Abstract
The giant knotweed Fallopia sachalinensis (Polygonaceae) synthesizes phenylacetonitrile (PAN) from l-phenylalanine when infested by the Japanese beetle Popillia japonica or treated with methyl jasmonate (MeJA). Here we identified (E/Z)-phenylacetaldoxime (PAOx) as the biosynthetic precursor of PAN and identified a cytochrome P450 that catalysed the conversion of (E/Z)-PAOx to PAN. Incorporation of deuterium-labelled (E/Z)-PAOx into PAN emitted from the leaves of F. sachalinensis was detected using gas chromatography–mass spectrometry. Further, using liquid chromatography–tandem mass spectrometry, we detected the accumulation of (E/Z)-PAOx in MeJA-treated leaves. These results showed that (E/Z)-PAOx is the biosynthetic precursor of PAN. MeJA-induced mRNAs were analysed by differential expression analysis using a next-generation sequencer. Of the 74,329 contigs obtained from RNA-seq and de novo assembly, 252 contigs were induced by MeJA treatment. Full-length cDNAs encoding MeJA-induced cytochrome P450s CYP71AT96, CYP82AN1, CYP82D125 and CYP715A35 were cloned using 5′- and 3′-RACE and were expressed using a baculovirus expression system. Among these cytochrome P450s, CYP71AT96 catalysed the conversion of (E/Z)-PAOx to PAN in the presence of NADPH and a cytochrome P450 reductase. It also acted on (E/Z)-4-hydroxyphenylacetaldoxime and (E/Z)-indole-3-acetaldoxime. The broad substrate specificity of CYP71AT96 was similar to that of aldoxime metabolizing cytochrome P450s. Quantitative RT-PCR analysis showed that CYP71AT96 expression was highly induced because of treatment with MeJA as well as feeding by the Japanese beetle. These results indicate that CYP71AT96 likely contributes the herbivore-induced PAN biosynthesis in F. sachalinensis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In response to attack by herbivores, plants emit a complex blend of volatile compounds (Unsicker et al. 2009). Herbivore-induced volatiles act as direct and indirect defensive agents to repel herbivores and to attract their natural enemies, respectively (Kessler and Baldwin 2001; De Moraes et al. 2001; Clavijo McCormick et al. 2012; Irmisch et al. 2014), as well as serve as cues for herbivores to locate hosts (Brilli et al. 2009). The biosynthesis of volatiles is triggered by elicitors derived from herbivores as well as by jasmonic acid and its derivatives, collectively designated as jasmonates (Rodriguez-Saona et al. 2001; Shah 2009) that function as downstream components in plant signal transduction pathways, which respond to stress induced by herbivore elicitors, and in plant growth and development (Hettenhausen et al. 2015).
Leaves of the giant knotweed Fallopia sachalinensis (Polygonaceae) emit volatiles in response to feeding by the Japanese beetles Popillia japonica or methyl jasmonate (MeJA) treatment but not in response to mechanical damage (Noge et al. 2011). Because the Japanese beetle is attracted to beetle-damaged leaves (Loughrin et al. 1995), the volatile blend emitted by F. sachalinensis is likely to play a role in attracting the Japanese beetles. Herbivore-induced volatiles are usually dominated by monoterpenes, sesquiterpenes and green leaf volatiles (Unsicker et al. 2009). The blend of F. sachalinensis volatiles comprises terpenes (E)-β-ocimene, linalool, (E)-4,8-dimethyl-1,3,7-nonatriene and (E,E)-α-farnesene as well as a characteristic nitrile compound, phenylacetonitrile (PAN) (Noge et al. 2011).
PAN and its derivatives are widely utilized in the chemical and pharmaceutical industries (Pollak et al. 2000). The chemical synthesis of PAN is performed under high-temperature and high-pressure conditions that require the use of cyanide compounds (Kato et al. 1999), which are highly toxic to animals (Hamel 2011). Conversely, the leaves of F. sachalinensis biosynthesize PAN from l-phenylalanine (L-Phe) (Noge and Tamogami 2013), and the enzymes that biosynthesize PAN in F. sachalinensis may produce PAN under mild conditions without using toxic compounds (Miki and Asano 2014). However, the intermediates and enzymes of the PAN biosynthetic pathway in F. sachalinensis are unknown.
In Populus trichocarpa (Salicaceae), which emits PAN, 2-methylbutyronitrile and 3-methylbutyronitrile in response to herbivore attack, CYP79D6 and CYP79D7 catalyses the conversion of amino acids to aldoximes, which are further converted by CYP71B40 and CYP40B41 to nitriles (Irmisch et al. 2013, 2014). In the cyanogenic Japanese apricot tree Prunus mume (Rosaceae), CYP79D16 catalyses the conversion of L-Phe to (E/Z)-phenylacetaldoxime (PAOx), which is converted by CYP71AN24 to mandelonitrile via PAN (Yamaguchi et al. 2014). In the aldoxime catabolic pathway (‘aldoxime–nitrile’ pathway of bacteria), PAN is converted from (E/Z)-PAOx by aldoxime dehydratase (Asano and Kato 1998; Kato et al. 2000; Yamaguchi and Asano 2015). Here, we show that (E/Z)-PAOx is the biosynthetic precursor of PAN in F. sachalinensis. Further, we used RNA-seq to identify genes that were differentially expressed in leaves treated with MeJA and to show that a cytochrome P450 catalysed the conversion of (E/Z)-PAOx to PAN.
Materials and methods
Plants and insects
Giant knotweeds (F. sachalinensis) were maintained at the Akita Prefectural University and Toyama Prefectural University at room temperature. Japanese beetles (P. japonica) were collected from areas surrounding the Toyama Prefectural University. Beetles were reared on the leaves of F. sachalinensis as described before (Yamaguchi et al. 2013).
