Abstract
Cotton fiber is a single cell that differentiates from the ovule epidermis and undergoes synchronous elongation with high secretion and growth rate. Apart from economic importance, cotton fiber provides an excellent single-celled model for studying mechanisms of cell-growth. Annexins are Ca2+- and phospholipid-binding proteins that have been reported to be localized in multiple cellular compartments and involved in control of vesicle secretions. Although several annexins have been found to be highly expressed in elongating cotton fibers, their functional roles in fiber development remain unknown. Here, 14 annexin family members were identified from the fully sequenced diploid G. raimondii (D5 genome), half of which were expressed in fibers of the cultivated tetraploid species G. hirsutum (cv. YZ1). Among them, GhAnn2 from the D genome of the tetraploid species displayed high expression level in elongating fiber. The expression of GhAnn2 could be induced by some phytohormones that play important roles in fiber elongation, such as IAA and GA3. RNAi-mediated down-regulation of GhAnn2 inhibited fiber elongation and secondary cell wall synthesis, resulting in shorter and thinner mature fibers in the transgenic plants. Measurement with non-invasive scanning ion-selective electrode revealed that the rate of Ca2+ influx from extracellular to intracellular was decreased at the fiber cell apex of GhAnn2 silencing lines, in comparison to that in the wild type. These results indicate that GhAnn2 may regulate fiber development through modulating Ca2+ fluxes and signaling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Annexins are Ca2+- and phospholipid-binding proteins with a broad taxonomic distribution covering prokaryotes, fungi, protists, plants and higher vertebrates (Gerke and Moss 2002; Morgan et al. 2004, 2006). The overall structure of annexins is evolutionary conserved. The C-terminal core of annexin consists of four annexin repeats and each repeat comprises five short α-helices (Laohavisit and Davies 2011). In the animal annexin, each of the four repeats contains a conserved endonexin fold (K-G-X-G-T-{38}-D/E) that can bind a single Ca2+ ion (Kourie and Wood 2000; Moss and Morgan 2004). Calcium enables the reversible binding of annexins to negatively charged phospholipids, and the Ca2+ requirement for binding can be reduced by acidic pH (Blackbourn et al. 1991). Differing from their animal counterparts, only the first and fourth repeated domains of plant annexins have the characteristic endonexin sequence. In addition, plant annexins have larger surface area due to extra grooves and clefts in comparison to mammalian annexins, suggesting that plant annexins may have wider range of interaction partners and a broad range of roles within the cell (Mortimer et al. 2008).
Plant annexins are expressed throughout the life cycle of a plant and are regulated by developmental and environmental signals. However, the functions of these proteins remain poorly understood. Most of what is described about plant annexin functions comes from in vitro studies. These include their involvement in exocytosis, actin binding, peroxidase activity, callose synthesis and ion transport (Laohavisit and Davies 2011). The expression of annexin has been found to be regulated by plant hormones such as abscisic acid (Xin et al. 2005), gibberellic acid (Lu et al. 2012), jasmonic acid (Kiba et al. 2005), auxin (Baucher et al. 2011), and salicylic acid (Gidrol et al. 1996; Konopka-Postupolska et al. 2009). Plant annexin genes can be induced by biotic stresses such as a pathogen attack (de Carvalho-Niebel et al. 2002), as well as by abiotic stresses including salt, drought, wounding, heat or cold, heavy metal and oxidative stresses (Cantero et al. 2006; Konopka-Postupolska et al. 2009; Divya et al. 2010; Jami et al. 2011). Of particular interest is that plant annexins are usually prominent at the apex of cells undergoing polar elongation, such as root hairs, pollen tubes and fern rhizoids (Mortimer et al. 2008), suggesting that plant annexins are also involved in cell elongation.
Cotton fibers (cotton lint) are single cells differentiated from the ovule epidermis that undergo rapid and synchronous elongation, which serve as the mainstay of the modern textile industry. Because of its unicellular and linear structures, cotton fiber is an ideal model for studies of plant cell elongation and cell wall biosynthesis (Kim and Triplett 2001). Cotton fiber development includes five overlapping stages: initiation, elongation, transitional wall thickening and primary wall remodeling, secondary wall synthesis and maturation (Haigler et al. 2012). The mature fiber can reach to 30–50 mm in length. It is hypothesized that cotton fiber may elongate via linear cell-growth mode, a combination of both tip-growth and diffuse-growth modes (Qin and Zhu 2011). Tip-growth is important for cell elongation, tip-localized Ca2+ gradient and active secretary vesicle trafficking are two important aspects of this mode of growth (Cole and Fowler 2006). Thus, genes such as annexins that associate with Ca2+ and active secretary vesicle trafficking will be good candidates for studying the mechanism of fiber cell elongation.
Early in vitro activity assay showed that cotton fiber annexins could inhibit β-glucansynthase activity (Andrawis et al. 1993). However, a recombinant cotton annexin expressed in Escherichia coli did not inhibit β-glucansynthase activity, instead it was capable of hydrolyzing ATP and GTP. The GTPase activity of this annexin was much greater than its ATPase activity. Northern-blot analysis showed that the annexin gene was highly expressed in the elongation stages of cotton fiber differentiation, suggesting a role of this annexin in cell elongation (Shin and Brown 1999). The ligon mutant of cotton (Gossypium hirsutum) is impaired in fiber elongation, and proteomic analysis revealed significant downregulation of five annexin isoforms compared to the wild type, also suggesting annexins may play roles in fiber development (Zhao et al. 2009). In the recent years, heterologous expression of cotton fiber annexins provided some cues for their molecular function in cell development. For example, GhAnx1, which could bind Ca2+ in vitro, was isolated from a cotton (G. hirsutum cv. CRI35) fiber cDNA library. E. coli cells expressing GhAnx1 were protected from tert-butyl hydroperoxide (tBH) stress, suggesting that it had a potential antioxidative role (Zhou et al. 2011). Overexpression of AnnGh3 in Arabidopsis resulted in a significant increase in trichome density and length on leaves of the transgenic plants, suggesting that AnnGh3 may be involved in fiber cell initiation and elongation of cotton (Li et al. 2013a, b). In addition, AnxGb6 is a G. barbadense annexin gene that was found to be specifically expressed in fiber. Yeast two hybridization and BiFC analysis revealed that a AnxGb6 homodimer interacted with a cotton fiber specific actin GbAct1. And ectopic-expression of AnxGb6 in Arabidopsis enhanced root elongation and resulted in more F-actin accumulation in the basal part of the root cell elongation zone. Therefore, it was suggested that AnxGb6 may be important in fiber elongation by potentially providing a domain for F-actin organization (Huang et al. 2013).
Despite the progress described above, the in planta role of annexins in fiber elongation still remains unclear and the answer to this relies on functional analyses in transgenic cotton. To achieve this goal, we identified 14 annexin family members from the fully sequenced G. raimondii (D5 genome), half of which were expressed in fibers of cultivated G. hirsutum (cv. YZ1). One of them, GhAnn2 was highly expressed in elongating fibers and was thus chosen for functional analyses by using a transgenic approach. The data show that RNAi silencing of GhAnn2 decreased the Ca2+ influx at the tips of fiber cells and reduced fiber elongation and cell wall thickness. We conclude that GhAnn2 may control fiber development through regulating Ca2+ fluxes and signaling.
