Abstract
A wide range of molecules are transported across membranes by the ATP binding cassette (ABC) transporters. Plants possess a collection of ABC proteins bearing similarities to the components of prokaryotic multi subunit ABC transporters, designed as ABC group I. However the functions of most of them are not well understood. Here, we characterized a naturally occurring rice mutant that exhibited albino phenotype under continuous rainy days in the field, but gradually recovered to normal green after the rainy season. Molecular and genetic analyses revealed that the phenotypes were caused by a mutation in the OsABCI8 that encoded a member of the ABCI family. Subcellular localization demonstrated that OsABCI8 is a chloroplast ABC transporter. Expression of OsABCI8 is significantly enhanced in rainy days compared to sunny days. Besides defects in chloroplast development and chlorophyll biosynthesis, the mutant phenotype is accompanied by a higher accumulation of iron, suggesting that OsABCI8 is involved in iron transportation and/or homeostasis in rice. Our results demonstrate that OsABCI8 represents a conserved ABCI protein involved in transition metals transportation and/or homeostasis and suggest an important role of the plastid-localized OsABCI8 for chloroplast development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chloroplasts are one of the most important plastids in plants. They not only are the site of photosynthesis, where solar energy is converted to oxygen and chemical energy stores in the form of sugars, but also the site for biosynthesis of chlorophyll, haem and other tetrapyrroles as well as initiation of abscisic acid, gibberellin and oxylipin biosynthesis (Neuhaus and Emes 2000). Chlorophyll biosynthesis shares early steps from the first committed precursor 5-aminolevulinic acid (ALA) to protoporphyrin IX with that of heme in the tetrapyrrole biosynthetic pathway (Tanaka and Tanaka 2007). Two molecules of ALA are condensed to form a porphobilinogen by ALA dehydratase. After sequential enzymatic conversions, the pathway is divided by metal chelation reactions of protoporphyrin IX, thereby directing the formation of the end products chlorophyll and heme (Tanaka and Tanaka 2007). It is evident that ALA formation is a key point for regulation of chlorophyll biosynthesis (Ilag et al. 1994; Tang et al. 2012).
To understand chloroplast biogenesis and development, various chloroplast-defective mutants have been identified and characterized from multiple plant species including rice, Arabidopsis, Oenothera (evening primrose) (Sakamoto et al. 2008; Hayashi-Tsugane et al. 2014; Massouh et al. 2016). Analyses of albino mutants provide important insights into the mechanisms of plastid development. Among them, green-revertible albino mutants are a new type of leaf-color mutants. These mutants are albino in the seedling stage, but turn to normal green color as the plants develop or as the environment changes, allowing the mutants to reach maturity and produce seeds. For instance, rice young seedling albino (YSA) mutant developed albino leaves before the three-leaf stage, but gradually turns green and recovers to normal green at the six-leaf stage (Su et al. 2012). The YSA mutant is associated with a change in chlorophyll content and chloroplast development (Su et al. 2012). Map-based cloning revealed that YSA encodes a pentatricopeptide-repeat protein (PPR). The v1 ~ 4 (virescent-1 ~ 4), wlp1 (white leaf and panicles1), and tcd9 (thermo-sensitive chloroplast development 9) are temperature-sensitive conditional chloroplast-deficient mutants (Kusumi and Iba 2014). They develop chlorotic leaves when germinated at low temperature but nearly normal green leaves at higher temperature. After they emerge, the leaf phenotype is no longer influenced by growth temperature. So far, more than 200 leaf-color mutants have been identified in rice, however, only about a quarter of the mutant genes have been identified (Deng et al. 2014).
The ATP-binding cassette (ABC) transporter proteins belong to a large, diverse and ubiquitous superfamily (Rea 2007). The main function of ABC transporter proteins is to transport a wide range of molecules across membranes. In plants, the ABC transporter genes are particularly abundant. The Arabidopsis and rice genomes encode 130 and 132 members, respectively, categorized into families A to G based on their homologies to the eukaryotic orthologs (Verrier et al. 2008). In addition, plants contain a collection of ABC proteins bearing similarities to the components of prokaryotic multisubunit ABC transporters, designed as ABC group I. The classical ABC transporters contain two units of transmembrane domain (TMDs) and nucleotide-binding domain (NBD) that contain conserved Walker motif and the ABC signature motif, the H loop and the Q loop, respectively. By contrast, members of ABCI subfamily encode only one single unit of TMD or NBD domain and/or accessory domains, which can assemble into multi-subunit complex in planta in a manner similar to the classical ABC transporters in prokaryotes (Verrier et al. 2008). There are 21 and 17 group I members in Arabidopsis and rice genomes, respectively. Among them, AtABC1 (also named as AtNAP1) has been characterized as a FR light-specific signaling factor involved in the phytochrome A signaling pathway (Moller et al. 2001). Furthermore, AtNAP1/AtABC1 can complex with AtNAP6/AtABCI6 and AtNAP7/AtABCI7 to form an ATP-driven energizer for Fe–S cluster assembly and regulate iron homeostasis in Arabidopsis (Xu et al. 2004). Recently, another Arabidopsis ABCI member, AtNAP14 (AtABCI11) was reported to play an important role in plastid transition metal homeostasis and in chloroplast development. Disruption of AtNAP14 results in over-accumulation of transition metals and aberrant chloroplast structures (Shimoni-Shor et al. 2010). These findings suggest that Arabidopsis ABCI members perform a wide variety of cellular functions including light signal transduction, Fe–S cluster assembly and the regulation of iron homeostasis. However, the functions of rice ABCI members are largely unknown.
