Abstract
Dendrobium officinale is a traditional Chinese medicinal plant. The stems of D. officinale contain mannan polysaccharides, which are promising bioactive polysaccharides for use as drugs. However, the genes involved in the biosynthesis of mannan polysaccharides in D. officinale have not yet been identified. In this study, four digital gene expression profiling analyses were performed on developing stems of greenhouse-grown D. officinale to identify such genes. Based on the accumulation of mannose and on gene expression levels, eight CELLULOSE SYNTHASE-LIKE A genes (CSLA), which are highly likely to be related to the biosynthesis of bioactive mannan polysaccharides, were identified from the differentially expressed genes database. In order to further analyze these DoCSLA genes, a full-length cDNA of each was obtained by RACE. The eight genes, belonging to the CSLA family of the CesA superfamily, contain conserved domains of the CesA superfamily. Most of the genes, which were highly expressed in the stems of D. officinale, were related to abiotic stress. Our results suggest that the CSLA family genes from D. officinale are involved in the biosynthesis of bioactive mannan polysaccharides.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Dendrobium genus is one of the largest in the Orchidaceae with an estimated 800–1400 species around the world (Jin et al. 2009). D. officinale, most likely the most prized Dendrobium species, is a perennial epiphytic herb that is widely distributed in Southeastern and South Asian countries (Wu et al. 2009). D. officinale stems are rich in active compounds, especially polysaccharides (Chen and Guo 2001).
Among higher plant polysaccharides, mannans are promising bioactive polysaccharides for use in drugs (Alonso-Sande et al. 2009). The family of mannans is the most widespread group of polysaccharides in higher plants (Buckeridge 2010; Hua et al. 2004; Moreira and Filho 2008; Petkowicz et al. 2001). Molecular, genetic and functional genomic studies have identified the involvement of the cellulose synthase (CESA) superfamily genes in the biosynthesis of mannan polysaccharides (Goubet et al. 2009; Lerouxel et al. 2006; Liepman et al. 2005). In higher plants, the family of CESA superfamily genes includes the authentic cellulose synthase (CESA) genes and nine additional cellulose synthase-like (CSL) genes, CSLA to CSLJ (Fincher 2009; Hazen et al. 2002; Richmond and Somerville 2000). The CESA genes encode an enzyme responsible for cellulose synthesis in monocots and dicots (Arioli et al. 1998; Tanaka et al. 2003; Taylor et al. 2003) while the CSL genes have been proposed to encode the enzyme that participates in the synthesis of the backbones of non-cellulosic polysaccharides (Richmond and Somerville 2000). Several studies have demonstrated that the CSLA family is involved in the biosynthesis of mannan polysaccharides in many plant species. For example, a β-mannan synthase (ManS) gene from guar (Cyamopsis tetragonolobus), belonging to the CSLA family, creates the β-1, 4-mannan backbone of galactomannan (Dhugga et al. 2004). The CSLA proteins from Arabidopsis and poplar (Populus trichocarpa) are capable of producing β-mannan polysaccharides (Goubet et al. 2009; Suzuki et al. 2006).
However, the genes involved in the biosynthesis of mannan polysaccharides in D. officinale have not yet been identified even though mannose is the major component of polysaccharides from Dendrobium species such as D. officinale, D. huoshanense, D. nobile, D. fimbriatum and D. chrysotoxum (Meng et al. 2013). This indicates that CSLA genes play an important role in the synthesis of bioactive polysaccharides in D. officinale and other Dendrobium species. In the present study, given the medicinal importance of mannan polysaccharides and of D. officinale, next-generation sequencing was initially used to explore whether genes coding for the biosynthesis of mannan polysaccharides existed in D. officinale. This work will be important for understanding the molecular mechanisms of the biosynthesis of bioactive polysaccharides in D. officinale, and even in other Dendrobium species. This work also has implications for the use of genetic engineering to obtain abundant bioactive mannan polysaccharides from existing crops, which would greatly benefit an understanding of their nutritional and health status.
Materials and methods
Plant materials and growth conditions
D. officinale plants used in this study for gene expression profiling analyses, and which sprouted in April, were grown and maintained in pots cultivated in a greenhouse (Guangzhou, China) under natural conditions. Four developmental stages of D. officinale stems: S1 (about 4 months after sprouting), S2 (about 10 months after sprouting), S3 (about 12 months after sprouting) and S4 (about 16 months after sprouting) were sequentially harvested. Details of the four stages are described in the results. Six stems from plants in different pots were sliced (about 1 cm of each), frozen rapidly in liquid nitrogen and kept at −80 °C until RNA extraction each time. The adult plants, about 8 months after sprouting, were harvested and used to determine the expression of DoCSLA genes in the roots, stems and leaves.
Water-soluble polysaccharides might be related to stress resistance (Li et al. 2012), including to drought (Clifford et al. 2002) and salt (de Lima et al. 2014). Thus, to explore the relationship between the expression of CSLA genes and stress resistance, D. officinale seedlings were subject to stress treatments to detect gene expression. D. officinale seedlings, which were cultured on half-strength Murashige and Skoog (MS) (Murashige and Skoog 1962) medium containing 0.1 % activated carbon, 2 % sucrose and 0.6 % agar (pH 5.4), were grown in a growth chamber (26 ± 1 °C, 40 µmol m−2 s−1, 12-h light/12-h dark, 60 % relative humidity). Seedlings (8–9 cm in height, about 10 months after germination, and with consistent growth) were selected to conduct stress treatments, applied as 15 % polyethylene glycol (PEG) 6000 (Sigma-Aldrich, Shanghai, China) or 250 mM NaCl (Guangzhou Chemical Reagent Factory, Guangzhou, China). After 6 h, roots were harvested, weighed and frozen in liquid nitrogen and stored immediately at −80 °C until RNA isolation. The same seedlings were also used to determine the expression of DoCSLA genes in the roots, stems and leaves of greenhouse plantlets.