Chemicals
(E/Z)-PAOx, (E/Z)-4-hydroxyPAOx, and (E/Z)-indole-3-acetaldoxime were synthesized according to the previous publications (Møller 1977; Asano and Kato 1998; Kato et al. 2000). [Ring-D5]-(E/Z)-PAOx was synthesized from bromobenzene-D5 via phenylmagnesium bromide-D5, 2-phenylethanol-D5, and 2-phenylacetaldehyde-D5 (Asano and Kato 1998; Yakhvarov et al. 2007; Shibata et al. 2012). All other chemicals were obtained from commercial suppliers.
Application of deuterium-labeled (E/Z)-PAOx and analysis of plant volatiles
To identify the precursor of PAN biosynthesis in F. sachalinensis, volatiles from leaves fed on deuterium-labelled (E/Z)-PAOx were analysed using a gas chromatography–mass spectrometry (GC–MS) system. Mature leaves of F. sachalinensis were treated with airborne MeJA, as described by Noge and Tamogami (2013), to induce PAN biosynthesis. Briefly, a mature leaf of F. sachalinensis was excised, and the petiole was placed in an Eppendorf tube containing 1.5 ml of 2 mM (E/Z)-PAOx in distilled water or [Ring-D5]-(E/Z)-PAOx. Each leaf was placed in a glass container (1000 ml) and a paper disk with or without 2 µl MeJA was introduced without any contact with the leaf. The container was kept at 25 °C under continuous LED light for 24 h. Volatiles emitted from leaves were collected using a solid-phase microextraction fibre (65-µm Stable Flex PDMS/DVB; Supelco, Bellefonte, PA, USA) for 30 min. The fibre was injected into a GC–MS system to identify the volatile compounds. GC–MS analysis performed using a GC–MS–QP2010 Plus (Shimadzu, Kyoto, Japan) equipped with a TC-70 (i.d., 60 m × 0.25 mm; film thickness, 0.25 µm; GL Science, Tokyo, Japan). Helium was used as the carrier gas and was applied at 30 ml min−1. The column temperature was increased by 10 °C min−1 from 80 to 250 °C.
Detection of (E/Z)-PAOx in F. sachalinensis
To detect (E/Z)-PAOx, 200 mg of leaf was disrupted using a Multi-beads Shocker (Yasui Kikai, Osaka, Japan). Plant powder was extracted with 300 µL of MeOH. The extract was diluted with water up to fivefold and analysed using a liquid chromatography–tandem mass spectrometry (LC–MS/MS) system equipped with a COSMOSIL 5C18-MS-II Packed Column (150 mm × 2.0 mm i.d.; Nacalai Tesuque, Kyoto, Japan) under the following conditions: mobile phase A, 0.1 % formic acid in water; mobile phase B, 0.1 % formic acid in acetonitrile and 5–50 % linear gradient of B for 25 min and 100 % linear gradient of B for 5 min delivered at 0.2 ml min−1. MS was simultaneously performed in positive-ion mode using a high-capacity ion-trap mass spectrometer (HCT-TK, Bruker Daltonics, Billerica, MA, USA) via electrospray ionization. Multiple reaction monitoring was used to identify (E/Z)-PAOx ions with m/z 136 [M + H]+→ 119. The amount of endogenous (E/Z)-PAOx was estimated from the peak area using the standard curve made from authentic compound.
RNA sequencing
Mature leaves of F. sachalinensis were treated with MeJA to induce PAN biosynthesis, as described above. Total RNA was prepared at 6 h after MeJA or control treatment of leaves using the Plant RNA Reagent (Invitrogen, Carlsbad, CA, USA). Total RNA was further purified using an RNeasy Mini Kit (Qiagen, Hilden, Germany). Purified total RNA was sequenced with no biological replicates at the Beijing Genomics Institute using with a Hiseq 2000 (Illumina, Hayward, CA, USA) equipped with a paired-end flow-cell. Raw RNA-seq data were deposited in the DNA Data Bank of JAPAN (DDBJ) Sequence Read Archive under the accession numbers DRR036752 and DRR036753.
Bioinformatics
RNA-seq data for MeJA-treated and untreated samples were assembled using Trinity r20140413p1 (Grabherr et al. 2011) and default parameters. Assembled sequences were annotated using BLASTx (Altschul et al. 1990) to search the non-redundant protein database (Nr) of the NCBI, and the E-value threshold was 1.0 × 10−3. Gene ontology (GO) terms were assigned using BLAST2GO software (Conesa et al. 2005) and were visualized using WEGO (Ye et al. 2006). To identify MeJA-induced transcripts, the analysis of differential expression was performed using the edgeR package (Robinson et al. 2010) of Bioconducter (Gentleman et al. 2004) that is included in Trinity r201404131p1 (http://trinityrnaseq.sourceforge.net/analysis/diff_expression_analysis.html). The false discovery rate method was employed to determine the p value threshold. The difference in expression levels of a transcript was considered to be significant when p value was ≤0.01 and the fold change was ≥4.
Molecular cloning of the cDNAs encoding MeJA-induced cytochrome P450s and FsCPR
Total RNA was prepared from MeJA-treated leaves as described above, and cDNA was synthesized using the GeneRacer Kit (Invitrogen, Carlsbad, CA, USA). The primers for 5′- and 3′-RACE were designed according to the assembled sequences. PCR was performed using Tks Gflex DNA Polymerase (Takara, Shiga, Japan). RACE products were cloned into the pCR-Blunt vector (Invitrogen) and sequenced. Coding sequences of MeJA-induced cytochrome P450s and FsCPR were amplified by PCR using Tks Gflex DNA Polymerase and gene-specific primers, which were designed according to the sequences of the RACE products. Amplified products were cloned into the pCR-Blunt vector. To avoid PCR-generated errors, more than four clones were sequenced. Sequence data were submitted to the DDBJ/EMBL/GenBank databases under the accession numbers LC060449, LC060452, LC060453, LC060456 and LC060457. Oligonucleotide primers used in this study are summarized in Table S1.
