some treatments, such as Fe(III)-EDTA with alkylglucoside2 and without adjuvants, induced increases in the percentage of green leaf area without leading to significant increases in the leaf Chl content. This would suggest that re-greening in these treatments was only superficial, in good agreement with the general belief of growers that in many cases only a “painting” effect is achieved after foliar Fe treatments. This finding also indicates that SPAD and image analysis are complementary methods to assess re-greening.
In conclusion, re-greening of Fe-deficient peach leaves was achieved in this study after foliar treatment with some optimized Fe-sprays, and addition of adjuvants to Fe sprays significantly improved leaf re-greening as compared to Fe-carrier solutions alone. Since the process of leaf penetration and subsequent Fe delivery to the cell is very complex, further trials to investigate the mechanisms involved in foliar Fe penetration and leaf cell uptake should be carried out in the future. These experiments should consider the possibility of stomatal and cuticular Fe penetration using Fe-deficient leaves, as well as introduce new techniques to investigate the spatial characteristics of the uptake of Fe-substances, including microanalysis of Fe-compounds and others. These studies will help optimizing Fe spray formulations to make foliar fertilization a reliable strategy in the future to control fruit tree Fe deficiency.
Abbreviations
- EDTA:
-
Ethylenediaminetetraacetic acid
- DTPA:
-
Diethylenetriamine pentaacetic acid
- IDHA:
-
Iminodisuccinic acid
- Chl:
-
Chlorophyll
- RH:
-
Relative humidity
References
Abadía J, Abadía A (1993) Iron and plant pigments. In: Barton LL, Hemming BC (eds) Iron chelation in plants and soil microorganisms. Academic Press, New York, pp 327–343. ISBN 0-12-079870-0
Abadía J, Álvarez-Fernández A, Morales F, Sanz M, Abadía A (2002a) Correction of iron chlorosis by foliar sprays. Acta Hortic 594:115–121
Abadía J, López-Millán A-F, Rombolà A D, Abadía A (2002b) Organic acids and Fe deficiency: a review. Plant Soil 241:75–86
Álvarez-Fernández A, Paniagua P, Abadía J, Abadía A (2003) Effects of Fe deficiency chlorosis on yield and fruit quality in peach (Prunus persica L. Batsch). J Agric Food Chem 51:5738–5744
Álvarez-Fernández A, García-Laviña P, Fidalgo J, Abadía J, Abadía A (2004) Foliar fertilization to control iron chlorosis in pear (Pyrus communis L.) trees. Plant Soil 263:5–15
Álvarez-Fernández A, Abadía J, Abadía A (2006) Iron deficiency, fruit yield and quality. In: Barton LL, Abadía J (eds) Iron nutrition in plants and rizospheric microorganisms. Springer, Dordrecht, The Netherlands, pp 85–101, ISBN-10 1-4020-4742-8
Barthlott W, Neinhuis C (1997) Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 202:1–8
Beyer M, Peschel S, Weichert H, Knoche M (2002) Studies on water transport through the sweet cherry fruit surface: VII Fe3+ and Al3+ reduce conductance for water uptake. J Agric Food Chem 50:7600–7608
Brüggemann W, Maas-Kantel K, Moog PR (1993) Iron uptake by leaf mesophyll cells: the role of the plasma-membrane bound ferric-chelate reductase. Planta 190:151–155
Currier HB, Dybing CD (1959) Foliar penetration of herbicides. Review and present status. Weeds 7:195–213
Domínguez E, Heredia A (1999) Water hydration in cutinized cell walls: a physico-chemical analysis. Biochim Biophys Acta 1426:168–176
Eichert T, Burkhardt J, Goldbach HE (2002) Some factors controlling stomatal uptake. Acta Hortic 594:85–90
Fernández V (2004) Investigations on foliar iron application to plants—a new approach. Shaker Verlag, Aachen, Germany, 171pp
Fernández V, Ebert G (2005) Foliar iron fertilization—a critical review. J Plant Nutr 28:2113–2124
Fernández V, Ebert G, Winkelmann G (2005) The use of microbial siderophores for foliar iron application studies. Plant Soil 272:245–252
Hazen JL (2000) Adjuvants—terminology, classification, and chemistry. Weed Technol 14:773–784
Horesh I, Levy Y (1981) Response of iron-deficient citrus trees to foliar iron sprays with a low-surface-tension surfactant. Sci Hortic 15:227–233
Jansen LL, Gentner WA, Shaw WC (1961) Effect of surfactants on the herbicidal activity of several herbicides in aqueous spray systems. Weeds 9:381–405
Jeffree CE (1996) Structure and ontogeny of plant cuticles. In: Kerstiens G (ed), Plant cuticles: an integrated functional approach. Bios Scientific Publishers, Oxford, UK, pp 33–82
Kadman A, Gazit S (1984) The problem of iron deficiency in mango trees and experiments to cure it in Israel. J Plant Nutr 7:283–290
Knoche M, Tamura H, Bukovac MJ (1991) Stability of the organosilicone surfactant Silwet L-77 in growth regulator sprays. HortScience 26:1498–1500