Analysis of water-soluble polysaccharide content
The roots, stems and leaves from seedlings (the same seedlings used in treatments) and from adult plants, also about 8 months after sprouting, as well as the stems from S1 to S4 were used to determine water-soluble polysaccharide content. Samples were crushed into a powder by a DFT-50 pulverizer (Xinno Instrument Equipment Inc., Shanghai, China) after drying in an oven at 105 °C for 10 h. The water-soluble polysaccharides were determined by the phenol–sulfuric acid method described by the Pharmacopoeia Committee of the People’s Republic of China (2010) and Dubois et al. (1956), with glucose solutions (10, 20, 40, 60, 80 and 100 μg/mL) as standards. Briefly, the powder (0.3 g) was pre-extracted in 80 % ethanol and kept in a static water bath for 2 h at 80 °C and filtered through Whatman filter paper No. 1. The residue was further extracted with 100 mL of distilled water for 2.5 h at 100 °C. After the residue was filtered out, distilled water was added to the supernatant and made up to 250 mL. From this stock, 200 µL was added to 1800 µL of distilled water. This dilute solution or 2000 µL of distilled water was rapidly vortexed, after adding 1 mL of 5 % phenol, then mixed with 5 mL of concentrated sulfuric acid. The absorbance of the sample solution was measured at 488 nm with a UV-6000 spectrophotometer (Shanghai Metash, Shanghai, China). The reaction solution which was added to 2000 µL of distilled water was used as the calibration standard. Each sample was assayed as three replicates.
Monosaccharide analysis
Powdered samples of the roots, stems and leaves from seedlings and stems (S1–S4) used for the analysis of water-soluble polysaccharide content were also used for the analysis of monosaccharides. High performance liquid chromatography (HPLC) was used to detect monosaccharides from among all polysaccharides according to the Pharmacopoeia Committee of the People’s Republic of China (2010) and Sun et al. (2013). Briefly, 0.12 g of powder was used to fractionate water-soluble polysaccharides. Ethanol-soluble materials were removed by pre-extracting twice with 80 % ethanol at 80 °C for 4 h. The water-soluble polysaccharides were extracted with 100 mL of double-distilled water at 100 °C for 2 h after adding 1 mL of internal standard (12 mg/mL, d-glucosamine hydrochloride, chromatographically pure, Sigma-Aldrich). One mL of polysaccharide solution generated by the previous step was loaded into a centrifuge tube, and then 3.0 M HCl (0.5 mL) was added for hydrolysis at 110 °C for 70 min. After hydrolysis, derivatization with 1-phenyl-3-methyl-5-pyrazolone (PMP) was conducted. For the PMP labeling reaction, 0.4 mL of the solution was used mixed with 0.3 M NaOH solution (0.4 mL) and 0.5 M PMP methanol solution (0.4 mL). The mixture was incubated at 70 °C for 110 min, then 0.3 M of HCl (0.5 mL) was added after the solution had cooled to room temperature. To remove proteins, the sample was extracted three times by 2 mL chloroform, mixing thoroughly for 2 min and centrifuging by universal 32R (Hettich, Tuttlingen, Germany) at 12,000 rpm for 5 min. The aqueous phase was collected and analyzed by HPLC under the following conditions: ZORBAX SB-Aq C (18) column (4.6 mm × 250 mm, 5 μm); acetonitrile–0.5 % ammonium acetate solution (20:80) as the mobile phase; flow rate = 1.0 mL min−1; detection wave length = 250 nm.
Digital gene expression library preparation, sequencing, and analysis
Four stages of D. officinale stems (S1, S2, S3 and S4; details described in the results) were chosen to establish four digital gene expression libraries named S1, S2, S3 and S4 for global analysis of polysaccharide metabolism (Fig. 1). Fifteen µg of total RNA was extracted from the four stages to prepare digital gene expression libraries using Column Plant RNAout2.0 (Tiandz, Inc., Beijing, China) according to the manufacturer’s protocol. Preparation of the cDNA library was described in detail in a previous study employed for another orchid, Cymbidium sinense (Zhang et al. 2013). The libraries were subjected to RNA-seq using an Illumina HiSeq™ 2000 platform at the Beijing Genomics Institute (Shenzhen, China). The raw data was cleaned by filtering out low quality reads (reads with an unknown nucleotide “N” or a quality value ≤5, which implies more than 50 % in a read) and reads in which more than 10 % had unknown bases as described previously by Blanca et al. (2011). Clean reads were mapped to in-house transcriptome reference database sequences using SOAP aligner/soap2 (Li et al. 2009), allowing no more than two nucleotide mismatches per read. The raw data of two transcriptomes sequencing using to establish the in-house transcriptome reference database have been submitted to the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) and are available under the accession numbers SRR1909493 and SRR1909494.
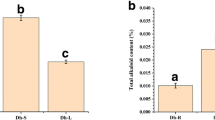
Detection of water-soluble polysaccharides contents in different organs of D. officinale in two stages (seedlings and adult plants) by phenol–sulfuric acid method. The seedlings were cultured on half-strength MS about 10 months after germination. Adult plants, about 8 months after sprouting, were maintained in pots cultivated in a greenhouse. DW dry weight. Each data bar represents the mean ± standard error (SE) (n = 3)
For gene expression analysis, the number of expressed sequence tags was calculated and then normalized to reads per Kb per million (RPKM) reads (Mortazavi et al. 2008). In this study, FDR ≤ 0.001, P ≤ 0.05 and an absolute value of the log2 ratio ≥1 were used as the threshold values to determine significant differences in gene expression between the four stages. The union of the differentially expressed genes (DEGs), generated by the four digital gene expression libraries, were assigned to a metabolic pathway using the Kyoto Encyclopedia of Genes and Genomes (KEGG, http://www.genome.jp/kegg/) annotation.