Materials and methods
Plant materials
Cotton G. hirsutum cv. YZ1 was used in this study. The plants were cultivated in the experimental field of Huazhong Agricultural University (Wuhan, China). Ovules and fibers were removed carefully from developing flower buds or bolls on selected days post anthesis (DPA). Petal, stigma and anther were separated from the flower in the field at day of anthesis. Roots, stems and leaves were collected from 21-day-old seedlings. All the samples were frozen immediately and stored at −70 °C before use.
Sequence analysis of annexin family members in Gossypium raimondii (D genome cotton)
Using annexin as keywords, annexin family members in Gossypium raimondii (D genome) and Arabidopsis were searched in the Phytozome 9.1 database (http://www.phytozome.net/). Phylogenetic analysis was performed with ClustalX program (version 1.83) (Thompson et al. 1997) and MEGA4 (Tamura et al. 2007) by the Neighbor-Joining method.
Gene cloning, sequence analysis, vector construction and transformation
Based on an EST sequence of GbAnn isolated from a normalized cDNA library of G. barbadense cv. 3–79 elongating fiber at the elongation stage (Tu et al. 2007), GhAnn2 was obtained by PCR amplification using 10 DPA cotton fiber cDNA of G. hirsutum cv. YZ1 as the template. Gene-specific primers are listed in Supplementary Table S7. In order to determine which subgenome GhAnn2 comes from, cotton annexin EST were obtained from the database of NCBI (http://www.ncbi.nlm.nih.gov/nucest/) and assembled by CAP3 (Huang and Madan 1999). Tablet (http://bioinf.scri.ac.uk/tablet/) was used to visualize the SNPs by sequence assemblies and alignments.
For the RNAi vector construction, a pair of primers with attB1 and attB2 adaptors was designed at the 1014th and 1124th nucleotides of the GhAnn2 sequence (Supplementary Table S7). The PCR product was cloned into pHellsgate4 (Helliwell et al. 2002) according to the manufacturer’s recommendations and then the constructed vectors were introduced into G. hirsutum YZ1 plants by A. tumefaciens using strains LBA4404 (Jin et al. 2006).
qRT-PCR, Southern and Northern bolts
Total RNA was extracted as previously described (Liu et al. 2006). For qRT-PCR, 3 μg RNA was reverse-transcribed to cDNA with the SuperScript III reverse transcriptase (Invitrogen, Carlsbad, CA, USA). Then qRT-PCR was performed using the ABI Prism 7500 (Applied Biosystems, Foster City, CA, USA). For Northern blotting, 12 μg RNA was denatured and electrophoresed on a 1.2 % agarose gel containing 6 % formaldehyde in 1× MOPS buffer. The RNA was then transferred to a Hybond-N+ nylon membrane (Amersham-Pharmacia, USA) and hybridized with a GhAnn2 probe fragment labeled by P32, with rRNA as the control.
Genomic DNA for Southern blots was extracted from leaves of cotton plants by the CTAB method with a plant genomic DNA kit DP305 (Tiangen Biotech, Beijing). Twenty micrograms genomic DNA was digested with HindIII, then separated by 0.8 % agarose gel electrophoresis and blotted onto Hybond-N+ nylon membranes (Amersham-Pharmacia, USA) and hybridized with the probe of NPTII fragment labeled by P32.
Ovule culture and La3+ treatment
Bolls were collected from cotton plants at 0 DPA (about 6:00 pm) and sterilized in 0.1 % (w/v) HgCl2 for about 15 min. After three washes with sterile distilled water, the ovules were removed from the bolls under sterile conditions, floated on liquid BT medium (0.5 μM GA3, 5 μM IAA) in a flask, and cultured in the dark at 30 °C (Beasley and Ting 1973). For La3+ treatment (Yuasa et al. 1998), the ovules from the RNAi lines and the wild-type were cultured in BT medium containing 7 or 28 μM LaCl3 (Sigma) for 10 days. More than 10 cultured ovules per treatment were used for fiber length measurement.
Length and quality measurements of cotton fiber
Fibers from the field were collected from the bolls at the same positions of the plants at the same time. The length of mature fiber was measured with a ruler after making the fiber straight by using a comb. For quality assessment of cotton fiber, more than 7 g fiber was sent to the Center for Cotton Fiber Quality Inspection and Testing at the Chinese Ministry of Agriculture (Anyang, Henan, China). Three biological replicates were performed. Data were analyzed using Student’s t test. For length measurement of the immature fibers (5, 10, 15 DPA) and in vitro cultured fibers, fiber bearing ovules were first boiled in the water to make the fibers dispersed and then clamped with forceps and flushed under the water tap to make the fiber straight, and then the fibers were dragged on the filter paper, and then kept at room temperature to dry for measurement.
Cotton fiber paraffin section
Fibers on the seeds were combed to make sure the fiber cells are straight. A bunch of fiber cells at the same position of the cotton seed were bundled with a fine thread and then stripped from the seed. Tissues were fixed with FAA for 24 h at room temperature, dehydrated with ethanol and trichloromethane (Wang et al. 2009). After paraffin bathing, tissues were embedded in paraffin blocks for sectioning (8 μm). Sections were dewaxed with xylene for 1 h, which was repeated for 0.5 h, washed for 10 s each in 100 % ethanol, 95 % ethanol, 85 % ethanol, 70 % ethanol, 50 % ethanol, 30 % ethanol and distilled water. Sections were stained with safranine (1 %) for 5 min, and then washed for 10 s with distilled water, 30 % ethanol, 50 % ethanol, 70 % ethanol and 85 % ethanol, then stained with Fast green (FCF, 0.5 %) for 20–25 s, washed for 10 s with 95 % ethanol, 100 % ethanol, 50 % ethanol + 50 % xylene, then placed in 100 % xylene. Finally, the samples were observed under the light microscope. The thickness of the fiber cell wall was measured with the software of Image-Pro Plus 6.0 (Media Cybernetics, Inc., USA). About 50 fiber cells from six seeds were measured and data were analyzed by the Student’s t test.
Measurement of extracellular Ca2+ influx
Fresh 5 DPA bolls of the RNAi lines and the wild-type were collected at the same position on the plant and the same time. Fiber bearing ovules in one chamber of the boll were carefully removed and equilibrated in 5 ml testing liquid (0.1 mM KCl, 0.1 mM CaCl2, 0.1 mM MgCl2, 0.5 mM NaCl, 0.3 mM MES, 0.2 mM Na2SO4, 0.1 % sucrose, pH 6.0) for 0.5 h. Then Ca2+ flux of the fiber cell apex was measured in another 5 ml testing liquid by Xu-Yue Science and Technology Co. (www.xuyue.net) using the non-invasive, scanning ion-selective electrode technique as described previously (Shabala et al. 1997). Four bolls in each line were analyzed, and at least three fiber cells per boll were tested. The generated data of potential difference was used for the calculation of Ca2+ flux. The average rates of influx were plotted on the graph.