Heterologous expression in insect cells of MeJA-induced cytochrome P450s together with FsCPR
To identify the enzymes involved in PAN biosynthesis, MeJA-induced cytochrome P450s and FsCPR were coexpressed in the microsome of Sf9 cells. Coding sequences of the cytochrome P450s and FsCPR were re-amplified by PCR and inserted into the appropriate cloning sites using the In-Fusin HD Cloning kit (Clontech Laboratories, Palo Alto, CA, USA), and expression was controlled by the polyhedron promoter or p10 promoter of pFastBac Dual (Invitrogen), respectively. After the confirmation of insert DNA sequences, plasmids were used to transform Escherichia coli DH10BAC (Invitrogen) to obtain recombinant bacmids. Sf9 cells maintained in Sf-900II-SFM medium (Invitrogen) containing 5 % fetal bovine serum (FBS) (Gibco BRL, Grand Island, NY, USA) at 28 °C were transfected with recombinant bacmids using X-tremeGENE 9 DNA Transfection Reagent (Roche Applied Science, Basel, Switzerland). To prepare high-titer stocks (P2 viruses), recombinant viruses were recovered at 3 days after transfection and were used to infect Sf9 cells; the virus preparation was stored at 4 °C.
To express cytochrome P450s and FsCPR, Sf9 cells (1.5 × 106 cells/ml) maintained in Sf-900II-SFM supplemented with 2 % FBS were infected with P2 viruses. The heme precursor hemin at a concentration of 2 µg/ml was added to the medium at 24 h after infection. Cells were harvested by centrifugation at 72 h after infection and resuspended in 50 mM HEPES–NaOH (pH 7.6) containing 20 % glycerol, 1 mM DTT and an appropriate volume of proteinase inhibitor cocktail (Roche Applied Science). After the disruption of cells using ultrasonication, the microsomal fraction was recovered by ultracentrifugation, as described previously (Yamaguchi et al. 2014). Protein concentrations were determined using a protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA) and bovine serum albumin as the standard.
Enzyme assay
A 200-µl reaction mixture containing 0.5-mg microsomal fraction, 50 mM HEPES–NaOH (pH 7.6), 2 mM NADPH, 1 mM DTT and 1 mM (E/Z)-PAOx was incubated at 30 °C for 16 h. PAN produced from (E/Z)-PAOx was extracted using twice the volume of n-hexane and analysed using a GC–MS system, as described above. To determine the substrate specificity of CYP71AT96, the reaction time was reduced to 30 min and 1 mM (E/Z)-PAOx, (E/Z)-4-hydroxyPAOx or (E/Z)-indole-3-aldoxime were used as substrates. The reaction products converted from aldoximes were quantified using a Prominence Ultra Fast Liquid Chromatography System (Shimadzu) equipped with a COSMOCIL 2.5C18-MS-II column (75 mm × 2.0 mm i.d.; Nacalai Tesque) under the following conditions: Condition A (PAN and indole-3-acetonitrile), column oven temperature of 40 °C; solvent, 0.1 % formic acid in 25 % acetonitrile and flow rate, 0.4 ml min−1, and Condition B (4-hydroxyPAN), column oven temperature of 30 °C; solvent, 0.1 % formic acid in 25 % acetonitrile and flow rate, 0.3 ml min−1. PAN, 4-hydroxyPAN and indole-3-acetonitrile were measured according to their absorbance at 210, 279 and 223 nm, respectively.
Quantitative real-time PCR (qRT-PCR) analysis
The expression level of CYP71AT96 after the leaves were fed upon by the Japanese beetle or after MeJA treatment was quantified using qRT-PCR. A leaf of F. sachalinensis was used to feed the Japanese beetles, as described by Noge et al. (2011). After 6 h of beetle feeding or MeJA treatment, total RNA was isolated, as described above. The integrity of the total RNA was ascertained using agarose gel electrophoresis (Fig. S5). cDNAs were synthesized using a PrimeScript RT reagent Kit with gDNA Eraser (Perfect Real Time; Takara). CYP71AT96 transcripts were quantified using an ABI 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) and SYBR premix Ex Taq II (Tli RNaseH Plus; Takara) with ROX as a reference dye. PCR amplification was performed as follows: at 95 °C for 30 s, 40 cycles at 95 °C for 5 s and at 60 °C for 40 s. To assess specificity of PCR amplification, PCR products were cloned into the pCRII Vector (Invitrogen) and fourteen individual clones were sequenced.
For calculation of expression levels, the relative standard curve method was used. Standard curves for each of the genes amplified were done using serial dilutions of cDNA (1/10, 1/100, 1/1000 and 1/10000). GAPDH mRNA (c24538_g1_i1) was selected as an endogenous control because the expression level of GAPDH was not affected by MeJA treatment. Samples from each of the four biological replicates were assayed in triplicates for a total of twelve measurements per data point.
Phylogenetic analysis of cytochrome P450s
Phylogenetic analysis was performed using the MeJA-induced cytochrome P450s of F. sachalinensis and previously reported aldoxime-metabolizing cytochrome P450s from the GenBank database. Multiple sequence alignment of cytochrome P450s was performed using the ClustalX program (Larkin et al. 2007) with default parameters. The phylogenetic tree was constructed using the Neighbor-Joining method using the MEGA 6 (Tamura et al. 2013). The significance level for the phylogenetic tree was assessed by bootstrap testing with 1000 replications.
Results
(E/Z)-PAOx is the biosynthetic precursor of PAN in F. sachalinensis
Because (E/Z)-PAOx is the precursor of PAN in Po. trichocarpa, cyanogenic plants and aldoxime-degrading bacteria (Kato et al. 2000; Yamaguchi et al. 2014; Irmisch et al. 2014; Yamaguchi and Asano 2015), PAN was proposed as the product of the conversion of L-Phe via (E/Z)-PAOx in F. sachalinensis. To support this hypothesis, [Ring-D5]-(E/Z)-PAOx was synthesized and applied to the leaves of F. sachalinensis with or without MeJA treatment. MeJA-treated leaves emitted PAN as opposed to untreated leaves (Fig. 1a). We used a GC–MS to analyse the incorporation of deuterium-labelled (E/Z)-PAOx into PAN and detected a mass shift of five atomic mass units of the molecular ions of PAN (m/z 117 → 122) (Fig. 1b) that was not detected in PAN emitted from unlabelled (E/Z)-PAOx-treated leaves (Fig. 1c). Further, we analysed endogenous (E/Z)-PAOx in the leaves of F. sachalinensis upon MeJA treatment. (E/Z)-PAOx was detected in MeOH extracts of the leaves at 6 and 24 h after MeJA treatment but was not detected in untreated leaves (Fig. S1). The amount of (E/Z)-PAOx at 6 and 24 h after MeJA treatment was trace level and 202.3 ± 62.4 ng/g fresh weight (n = 3), respectively. These results are in agreement with previous findings that PAN emission is detectable at 6 h after MeJA treatment and that the amount of PAN increases with time (Noge et al. 2011). These results indicate that (E/Z)-PAOx is the biosynthetic precursor of PAN in F. sachalinensis.