Here we report a naturally occurring novel rice green-revertible albino mutant abci8, caused by a 153-bp deletion in the first exon of the OsABCI8 gene. OsABCI8 is chloroplast-localized and is required for proper formation of chloroplast structure and bio-synthesis of chlorophyll precursor under continuous rainy days. Interestingly, abci8 mutants are almost indistinguishable from wild type plants grown under white light conditions. Thus OsABCI8 is dispensable during normal growth condition, but is required for chloroplast development and chlorophyll biosynthesis under continuing cloudy weather.
Materials and methods
Plant materials and growth condition
A spontaneously occurring rice green-revertible albino mutant abci8 was isolated from the Oryza sativa L. ssp. Japonica cultivar Zhonghua 11. A T-DNA insertion line for the OsABCI8 gene, ATL_03Z11JN90_LBT2, was obtained from the National Center of Plant Gene Research (Wuhan) at Huazhong Agricultural University (Zhang et al. 2006). The abci8 homozygous mutant plants were screened by PCR amplification.
To investigate the effect of different light intensities on seedling growth, the WT and abci8 seeds were grown on 1/2 MS agar without sucrose at 28 °C/25 °C day/night temperatures with a 13 h-light/11 h-dark regime in culture pots covered with 0, 3, 5, 7 layers of print paper (to achieve different light irradiance of 100, 14, 1.5, 0.2 μmol m−2 s−1, respectively) in a growth chamber for 12 days.
For complementation analysis, abci8 mutant and wild-type plants were grown in a paddy field for 12 sunny days after germination followed by 18 day of cloudy/rainy conditions.
Transmission electron microscopy
Transmission electron microscopy (TEM) was performed according to the method previously described (Peng et al. 2006) with minor modifications. Briefly, pieces of leaf tissue were fixed immediately with 2.5% glutaraldehyde in 0.2 M phosphate buffer saline (PBS, pH7.2) for 4 h at 4 °C, rinsed 3× with the same PBS and incubated in 1% OsO4 (in 0.2 M PBS, pH 7.2) overnight at 4 °C, rinsed again with PBS (pH 7.2), dehydrated in an ethanol series (30, 50, 70, 90 and 100%), infiltrated with a graded series of epoxy resin in epoxy propane, and then embedded in Epon 812 resin. Thin sections were obtained using a diamond knife and a Reichert OM2 ultramicrotome, stained in 2% uranyl acetate, pH 5.0, followed by 10 mM lead citrate, pH 12, then imaged with a transmission electron microscope (JEM-1230; JEOL).
Map-based cloning
For map-based cloning of the OsABCI8 gene, 425 individual plants showing albino leaf were selected from an F2 population derived from a cross between the abci8 mutant and indica var Huajingxian74. Simple sequence repeat (SSR) markers and InDel markers on chromosome 11 were used for fine mapping. The OsABCI8 gene was selected from an approximately 17.6-kb region as the candidate gene. To find out the mutation site, we amplified the corresponding fragments from the abci8 mutant and wild-type plants, respectively. Primers used for the map-based cloning are listed in Supplementary Table 1.
Rice transformation
For complementation of the abci8 mutation, a full-length genomic sequence of OsABCI8 containing its promoter was cloned into the p2300 binary vector. The resulting plasmid was introduced into the calli generated from the mature seed embryos of the abci8 mutants through the Agrobacterium (strain EHA105)-mediated method (Hiei et al. 1994).
RT-PCR
Total RNA was extracted from frozen samples with TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. The RNA was pre-treated with DNase I, and first-strand cDNA was generated using the RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific). The primers used for RT-PCR and quantitative qRT-PCR were listed in Supplementary Table 1. For qRT-PCR analysis, leaf samples were collected from 32-day old seedlings grown in paddy field that were subsequently subjected to continuous 10 sunny days, followed by 4 more rainy days, then 2 rainy days, then 2 more sunny days. The rice ACTIN gene was used as an internal control for the RT-PCR analysis.
Subcellular localization
To determine subcellular localization of OsABCI8, an 867-bp coding sequence of OsABCI8.1 was amplified with the primers ABC-GEXANF and ABC-GEXANR (Table S1) and ligated upstream to the GFP coding sequence of pAB580 vector at the SpeI/XbaI sites. The resulting pOsABCI8.1-GFP construct was transformed into rice protoplasts as reported previously (Zhang et al. 2011). Similarly, an 828-bp coding sequence of OsABCI8.2 was amplified and fused upstream of the GFP sequence at the XbaI/BamHI sites of the pAN580 vector to generate OsABCI8.2-GFP fusion transgene for rice protoplast transformation.
Phylogenetic analysis
Protein sequences of OsABCI8 members in rice and Arabidopsis were obtained from RGAP (http://rice.plantbiology.msu.edu) and TAIR (http://www.arabidopsis.org), respectively. The phylogenetic unrooted tree was constructed by neighbor-joining (NJ) method based on the amino acid pairwise distance with the Poisson-correction method using the Mega6 software (Tamura et al. 2013). Bootstrap values were estimated (with 1000 replicates) to assess the relative support for each branch. All positions containing alignment gaps were eliminated in pairwise sequence comparisons during NJ analyses.