Results
GhAnn2 is preferentially expressed in fast elongating cotton fibers
When we analyzed the gene expression profiles of G. barbadense cv. 3–79 fibers, an annexin gene expressed preferentially during elongation stage was isolated (Tu et al. 2007, KC316004.1). Due to technical difficulties in transforming G. barbadense, we identified its homolog from the transformable G. hirsutum for determining its in vivo function. Based on primes for the sequence of this Gb annexin, we isolated a full-length cDNA clone consisting of 1,269 nucleotides, encoding a polypeptide of 316 amino acids (Fig. S1). This protein, named GhAnn2, shared 99 % identity with the previously identified, Gb annexin (Tu et al. 2007). Its predicted amino acid sequence contained a typical annexin domain, which consisted of four repeats (Repeat 1–4). Repeat 1 contained a type-II Ca2+-binding site (G-X-G-T-{38}-E). Trp27 (W) and His40 (H) in Repeat 1 is important for phospholipid-binding (Delmer and Potikha 1997) and peroxidase activity of annexins (Mortimer et al. 2008), respectively. IRI in Repeat 3 was an F-actin-binding site, and in Repeat 4, there was a GTP-binding site (position 298–301 DXXG; Fig. S1). Multiple sequence alignment analysis showed the predicted amino acid sequence of this annexin had only two amino acids difference with AnnGh2 (AAB67994; Potikha and Delmer 1997). Moreover, the nucleotide sequence of ORF in this annexin had 99 % identity with AnnGh2 (Table S4). As a result, this annexin was named GhAnn2 according to the gene nomenclature conventions.
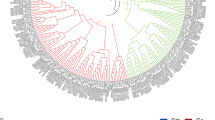
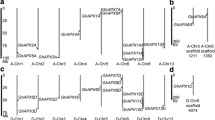
Annexins are highly expressed in cotton fiber, have therefore drawn attention from many researchers (Shin and Brown 1999; Tu et al. 2007; Wang et al. 2010a, b; Zhou et al. 2011; Huang et al. 2013; Li et al. 2013a). And as a result, many annexins have been cloned and named. In order to rationalize the relationship among these annexins, we attempted to identify all the annexins in cotton. Firstly, by searching the database of Phytozome v 9.1 (http://phytozome.net/) using annexin as a keyword, 14 annexin family members were identified in G. raimondii (D5 subgenome), most of which contained typical four conserved annexin repeats (Fig. S2). Then using GhAnn2 as a BLAST query sequence in NCBI, we obtained a total of 250 ESTs from four cotton species (Table S1), including 156 ESTs from G. hirsutum ((AD)1 genome), 15 ESTs from G. barbadense ((AD)2 genome), 34 ESTs from G. arboretum (A2 genome) and 45 ESTs from G. raimondii (D5 genome). ESTs of each species were assembled respectively and 10 contigs were obtained, including two contigs of Gh (Ghcontig1, 2), 3 contigs of Gb (Gbcontig1, 2, 3), 2 contigs of Ga (Gacontig1, 2) and 3 contigs of Gr (Grcontig1, 2, 3) (Table S2 and S3). Then phylogenetic analysis was performed to examine the evolutionary relationship between the reported annexins and the annexins we found (14 Gr annexins and 10 annexin contigs). From the sequence analysis it became clear that GhAnn2, Ghcontig1, Gbcontig1, Grcontig1, Gacontig1 and Gorai.007G060900, two reported annexins AnnGh2 and AnnGh6 should be the orthologous of the same annexin ancestor in different cotton species (Fig. 1a; Table S4). According to this, contig2s, Gorai.007G239000 and the other three reported annexins (GhAnx1, AnxGb1-2) showed at least 99 % identity which should also be orthologous in different cotton species (Fig. 1a, Table S5). Contig3, Gorai.009G237900 and the other four reported annexins (AnxGh1, AnnGh1, AnnGh3 and AnxGb3) showed at least 98 % identity and formed another potential annexin family member in different cotton species (Fig. 1a, Table S6).
Phylogenetic analysis and subgenomic location of GhAnn2 and relative expression levels of cotton annexins in cotton fibers. a Phylogenetic analysis of annexins identified in this study and all the previously reported annexins. Phylogenetic analysis was performed with ClustalX program (version 1.83) (Thompson et al. 1997) and MEGA4 (Tamura et al. 2007) by the Neighbor-Joining method. GhAnx1, AY351650; AnxGh1, AAC33305; GhFAnnx, ACJ11719; AnnGh1, AAB67993; AnnGH2, AAB67994; AnnGh3-6, JX897059-62; AnxGb1-6, KC316004-9. b Subgenomic location of GhAnn2. ESTs of Ghcontig1 could be divided into two groups (Ghcontig1-1 and Ghcontig1-2) according to the SNP loci between Grcontig1 and Gacontig1. According to these SNP loci, GhAnn2 was similar to Grcontig1 with only one nucleotide difference in the position 718, indicating that GhAnn2 might come from DT genome. The numbers such as 45, 412, 562 et al. represent the position of nucleotide in ORF of GhAnn2 sequence. c The numbers of annexin ESTs from NCBI that assemble contigs 1, 2 and 3. d–f qRT-PCR analysis of the seven annexin family members expressed in fibers. According to the expression model in fiber, these members are divided into three groups. d The first group had highest levels in fiber cells at fast elongating phases (5–15 DPA). e The second group expressed to highest levels at 5 DPA. f The third group had highest expression levels in 20 DPA but also expressed to relatively high level in fiber cells at 5–15 DPA. Gorai.007G060900, Gorai.007G239000 and Gorai.009G237900 were the three members that had the highest expression levels in these three groups, respectively. Gh UBQ7 (DQ116441) was included as the template control. The qRT-PCR results were obtained from three independent RNA extractions. Error bars are the SDs of four technical replicates. 0, ovules at 0 DPA; 5–20 DPA, fibers at 5–20 DPA. g qRT-PCR to show the expression of AnnGh2 is induced by exogenous 5 μM IAA and 0.5 μM GA3. Zero DPA ovules cultured in BT medium with 5 μM IAA and 0.5 μM GA3 for 12 h were sampled for RNA extraction. Cotton UBQ7 was included as the template control. The qRT-PCR results were obtained from three independent RNA extractions. Error bars are the SDs of four technical replicates
Sequence analysis showed GhAnn2 had the best match with contig1 compared to contig2 and contig3. In order to survey the subgenomic location of GhAnn2, Grcontig1 and Gacontig1 were aligned first and some distinctive SNP loci were discovered (Fig. 1b). Then Ghcontig1 and its assembled ESTs were analyzed in detail and we found the ESTs could be divided into two groups (Ghcontig1-1 and Ghcontig1-2) according to the SNP loci between Grcontig1 and Gacontig1. Ghcontig1-1 matched Grcontig1 and Ghcontig1-2 matched Gacontig1 (Fig. 1b). The EST number ratio of the two groups was about 5:1. These results inferred that Ghcontig1 contained two genes from A and D sub-genome respectively and the one from Dt genome expressed in higher level than the one from At genome. According to SNP loci, GhAnn2 was found to be similar to Grcontig1 with only one nucleotide difference in the position of 718 and might come from Dt genome (Fig. 1b). And AnnGh2 and AnnGh6 were also from Dt genome. Ghcontig1, Gbcontig1, Gacontig1 and Grcontig1 were assembled by a total of 91, contig2 by 151 ESTs and contig3 by only 20 ESTs from Gb and Gr, respectively (Fig. 1c; Table S3). Because these ESTs were mainly from fiber, the number of assembled ESTs could reflect the relative expression level of the annexin gene represented by the contigs in fiber to some extent. According to this, the annexin genes represented by contig1, contig2 and contig3 would have relatively high expression level in cotton fiber. Therefore, besides GhAnn2, it is possible there are other annexin family members with relatively high expression level in cotton fiber.