Incorporation of [Ring-D5]-(E/Z)-PAOx into MeJA-induced PAN. a Typical gas chromatogram of volatiles from a MeJA-treated leaf treated with deuterium-labelled (E/Z)-PAOx (1) or unlabeled (E/Z)-PAOx (2) and volatiles from an untreated leaf treated with (E/Z)-PAOx (3). b Mass spectrum of PAN emitted from a leaf treated with deuterium-labelled (E/Z)-PAOx. c Mass spectrum of PAN emitted from a leaf treated with unlabelled (E/Z)-PAOx
Detection of MeJA-induced transcripts by RNA-seq
To identify the enzyme(s) involved in the PAN biosynthesis of F. sachalinensis, we used RNA-seq to identify MeJA-induced transcripts at 6 h after MeJA treatment when (E/Z)-PAOx and emission of PAN were detectable. As a negative control total RNA was prepared from an untreated leaf. RNAs isolated from MeJA-treated and -untreated samples were sequenced to produce 90 bp paired-end clean reads (~50 million reads for both samples). Using Trinity, the assembly of reads generated 74,329 contigs with a mean size of 696 bp and an N50 of 1680 bp (Table 1). The raw reads of RNA-seq data were deposited in the DDBJ Sequence Read Archive (DRR036752 and DRR036753).
To annotate the contigs, we used them to query the non-redundant protein database (Nr) of the NCBI. Sixty-six percent of the contigs yielded significant matches with sequences of various plant species, and the highest number of sequences were those representing Vitis vinifera (21 %), Theobroma cacao (9 %), Jatropha curcas (6 %), Po. trichocarpa (5 %) and Citrus sinensis (4 %) (Fig. S2). Of the contigs with significant identities, 70.5 % were assigned GO terms. The annotated GO terms were classified into three categories as follows: cellular component, biological process and molecular function. The classification of annotated contigs is summarized in Fig. S3.
The 252 and 352 contigs that were induced or reduced, respectively, in response to MeJA (Fig. 2) are summarized in Table S2. Among the MeJA-induced contigs, c17497_g1_i1 and c25152_g1_i1 (annotated as (E)-β-ocimene/(E,E)-α-farnesene synthases) as well as c18512_g1_i1 (annotated as nerol dehydratase) are likely to be involved in the biosynthesis of MeJA-induced terpenes in F. sachalinensis, which include (E)-β-ocimene, linalool, (E)-4,8-dimethyl-1,3,7-nonatriene and (E,E)-α-farnesene (Noge et al. 2011). These findings indicate the successful identification of MeJA-induced transcripts.
Cloning of cDNAs encoding candidates enzymes that catalyses the synthesis of PAN
Nitriles are formed from amino acids via aldoximes with the aid of CYP79s and CYP71s in Po. trichocarpa and cyanogenic plants (Bak et al. 1998; Jørgensen et al. 2011; Irmisch et al. 2013; Gleadow and Møller 2014; Yamaguchi et al. 2014; Irmisch et al. 2014). Therefore, we reasoned that MeJA-induced cytochrome P450s are involved in PAN biosynthesis in F. sachalinensis. Among the contigs induced by MeJA treatment, five (c21714_g1_i2, c11561_g1_i1, c15589_g2_i2, c15589_g3_i1 and c8278_g1_i1) are annotated as cytochrome P450s. Because c15589_g2_i2 and c15589_g3_i1 were combined after 5′- and 3′-RACE, four full-length cDNAs encoding MeJA-induced cytochrome P450s were cloned as putative components of the PAN biosynthetic pathway. According to the conventions of cytochrome P450 nomenclature, these cytochrome P450s were designated as CYP71AT96 (c21714_g1_i1), CYP82AN1 (c11561_g1_i1), CYP82D125 (c15589_g2_i2 and c15589_g3_i1) and CYP715A35 (c8278_g1_i1) (DDBJ accession numbers: LC060449, LC060452, LC060453 and LC060456, respectively). CYP71AT96 showed 57, 55, 42, 50 and 45 % identities at the amino acid level to CYP71AT3 from V. vinifera, CYP83F21 from Citrus clementina, CYP71AN24 from Pr. mume, CYP71B40v3 from Po. trichocarpa and CYP71B41v2 from Po. trichocarpa, respectively. CYP82AN1, CYP82D125 and CYP715A35 showed 47, 57 and 52 % identities at the amino acid level to CYP82S8 from V. vinifera, CYP82D4 from V. vinifera and CYP715A9 from Ricinus communis, respectively. CYP79 sequences were not represented in any of the contigs; this result suggested that the levels of CYP79 mRNAs were lower than the limit of detection of RNA-seq, or that (E/Z)-PAOx is synthesized by CYP79-independent pathway(s). The predicted amino acid sequences of the abovementioned cytochrome P450s include conserved motifs of the cytochrome P450 family such as the PERF, G-x-E/D-T-T/S motif and heme-binding motif (PFGXGXRCXG) (Bak et al. 2011).
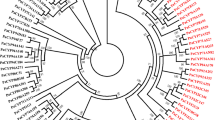
Phylogenetic analysis revealed a closer relationship of CYP71AT96 to CYP83s rather than aldoxime-metabolizing CYP71s (Fig. 3). Considering that CYP83A1 and CYP83B1 act on aldoximes in glucosinolate biosynthesis (Hansen et al. 2001; Bak and Feyereisen 2001; Naur et al. 2003), we reasoned that the conversion of (E/Z)-PAOx to PAN is likely to be catalysed by CYP71AT96.