Screening the CELLULOSE SYNTHASE-LIKE A (CSLA) genes from the DEG databases and cloning
According to the annotation of unigenes and the RPKMs of the four digital gene expression databases, unigenes that were associated with the biosynthesis of mannan polysaccharides were obtained. The SMARTer™ RACE cDNA Amplification Kit (Clontech Laboratories) was used to generate both 5′ and 3′ cDNA ends. The 5′ and 3′ RACE PCR products were purified by a Gel Extraction Kit (Dongsheng Biotech, Guangzhou, China), cloned into the pMD18-T vector (Takara Bio Inc., Dalian, China) and sequenced at the Beijing Genomics Institute. The full-length cDNA were amplified with primers designed from 5′ and 3′ untranslated regions (UTR) by PCR using the LA PCR Amplification Kit (Takara Bio Inc.). The primers, designed by Primer 5.0 and produced at the Beijing Genomics Institute, were used for amplification of 3′ and 5′ UTR regions listed in Supplementary Table 1 and Supplementary Table 2, respectively. The primer pairs for full-length cDNAs listed in Supplementary Table 3.
RNA extraction, cDNA synthesis and quantitative real-time PCR (qRT-PCR) analysis
Total RNA was extracted as indicated above. Two μg of total RNA isolated from samples (S1–S4), as well as from roots, stems and leaves of seedlings and adult plants (about 8 months after sprouting) were reverse transcribed for the first-strand cDNA, using M-MLV reverse transcriptase (Promega, Madison, USA) according to the manufacturer’s instructions. The gene-specific primer pairs were designed by online Primerquest software (listed in Supplementary Table 4). qRT-PCR was performed using the SYBR Premix Ex Taq™ Kit (TaKaRa Bio Inc.) according to the manufacturer’s protocol in an ABI 7500 Real-time system (ABI, California, USA). Amplification conditions were: 95 °C for 2 min, followed by 40 cycles of amplification (95 °C for 15 s, 60 °C for 1 min) and plate reading after each cycle. The constitutive expressed gene, D. officinale actin (cloned by our laboratory; NCBI accession number: JX294908), was used as the internal control.
Results
Changes to water-soluble polysaccharides and mannose content during four stem developmental stages
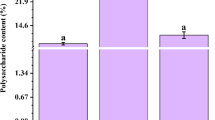
To compare the content of water-soluble polysaccharides, the roots, stems and leaves of D. officinale from seedlings and adult plants were used to detect the content of polysaccharides. Both seedlings and adult plants had the lowest content of water-soluble polysaccharides in roots (about 104.56 mg/g in seedlings and 92.88 mg/g in adult plants), but had the highest content in stems (about 198.69 and 255.25 mg/g, respectively; Fig. 1). To understand the global changes in water-soluble polysaccharides in D. officinale stems, the content of water-soluble polysaccharides was assessed every month for 24 months. Stems in the four stages of development showed significant changes in polysaccharide content (Fig. 2a): lowest in S1, higher in S2, peaking in S3, and low (equivalent to degradation) in S4 (Fig. 2b).
Four stages of D. officinale stems with different contents of polysaccharides and mannose were used to prepare digital gene expression libraries for Illumina sequencing. a The four stages: plant in vegetative growth with few polysaccharides (stage 1, S1); plant accumulates polysaccharides rapidly (stage 2, S2); plant develops into a mature stage with the highest polysaccharide content (stage 3, S3); plant begins to die and the polysaccharide content decreases rapidly (stage 4, S4). b Polysaccharide content in the stems of four stages. Each data bar represents the mean ± SE (n = 3). c The content of mannose increased gradually over the four stages. Each data bar represents the mean ± SE (n = 3). DW dry weight
In order to determine the monosaccharide composition of the stems at the four stages, neutral monosaccharide composition was analyzed by HLPC. Mannose was the most abundant neutral monosaccharide in the stems of D. officinale (Fig. 2c). Its content increased gradually during D. officinale development, corresponding with the changes in soluble polysaccharides from S1 to S3. However, unlike soluble polysaccharides, which decreased in S4, mannose content stayed level at this developmental stage. As is clear from Fig. 2c, the content of glucose decreased in S4 causing a decrease in the total content of polysaccharides.
General features of gene expression profiles and KEGG analysis of DEGs
To identify the genes involved in the biosynthesis of bioactive polysaccharides, next-generation sequencing-based digital gene expression tag profiling was conducted to reveal the molecular events underlying the changes in polysaccharides (including mannose) during the four developmental stages of D. officinale stems (S1–S4). Illumina sequencing generated 24589704, 34136798, 21003674 and 19779982 raw reads with about 200 bp for S1, S2, S3 and S4, respectively. The sequenced raw reads have been submitted to the SRA at NCBI with the following accession numbers: S1, SRR1917040; S2, SRR1917041; S3, SRR1917042; S4, SRR1917043. The clean reads of the four digital gene expression libraries, which accounted for more than 97 % of total reads, were mapped to the D. officinale reference transcriptome sequence database. By using a stringent value of FDR ≤0.001, a P value ≤0.05 and an absolute value of the log2 ratio ≥1 as the threshold, a total of 21426 DEGs were identified. Preliminary screening was used to compare the results between any two digital gene expression libraries shown in Fig. 3a. The comparative analysis between S1 and S2 libraries showed that 5254 genes were highly expressed in S2 whereas the expression of 4409 genes was down-regulated. The relatively smaller changes in gene expression between S2 and S3 revealed that most genes exhibited a similar expression level in S3 and in S2. The expression pattern of these genes could be classified into eight groups (Fig. 3b). The genes in cluster 6 were up-regulated at S2 and S3 but decreased at S4, consistent with the changes in polysaccharides.