Measurement of photosynthetic pigments
For total chlorophyll and carotenoid concentration measurememt, pigments were extracted from leaf tissues of wild-type and abci8 plants with 95% ethanol. The concentrations of chlorophyll and carotenoid were determined by UV/Vis spectrophotometer as described previously (Lichtenthaler 1987). Protoporphyrin IX and protochlorophyllide (Pchlide) concentrations were determined as previously described (Hodgins and Van Huystee 1986). The concentrations of 5-aminolevulinic acid (ALA) in 10 days-old WT and abci8 leaves grown under darkness and light conditions were performed spectrophotometrically at the wavelengths 553 nm as described (Dei 1985).
Elemental analysis
Elemental analysis was performed as described (Cheng et al. 2007) with minor modifications. The field grown abci8 albino leaves and wild type normal leaves were collected at six-leaf stage. All the rice samples were dried and milled into powder with a mixer mill (Retsch MM301, German) and oven-dried at 65 °C for 72 h. Dried powders (0.2 g) were digested with 4.0 mL of HNO3 (reagent grade) and 1.0 mL of H2O2 (30%, analytical reagent, Beijing Chemical Works, China) in a screw cap polypropylene sample tube (Corning Incorporated, Corning, NY, USA) using a Hot Block Digestion System (Model SC154, Environmental Express, Mt. Pleasant, SC, USA). The concentrations of elements in each sample (i.e., Ca, Mn, Fe, Zn, and Mg) were determined using inductively coupled plasma mass spectrometry (ICP-MS; Agilent 7500a, Agilent Technologies, Palo Alto, CA, USA). The concentrations of nickel elements in the samples were determined by graphite furnace atomic absorption spectrometry (Analytik Jena AG, Zeenit 60).
Results
Phenotypic characterization of rice abci8 mutant
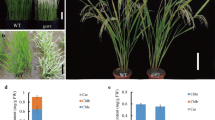
A naturally occurring rice mutant with green-revertible albino phenotype, named abci8, was isolated from a japonica variety Zhonghua 11 in Guangzhou, South China during a 2-month long rainy season (2006). In paddy field, the leaves of the abci8 mutant exhibited normal green color under normal daylight conditions (Supplementary Fig. 1A), but turned albino during a continuous rainy period of 10 days or longer (Fig. 1, Supplementary Fig. 1B), and returned to normal green color when the weather turns to normal sunny days (Fig. 1). In addition, the albino phenotype can also be observed in the tillering (Supplementary Fig. 1C) and heading stages (Supplementary Fig. 1D) if mutant plants are exposed to prolonged cloudy/rainy days in paddy field. Most of these mutant seedlings died before flowering under a continuous rainy period of more than 6 weeks. However, some mutants (less than 10%) could survive and set seeds, despite the fact that plant height and tiller numbers are reduced compared to wild type plants (Supplementary Fig. 1E, F). These results indicated that the rice abci8 albino phenotype is not developmental stage-specific.
The abci8 albino phenotype under continuous cloudy/rainy days (Fig. 1a), but not in sunny days (Supplementary Fig. 1A), could be due to light conditions. We examined the effects of light intensities on the phenotype of the abci8 mutant. As shown in Supplementary Fig. 2A, the abci8 plant was similar to the wild-type plant under different intensities of white light. In addition, abci8 mutant had no significant difference in chlorophyll and carotenoid contents compared to WT seedlings (Supplementary Fig. 2B). The results suggested that the albino phenotype of abci8 did not depend on light intensities.
Defect in OsABCI8 impairs chloroplast development and chlorophyll precursor synthesis
To investigate the effects of OsABCI8 on chloroplast development, we examined the ultrastructure of chloroplasts in both wild-type and the albino abci8 plants grown in the field at the albino-stage by transmission electron microscopy (TEM). A typical leaf blade of abci8 mutant at the albino-stage could be divided into green, chlorotic and albino three sections from leaf tip to leaf base (Fig. 2a). At the green section of leaf blade, the chloroplasts in the abci8 plants displayed well-developed lamellar structures equipped with normally stacked grana and thylakoid membranes, which is comparable to those of wild-type plants (Fig. 2b, c-I). However, the thylakoid membranes were much less abundant in the chloroplasts of the abci8 mutants at the chlorotic section of leaf blade (Fig. 2c-II). As far as the albino section of leaf blade was considered, the abci8 contained only rudimentary plastids that lack internal membrane structure and are devoid of grana thylakoids (Fig. 2c-III). Moreover, the development of grana thylakoids was severely disturbed in mutant plastids. Thus, the results suggest that the albino abci8 has a dramatic distortion in chloroplast development, characterized by inchoate or absent grana thylakoids.