In order to know the expression level of each of the annexin members, RT-PCR was firstly performed to analyze Gr annexin expression levels in various tissues of upland cotton plants. The results showed that about half of the annexin members were expressed in developing fiber cells (Fig. S3). These fiber-expressed annexins were further analyzed by qRT-PCR. GhAnn2/Gorai.007G060900, the other two annexin family members Gorai.007G239000 and Gorai.009G237900 were found to have the most abundant transcripts in fibers compared to the other cotton annexins. These three annexin genes showed highest similarities with contig1, contig2 and contig3 (Fig. 1a) and the expression levels measured by qRT-PCR of these three annexins in fibers were consistent with the ESTs redundancies (number of ESTs) of contig1, contig2 and contig3 (Fig. 1c). We divided all the Gr annexin members into three groups dependent on their expression model in fibers. The first group as shown in Fig. 1d contained three annexin members (Gorai.007G060900, Gorai.011G212700 and Gorai.001G068900) had highest level in fiber cells at fast elongating phase (5–15 DPA; Fig. 1d). Two members (Gorai.007G239000 and Gorai.006G190800) with highest levels at 5 DPA formed the second group (Fig. 1e). The last group contained Gorai.009G237900 and Gorai.001G212900 which had highest expression level at 20 DPA but also expressed to relatively high level in fiber cells at 5–15 DPA (Fig. 1f). Due to the relatively high expression levels in fibers at the fast elongating stage, GhAnn2/Gorai.007G060900 would be a good candidate for studying the role of annexin in fiber elongation.
Plant phytohormones such as IAA, GA3 play essential roles in fiber development (Beasley and Ting 1973; Xiao et al. 2010; Zhang et al. 2011). To study the potential effects of these hormones on the expression of cotton annexin genes, we measured the expression level of GhAnn2 in 0 DPA ovules cultured for 12 min in BT medium (Beasley and Ting 1973) with or without 5 μM IAA or 0.5 μM GA3. qRT-PCR showed that when treated with IAA or GA3 the relative expression level of GhAnn2 was nearly double and triple that of the control, respectively (Fig. 1g).
RNAi silencing of GhAnn2 results in shorter and thinner fibers
GhAnn2 was highly expressed in elongating fibers and could be induced by IAA and GA3, which implied that it may play a role in fiber development. To examine the exact effect of GhAnn2 on fiber development, an RNAi silencing construct against GhAnn2 was transformed into upland cotton variety YZ1. Three independent transgenic GhAnn2 RNAi cotton lines were obtained for further analysis. After analysis by qRT-PCR and Northern blots, two RNAi lines (i21 and i34) were found to have much lower expression level of GhAnn2, compared with wild type (Fig. 2a, b). Southern blotting confirmed that they are the positive transforms (T2) (Fig. S4). Because there were other annexin members expressed to high levels in fiber, RT-PCR was also carried out to check whether those, other than GhAnn2, were affected in their expression in the RNAi lines. The analyses revealed no obvious changes in the expression of other annexins (Fig. S5).
Down-regulating GhAnn2 inhibits fiber elongation. a qRT-PCR analysis of GhAnn2 in 10 DPA fibers of GhAnn2 RNAi lines and wild-type. The expression level of UBQ7 was used as the internal control to standardize the RNA samples for each reaction. Error bars are the SDs of four technical replicates. b Northern blotting analysis of GhAnn2 with 10 DPA fibers. rRNA was used as an internal standard. c Straight fibers of 5 DPA, 10 DPA, 20 DPA and mature fibers from two RNAi lines and wild type. 5 DPA, bar = 1 mm; 10 DPA, 15 DPA and mature, bar = 10 mm. d Fiber length measurements for the whole periods of fiber development. The bar represents the SD of at least 40 ovules in 4 bolls. **P < 0.01; *P < 0.05. e qRT-PCR analysis of fiber elongation related genes in 5 and 10 DPA fibers from two RNAi lines and wild type. The expression level of UBQ7 was used as the internal control to standardize the RNA samples for each reaction. Error bars are the SDs of four technical replicates. WT, wild type; i21, i34, two independent RNAi lines
Fiber length, a key determinant of fiber quality and output, was compared between the RNAi lines and wild-type. As shown in Fig. 2c, d, the development of GhAnn2 RNAi fiber cells was significantly inhibited. The length of 5 DPA fiber cells of i21 and i34 lines were 2.9 ± 0.3 and 3.1 ± 0.2 mm, decreased by 14.7 and 10.1 %, respectively, in comparison with that of wild type. At 10 DPA, fiber cells of i21 and i34 lines only elongated to 13.0 ± 1.1 and 12.6 ± 0.8 mm, 12.9 and 15.6 % shorter than the wild-type, respectively. The length of fiber cells at 15 DPA (21.0 ± 1.0 and 21.6 ± 1.0 mm) was also significantly shorter for i21 and i34 lines, respectively, than the wild-type (23.3 ± 0.8 mm), showing a decrease of 9.8 and 7.1 %, respectively. This reduced fiber length phenotype was also observed at maturity. The mature fiber final length of i21 and i34 lines were only 26.4 ± 0.5 and 26.2 ± 0.7 mm, which is 8.7 and 9.4 % shorter than the wild-type (29.0 ± 0.6 mm). qRT-PCR was performed to measure the relative expression levels of several fiber elongation related genes such as GhEXPA1 (GenBank: ABD48785; Xu et al. 2013); GhPEL (GenBank: DQ073046.1; Wang et al. 2010a, b), GhPME1 (GenBank: JQ340871.1; Liu et al. 2013). These genes’ expression levels were decreased both in 5 DPA and 10 DPA fibers of GhAnn2 RNAi lines compared to the wild-type, especially the GhEXPA1 gene (Fig. 2e). The results above indicate that GhAnn2 expression is required for normal fiber elongation. It is worth to mention that the RNAi lines showed normal vegetative phenotypes (Fig. S6), suggesting that the silencing effect appears to be fiber specific.