Phylogenetic analysis of MeJA-induced cytochrome P450s of F. sachalinenesis and aldoxime metabolizing cytochrome P450s. The protein sequences of aldoxime metabolizing cytochrome P450s were obtained from the GenBank. A phylogenetic tree was constructed by the neighbour-joining method using a 1000-replicate bootstrap test. MeJA-induced cytochrome P450s of F. sachalinensis were indicated with green circles
CYP71AT96 catalyses the conversion of (E/Z)-PAOx to PAN
To identify enzymes that catalyse the conversion of (E/Z)-PAOx to PAN, four MeJA-induced cytochrome P450s were coexpressed with their redox partner cytochrome P450 reductase (FsCPR: LC060457) in the microsomes of Sf9 cells. In our enzyme assay using a GC–MS, a back ground level of PAN was detected (Fig. 4) because of thermal dehydration of the aldoxime during the high-temperature GC injection (Ouédraogo et al. 2009). Among the cytochrome P450s, CYP71AT96 generated PAN in amounts significant above back ground level after incubation with (E/Z)-PAOx and NADPH (Fig. 4).
CYP71AN24 from Pr. mume catalyses the sequential dehydration of (E/Z)-PAOx and the hydroxylation of PAN to form mandelonitrile in the pathway of prunasin and amygdalin biosynthesis (Yamaguchi et al. 2014). Mandelonitrile decomposes spontaneously to benzaldehyde and HCN in a GC–MS (Yamaguchi et al. 2014); however, CYP71AT96 did not produce benzaldehyde (Fig. S4), indicating that CYP71AT96 catalyses the conversion of (E/Z)-PAOx to PAN but not to mandelonitrile.
CYP71AT96 not only acted on (E/Z)-PAOx but also on l-tryptophan-derived (E/Z)-indole-3-acetaldoxime and L-tyrosine-derived (E/Z)-4-hydroxyPAOx with relative activity of 28.8 and 2.8 % of (E/Z)-PAOx, respectively (Table 2). The broad substrate specificity of CYP71AT96 is similar to that of known aldoxime-metabolizing CYP71s (Kahn et al. 1999; Jørgensen et al. 2011; Yamaguchi et al. 2014; Irmisch et al. 2014).
CYP71AT96 expression is induced by feeding by Japanese beetles
We performed qRT-PCR to analyse the expression of the gene encoding CYP71AT96 when the leaves of F. sachalinensis were infested by the Japanese beetle or treated with MeJA. At first, we evaluated our qRT-PCR system. There was no difference in quality of total RNA isolated from untreated leaf and PAN biosynthesis induced leaf (Fig. S5). The R2 values for standard curves for CYP71AT96 and GAPDH were 0.999 and 0.994, respectively. Sequencing of qRT-PCR fragments obtained from cDNA made from MeJA-treated leaves did not detect amplification of non-targeted genes. These results indicated that our qRT-PCR system could accurately quantify CYP71AT96 expression.
CYP71AT96 mRNA level was strongly increased when treated with MeJA or infested with the Japanese beetle compared with its levels in untreated leaves (MeJA, p < 0.05; beetle feeding, p < 0.01; n = 4) (Fig. 5). The results in agreement with that MeJA-untreated leaves of F. sachalinensis-treated (E/Z)-PAOx did not emit PAN (Fig. 1a-3).
Relative levels of CYP71AT96 mRNA induced by beetle feeding and MeJA treatment. RNA was extracted from beetle-infested, MeJA-treated, or untreated leaves. The expression level of CYP71AT96 was normalized to that of GAPDH. Error bars represent the standard error of the mean of four biological replicates. Asterisks indicate statistical significance (*p < 0.05, **p < 0.01, two-tailed Welch’s t test (n = 4) relative to controls)
Discussions
After the Japanese beetle feeding or MeJA treatment, F. sachalinensis synthesizes PAN from L-Phe (Noge et al. 2011; Noge and Tamogami 2013). However, the biosynthetic intermediate and enzymes involved in the pathway have remained unclear. In this study, we showed that (E/Z)-PAOx was an intermediate in herbivore-induced PAN biosynthesis, and that CYP71AT96 converted (E/Z)-PAOx to PAN in leaves in response to feeding by beetles and MeJA treatment.
Several members of CYP71 family, which represents more than half of all cytochrome P450s in higher plants (Nelson and Werck-Reichhart 2011), are known to act on aldoximes. Nitrile forming CYP71s are well studied in cyanogenic plants. CYP71s catalyse sequential dehydration and hydroxylation of aldoximes to form hydroxynitriles (Bak et al. 1998; Jørgensen et al. 2011), which are subsequently converted to cyanogenic glucosides as storage form (Jones et al. 1999; Nielsen et al. 2008). For example, CYP71AN24 from Pr. mume catalyses dehydration of (E/Z)-PAOx to PAN and hydroxylation of PAN to mandelonitrile. These reactions require cytochrome P450 reductase-mediated electron transfer from NADPH to CYP71AN24 (Yamaguchi et al. 2014). Some plants are known to release nitriles as volatile (Takabayashi et al. 1991, 1994; Van Poecke et al. 2001; Irmisch et al. 2013; Kuwahara et al. 2014). However, enzymes involved in volatile nitrile biosynthesis have been reported only in Po. trichocarpa (Irmisch et al. 2013, 2014). CYP71B40v3 and CYP71B41v2 from the plant catalyse dehydration of aldoximes to nitriles without the aid of cytochrome P450 reductase and NADPH, when expressed in E. coli (Irmisch et al. 2014). Although the conversion of (E/Z)-PAOx to PAN by CYP71AT96 identified in this study was identical to that by CYP71B40v3 and CYP71B41v2, CYP71AT96 required cytochrome P450 reductase and NADPH to its catalysis (Fig. 4), indicating that reduction of the heme group is required for dehydration of (E/Z)-PAOx. This observation was in agreement with the previous reports that dehydration of aldoximes requires an initial reduction of Fe(III) to Fe(II) in the heme group of enzyme to enable binding of the nitrogen atom of aldoximes to Fe(II) (Hart-Davis et al. 1998; Sawai et al. 2009). Mutagenic analysis of CYP71Bs showed that last Ser residue of the conserved G-x-E/D-T-T/S motif, which corresponds to the proton transfer groove on the distal side of heme (Jia et al. 2014), is crucial for the conversion of aldoximes to nitriles (Irmisch et al. 2014). The motif of CYP71AT96 is A305TETT309, which is dissimilar to G308VDTS312 of CYP71Bs. The motif may cause the difference of electron transfer dependencies between CYP71AT96 and CYP71Bs, although the mechanism of dehydration catalysed by cytochrome P450s is not clear.