Transmission electron microscopy (TEM) analysis of plastids in different section of abci8 leaves. a Leaves of green (I), chlorotic (II), albino (III) sections in the abci8 mutant; b TEM analysis of plastids in wild-type rice leaves; c TEM analysis of plastids in the three section of the abci8 mutant leaves. C chloroplast; N nucleus; G grana thylakoid; PG plastoglobule
To examine the effects of the abci8 mutation on rice chlorophyll synthesis, we analyzed the pigment contents and precursor for chlorophyll synthesis in wild-type and abci8 plants. At the green section of leaf blade, the amount of pigment in the abci8 is similar to those of wild-type seedlings in spite of the fact that the abci8 mutant tend to overaccumulate 5-Aminolevulinic acid (ALA). However, the albino section of leaf blade in abci8 mutants contained no measurable pigment. Analysis of ALA content revealed that the abci8 mutant had a great reduction in ALA content (Fig. 3). Moreover, the abci8 mutant exhibited extremely reduced protoporphyrin IX and protochlorophyllide contents at the albino section of leaf blade (Fig. 3). 5-Aminolevulinic acid (ALA) is the universal precursor for tetrapyrrole biosynthesis, including chlorophyll and heme (Tanaka and Tanaka 2007). The results indicate that loss of OsABCI8 function impairs bio-synthesis of chlorophyll precursor under continuous cloudy/rainy days.
Determination of pigment contents in leaves of green and albino sections of the abci8 and wild-type plants. Leaf samples were collected from 30-day-old wild-type (WT) and abci8 mutant plants grown in a paddy field for 12 sunny days after germination followed by 18 day of cloudy/rainy conditions. Chla chlorophyll a; Chlb chlorophyll b; Car carotenoid; ALA δ-Aminolevulinic acid; Proto IX protoporphyrin IX; Pchl protochlorophyllide. In each graph, statistically significant differences are indicated by the asterisks **P < 0.01
Map-based cloning of the OsABCI8 gene
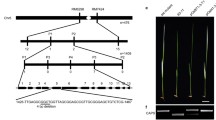
To determine the inheritance of abci8, we examined the phenotypes of progeny derived from a cross between abci8 and wild type (Zhonghua11). All F1 plant displayed wild-type phenotype, and their F2 progenies showed a segregation ratio of 3:1 (normal:albino = 320:102; χ2 = 0.11 < χ2 0.05 = 3.84), indicating that the mutant phenotype was controlled by a single recessive nuclear locus. To identify the mutated gene responsible for the green-revertible albino phenotype, map-based cloning method was employed. Using an F2 mapping population derived from a cross between indica var Huajingxian74 and the abci8 mutant, the mutated gene locus was mapped to a 17.6 kb DNA region on chromosome 11 between marker RM26739 and IDM-2 (Fig. 4a). Within this 17.6-kb interval, there are two predicted ORFs: LOC_Os11g29840 and LOC_Os11g29850. DNA sequence comparison revealed a 153-bp deletion in the first exon of LOC_Os11g29850 in the abci8 and no sequence difference was found in LOC_Os11g29840. The 153-bp deletion in the abci8 mutant occurred at 12 bp upstream from the initiation codon ATG and is predicted to produce a truncated protein product. LOC_Os11g29850 encodes a protein with an ABC transporter domain, and was named OsABCI8 according to the nomenclature of plant ABC transporter proteins (Verrier et al. 2008). The OsABCI8 contained two alternatively spliced isoforms, OsABCI8.1 and OsABCI8.2, encoding predicted 289 and 276 amino acid polypeptides, respectively (Fig. 4a).
Map-based cloning of the OsABCI8 gene. a The OsABCI8 locus was mapped to the long arm of rice chromosome 11. Black boxes indicate the coding sequence, grey boxes indicate the 5′ and 3′ untranslated regions, and lines between boxes indicate introns. Deletion site identified in the abci8 are indicated by dashed lines around ATG. b Complementation analysis of the abci8 mutant. Seedling phenotype of 30-day-old of WT, abci8, abci8 transformed with an empty vector p2300, or with the OsABCI8 genomic fragment grown in a paddy field for 12 sunny days after germination followed by 18 day of cloudy/rainy conditions. Bar 10 cm. c Expression analysis of OsABCI8 in WT, abci8, or p2300 and pABCI8 transgenic plants in the abci8 background. RT-PCR analysis of OsABCI8 showed that complementation of abci8 by the pABCI8 transgene was accompanied by expression of full length OsABCI8 transcript. These plants tested were grown in a paddy field under 12 sunny days after germination followed by 18 day of cloudy/rainy conditions. ACTIN was used as a control
To confirm the mutation in OsABCI8 was responsible for the mutant phenotype, a transgenic construct, pABCI8, containing the entire OsABCI8 coding region and 1264 bp 5′ upstream sequences was introduced into the binary vector pCAMBIA2300, and transformed into the abci8 background. As a control, the pCAMBIA2300 empty vector p2300 was also introduced into the abci8. We found that the abci8 mutant phenotype was rescued in pABCI8 transgenic plants (Fig. 4b). Expression analysis by RT-PCR showed that OsABCI8 was truncated in the abci8 plants, and normal length of OsABCI8 transcript was detected in the complementing transgenic plants (Fig. 4c). Furthermore, plants homozygous for an independent T-DNA insertion mutant in the OsABCI8 coding region (abci8-t) exhibited identical phenotype to that of the abci8 (Supplementary Fig. 3). These findings confirmed that mutation in OsABCI8 is responsible for the mutant phenotype.