Micronaire is an indicator of fiber maturity and fineness, which is one of the most important fiber characteristics for international cotton classers and spinners. It is a measure of the rate at which air flows under pressure through a plug of lint of known weight compressed into a chamber of fixed volume. The rate of air flow depends on the resistance offered by the total surface area of the fibers which is related to the linear density as well as the thickness of the fiber walls. A reduction in linear density, wall thickness or fiber perimeter decreases the micronaire reading (Montalvo and Hoven 2005). Mature fibers of GhAnn2 RNAi lines and wild type were collected at the same time and the same position of the plants and used for micronaire measurement. The result showed that the fiber micronaire values were considerably lower in GhAnn2 RNAi transgenic lines (5.01 ± 0.31 and 4.97 ± 0.28 for i21 and i34, respectively) than the wild-type (5.64 ± 0.24) (Fig. 3a). In order to confirm the result above, paraffin histological sections were made of mature fibers from wild-type and transgenic lines and observed under the light microscope. The cell walls of mature fibers in RNAi lines appeared to be thinner than those of wild-type. Measurements of the fiber wall thickness showed that fiber cell wall of GhAnn2 RNAi lines were 3.31 ± 0.25 and 2.76 ± 0.27 μm, significantly thinner than that of wild type (3.85 ± 0.33 μm) (Fig. 3b). To investigate the molecular basis of this, qRT-PCR was performed to analyze the expression levels of genes associated with the deposition of the secondary cell wall cellulose including GhCelA1 (cellulose synthase; Shimizu et al. 1997; Li et al. 2013a) and GhCTL1 (chitinase-like proteins; Zhang et al. 2004). These genes were found to be down-regulated both in 15 and 20 DPA fibers of GhAnn2 RNAi lines (Fig. 3c, d), which is consistent with the reduced cell wall thickness phenotypes.
Down-regulating GhAnn2 inhibits fiber secondary cell wall synthesis. a Micronaire values of mature fiber in GhAnn2 RNAi lines and wild type. Error bars indicate the SD of three biological replicates. b Paraffin sections of mature fiber cell wall. The data below show the thickness of the fiber cell wall (μm). Bar = 10 μm. **P < 0.01. c qRT-PCR analysis of GhCelA1 in 15 and 20 DPA fibers from GhAnn2 RNAi lines and wild type. The expression level of UBQ7 was used as the internal control to standardize the RNA samples for each reaction. Error bars are the standard deviations of four technical replicates. d qRT-PCR analysis of GhCTL1 in 15 and 20 DPA fibers from GhAnn2 RNAi lines and wild type. The expression level of UBQ7 was used as the internal control to standardize the RNA samples for each reaction. Error bars are the SDs of four technical replicates
RNAi silencing of GhAnn2 decreases Ca2+ influx at the apex of fiber cells
In vitro studies of certain plant annexins have revealed their potential to act directly as calcium channels (Mortimer et al. 2008). In order to test whether GhAnn2 is this kind of annexin, the non-invasive, scanning ion-selective electrode technique was used to measure the Ca2+ influx at the fiber cell apex from the extracellular into intracellular environment. The result showed that at 5 DPA, there was a Ca2+ influx at the tips of wild type fiber cells (Fig. 4a). The rate was 31.1 ± 6.4 pmol. cm−2. s−1. However, there was no Ca2+ influx at the tip of GhAnn2 RNAi fibers, instead, there was Ca2+ efflux from the intracellular into extracellular environment (Fig. 4a). The efflux rates were 29.2 ± 4.4 and 58.6 ± 6.4 pmol. cm−2. s−1 for i21 and i34 lines, respectively (Fig. 4b). A previous study has shown that Ca2+ influx is important for fiber elongation (Tang et al. 2014). In order to confirm the essential role of tip Ca2+ influx in cotton fiber cell elongation, an ovule culture assay was performed and the results showed that when 7 and 28 μM Ca2+ channel blocker La3+ (Yuasa et al. 1998) was applied, fiber elongation was significantly inhibited (Fig. S7). Then we examined the effect of La3+ on RNAi lines fibers. When ovules were cultured in BT medium (containing 5 μM IAA and 0.5 μM GA3) for 10 days, the wild type fiber cells could elongate to 10.5 ± 0.3 mm, whereas the fiber of RNAi lines could only elongate to 8.3 ± 0.5 mm, 7.9 ± 0.3 mm for i21 and i34, respectively, consistent with the length data of mature fiber in vivo. Upon addition of 14 μM La3+ to the medium, the lengths of both wild-type and RNAi fibers were decreased. However, the fiber lengths of i21 and i34 lines decreased 63.9 and 68.4 %, respectively, which were significantly shorter than that of wild-type (decreased 34.3 %). The results suggest that GhAnn2 fibers of RNAi lines were more sensitive to La3+ (Fig. 4c).
RNAi silencing of GhAnn2 decreases the rate of Ca2+ influx at the fiber cell apex. a Tip Ca2+ flux of 5 DPA fiber cells was measured by noninvasive scanning ion-selective electrode. Four bolls in one line were analyzed, and at least three fiber cells in one boll were tested. b Average rate of Ca2+ flux of 5 DPA fiber cells. Error bars indicate the SD of three biological replicates. c La3+ treatments in ovule cultures of GhAnn2 RNAi and wild type. 0 DPA ovules of GhAnn2 RNAi lines and the wild type were cultured in BT medium and BT medium containing 28 μM La3+ for 10 days. More than 10 cultured ovules per treatment were used for fiber length measurement. Different letters (a, b, c, d) in the histograms indicate statistically significant differences at p < 0.05 based on ANOVA (Student–Newman–Keuls method)
RNAi silencing of GhAnn2 down-regulates expression of Ca2+ sensors in elongating fiber cells
Ca2+ signaling plays crucial roles in a wide array of growth and developmental processes (Jones and Lunt 1967). Ca2+ influx can contribute to the generation of Ca2+ signals which can be sensed by Ca2+ sensors, including CaMs (calmodulins)/CMLs (CaM-like proteins), CDPKs (Ca2+-dependent protein kinases)/CCaMK (calcium/calmodulin-dependent protein kinases), and CBLs (calcineurin B-like proteins; Kudla et al. 2010). To test if silencing GhAnn2 may affect Ca2+ signaling, we searched the D genome Phytozome 9.1 database for genes encoding Ca2+ sensors. The exercise identified 18 Ca2+ sensor genes that had relatively high expression level in elongating fibers (data not shown). We examined the expression levels of these genes in 15 DPA fibers of GhAnn2 RNAi lines and wild type. The results showed that the expression levels of 2 CaMs, 4 CMLs, 4 CCaMKs and 2 CBLs were down-regulated in GhAnn2 RNAi fibers compared to the wild type, especially regarding to the two CaMs and CBLs. The data provided further evidence that GhAnn2 may have an effect on Ca2+ influx and signaling during fiber development (Fig. 5).