Analysis of changes in gene expression among different developmental stages. a The number of differentially expressed genes (DEGs) was obtained from comparisons of S1 versus S2, S1 versus S3, S1 versus S4, S2 versus S3, S2 versus S4 and S3 versus S4. b All DEGs were classified into eight clusters by short time-series expression miner (STEM, P value < 0.05). c KEGG analysis of the DEGs related to carbohydrate metabolism pathways
To gain insight into the metabolic pathways of the DEGs, the annotated sequences were mapped to the KEGG database to define the cellular pathways containing these DEGs. In total, 4257 unigenes were assigned to 123 KEGG pathways, including ‘metabolism’, ‘environmental information processing’, ‘genetic information processing’ and ‘cellular processes’. Figure 3c shows the differential genes in carbohydrate metabolism pathways. Notably, 107 differential genes were predicted to be involved in ‘glycolysis/gluconeogenesis’ and 99 genes in ‘starch and sucrose metabolism’. It was predicted that 66 genes were involved in ‘amino sugar and nucleotide sugar metabolism’ and 44 genes in ‘fructose and mannose metabolism’, both pathways related to nucleotide sugar biosynthesis.
Identifying DoCSLA genes
As indicated above, mannose was the major monosaccharide in D. officinale stems. Thus, genes involved in the biosynthetic pathway of mannan polysaccharides are of interest. Based on the changes in mannose content throughout the four developmental stages, eight genes encoding proteins that catalyze the biosynthesis of mannan polysaccharides were identified from the DEG database. These genes were named DoCSLA1-8. Most of these genes showed significantly different expression patterns among the four developmental phases, but were consistent with the changes in mannose content. To further validate transcript abundance, the genes were used to design gene-specific primers for qPCR analysis, in triplicate. The expression of these genes showed that they were up-regulated at S2 or S3 and down-regulated at S4 (Fig. 4), corresponding to the increase, then lack of change, in mannose content in the same developmental stages (Fig. 2c). The eight candidate genes could be divided into two groups based on the highest relative expression level at S2 (DoCSLA1, DoCSLA2, DoCSLA3, DoCSLA4, DoCSLA6 and DoCSLA8) or S3 (DoCSLA5 and DoCSLA7). A total of eight cDNAs corresponding to these eight DoCSLA genes were obtained and have been submitted to GenBank with the following accession numbers: DoCSLA1, KM980199; DoCSLA2, KM980200; DoCSLA3, KP003920; DoCSLA4, KM980201; DoCSLA5, KM980202; DoCSLA6, KF195561, DoCSLA7, KP205040; DoCSLA8, KP205041.
Cloning eight DoCSLA genes and analysis
To further analyze the identified DoCSLA genes, the full length of the eight genes was obtained by RACE. Homology searches indicated that the encoded proteins were members of the glycosyltransferase superfamily (GT2). Based on sequence similarities, the plant GT2 superfamily, namely the CesA superfamily, is classified into nine subfamilies: CesA, CSLA, CSLB, CSLC, CSLD, CSLE, CSLF, CSLG, and CSLH (Hazen et al. 2002; Richmond and Somerville 2000). To examine the relationship between the eight proteins and CesA superfamily numbers, the amino acid sequence of Arabidopsis were downloaded from NCBI (http://www.ncbi.nlm.nih.gov/) to construct a phylogenetic tree. The eight proteins belonged to the CSLA family (Fig. 5) and contained the DxD motif and the D,D,D,QXXRW motif (Figure S1), which is a characteristic motif of the CesA superfamily (Goubet et al. 2003; Lerouxel et al. 2006).
Molecular phylogenetic tree of the amino acid sequences of the GT2 family of Arabidopsis and eight DoCSLA proteins from D. officinale. The tree was constructed using MEGA 4 by the neighbor-joining method. Enzymes used for alignment are as follows: AtCesA1, AAC39334.1; AtCesA10, AAD20713.1; AtCesA2, AAC39335.1; AtCesA3, AAC39336.1; AtCesA4, AAO15532.1; AtCesA5, BAB09408.1; AtCesA6, BAB10307.1; AtCesA7, AAD32031.1; AtCesA8, AAM20487.1; AtCesA9, AAD20396.1; AtCSLA1, AAO42230.1; AtCSLA10, NP_173818.1; AtCSLA11, NP_197123.2; AtCSLA14, NP_191159.2; AtCSLA15, NP_193077.2; AtCSLA2, BAB11680.1; AtCSLA3, AAN15522.1; AtCSLA7, AAL24081.1; AtCSLA9, CAB82941.1; AtCSLB2, AAC25944.1; AtCSLB3, AAL90907.1; AtCSLB4, AAC25936.1; AtCSLB5, NP_193264.3; AtCSLB6, CAB78574.1; AtCSLC12, AAD15482.1; AtCSLC4, BAB01433.1; AtCSLC5, CAB79877.1; AtCSLC6, AAF02144.1; AtCSLC8, AAD23884.1; AtCSLD1, AAC04910.1; AtCSLD2, CAC01704.1; AtCSLD3, AAF26119.1; AtCSLD4, CAB37559.1; AtCSLD5, AAF02892.1; AtCSLE1, AAF79313.1; AtCSLG1, AAB63622.1; AtCSLG2, AAM20086.1; AtCSLG3, NP_194130.2
Expression pattern of the DoCSLA genes in different organs
Based on the understanding that D. officinale stems are the principal vessel for the storage of polysaccharides, the roots, stems and leaves from D. officinale seedlings and adult plants were used to determine the relative expression level of the eight DoCSLA genes by qRT-PCR. All eight DoCSLA genes were expressed in all three organs. However, the transcripts of many DoCSLA genes displayed tissue-specific abundance patterns. For example, DoCSLA3, DoCSLA4, DoCSLA6, DoCSLA7 and DoCSLA8 showed high expression in the stems of both stages. The transcripts of the DoCSLA2 gene were most abundant in leaves while those of DoCSLA1 were most abundant in roots in both stages (Fig. 6). To determine the relationship between gene expression and mannose content, the D. officinale seedlings with a low content of water-soluble polysaccharides in stems were used to determine the contents of mannose and glucose. Mannose was the main monosaccharide in the stems of D. officinale seedlings and showed the highest content in stems (Fig. 7).