OsABCI8 is homologous to a putative iron transporter from Arabidopsis
The ABCI subfamily consists of 17 members in rice. Among them, the CBY/Y179 subgroup has three members including OsABCI8 (Verrier et al. 2008). Phylogenetic analysis showed that the OsABCI8 (encoded by Os11g29850) is more closely related to AtNAP14 (encoded by At5g14100) than to others in the CBY/Y179 subfamily (Fig. 5a). Comparison of deduced amino acid sequences showed that OsABCI8 shares a high degree of sequence identity with the AtNAP14 (Fig. 5b).
Evolutionary relationship between the rice OsABCI8 and the Arabidopsis AtNAP14. a Phylogenetic relationship of the CBY/Y179 subgroup of ABCI subfamily proteins in rice and Arabidopsis. The phylogenetic tree was constructed using MEGA6 software. Bootstrap analysis was performed with 1000 replicates. The numbers at the branches are confidence values (percent). b Amino acid sequences alignment of OsABCI8 and AtNAP14. Identical amino acids are shown as white letters on a black background. OsABCI8 protein is characterized by the possession of an ATP-binding cassette, also known as the nucleotide-binding domain (NBD), which contains several highly conserved motifs, including the Walker A/P, Q-loop/lid, ABC transporter signature motif, walker B loop, walker D loop, H loop/switch region
The AtNAP14 has been demonstrated to play an important role in plastid iron homeostasis (Shimoni-Shor et al. 2010). In addition, it was shown that Ni2+can be absorbed via the Fe2+ uptake system in Arabidopsis (Nishida et al. 2011). These suggest that OsABCI8 may be involved in iron/nickel transportation and/or homeostasis. We measured the transition metal concentration, including Fe, Ni, Ca, Mg and Mn elements in the abci8 and wild-type plants grown in the field at the albino stage (Fig. 6). The largest effect observed in the abci8 shoots was the Fe concentration, which was 2.78-fold higher in the abci8 than that in wild-type shoots. A significant increase in Ni concentrations was observed as well (2.23-fold). In addition, small differences in Mn (1.29-fold) and Ca (0.77-fold) were also detected between abci8 and wild-type plants. The concentration of transition metals Mg and Zn distribution in the abci8 was not significantly different from that of wild-type plants. As expected, OsABCI8 T-DNA mutant plant also accumulated more Iron than WT plants (Supplementary Fig. 4). In addition, we examined the iron content of the abci8 mutant grown under white light conditions. The results showed that Fe content of seedlings of abci8 mutant were similar to that of WT (Supplementary Fig. 5). The results suggest that OsABCI8 might function as an iron/nickel transporter to regulate iron/nickel homeostasis in cloudy/rainy days.
Elevated levels of iron and nickel accumulation in the abci8. To determine element content, the leaves from the abci8 mutant developing albino leaves and wild type plants grown in field were collected at six-leaf stage. abci8 mutant plants accumulate significantly higher levels of iron and nickel compared to WT plants. Mean and SD of three biological replicates are shown (Relative content, percentages). The actual concentrations of the elements were shown in Supplementary Table 2. Significant difference from the corresponding wild-type value, based on the Student’s t-test, are marked by *P < 0.05 or **P < 0.01
Subcellular localization of OsABCI8
A chloroplast signal peptide was identified in OsABCI8 by TargetP and SignalP prediction servers (Emanuelsson et al. 2000). In addition, OsABCI8 exhibits a high degree of sequence identity with the chloroplast-localized Arabidopsis ABC transporter AtNAP14 (Fig. 5b) (Shimoni-Shor et al. 2010), suggesting they might share the same subcellular localization. To ascertain this hypothesis, we fused the GFP protein to the C terminus of OsABCI8.1 and expressed this fusion protein in rice protoplasts. The green GFP signal merged with red auto-fluorescence of chloroplast, as seen in the merged regions (yellowish orange) in the overlay (Fig. 7). Furthermore, the green fluorescence from the OsABCI8.2-GFP fusion protein was also co-localized with chlorophyll auto fluorescence. The results indicated that both OsABCI8 protein isoforms are chloroplast-localized.
Co-localization of OsABCI8 proteins with chlorophyll auto fluorescence in rice protoplasts. ABCI8.1/2-GFP, green fluorescence from OsABCI81/2-GFP fusion protein; Chl-AF chlorophyll auto fluorescence; Bright field bright-field image under transmitted light; Merge merged image of OsABCI8-GFP, Chl-AF, and Bright field. Excitation/emission wavelengths were 488/516 nm for GFP, and 488/620 nm for the auto fluorescence. Bar 5 μm
OsABCI8 is sensitive to variable weather conditions
Given the abci8’s conditional albino phenotype (Fig. 1), we speculated that the expression of OsABCI8 might be sensitive to variable weather conditions. To test this hypothesis, we conducted expression analysis of OsABCI8 when plants are exposed to sunny and rainy in paddy field. The OsABCI8 contained two alternatively spliced isoforms (Fig. 8a), OsABCI8.1 and OsABCI8.2. The latter, arising from intron IX retention, contained a stop codon that produces a shorter protein. Expression analysis showed that the transcript abundances of the variants were significant higher when plants were exposed to rainy days compared to sunny days (Fig. 8). Interestingly, the transcripts of both variants were remarkably down-regulated when plants were returned to two more sunny days. The results demonstrate that the transcripts of OsABCI8 is sensitive to variable weather conditions, suggesting the possibility that OsABCI8 functions mainly in rainy days rather than in sunny days.