RNAi silencing of GhAnn2 down-regulated expression levels of Ca2+ sensors in elongating fiber cells. CaMs (calmodulins)/CMLs (CaM-like proteins), CDPKs (Ca2+-dependent protein kinases)/CCaMK (calcium/calmodulin-dependent protein kinases), and CBLs (calcineurin B-like proteins) are the three largest categories of Ca2+ sensors in plants. The numbers begin with Gorai are the Ca2+ sensor genes in G. raimondii. The expression level of UBQ7 was used as the internal control to standardize the RNA samples for each reaction. Error bars are the SDs of four technical replicates
Discussion
Annexins typically form a big gene family in all multicellular organisms. Genome sequencing revealed that there are eight annexin genes in Arabidopsis (Cantero et al. 2006; Mortimer et al. 2008), nine in rice (Moss and Morgan 2004) and ten in Medicago (Talukdar et al. 2009). In cotton, a draft genome of G. raimondii (D5 subgenome) has been sequenced and assembled recently (Wang et al. 2012). We have integrated the genome and identified 14 annexin genes in G. raimondii (D5 genome; Fig. 1a, S2). We found that many reported annexin genes with different names were in fact the same genes in different cotton species or cultivars (Fig. 1a; Table S4-6) and some small differences in nucleotide sequence might have come from SNP between the A and D genome or sequencing errors. The observation that annexin proteins constitute up to 0.1 % of total plant proteins (Delmer and Potikha 1997; Clark et al. 2001; Moss and Morgan 2004; Mortimer et al. 2008) indicates their essential role in plant cell development. However, definitive evidence of the in planta role of annexin are still lacking thus far.
Developing cotton fiber is a fast-growing single cell which may be enriched for annexins. This was observed in our RT-PCR and qRT-PCR studies. About half of the annexin family members were expressed in cotton fiber and three members including Gorai.007G239000, Gorai.007G060900 and Gorai.009G237900 had relatively high expression levels compared to other members (Fig. 1d–f). Although several studies have been conducted to examine their expression levels in cotton and potential roles in Arabidopsis (Shin and Brown 1999; Tu et al. 2007; Wang et al. 2010a, b; Zhou et al. 2011; Li et al. 2013a; Huang et al. 2013), the mechanism by which annexins regulate cotton fiber development is still unknown.
In this study, by using transgenic and cell biology approaches, we provide evidence that GhAnn2 may contribute to Ca2+ influx at the tip of fiber cells (Fig. 4; Fig. S7). Ca2+ plays crucial roles in a wide array of growth and developmental processes (Jones and Lunt 1967) and is especially important in tip-growth (Fan et al. 2004; Yoon et al. 2006; Kudla et al. 2010). The tip-high Ca2+ gradient achieved by Ca2+ influx from the extracellular environment maintains the rate of cell elongation in pollen tubes (Hepler et al. 2001; Holdaway-Clarke and Hepler 2003). Ca2+ has been reported to play important roles in fiber elongation (Lee et al. 2007; Taliercio and Boykin 2007; Padmalatha et al. 2012; Walford et al. 2012; Tang et al. 2014). A high Ca2+ gradient is found in the cytoplasm of fast elongating cotton fiber cells near the growing tip (Huang et al. 2008). During fiber development, there is a sustained Ca2+ influx at the tip of fiber cells and the flux rate peaks during fiber rapid elongating at between 10 and 15 DPA (Tang et al. 2014) when plasmodesmata close and all solutes including ions such as Ca2+ must move into fibers across plasma membrane (Ruan 2007). We found inhibition of Ca2+ influx by Ca2+ channel blocker La3+ (Yuasa et al. 1998) and Ca2+ pool release channel blocker 2-APB (Bootman et al. 2002) strongly suppresses fiber cell elongation (Fig. 4c, S6; Tang et al. 2014), supporting an essential role for Ca2+ in fiber development. On the other hand, Ca2+ influx contributes to the generation of Ca2+ signal which should be sensed by Ca2+ sensors, such as CaMs/CMLs, CDPKs/CCaMKs, and CBLs (Kudla et al. 2010). There are several reports that genes associated with calmodulin and calmodulin-binding proteins are up-regulated in fiber initials and during fiber elongation by expression profile analysis (Lee et al. 2007; Taliercio and Boykin 2007). Consistent with these reports, two calmodulins including GhCaM7 (Gorai.009G080400.1) and Gorai.006G118400.1 were down-regulated in the GhAnn2 RNAi fibers. GhCaM7 had the highest expression level in elongating fibers among all the GhCaM genes. Similar to GhAnn2, suppression of GhCaM7 by RNAi also inhibits fiber elongation (Tang et al. 2014) and cell wall synthesis (Fig. S8). Except for calmodulin, other Ca2+ sensors that had relatively high expression levels in elongating fibers are also down-regulated in GhAnn2 RNAi fibers. Although the function of these CDPKs/CCaMKs, and CBLs in cotton fiber development remains to be determined, studies in pollen tubes shows that these Ca2+ sensors play important roles in cell tip-growth (Zhao et al. 2013).
Annexins are small proteins that possess structural motifs that could support calcium transport. Several animal annexins have been reported to function in vitro as Ca2+ channels, such as the vertebrate annexins A1, 2, 5–7, and 12 (Burger et al. 1994; Liemann et al. 1996; Kourie and Wood 2000). Some loss-of-function mutants of annexin have impaired ability to regulate cytoplasmic Ca2+. These include, for example, A5 (2/2) chicken DT40 cells (Kubista et al. 1999), A7 (+/2) murine brain cells (Watson et al. 2004), and A7 (2/2) murine cardiomyocytes (Schrickel et al. 2007). In plants, a Zea mays annexin could increase [Ca2+]cyt of Arabidopsis protoplasts when the recombinant annexin was added to the root protoplasts expressing the calcium sensor protein, aequorin (Demidchik et al. 2002; Dodd et al. 2006). The pharmacological profile was consistent with annexin activation (at the extracellular plasma membrane face) of Arabidopsis Ca2+-permeable nonselective cation channels. It is suggested that annexins create a Ca2+ influx pathway directly, particularly during stress responses involving acidosis (Laohavisit et al. 2009). In addition, the Arabidopsis loss-of-function mutant (Atann1) that lacks root hairs was found to be impaired in OH· activated Ca2+- and K+-permeable conductance. And the OH·-activated Ca2+ conductance could be reconstituted by recombinant ANN1 in planar lipid bilayers. ANN1 therefore presents as a novel Ca2+-permeable transporter in plants (Laohavisit et al. 2012). In cotton, we found that GhAnn2 mediates the tip Ca2+ influx (Fig. 4a, b). Together with previous studies, it is suggested that GhAnn2 might also act as a Ca2+ channel and have a positive role in fiber elongation.
In summary, by taking a transgenic approach, we show that GhAnn2 is required for fiber cell elongation through mediating Ca2+ influx at the tip of fiber cells and possibly contributes to the generation of Ca2+ signal.