In response to herbivore attack, Po. trichocarpa biosynthesizes L-Phe-derived PAN, l-isoleucine-derived 2-methylbutyronitrile and l-leucine-derived 3-methylbutyronitrile with the aid of CYP79D6v3, CYP79D7v2, CYP71B40v3 and CYP71B41v2, because CYP79Ds and CYP71Bs accept various amino acids and aldoximes, respectively (Irmisch et al. 2013, 2014). The cyanogenic Japanese apricot produces only L-Phe-derived cyanogenic glucosides, prunasin and amygdalin, because CYP79D16 accepts L-Phe but not other amino acids, whereas CYP71AN24 act on various aldoximes (Yamaguchi et al. 2014). Because previously reported aldoxime metabolising CYP71s are known to have broad substrate specificity (Kahn et al. 1999; Jørgensen et al. 2011; Yamaguchi et al. 2014; Irmisch et al. 2014), nitrile biosynthesis is likely specified by aldoxime forming enzymes. F. sachalinensis emits L-Phe-derived PAN but not other amino acid-derived nitriles (Noge and Tamogami 2013), although CYP71AT96 catalyses the conversion of various aldoximes to their corresponding nitriles (Table 2). Because the leaves of F. sachalinensis accumulate amino acids including L-Phe, l-tyrosine and l-tryptophan (Noge and Tamogami 2013), the aldoxime forming enzyme(s) of F. sachalinensis is likely L-Phe-specific and provides CYP71AT96 with (E/Z)-PAOx to catalyse PAN synthesis.
Herbivore-induced volatile emission is often controlled at the transcriptional level of the biosynthetic genes involved. CYP71AT96 expression was highly induced by the MeJA treatment or the Japanese beetle feeding (Fig. 5). Feeding of (E/Z)-PAOx to MeJA-untreated leaves of F. sachalinensis did not result in PAN emission (Fig. 1a-3), thereby indicating that the conversion of (E/Z)-PAOx to PAN is controlled at the level of CYP71AT96 transcription when leaves are fed upon by beetles or are treated with MeJA. Taken together, we propose that the herbivore-induced PAN biosynthetic pathway of F. sachalinensis is mediated by CYP71AT96, as depicted in Fig. 6.
Cytochrome P450s involved in secondary metabolism of plants have potential for industrial applications (Chemler and Koffas 2008). Although the utilization of plant cytochrome P450s has been limited because of low protein expression levels in heterologous host, a number of strategies to improve expression levels of cytochrome P450s have been developed (Zelasko et al. 2013; Miki and Asano 2014). CYP71AT96 from F. sachalinensis catalysed the conversion of aldoximes to nitriles under mild conditions, while chemical synthesis of nitriles from aldoximes requires high-temperature and high-pressure conditions (Asano and Kato 1998). Thus, CYP71AT96 could be utilized as a biocatalyst for production of nitriles, which are generally used in industries.
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410. doi:10.1016/S0022-2836(05)80360-2
Asano Y, Kato Y (1998) Z-phenylacetaldoxime degradation by a novel aldoxime dehydratase from Bacillus sp. strain OxB-1. FEMS Microbiol Lett 158:185–190. doi:10.1016/S0378-1097(97)00520-X
Bak S, Feyereisen R (2001) The involvement of two p450 enzymes, CYP83B1 and CYP83A1, in auxin homeostasis and glucosinolate biosynthesis. Plant Physiol 127:108–118
Bak S, Kahn RA, Nielsen HL, Møller BL, Halkier BA (1998) Cloning of three A-type cytochromes P450, CYP71E1, CYP98, and CYP99 from Sorghum bicolor (L.) Moench by a PCR approach and identification by expression in Escherichia coli of CYP71E1 as a multifunctional cytochrome P450 in the biosynthesis of the cyanogenic glucoside dhurrin. Plant Mol Biol 36:393–405. doi:10.1023/A:1005915507497
Bak S, Beisson F, Bishop G, Hamberger B, Höfer R, Paquette S, Werck-Reichhart D (2011) Cytochromes P450. Arabidopsis Book 9:e0144. doi:10.1199/tab.0144
Brilli F, Ciccioli P, Frattoni M, Prestininzi M, Spanedda AF, Loreto F (2009) Constitutive and herbivore-induced monoterpenes emitted by Populus x euroamericana leaves are key volatiles that orient Chrysomela populi beetles. Plant Cell Environ 32:542–552. doi:10.1111/j.1365-3040.2009.01948.x
Chemler JA, Koffas MAG (2008) Metabolic engineering for plant natural product biosynthesis in microbes. Curr Opin Biotechnol 19:597–605. doi:10.1016/j.copbio.2008.10.011
Clavijo McCormick A, Unsicker SB, Gershenzon J (2012) The specificity of herbivore-induced plant volatiles in attracting herbivore enemies. Trends Plant Sci 17:303–310. doi:10.1016/j.tplants.2012.03.012
Conesa A, Götz S, García-Gómez JM, Terol J, Talón M, Robles M (2005) Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 21:3674–3676. doi:10.1093/bioinformatics/bti610
De Moraes CM, Mescher MC, Tumlinson JH (2001) Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature 410:577–580. doi:10.1038/35069058
Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JYH, Zhang J (2004) Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5:R80. doi:10.1186/gb-2004-5-10-r80