Expression analysis of OsABCI8 gene under variable weather conditions. a Structures of the OsABCI8 transcription. Arrows indicate primers used for the RT-PCR analysis in (b). b Quantity RT-PCR analysis of OsABCI8 transcripts in rice seedlings under variable weather conditions. After continuous 10 sunny days, leaf samples were collected from 32-day-old seedlings grown in paddy field, followed by 4 more rainy days, then 2 rainy days, and then 2 sunny days, respectively. Data are means ± SD of three biological replicates
Discussion
Rice abci8 is a conditional, chloroplast-deficient mutant
Rice leaf-color mutants are important materials for studying the molecular mechanism of chlorophyll biosynthesis or chloroplast development. To date, more than 200 rice leaf-color mutants have been identified, such as v1(virescent 1), v2, v3, v4, st1(stripe1), ysa (young seedling albino), ylc1(young leaf chlorosis1), fdl (faded green leaf), wlp1(white leaf and panicles1), and tcd9 (thermo-sensitive chloroplast development 9) (Yoo et al. 2009; Zhou et al. 2013; Gong et al. 2014; Jiang et al. 2014; Kusumi and Iba 2014; Song et al. 2014). Some of these mutants with albino phenotype are stage-dependent or low-temperature responsive during early leaf development stage. In this study, we isolated and characterized a novel conditional green-revertible albino mutant abci8. Our results showed the rice abci8 mutant phenotype was not developmental stage-specific (Supplementary Fig. 1). And light intensities was not associated with the albino leaves of abci8. Aside from raining day, the major factors affecting the abci8 phenotype remain to be determined.
Unlike Arabidopsis nap14 mutant, which exhibited albino phenotype in white light (Shimoni-Shor et al. 2010), abci8 mutants were almost indistinguishable from wild type plants grown under white light condition, suggesting that Arabidopsis might be more sensitive to the loss of chloroplast-related gene than monocots rice. Ultra structural analysis revealed that the abci8 albino leaf contained aberrant chloroplasts that completely lacked the thylakoid membrane (Fig. 2), suggesting OsABCI8 is required for plastid development and internal membrane organization during suboptimal light conditions such as continuous cloudy/rainy days. Thus analysis of rice abci8 has the potential to uncover additional gene functions that were not revealed in Arabidopsis research. It is interesting to note that the OsABCI8 has two alternatively-spliced isoforms (Fig. 4a) while no splicing isoforms were detected for AtNAP14 gene in Arabidopsis (Shimoni-Shor et al. 2010). Whether or not the isoforms in rice contributes to the difference of phenotype in chloroplast development between the rice abci8 and Arabidopsis nap14 mutant remains to be investigated.
abci8 has higher accumulation of iron
In this study, elemental analysis showed that iron and nickel were significantly increased in the abci8 plants compared to wild-type plants at albino stage, while no significant change in the other elements such as Mg and Zn (Fig. 6). Thus, our results suggest that the OsABCI8 is involved in iron and nickel transportation and/or homeostasis. Similar to the transport mechanism of iron-nickel in the dicot Arabidopsis (Nishida et al. 2011), our results also support that nickel can be absorbed through iron transport systems in rice, implying that the molecular mechanism might be conserved among plants. The findings are helpful to understand the competitive absorption of iron and nickel in plants.
Studies with the dgl and brz mutants of pea showed that there is a shoot-to-root signal transduction regulating Fe homeostasis in plants (Grusak and Pezeshgi 1996). The dgl and brz mutants have been shown to have the capacity to over accumulate Fe. Reciproca1 grafting experiments suggested that the dgl and brz shoots transmits a signal compound that acts as a promoter in this root response and resulted in Fe hyper accumulating phenotype in the mutants. It has been reported that FRO1 gene from pea (Pisum sativum) encodes a ferric-chelate reductase, which reduce soil Fe (III) to Fe (II) and is involved in root iron acquisition (Waters et al. 2002). FRO1 transcription and its reductase activity were detected only under Fe-deficient conditions in pea (Waters et al. 2002), whereas both dgl and brz mutants show constitutive root Fe (III)-chelate reductase activity and overaccumulate Fe (Waters et al. 2002). These findings suggest the possibility that FRO1 might be involved in transmission of a shoot-derived signal of iron status in pea whereas overaccumulation of iron in dgl and brz mutants may be due to increased activity of ferric-chelate reductase. Similarly, our results showed that the abci8 mutant over accumulates Fe (Fig. 6). Thus, it is possible that loss of OsABCI8 activity might cause a Fe deficiency signal and promote this root response to over accumulate Fe.
In addition to defects in pigment biosynthesis causing an albino mutation (Jung et al. 2003), there are a number of factors that can lead to an albino phenotype in plants. It has been shown that knockout plants for the Fe transporter localized in chloroplast exhibit severe chlorotic phenotype in Arabidopsis (Duy et al. 2007; Jeong et al. 2008; Shimoni-Shor et al. 2010). Arabidopsis ferric reductase oxidase 7 (FRO7) is essential for seedling viability under iron limiting conditions (Jeong et al. 2008). The fro7 seedlings showed severe chlorosis and growth defects in alkaline soil (pH 8) where the availability of iron is limited, whereas the wild-type plants were only slightly chlorotic. The phenotype was rescued by watering with excess soluble iron (Jeong et al. 2008). Since the albino phenotype of the abci8 mutant is accompanied by an elevated accumulation of Fe, it is tempting to speculate that the albino phenotype might have resulted from unusual Fe homeostasis in abci8 mutants.