References
Andrawis A, Solomon M, Delmer DP (1993) Cotton fiber annexins: a potential role in the regulation of callose synthase. Plant J 3:763–772
Baucher M, Lowe YO, Vandeputte OM et al (2011) Ntann12 annexin expression is induced by auxin in tobacco roots. J Exp Bot 62:4055–4065
Beasley C, Ting IP (1973) The effects of plant growth substances on in vitro fiber development from fertilized cotton ovules. Am J Bot 60:130–139
Blackbourn HD, Walker JH, Battey NH (1991) Calcium-dependent phospholipid-binding proteins in plants—their characterization and potential for regulating cell-growth. Planta 184:67–73
Bootman MD, Collins TJ, MacKenzie L, Roderick HL, Berridge MJ, Peppiatt CM (2002) 2-aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J 16:1145–1150
Burger A, Voges D, Demange P et al (1994) Structural and electrophysiological analysis of annexin V mutants: mutagenesis of human annexin V, an in vitro voltage-gated calcium channel, provides information about the structural features of the ion pathway, the voltage sensor and the ion selectivity filter. J Mol Biol 237:479–499
Cantero A, Barthakur S, Bushart TJ, Chou S, Morgan RO, Fernandez MP, Clark GB, Roux SJ (2006) Expression profiling of the Arabidopsis annexin gene family during germination, de-etiolation and abiotic stress. Plant Physiol Biochem 44:13–24
Clark GB, Sessions A, Eastburn DJ, Roux SJ (2001) Differential expression of members of the annexin multigene family in Arabidopsis. Plant Physiol 126:1072–1084
Cole RA, Fowler JE (2006) Polarized growth: maintaining focus on the tip. Curr Opin Plant Biol 9:579–588
de Carvalho-Niebel F, Timmers ACJ, Chabaud M, DefauxPetras A, Barker DG (2002) The Nod factor-elicited annexin MtAnn1 is preferentially localised at the nuclear periphery in symbiotically activated root tissues of Medicago truncatula. Plant J 32:343–352
Delmer DP, Potikha TS (1997) Structures and functions of annexins in plants. Cell Mol Life Sci 53:546–553
Demidchik V, Bowen HC, Maathuis FJM et al (2002) Arabidopsis thaliana root non-selective cation channels mediate calcium uptake and are involved in growth. Plant J 32:799–808
Divya K, Jami SK, Kirti PB (2010) Constitutive expression of mustard annexin, AnnBj1 enhances abiotic stress tolerance and fiber quality in cotton under stress. Plant Mol Biol 73:293–308
Dodd AN, Jakobsen MK, Baker AJ et al (2006) Time of day modulates low-temperature Ca2+ signals in Arabidopsis. Plant J 48:962–973
Fan X, Hou J, Chen X, Chaudhry F, Staiger CJ, Ren H (2004) Identification and characterization of a Ca2+-dependent actin filament-severing protein from lily pollen. Plant Physiol 136:3979–3989
Gerke V, Moss SE (2002) Annexins: from structure to function. Physiol Rev 82:331–371
Gidrol X, Sabelli PA, Fern YS, Kush AK (1996) Annexin-like protein from Arabidopsis thaliana rescues DoxyR mutant of Escherichia coli from H2O2 stress. Proc Natl Acad Sci USA 93:11268–11273
Haigler CH, Betancur L, Stiff MR et al (2012) Cotton fiber: a powerful single-cell model for cell wall and cellulose research. Front Plant Sci 3:104
Helliwell CA, Wesley SV, Wielopolska AJ, Waterhouse PM (2002) High-throughput vectors for efficient gene silencing in plants. Funct Plant Biol 29:1217–1225
Hepler PK, Vidali L, Cheung AY (2001) Polarized cell growth in higher plants. Annu Rev Cell Dev Biol 17:159–187
Holdaway-Clarke TL, Hepler PK (2003) Control of pollen tube growth: role of ion gradients and fluxes. New Phytol 159:539–563
Huang X, Madan A (1999) CAP3: A DNA sequence assembly program. Genome Res 9:868–877
Huang QS, Wang HY, Gao P et al (2008) Cloning and characterization of a calcium dependent protein kinase gene associated with cotton fiber development. Plant Cell Rep 27:1869–1875
Huang Y, Wang J, Zhang L et al (2013) A cotton annexin protein AnxGb6 regulates fiber elongation through its interaction with actin 1. PLoS ONE 8:e66160
Jami SK, Clark GB, Ayele BT, Roux SJ, Kirti PB (2011) Identification and characterization of annexin gene family in rice. Plant Cell Rep 31:813–825
Jin S, Zhang X, Nie Y, Guo X, Liang S, Zhu H (2006) Identification of a novel elite genotype for in vitro culture and genetic transformation of cotton. Biol Plant 50:519–524
Jones RG, Lunt OR (1967) The function of calcium in plants. Bot Rev 33:407–426
Kiba T, Naitou T, Koizumi N, Yamashino T, Sakakibara H, Mizuno T (2005) Combinatorial microarray analysis revealing arabidopsis genes implicated in cytokinin responses through the His → Asp phosphorelay circuitry. Plant Cell Physiol 46:339–355
Kim HJ, Triplett BA (2001) Cotton fiber growth in planta and in vitro. Models for plant cell elongation and cell wall biogenesis. Plant Physiol 127:1361–1366
Konopka-Postupolska D, Clark G, Goch G, Debski J, Floras K, Cantero A, Fijolek B, Roux S, Hennig J (2009) The role of annexin1 in drought stress in Arabidopsis. Plant Physiol 150:1394–1410
Kourie JI, Wood HB (2000) Biophysical and molecular properties of annexin-formed channels. Prog Biophys Mol Biol 73:91–134
Kubista H, Hawkins TE, Patel DR, Haigler HT, Moss SE (1999) Annexin 5 mediates a peroxide-induced Ca (2+) influx in B cells. Curr Biol 9:1403–1406
Kudla J, Batistič O, Hashimoto K (2010) Calcium signals: the lead currency of plant information processing. Plant Cell 22:541–563
Laohavisit A, Davies JM (2009) Multifunctional annexins. Plant Sci 177:532–539
Laohavisit A, Davies JM (2011) Annexins. New Phytol 189:40–53
Laohavisit A, Mortimer JC, Demidchik V et al (2009) Zea mays annexins modulate cytosolic free Ca2+ and generate a Ca2+-permeable conductance. Plant Cell 21:479–493
Laohavisit A, Shang Z, Rubio L et al (2012) Arabidopsis annexin1 mediates the radical-activated plasma membrane Ca2+- and K+-permeable conductance in root cells. Plant Cell 24:1522–1533
Lee JJ, Woodward AW, Chen ZJ (2007) Gene expression changes and early events in cotton fibre development. Ann Bot (Lond) 100:1391–1401
Li A, Xia T, Xu W et al (2013a) An integrative analysis of four CESA isoforms specific for fiber cellulose production between Gossypium hirsutum and Gossypium barbadense. Planta 237:1585–1597
Li B, Li DD, Zhang J, Xia H, Wang XL, Li Y, Li XB (2013b) Cotton AnnGh3 encoding an annexin protein is preferentially expressed in fibers and promotes initiation and elongation of leaf trichomes in transgenic Arabidopsis. J Integr Plant Biol 55:902–916