qRT-PCR analyzed the expression of DoCSLA genes in different organs of D. officinale. a Roots, stems and leaves from seedlings, which were cultured on half-strength MS about 10 months after germination. b Roots, stems, leaves from adult plants, about 8 months after sprouting, were maintained in pots cultivated in a greenhouse. R roots, S stems, L leaves. Each data bar represents the mean ± SE (n = 3)
Response of DoCSLA genes to PEG and salt stress
Previous studies demonstrated that polysaccharides from higher plants have antioxidant activities (Tsai et al. 2007; Zhao et al. 2012). To check the expression pattern of DoCSLA genes in an abiotic stress environment, qRT-PCR was used to detect the expression level in PEG and salinity (NaCl) stress treatments (Fig. 8). Most of the DoCSLA genes were inducible by PEG and salt stress, which indicates that DoCSLA genes may play an important role in stress tolerance in D. officinale. More specifically, six genes (DoCSLA1, DoCSLA2, DoCSLA5, DoCSLA6, DoCSLA7 and DoCSLA8) were induced by salinity and strongly induced by PEG. Conversely, DoCSLA3 was strongly induced by salinity, but down-regulated by PEG. In contrast to these observations, DoCSLA4 was down-regulated in both PEG and salt stress.
Discussion
Mannan polysaccharides are abundant in D. officinale stems
D. officinale, an important traditional Chinese herb, has an abundance of polysaccharides that are useful for human health. Among them, mannan polysaccharides are promising polysaccharides for biopharmaceutical purposes. The family of mannan polysaccharides is the most widespread among higher plants, for example, present in Arabidopsis inflorescence stems and leaves (Handford et al. 2003), in the seed endosperms of fenugreek (Wang et al. 2012b), even in poplar (Populus trichocarpa) stem tissues (Wang et al. 2012a). Mannan polysaccharides isolated from many higher plants are classified into four subfamilies: pure mannan, glucomannan, galactomannan, and galactoglucomanan (Buckeridge 2010; Moreira and Filho 2008, Petkowicz et al. 2001). A glucomannan polysaccharide from the stems of Dendrobium huoshanense could activate murine splenocytes to produce several cytokines (Hsieh et al. 2008). A glucomannan (DOP-1-A1) has a backbone of (1 → 4)-linked β-d-mannopyranosyl residues and β-d-glucopyranosyl residues was isolated from D. officinale (Hua et al. 2004). In recent years, the other O-acetyl-glucomannan from D. officinale was described by Xing et al. (2014, 2015). In the present study, polysaccharides accumulated in D. officinale stems at both the juvenile and adult stages. These polysaccharides were mainly composed of a monosaccharide, mannose. Our results suggest that mannan polysaccharides are abundant in the stems of D. officinale. Polysaccharides in the stems of D. officinale may serve as a form of energy storage for providing energy for flowering and sprouting. Several monocotyledonous species produced storage mannan polysaccharides that are located in non-reproductive organs. Three examples include Lilium testaceum, which produces large quantities of storage β-1,4-glucomannan in bulbs (Wozniewski et al. 1992), aloe vera (Aloe barbadensis Miller), which stores a mannan polysaccharide within the protoplast of parenchymatous cells (Femenia et al. 1999), or a member of another Orchidaceae genus, Oncidium (cv. Gower Ramsey), in which pure mannan—that is stored in the pseudobulbs—is considered to be a reserve polysaccharide for florescence development (Wang et al. 2008).
All these studies provide biochemical insight into understanding the genes related to the synthesis of polysaccharides in D. officinale. Together with data of the four developmental stages, eight candidate genes involved in the biosynthesis of mannan polysaccharides were identified in this study.
DoCSLA genes are members of the CSLA family
The CSL genes, which belong to the CesA superfamily, encode enzymes that polymerize the backbones of β-linked non-cellulosic polysaccharides (Sandhu et al. 2009). The CSLA family members are located in the Golgi membrane (Davis et al. 2010; Goubet et al. 2003) and are involved in the synthesis of mannan polysaccharides. Not all of nine CSLA genes in Arabidopsis have been demonstrated to encode mannan synthases, only AtCSLA2, AtCSLA3, AtCSLA7 and AtCSLA9 have been demonstrated to make (gluco)mannan polysaccharides (Dhugga 2012; Sandhu et al. 2009). AtCSLA2 incorporated GDP-mannose into a β-mannan or glucomannan and also incorporated GDP-glucose into a glucomannan (De Caroli et al. 2014; Liepman et al. 2005). AtCSLA2 was found to synthesize glucomannan in stems and was involved in the biosynthesis of mucilage glucomannan in the seed coat, which is related to maintenance of the normal structure of the adherent mucilage layer (Goubet et al. 2009; Yu et al. 2014). AtCSLA3 was responsible for the synthesis of glucomannan in Arabidopsis stems (Goubet et al. 2009). AtCSLA7 used GDP-mannose as a substrate for the biosynthesis of β-mannan or glucomannan but not GDP-glucose and the product was located in embryos, influencing the progression of embryogenesis (Goubet et al. 2003, 2009; Liepman et al. 2005). AtCSLA9 exhibited properties similar to AtCSLA2 and catalyzed the biosynthesis of β-mannan or glucomannan (Goubet et al. 2009; Liepman et al. 2005). CSLA genes in other species have been identified and all exhibit the function of β-mannan synthase. For example, the CSLA gene from guar (Cyamopsis tetragonolobus) and AkCSLA3 from corm (Amorphophallus konjac) encode a β-mannan synthase in vitro (Dhugga et al. 2004; Gille et al. 2011). The eight DoCSLA genes are members of the CSLA family of the CesA superfamily belonging to the glycosyltransferases 2 (GT2) family (Cantarel et al. 2009). Glycosyltransferases are enzymes that catalyze the formation of the glycosidic linkage to form a glycoside by utilizing ‘activated’ sugar (nucleotide sugar) as glycosyl donors (Lairson et al. 2008). The eight CSLA proteins were likely to have the same catalytic function as the other members of the CSLA family.