Gleadow RM, Møller BL (2014) Cyanogenic glycosides: synthesis, physiology, and phenotypic plasticity. Annu Rev Plant Biol 65:155–185. doi:10.1146/annurev-arplant-050213-040027
Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, Adiconis X, Fan L, Raychowdhury R, Zeng Q, Chen Z, Mauceli E, Hacohen N, Gnirke A, Rhind N, di Palma F, Birren BW, Nusbaum C, Lindblad-Toh K, Friedman N, Regev A (2011) Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol 29:644–652. doi:10.1038/nbt.1883
Hamel J (2011) A review of acute cyanide poisoning with a treatment update. Crit Care Nurse 31:72–81. doi:10.4037/ccn2011799 quiz 82
Hansen CH, Du L, Naur P, Olsen CE, Axelsen KB, Hick AJ, Pickett JA, Halkier BA (2001) CYP83b1 is the oxime-metabolizing enzyme in the glucosinolate pathway in Arabidopsis. J Biol Chem 276:24790–24796. doi:10.1074/jbc.M102637200
Hart-Davis J, Battioni P, Boucher JL (1998) New catalytic properties of iron porphyrins: model systems for cytochrome P450-catalyzed dehydration of aldoximes. J Am Chem Soc. doi:10.1021/ja981805y
Hettenhausen C, Schuman MC, Wu J (2015) MAPK signaling: a key element in plant defense response to insects. Insect Sci 22:157–164. doi:10.1111/1744-7917.12128
Irmisch S, McCormick AC, Boeckler GA, Schmidt A, Reichelt M, Schneider B, Block K, Schnitzler J-P, Gershenzon J, Unsicker SB, Köllner TG (2013) Two herbivore-induced cytochrome P450 enzymes CYP79D6 and CYP79D7 catalyze the formation of volatile aldoximes involved in poplar defense. Plant Cell 25:4737–4754. doi:10.1105/tpc.113.118265
Irmisch S, Clavijo McCormick A, Günther J, Schmidt A, Boeckler GA, Gershenzon J, Unsicker SB, Köllner TG (2014) Herbivore-induced poplar cytochrome P450 enzymes of the CYP71 family convert aldoximes to nitriles which repel a generalist caterpillar. Plant J 80:1095–1107. doi:10.1111/tpj.12711
Jia S, Wan P-J, Li G-Q (2014) Molecular cloning and characterization of the putative Halloween gene Phantom from the small brown planthopper Laodelphax striatellus. Insect Sci 22:707–718. doi:10.1111/1744-7917.12147
Jones PR, Møller BL, Hoj PB (1999) The UDP-glucose:p-hydroxymandelonitrile-O-glucosyltransferase that catalyzes the last step in synthesis of the cyanogenic glucoside dhurrin in Sorghum bicolor. Isolation, cloning, heterologous expression, and substrate specificity. J Biol Chem 274:35483–35491. doi:10.1074/jbc.274.50.35483
Jørgensen K, Morant AV, Morant M, Jensen NB, Olsen CE, Kannangara R, Motawia MS, Møller BL, Bak S (2011) Biosynthesis of the cyanogenic glucosides linamarin and lotaustralin in cassava: isolation, biochemical characterization, and expression pattern of CYP71E7, the oxime-metabolizing cytochrome P450 enzyme. Plant Physiol 155:282–292. doi:10.1104/pp.110.164053
Kahn RA, Fahrendorf T, Halkier BA, Møller BL (1999) Substrate specificity of the cytochrome P450 enzymes CYP79A1 and CYP71E1 involved in the biosynthesis of the cyanogenic glucoside dhurrin in Sorghum bicolor (L.) Moench. Arch Biochem Biophys 363:9–18. doi:10.1006/abbi.1998.1068
Kato Y, Ooi R, Asano Y (1999) A new enzymatic method of nitrile synthesis by Rhodococcus sp. strain YH3-3. J Mol Catal B-Enzym 6:249–256
Kato Y, Ooi R, Asano Y (2000) Distribution of aldoxime dehydratase in microorganisms. Appl Environ Microbiol 66:2290–2296. doi:10.1128/AEM.66.6.2290-2296.2000
Kessler A, Baldwin IT (2001) Defensive function of herbivore-induced plant volatile emissions in nature. Science 291:2141–2144. doi:10.1126/science.291.5511.2141
Kuwahara Y, Ichiki Y, Morita M, Asano Y (2014) (2-Nitroethyl)benzene: a major flower scent from the Japanese loquat Eriobotrya japonica [Rosales: Rosaceae]. Biosci Biotechnol Biochem 78:1320–1323. doi:10.1080/09168451.2014.921558
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948. doi:10.1093/bioinformatics/btm404
Loughrin JH, Potter DA, Hamilton-Kemp TR (1995) Volatile compounds induced by herbivory act as aggregation kairomones for the Japanese beetle (Popillia japonica Newman). J Chem Ecol 21:1457–1467. doi:10.1007/BF02035145
Miki Y, Asano Y (2014) Biosynthetic pathway for the cyanide-free production of phenylacetonitrile in Escherichia coli by utilizing plant cytochrome P450 79A2 and bacterial aldoxime dehydratase. Appl Environ Microbiol 80:6828–6836. doi:10.1128/AEM.01623-14
Møller BL (1977) Chemical synthesis of labelled intermediates in cyanogenic glucoside biosynthesis. J Label Compd Radiopharm 14:663–671. doi:10.1002/jlcr.2580140504
Naur P, Petersen BL, Mikkelsen MD, Bak S, Rasmussen H, Olsen CE, Halkier BA (2003) CYP83A1 and CYP83B1, two nonredundant cytochrome P450 enzymes metabolizing oximes in the biosynthesis of glucosinolates in Arabidopsis. Plant Physiol 133:63–72. doi:10.1104/pp.102.019240
Nelson D, Werck-Reichhart D (2011) A P450-centric view of plant evolution. Plant J 66:194–211. doi:10.1111/j.1365-313X.2011.04529.x
Nielsen KA, Tattersall DB, Jones PR, Møller BL (2008) Metabolon formation in dhurrin biosynthesis. Phytochemistry 69:88–98. doi:10.1016/j.phytochem.2007.06.033