In conclusion, we characterized a naturally occurring conditional albino rice mutant in this study. Interestingly, abci8 mutants were almost indistinguishable from wild type plants under sunny conditions, but exhibited albino phenotype in continuous rainy days, which is caused by the mutation of an ABC transporter family gene, OsABCI8. OsABCI8 transcript levels in rainy days are dramatically higher than that in sunny days. This study provides an important evidence for a key role of chloroplast-localized OsABCI8 in rice chloroplast development under suboptimal light conditions.
References
Cheng L, Wang F, Shou H, Huang F, Zheng L, He F, Li J, Zhao FJ, Ueno D, Ma JF, Wu P (2007) Mutation in nicotianamine aminotransferase stimulated the Fe(II) acquisition system and led to iron accumulation in rice. Plant Physiol 145:1647–1657
Dei M (1985) Benzyladenine-induced stimulation of 5-aminolevulinic acid accumulation under various light intensities in levulinic acid-treated cotyledons of etiolated cucumber. Physiol Plant 64:153–160
Deng XJ, Zhang HQ, Wang Y, He F, Liu JL, Xiao X, Shu ZF, Li W, Wang GH, Wang GL (2014) Mapped clone and functional analysis of leaf-color gene Ygl7 in a rice hybrid (Oryza sativa L. ssp. indica). PLoS ONE 9:e99564
Duy D, Wanner G, Meda AR, von Wirén N, Soll J, Philippar K (2007) PIC1, an ancient permease in Arabidopsis chloroplasts, mediates iron transport. Plant Cell 19:986–1006
Emanuelsson O, Nielsen H, Brunak S, von Heijne G (2000) Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J Mol Biol 300:1005–1016
Gong X, Su Q, Lin D, Jiang Q, Xu J, Zhang J, Teng S, Dong Y (2014) The rice OsV4 encoding a novel pentatricopeptide repeat protein is required for chloroplast development during the early leaf stage under cold stress. J Integr Plant Biol 56:400–410
Grusak MA, Pezeshgi S (1996) Shoot-to-root signal transmission regulates root Fe(III) reductase activity in the dgl mutant of pea. Plant Physiol 110:329–334
Hayashi-Tsugane M, Takahara H, Ahmed N, Himi E, Takagi K, Iida S, Tsugane K, Maekawa M (2014) A mutable albino allele in rice reveals that formation of thylakoid membranes requires the SNOW-WHITE LEAF1 Gene. Plant Cell Physiol 55:3–15
Hiei Y, Ohta S, Komari T, Kumashiro T (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6:271–282
Hodgins RR, Van Huystee RB (1986) Rapid simultaneous estimation of protoporphyrin and Mg-porphyrins in higher plants. J Plant Physiol 125:311–323
Ilag LL, Kumar AM, Söll D (1994) Light regulation of chlorophyll biosynthesis at the level of 5-aminolevulinate formation in Arabidopsis. Plant Cell 6:265–275
Jeong J, Cohu C, Kerkeb L, Pilon M, Connolly EL, Guerinot ML (2008) Chloroplast Fe(III) chelate reductase activity is essential for seedling viability under iron limiting conditions. Proc Natl Acad Sci U S A 105:10619–10624
Jiang Q, Mei J, Gong XD, Xu JL, Zhang JH, Teng S, Lin DZ, Dong YJ (2014) Importance of the rice TCD9 encoding α subunit of chaperonin protein 60 (Cpn60α) for the chloroplast development during the early leaf stage. Plant Sci 215–216:172–179
Jung K-H, Hur J, Ryu C-H, Choi Y, Chung Y-Y, Miyao A, Hirochika H, An G (2003) Characterization of a rice chlorophyll-deficient mutant using the T-DNA gene-trap system. Plant Cell Physiol 44:463–472
Kusumi K, Iba K (2014) Establishment of the chloroplast genetic system in rice during early leaf development and at low temperatures. Front Plant Sci 5:386
Lichtenthaler HK (1987) [34] Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol 148: 350–382
Massouh A, Schubert J, Yaneva-Roder L, Ulbricht-Jones ES, Zupok A, Johnson MTJ, Wright SI, Pellizzer T, Sobanski J, Bock R, Greiner S (2016) spontaneous chloroplast mutants mostly occur by replication slippage and show a biased pattern in the plastome of Oenothera. Plant Cell 28:911–929
Moller SG, Kunkel T, Chua NH (2001) A plastidic ABC protein involved in intercompartmental communication of light signaling. Genes Dev 15:90–103
Neuhaus HE, Emes MJ (2000) Non photosynthetic metabolism in plastids. Annu Rev Plant Physiol Plant Mol Biol 51:111–140
Nishida S, Tsuzuki C, Kato A, Aisu A, Yoshida J, Mizuno T (2011) AtIRT1, the primary iron uptake transporter in the root, mediates excess nickel accumulation in Arabidopsis thaliana. Plant Cell Physiol 52:1433–1442
Peng L, Ma J, Chi W, Guo J, Zhu S, Lu Q, Lu C, Zhang L (2006) LOW PSII ACCUMULATION1 is involved in efficient assembly of photosystem II in Arabidopsis thaliana. Plant Cell 18:955–969