Each of the eight DoCSLA members contained DAD motifs and the D,D,D,QXXRW motif, both of which are conserved in all CSLA members (Lerouxel et al. 2006). The DAD residues among the D,D,D,QXXRW motif are thought to be important in the coordination of a divalent metal ion that is required for binding of the nucleotide sugar (Breton and Imberty 1999; Breton et al. 2006). Nucleotide sugars, the activated forms of monosaccharides, serve as a sugar donor by providing sugar for glycosyltransferases involved in polysaccharide biosynthetic pathways (Reyes and Orellana 2008). A D,D,D,QXXRW motif has been proposed to define the nucleotide sugar-binding domain and the catalytic site of the CesA superfamily enzymes (Richmond and Somerville 2000). The D,D,D,QXXRW motif, which is conserved in β-glycosyltransferases, has been proposed to be involved in their catalytic activity and processivity (Saxena and Brown 1995).
Expression patterns of DoCSLA genes and responses to abiotic stresses
Based on the accumulation of polysaccharides and mannose in stems, eight genes predicted to encode mannan synthases were identified. These genes showed high expression at S2 or S3, which corresponded to the rapid accumulation or the peak of polysaccharides, respectively. Mannose was the most abundant neutral monosaccharide in the stems and leaves of D. officinale, but was the highest in stems (Figs. 2c, 7). Interestingly, DoCSLA3, DoCSLA4, DoCSLA6, DoCSLA7 and DoCSLA8 genes showed high expression in the stems of D. officinale seedlings and adult plants with a perfect correlation with mannose content. DoCSLA6 showed high homology to the AtCSLA9 gene and was highly expressed in stems. These results suggest that these genes are related to the biosynthesis of mannan polysaccharides in D. officinale.
Most of the D. officinale DoCSLA genes were up-regulated in the PEG and salt stress treatments, suggesting that they play important roles in the regulation associated with abiotic stress responses. Abiotic stresses induce the overproduction of reactive oxygen species (ROS) in plants, and ROS cause wide-scale damage to proteins, lipids, DNA and carbohydrates (Gill and Tuteja 2010). Evidence exists that polysaccharides act as a reducing agent and scavenge ROS (Duan and Kasper 2011; Fry et al. 2001). By scavenging ROS induced by abiotic stresses, Dendrobium polysaccharides, which have antioxidant activity (Fan et al. 2009; Luo et al. 2010), may be able to enhance resistance. Moreover, mannan polysaccharides located in the cell wall may help to maintain cell wall integrity and thus minimize cellular damage during abiotic stress.
Both salt and water stress reduce the ability of plants to take up water from soil (Munns 2002; Munns and Tester 2008). Plants accumulate compatible solutes, included proline, glycine betaine, polyols and sugars to reduce the cell water potential and absorb water (Parida and Das 2005). Some carbohydrates serve as solutes and are involved in osmotic adjustment in plants. Kerepesi and Galiba (2000) found that tolerant wheat (Triticum aestivum L.) genotypes accumulated more soluble carbohydrate than sensitive ones and suggested soluble carbohydrates as markers for selecting drought- or salt-tolerant plants. Water or salinity stress induces the accumulation of soluble sugars in many plant species, such as in Oryza sativa L. (Dubey and Singh 2006; Aquino et al. 2011), Lupinus angustifolius (Comino et al. 1997) and even a halophytic seagrass, Ruppia maritima L. (Aquino et al. 2011). Water deficit caused by PEG and salt stress may also be a signal that can trigger responsive gene expression to modulate plants to adapt to such adverse environments. The DoCSLA genes, induced by such factors, synthesized polysaccharides involved in osmotic adjustment. Moreover, the soluble carbohydrates that accumulated during osmotic adjustment may be species-specific. The concentration of sulfated polysaccharides was positively correlated with salinity in R. maritima: salt stress did not induce the biosynthesis of sulfated polysaccharides but increased the concentration of carboxylated polysaccharides in O. sativa (Aquino et al. 2011). D. officinale is a plant that accumulates mannan polysaccharides to deal with water deficit.
In conclusion, D. officinale is an important traditional Chinese herb whose stems are abundant in polysaccharides. These polysaccharides are composed of mannose and glucose. This study paves the way for the search for genes involved in the biosynthesis of bioactive polysaccharides. Digital gene expression analysis provided comprehensive information on gene expression and is an effective approach to identify candidate DoCSLA genes and other genes involved in the biosynthesis of bioactive polysaccharides. DoCSLA genes could also be used to investigate the relationship with mannan polysaccharides.