Noge K, Tamogami S (2013) Herbivore-induced phenylacetonitrile is biosynthesized from de novo-synthesized l-phenylalanine in the giant knotweed, Fallopia sachalinensis. FEBS Lett 587:1811–1817. doi:10.1016/j.febslet.2013.04.038
Noge K, Abe M, Tamogami S (2011) Phenylacetonitrile from the giant knotweed, Fallopia sachalinensis, infested by the Japanese beetle, Popillia japonica, is induced by exogenous methyl jasmonate. Molecules 16:6481–6488. doi:10.3390/molecules16086481
Ouédraogo IW, Boulvin M, Flammang R, Gerbaux P, Bonzi-Coulibaly YL (2009) Conversion of natural aldehydes from Eucalyptus citriodora, Cymbopogon citratus, and Lippia multiflora into oximes: GC-MS and FT-IR analysis. Molecules 14:3275–3285. doi:10.3390/molecules14093275
Pollak P, Romeder G, Hagedorn F, Gelbke H-P (2000) Nitriles. Wiley-VCH Verlag GmbH & Co, KGaA, Weinheim
Robinson MD, McCarthy DJ, Smyth GK (2010) edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26:139–140. doi:10.1093/bioinformatics/btp616
Rodriguez-Saona C, Crafts-Brandner SJ, Paré PW, Henneberry TJ (2001) Exogenous methyl jasmonate induces volatile emissions in cotton plants. J Chem Ecol 27:679–695
Sawai H, Sugimoto H, Kato Y, Asano Y, Shiro Y, Aono S (2009) X-ray crystal structure of michaelis complex of aldoxime dehydratase. J Biol Chem 284:32089–32096. doi:10.1074/jbc.M109.018762
Shah J (2009) Plants under attack: systemic signals in defence. Curr Opin Plant Biolo 12:459–464. doi:10.1016/j.pbi.2009.05.011
Shibata K, Hasegawa N, Fukumoto Y, Chatani N (2012) Ruthenium-catalyzed carbonylation of ortho C–H bonds in arylacetamides: C–H bond activation utilizing a bidentate-chelation system. ChemCatChem 4:1733–1736. doi:10.1002/cctc.201200352
Takabayashi J, Dicke M, Posthumus MA (1991) Variation in composition of predator-attracting allelochemicals emitted by herbivore-infested plants: relative influence of plant and herbivore. Chemoecology 2:1–6. doi:10.1007/BF01240659
Takabayashi J, Dicke M, Posthumus MA (1994) Volatile herbivore-induced terpenoids in plant-mite interactions: variation caused by biotic and abiotic factors. J Chem Ecol 20:1329–1354. doi:10.1007/BF02059811
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729. doi:10.1093/molbev/mst197
Unsicker SB, Kunert G, Gershenzon J (2009) Protective perfumes: the role of vegetative volatiles in plant defense against herbivores. Curr Opin Plant Biolo 12:479–485. doi:10.1016/j.pbi.2009.04.001
Van Poecke RM, Posthumus MA, Dicke M (2001) Herbivore-induced volatile production by Arabidopsis thaliana leads to attraction of the parasitoid Cotesia rubecula: chemical, behavioral, and gene-expression analysis. J Chem Ecol 27:1911–1928
Yakhvarov DG, Ganushevich YS, Sinyashin OG (2007) Direct formation of P–C and P–H bonds by reactions of organozinc reagents with white phosphorus. Mendeleev Commun 17:197–198
Yamaguchi T, Asano Y (2015) Complete genome sequence of an aldoxime degrader, Bacillus sp. OxB-1. Genome Announc 3:e00025-15. doi:10.1128/genomeA.00025-15
Yamaguchi T, Bando H, Asano S-I (2013) Identification of a Bacillus thuringiensis Cry8Da toxin-binding glucosidase from the adult Japanese beetle, Popillia japonica. J Invertebr Pathol 113:123–128. doi:10.1016/j.jip.2013.03.006
Yamaguchi T, Yamamoto K, Asano Y (2014) Identification and characterization of CYP79D16 and CYP71AN24 catalyzing the first and second steps in l-phenylalanine-derived cyanogenic glycoside biosynthesis in the Japanese apricot, Prunus mume Sieb. et Zucc. Plant Mol Biol 86:215–223. doi:10.1007/s11103-014-0225-6
Ye J, Fang L, Zheng H, Zhang Y, Chen J, Zhang Z, Wang J, Li S, Li R, Bolund L, Wang J (2006) WEGO: a web tool for plotting GO annotations. Nucleic Acids Res 34:W293–W297. doi:10.1093/nar/gkl031
Zelasko S, Palaria A, Das A (2013) Optimizations to achieve high-level expression of cytochrome P450 proteins using Escherichia coli expression systems. Protein Expr Purif. doi:10.1016/j.pep.2013.07.017
Acknowledgments
We thank Dr. D. Nelson for naming our cytochrome P450s, Dr. Y. Miki for preparing RNAs for RNA-seq and Dr. Y. Kuwahara and M. Morita for synthesizing deuterium-labelled compounds. This study was supported by the Exploratory Research for Advanced Technology Program of the Japan Science and Technology Agency.
Author Contributions
Conceived and designed the experiments: TY KN YA. Performed the experiments: TY KN. Analyzed the data: TY KN YA. Contributed reagents/materials/analysis tools: TY KN YA. Wrote the paper: TY KN YA. Revising the manuscript: TY KN YA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yamaguchi, T., Noge, K. & Asano, Y. Cytochrome P450 CYP71AT96 catalyses the final step of herbivore-induced phenylacetonitrile biosynthesis in the giant knotweed, Fallopia sachalinensis . Plant Mol Biol 91, 229–239 (2016). https://doi.org/10.1007/s11103-016-0459-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-016-0459-6