Rea PA (2007) Plant ATP-binding cassette transporters. Annu Rev Plant Biol 58:347–375
Sakamoto W, Miyagishima S-y, Jarvis P (2008) Chloroplast biogenesis: control of plastid development, protein import, division and inheritance. Arabidopsis Book/Am Soc Plant Biol 6:e0110
Shimoni-Shor E, Hassidim M, Yuval-Naeh N, Keren N (2010) Disruption of Nap14, a plastid-localized non-intrinsic ABC protein in Arabidopsis thaliana results in the over-accumulation of transition metals and in aberrant chloroplast structures. Plant Cell Environ 33:1029–1038
Song J, Wei X, Shao G, Sheng Z, Chen D, Liu C, Jiao G, Xie L, Tang S, Hu P (2014) The rice nuclear gene WLP1 encoding a chloroplast ribosome L13 protein is needed for chloroplast development in rice grown under low temperature conditions. Plant Mol Biol 84:301–314
Su N, Hu ML, Wu DX, Wu FQ, Fei GL, Lan Y, Chen XL, Shu XL, Zhang X, Guo XP, Cheng ZJ, Lei CL, Qi CK, Jiang L, Wang H, Wan JM (2012) Disruption of a rice pentatricopeptide repeat protein causes a seedling-specific albino phenotype and its utilization to enhance seed purity in hybrid rice production. Plant Physiol 159:227–238
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Tanaka R, Tanaka A (2007) Tetrapyrrole biosynthesis in higher plants. Annu Rev Plant Biol 58:321–346
Tang W, Wang W, Chen D, Ji Q, Jing Y, Wang H, Lin R (2012) Transposase-derived proteins FHY3/FAR1 interact with PHYTOCHROME-INTERACTING FACTOR1 to regulate chlorophyll biosynthesis by modulating HEMB1 during deetiolation in Arabidopsis. Plant Cell 24:1984–2000
Verrier PJ, Bird D, Burla B, Dassa E, Forestier C, Geisler M, Klein M, Kolukisaoglu U, Lee Y, Martinoia E, Murphy A, Rea PA, Samuels L, Schulz B, Spalding EJ, Yazaki K, Theodoulou FL (2008) Plant ABC proteins–a unified nomenclature and updated inventory. Trends Plant Sci 13:151–159
Waters BM, Blevins DG, Eide DJ (2002) Characterization of FRO1, a pea ferric-chelate reductase involved in root iron acquisition. Plant Physiol 129:85–94
Xu XM, Adams S, Chua NH, Moller SG (2004) AtNAP1 represents an atypical SufB protein in Arabidopsis plastids. J Biol Chem 280:6648–6654
Yoo SC, Cho SH, Sugimoto H, Li J, Kusumi K, Koh HJ, Iba K, Paek NC (2009) Rice Virescent3 and Stripe1 encoding the large and small subunits of ribonucleotide reductase are required for chloroplast biogenesis during early leaf development. Plant Physiol 150:388–401
Zhang J, Li C, Wu C, Xiong L, Chen G, Zhang Q, Wang S (2006) RMD: a rice mutant database for functional analysis of the rice genome. Nucleic Acids Res 34:D745–D748
Zhang Y, Su J, Duan S, Ao Y, Dai J, Liu J, Wang P, Li Y, Liu B, Feng D, Wang J, Wang H (2011) A highly efficient rice green tissue protoplast system for transient gene expression and studying light/chloroplast-related processes. Plant. Methods 7:30–30
Zhou K, Ren Y, Lv J, Wang Y, Liu F, Zhou F, Zhao S, Chen S, Peng C, Zhang X, Guo X, Cheng Z, Wang J, Wu F, Jiang L, Wan J (2013) Young Leaf Chlorosis 1, a chloroplast-localized gene required for chlorophyll and lutein accumulation during early leaf development in rice. Planta 237:279–292
Acknowledgements
We thank the National Center of Plant Gene Research (Huazhong Agricultural University, China) for providing the T-DNA insertion line, ATL_03Z11JN90_LBT2, for the OsABCI8 gene. This work was supported by the National Natural Science Foundation of China (Grants Nos. 31671594 to XQ. Zhang and 31272491 to XM. Xie), the Natural Science Foundation of Anhui Province, China (Grant No. 1508085MC46 to B. Teng), Natural Science Foundation of Guangdong Province, China (Grant Nos. 2014A030313457 and 2015A020209118 to XQ. Zhang).
Author contributions
The author(s) have made the following declarations about their contributions: Conceived and designed the experiments: XQZ TFH. Performed the experiments: XZ, RT, HG and SK. Analyzed the data: BT YHH XZ. Contributed reagents/materials/analysis tools: ZX XMX. Wrote the paper: XQZ TFH. All authors discussed the results and commented on the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Xiuyu Zeng and Ran Tang have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zeng, X., Tang, R., Guo, H. et al. A naturally occurring conditional albino mutant in rice caused by defects in the plastid-localized OsABCI8 transporter. Plant Mol Biol 94, 137–148 (2017). https://doi.org/10.1007/s11103-017-0598-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-017-0598-4