References
Alonso-Sande M, Teijeiro-Osorio D, Remuñán-López C, Alonso MJ (2009) Glucomannan, a promising polysaccharide for biopharmaceutical purposes. Eur J Pharm Biopharm 72:453–462
Aquino RS, Grativol C, Mourão PA (2011) Rising from the sea: correlations between sulfated polysaccharides and salinity in plants. PLoS One 6:e18862
Arioli T, Peng L, Betzner AS, Burn J, Wittke W, Herth W, Camilleri C, Höfte H, Plazinski J, Birch R, Cork A, Glover AJ, Redmond J, Williamson RE (1998) Molecular analysis of cellulose biosynthesis in Arabidopsis. Science 279:717–720
Blanca JM, Pascual L, Ziarsolo P, Nuez F, Cañizares J (2011) ngs_backbone: a pipeline for read cleaning, mapping and SNP calling using next generation sequence. BMC Genomics 12:285
Breton C, Imberty A (1999) Structure/function studies of glycosyltransferases. Curr Opin Struct Biol 9:563–571
Breton C, Snajdrova L, Jeanneau C, Koca J, Imberty A (2006) Structures and mechanisms of glycosyltransferases. Glycobiology 16:29R–37R
Buckeridge MS (2010) Seed cell wall storage polysaccharides: models to understand cell wall biosynthesis and degradation. Plant Physiol 154:1017–1023
Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B (2009) The Carbohydrate-Active EnZymes database (CAZy): an expert resource for glycogenomics. Nucleic Acids Res 37:D233–D238
Chen X, Guo S (2001) Advances in the research of constituents and pharmacology of Dendrobium. Nat Prod Res Dev 13:70–75
Clifford SC, Arndt SK, Popp M, Jones HG (2002) Mucilages and polysaccharides in Ziziphus species (Rhamnaceae): localization, composition and physiological roles during drought-stress. J Exp Bot 53:131–138
Comino ML, de Felipe MR, Fernandez-Pascual M, Martin L (1997) Effect of drought stress on carbohydrate metabolism in nodules of Lupinus angustifolius. In: Eukaryotism Symbiosis. Springer, pp 449–456
Davis J, Brandizzi F, Liepman AH, Keegstra K (2010) Arabidopsis mannan synthase CSLA9 and glucan synthase CSLC4 have opposite orientations in the Golgi membrane. Plant J 64:1028–1037
De Caroli M, Lenucci MS, Di Sansebastiano G-P, Tunno M, Montefusco A, Dalessandro G, Piro G (2014) Cellular localization and biochemical characterization of a chimeric fluorescent protein fusion of Arabidopsis Cellulose Synthase-Like A2 inserted into Golgi membrane. Scientific World J 2014:7
de Lima RB, dos Santos TB, Vieira LG, Ferrarese MDLL, Ferrarese-Filho O, Donatti L, Boeger MR, de Oliveira Petkowicz CL (2014) Salt stress alters the cell wall polysaccharides and anatomy of coffee (Coffea arabica L.) leaf cells. Carbohydr Polym 112:686–694
Dhugga KS (2012) Biosynthesis of non-cellulosic polysaccharides of plant cell walls. Phytochemistry 74:8–19
Dhugga KS, Barreiro R, Whitten B, Stecca K, Hazebroek J, Randhawa GS, Dolan M, Kinney AJ, Tomes D, Nichols S, Anderson P (2004) Guar seed beta-mannan synthase is a member of the cellulose synthase super gene family. Science 303:363–366
Duan J, Kasper DL (2011) Oxidative depolymerization of polysaccharides by reactive oxygen/nitrogen species. Glycobiology 21:401–409
Dubey RS, Singh AK (2006) Salinity induces accumulation of soluble sugars and alters the activity of sugar metabolising enzymes in rice plants. Biol Plant 42:233–239
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Fan YJ, He XJ, Zhou SD, Luo AX, He T, Chun Z (2009) Composition analysis and antioxidant activity of polysaccharide from Dendrobium denneanum. Int J Biol Macromol 45:169–173
Femenia A, Sánchez ES, Simal S, Rosselló C (1999) Compositional features of polysaccharides from Aloe vera (Aloe barbadensis Miller) plant tissues. Carbohydr Polym 39:109–117
Fincher GB (2009) Revolutionary times in our understanding of cell wall biosynthesis and remodeling in the grasses. Plant Physiol 149:27–37
Fry SC, Dumville JC, Miller JG (2001) Fingerprinting of polysaccharides attacked by hydroxyl radicals in vitro and in the cell walls of ripening pear fruit. Biochem J 357:729–737
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gille S, Cheng K, Skinner ME, Liepman AH, Wilkerson CG, Pauly M (2011) Deep sequencing of voodoo lily (Amorphophallus konjac): an approach to identify relevant genes involved in the synthesis of the hemicellulose glucomannan. Planta 234:515–526
Goubet F, Misrahi A, Park SK, Zhang Z, Twell D, Dupree P (2003) AtCSLA7, a cellulose synthase-like putative glycosyltransferase, is important for pollen tube growth and embryogenesis in Arabidopsis. Plant Physiol 131:547–557
Goubet F, Barton CJ, Mortimer JC, Yu X, Zhang Z, Miles GP, Richens J, Liepman AH, Seffen K, Dupree P (2009) Cell wall glucomannan in Arabidopsis is synthesised by CSLA glycosyltransferases, and influences the progression of embryogenesis. Plant J 60:527–538
Handford MG, Baldwin TC, Goubet F, Prime TA, Miles J, Yu X, Dupree P (2003) Localisation and characterisation of cell wall mannan polysaccharides in Arabidopsis thaliana. Planta 218:27–36
Hazen SP, Scott-Craig JS, Walton JD (2002) Cellulose synthase-like genes of rice. Plant Physiol 128:336–340
Hsieh YSY, Chien C, Liao SKS, Liao SF, Hung WT, Yang WB, Lin CC, Cheng TJR, Chang CC, Fang JM, Wong CH (2008) Structure and bioactivity of the polysaccharides in medicinal plant Dendrobium huoshanense. Bioorg Med Chem. 16:6054–6068
Hua YF, Zhang M, Fu CX, Chen ZH, Chan GY (2004) Structural characterization of a 2-O-acetylglucomannan from Dendrobium officinale stem. Carbohydr Res 339:2219–2224
Jin X, Chen S, Luo Y (2009) Taxonomic revision of Dendrobium moniliforme complex (Orchidaceae). Sci Hortic 120:143–145
Kerepesi I, Galiba G (2000) Osmotic and salt stress-induced alteration in soluble carbohydrate content in wheat seedlings. Crop Sci 40:482–487
Lairson LL, Henrissat B, Davies GJ, Withers SG (2008) Glycosyltransferases: structures, functions, and mechanisms. Annu Rev Biochem 77:521–555
Lerouxel O, Cavalier DM, Liepman AH, Keegstra K (2006) Biosynthesis of plant cell wall polysaccharides—a complex process. Curr Opin Plant Biol 9:621–630