Koch K, Neinhuis C, Ensikat HJ, Barthlott W (2004) Self assembly of epicuticular waxes on living plant surfaces imaged by atomic force microscopy (AFM). J Exp Bot 55:711–718
Kosegarten H, Hoffmann B, Mengel K (2001) The paramount influence of nitrate in increasing apoplastic pH of young sunflower leaves to induce Fe deficiency chlorosis, and the re-greening effect brought about by acidic foliar sprays. J Plant Nutr Soil Sci 164:155–163
Larbi A, Morales F, López-Millán AF, Gogorcena Y, Abadía A, Moog PR, Abadía J (2001) Technical advance: reduction of Fe(III)-chelates by mesophyll leaf disks of sugar beet. Multi-component origin and effects of Fe deficiency. Plant Cell Physiol 42:94–105
López-Millán AF, Morales F, Abadía A, Abadía J (2001) Changes induced by iron deficiency in the composition of the leaf apoplastic fluid from field-grown pear (Pyrus communis L.) trees. J Exp Bot 52:1489–1498
Neumann M, Prinz R (1975) The reduction by surfactants of leaf burn resulting from foliar sprays and a salt-induced inhibition of the effect. J Sci Food Agric 26:909–914
Nikolic M, Römheld V (2003) Nitrate does not result in iron inactivation in the apoplast of sunflower leaves. Plant Physiol 132:1303–1314
Nonomura AM, Nishio JN, Benson AA (1995) Stimulated growth and correction of Fe-deficiency with trunk- and foliar-applied methanol-soluble nutrient amendments. In: Abadía J (ed) Iron nutrition of soils and plants. Kluwer Academic Publishers, Dordrecht, pp 329–333
Popp C, Burghardt M, Friedmann A, Rieder M (2005) Characterization of hydrophilic and lipophilic pathways of Hedera helix L. cuticular membranes: permeation of water and uncharged organic compounds. J Exp Bot 56:2797–2806
Rombolà AD, Brüggemann W, Tagliavini M, Marangoni B, Moog PR (2000) Iron source affects iron reduction and re-greening of kiwifruit (Actinidia deliciosa) leaves. J Plant Nutr 23:1751–1765
Sanz M, Cavero J, Abadía J (1992) Iron chlorosis in the Ebro River Basin, Spain. J Plant Nutr 15:1971–1981
Schols P, Dessein S, D’Hondt K, Huysmans S, Smets E (2002) Carnoy: a new digital measurement tool for palynology. Grana 41:124–126
Schönherr J (1976) Water permeability of isolated cuticular membranes: the effect of pH and cations on diffusion, hydrodynamic permeability and size of polar pores in the cutin matrix. Planta 128:113–126
Schönherr J (2000) Calcium chloride penetrates plant cuticles via aqueous pores. Planta 212:112–118
Schönherr J (2001) Cuticular penetration of calcium salts: effects of humidity, anions, and adjuvants. J Plant Nutr Soil Sci 164:225–231
Schönherr J (2002) A mechanistic analysis of penetration of glyphosate salts across astomatous cuticular membranes. Pest Manag Sci 58:343–351
Schönherr J, Bukovac M (1972) Penetration of stomata by liquids. Dependence on surface tension, wettability and stomatal morphology. Plant Physiol 49:813–819
Schönherr J, Schreiber L (2004) Size selectivity of aqueous pores in astomatous cuticular membranes isolated from Populus canescens (Aiton) Sm. leaves. Planta 219:405–411
Schönherr J, Fernández V, Schreiber L (2005) Rates of cuticular penetration of chelated FeIII: role of humidity, concentration, adjuvants, temperature and type of chelate. J Agric Food Chem 53:4484–4492
Schreiber L, Elshatshat S, Koch K, Lin J, Santrucek J (2006) AgCl precipitates in isolated cuticular membranes reduce rates of cuticular transpiration. Planta 223:283–290
Weichert H, Von Jagemann C, Peschel S, Knoche M, Neumann D, Erfurth W (2004) Studies on water transport through the sweet cherry fruit surface: VIII. Effect of selected cations on water uptake and fruit cracking. J Am Soc Hortic Sci 129:781–788
Acknowledgements
This study was supported by the Spanish Ministry of Science and Education (Projects AGL2003-1999 and AGL2004-0194, co-financed with FEDER) and the Commission of European Communities (project Isafruit). V. Fernández was supported by a “I3P” post-doctoral contract financed by the CSIC, co-financed by the European Social Fund. We would like to thank L.M. Cerecedo (Centro Politécnico Superior, University of Zaragoza, Spain) and S. Jiménez-Tarodo for their support to carry out surface tension measurements and statistical analyses, respectively. Thanks are given to Lanxess and Cognis for providing free sample products for experimental purposes.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fernández, V., Del Río, V., Abadía, J. et al. Foliar Iron Fertilization of Peach (Prunus persica (L.) Batsch): Effects of Iron Compounds, Surfactants and Other Adjuvants. Plant Soil 289, 239–252 (2006). https://doi.org/10.1007/s11104-006-9132-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-006-9132-1