Li R, Yu C, Li Y, Lam TW, Yiu SM, Kristiansen K, Wang J (2009) SOAP2: an improved ultrafast tool for short read alignment. Bioinformatics 25:1966–1967
Liepman AH, Wilkerson CG, Keegstra K (2005) Expression of cellulose synthase-like (Csl) genes in insect cells reveals that CslA family members encode mannan synthases. Proc Natl Acad Sci USA 102:2221–2226
Li JJ, Bi HT, Yan JH, Sun F, Fan SS, Cao G, Zhou YF, Chen XG (2012) Comparative analysis of polysaccharides from two ecological types of Leymus chinensis. Chem Res Chin Univ 28:677–681
Luo AX, He XJ, Zhou SD, Fan YJ, Luo AS, Chun Z (2010) Purification, composition analysis and antioxidant activity of the polysaccharides from Dendrobium nobile Lindl. Carbohydr Polym 79:1014–1019
Meng LZ, Lv GP, Hu DJ, Cheong KL, Xie J, Zhao J, Li SP (2013) Effects of polysaccharides from different species of Dendrobium (Shihu) on macrophage function. Molecules 18:5779–5791
Moreira LR, Filho EX (2008) An overview of mannan structure and mannan-degrading enzyme systems. Appl Microbiol Biotechnol 79:165–178
Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B (2008) Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 5:621–628
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25:239–250
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Parida AK, Das AB (2005) Salt tolerance and salinity effects on plants: a review. Ecotoxicol Environ Saf 60:324–349
Petkowicz CLdO, Reicher F, Chanzy H, Taravel FR, Vuong R (2001) Linear mannan in the endosperm of Schizolobium amazonicum. Carbohydr Polym 44:107–112
Reyes F, Orellana A (2008) Golgi transporters: opening the gate to cell wall polysaccharide biosynthesis. Curr Opin Plant Biol 11:244–251
Richmond TA, Somerville CR (2000) The cellulose synthase superfamily. Plant Physiol 124:495–498
Sandhu AP, Randhawa GS, Dhugga KS (2009) Plant cell wall matrix polysaccharide biosynthesis. Mol Plant 2:840–850
Saxena I, Brown JR (1995) Identification of a second cellulose synthase gene (acsAII) in Acetobacter xylinum. J Bacteriol 177:5276–5283
Sun YD, Wang ZH, Ye QS (2013) Composition analysis and anti-proliferation activity of polysaccharides from Dendrobium chrysotoxum. Int J Biol Macromol 62:291–295
Suzuki S, Li L, Sun YH, Chiang VL (2006) The cellulose synthase gene superfamily and biochemical functions of xylem-specific cellulose synthase-like genes in Populus trichocarpa. Plant Physiol 142:1233–1245
Tanaka K, Murata K, Yamazaki M, Onosato K, Miyao A, Hirochika H (2003) Three distinct rice cellulose synthase catalytic subunit genes required for cellulose synthesis in the secondary wall. Plant Physiol 133:73–83
Taylor NG, Howells RM, Huttly AK, Vickers K, Turner SR (2003) Interactions among three distinct CesA proteins essential for cellulose synthesis. Proc Natl Acad Sci USA 100:1450–1455
The State Pharmacopoeia Commission of People’s Republic of China (2010) Pharmacopoeia of the People’s Republic of China, vol 1. Chemical Industry Press, Beijing, pp 265–266 (Chinese edn)
Tsai MC, Song TY, Shih PH, Yen GC (2007) Antioxidant properties of water-soluble polysaccharides from Antrodia cinnamomea in submerged culture. Food Chem 104:1115–1122
Wang CY, Chiou CY, Wang HL, Krishnamurthy R, Venkatagiri S, Tan J, Yeh KW (2008) Carbohydrate mobilization and gene regulatory profile in the pseudobulb of Oncidium orchid during the flowering process. Planta 227:1063–1077
Wang HT, Liu IH, Yeh TF (2012a) Immunohistological study of mannan polysaccharides in poplar stem. Cellul Chem Technol 46:149–155
Wang Y, Alonso AP, Wilkerson CG, Keegstra K (2012b) Deep EST profiling of developing fenugreek endosperm to investigate galactomannan biosynthesis and its regulation. Plant Mol Biol 79:243–258
Wozniewski T, Blaschek W, Franz G (1992) Isolation and characterization of an endo-β-mannanase of Lilium testaceum bulbs. Phytochemistry 31:3365–3370
Wu ZY, Raven PH, Hong DY (eds) (2009) Flora of China (Orchidaceae), vol 25. Science Press and St. Louis: Missouri Botanical Garden Press, Beijing, pp 382–383
Xing X, Cui SW, Nie S, Phillips GO, Goff HD, Wang Q (2014) Study on Dendrobium officinale O-acetyl-glucomannan (Dendronan®): part I. Extraction, purification, and partial structural characterization. Bioact Carbohydr Diet Fibre 4:74–83
Xing X, Cui SW, Nie S, Phillips GO, Goff HD, Wang Q (2015) Study on Dendrobium officinale O-acetyl-glucomannan (Dendronan®): part II. Fine structures of O-acetylated residues. Carbohydr Polym 117:422–433
Yu L, Shi D, Li J, Kong Y, Yu Y, Chai G, Hu R, Wang J, Hahn MG, Zhou G (2014) CELLULOSE SYNTHASE-LIKE A2, a glucomannan synthase, is involved in maintaining adherent mucilage structure in Arabidopsis seed. Plant Physiol 164:1842–1856
Zhang J, Wu K, Zeng S, Teixeira da Silva JA, Zhao X, Tian CE, Xia H, Duan J (2013) Transcriptome analysis of Cymbidium sinense and its application to the identification of genes associated with floral development. BMC Genom 14:279
Zhao Q, Xie B, Yan J, Zhao F, Xiao J, Yao L, Zhao B, Huang Y (2012) In vitro antioxidant and antitumor activities of polysaccharides extracted from Asparagus officinalis. Carbohydr Polym 87:392–396
Acknowledgments
This work was supported by the National Science Foundation of China Projects (Grant number 31370365), the Transformation of Agricultural Science and Technology Achievement Fund (Contract number 2013GB24910676), the Forestry Science and Technology Innovation Fund Project of Guangdong province (Project number 2013KJCX014-06), and the Science and Technology Planning Project of Guangdong Province (Project number 2012A020602100).
Conflict of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
He, C., Zhang, J., Liu, X. et al. Identification of genes involved in biosynthesis of mannan polysaccharides in Dendrobium officinale by RNA-seq analysis. Plant Mol Biol 88, 219–231 (2015). https://doi.org/10.1007/s11103-015-0316-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-015-0316-z