Liemann S, Benz J, Burger A, Voges D, Hofmann A, Huber R, Göttig P (1996) Structural and functional characterization of the voltage sensor in the ion channel human annexin V. J Mol Biol 258:555–561
Liu D, Zhang X, Tu L, Zhu L, Guo X (2006) Isolation by suppression-subtractive hybridization of genes preferentially expressed during early and late fiber development stages in cotton. Mol Biol 40:825–834
Liu Q, Talbot M, Llewellyn DJ (2013) Pectin methylesterase and pectin remodelling differ in the fibre walls of two gossypium species with very different fibre properties. PLoS ONE 8:e65131
Lu Y, Ouyang B, Zhang J, Wang T, Lu C, Han Q, Zhao S, Ye Z, Li H (2012) Genomic organization, phylogenetic comparison and expression profiles of annexin gene family in tomato (Solanum lycopersicum). Gene 499:14–24
Montalvo JG Jr, Hoven TMV (2005) Relationship between micronaire, fineness and maturity. Part II. Experimental. J Cotton Sci 9:89–96
Morgan RO, Martin-Almedina S, Iglesias JM, Gonzalez-Florez MI, Fernandez MP (2004) Evolutionary perspective on annexin calcium-binding domains. Biochim Biophys Acta 1742:133–140
Morgan RO, Martin-Almedina S, Garcia M, Jhoncon-Kooyip J, Fernandez M (2006) Deciphering function and mechanism of calcium-binding proteins from their evolutionary imprints. Biochim Biophys Acta 1763:1238–1249
Mortimer JC, Laohavisit A, Macpherson N, Webb A, Brownlee C, Battey NH, Davies JM (2008) Annexins: multifunctional components of growth and adaptation. J Exp Bot 59:533–544
Moss SE, Morgan RO (2004) The annexins. Genome Biol 5:1–8
Padmalatha KV, Patil DP, Kumar K et al (2012) Functional genomics of fuzzless-lintless mutant of Gossypium hirsutum L. cv. MCU5 reveal key genes and pathways involved in cotton fibre initiation and elongation. BMC Genom 13:624
Potikha TS, Delmer DP (1997) cDNA clones for annexin AnnGh1 (accession no. U73746) and AnnGh2 (accession no. U73747) from Gossypium hirsutum (cotton) 1 (PGR 97-003). Plant Physiol 113:305
Qin YM, Zhu YX (2011) How cotton fibers elongate: a tale of linear cell-growth mode. Curr Opin Plant Biol 14:106–111
Ruan YL (2007) Rapid cell expansion and cellulose synthesis regulated by plasmodesmata and sugar: insights from the single-celled cotton fibre. Funct Plant Biol 34:1–10
Schrickel JW, Brixius K, Herr C et al (2007) Enhanced heterogeneity of myocardial conduction and severe cardiac electrical instability in annexin A7-deficient mice. Cardiovasc Res 76:257–268
Shabala SN, Newman IA, Morris J (1997) Oscillations in H+ and Ca2+ ion fluxes around the elongation region of corn roots and effects of external pH. Plant Physiol 113:111–118
Shimizu Y, Aotsuka S, Hasegawa O et al (1997) Changes in levels of mRNAs for cell wall-related enzymes in growing cotton fiber cells. Plant Cell Physiol 38:375–378
Shin H, Brown RM (1999) GTPase activity and biochemical characterization of a recombinant cotton fiber annexin. Plant Physiol 119:925–934
Taliercio EW, Boykin D (2007) Analysis of gene expression in cotton fiber initials. BMC Plant Biol 7:22
Talukdar T, Gorecka KM, de Carvalho-Niebel F, Downie JA, Cullimore J, Pikula S (2009) Annexins calcium- and membrane-binding proteins in the plant kingdom. Potential role in nodulation and mycorrhization in Medicago truncatula. Acta Biochim Polonia 56:199–210
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Tang WX, Tu LL, Yang XY, Tan JF, Deng FL, Hao J, Guo K, Lindsey K, Zhang XL (2014) The calcium sensor GhCaM7 promotes cotton fiber elongation by modulating ROS production. New Phytol 202:509–520
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucl Acids Res 25:4876–4882
Tu LL, Zhang XL, Liang SG et al (2007) Genes expression analyses of sea-island cotton (Gossypium barbadense L.) during fiber development. Plant Cell Rep 26:1309–1320
Walford SA, Wu Y, Llewellyn DJ, Dennis ES (2012) Epidermal cell differentiation in cotton mediated by the homeodomain leucine zipper gene, GhHD-1. Plant J 71:464–478
Wang HY, Wang J, Gao P, Jiao GL, Zhao PM, Li Y, Wang GL, Xia GX (2009) Down-regulation of GhADF1 gene expression affects cotton fibre properties. Plant Biotechnol J 7:13–23
Wang H, Guo Y, Lv F, Zhu H, Wu S, Jiang Y, Li F, Zhou B, Guo W, Zhang T (2010a) The essential role of GhPEL gene, encoding a pectate lyase, in cell wall loosening by depolymerization of the de-esterified pectin during fiber elongation in cotton. Plant Mol Biol 72:397–406
Wang LK, Niu XW, Lv YH et al (2010b) Molecular cloning and localization of a novel cotton annexin gene expressed preferentially during fiber development. Mol Biol Rep 37:3327–3334
Wang K, Wang Z, Li F et al (2012) The draft genome of a diploid cotton Gossypium raimondii. Nat Genet 44:1098–1103
Watson WD, Srivastava M, Leighton X, Glasman M, Faraday M, Fossam LH, Pollard HB, Verma A (2004) Annexin 7 mobilizes calcium from endoplasmic reticulum stores in brain. Biochim Biophys Acta 1742:151–160
Xiao YH, Li DM, Yin MH et al (2010) Gibberellin 20-oxidase promotes initiation and elongation of cotton fibers by regulating gibberellin synthesis. J Plant Physiol 167:829–837
Xin Z, Zhao Y, Zheng ZL (2005) Transcriptome analysis reveals specific modulation of abscisic acid signaling by ROP10 small GTPase in Arabidopsis. Plant Physiol 139:1350–1365
Xu B, Gou J, Li F, Shangguan X, Zhao B, Yang C, Wang L, Yuan S, Liu C, Chen X (2013) A cotton BURP domain protein interacts with α-Expansin and their co-expression promotes plant growth and fruit production. Mol Plant 6:945–958
Yoon GM, Dowd PE, Gilroy S, McCubbin AG (2006) Calcium-dependent protein kinase isoforms in Petunia have distinct functions in pollen tube growth, including regulating polarity. Plant Cell 18:867–878
Yuasa K, Takahashi K, Katou K (1998) Calcium chelator and channel blockers suppress the IAA-induced membrane hyperpolarization without inhibiting the following growth promotion in hypocotyl sections of Vigna unguiculata under xylem perfusion. Plant Cell Physiol 39:978–986
Zhang D, Hrmova M, Wan CH et al (2004) Members of a new group of chitinase-like genes are expressed preferentially in cotton cells with secondary walls. Plant Mol Biol 54:353–372
Zhang M, Zheng X, Song S et al (2011) Spatiotemporal manipulation of auxin biosynthesis in cotton ovule epidermal cells enhances fiber yield and quality. Nat Biotechnol 29:453–458
Zhao PM, Wang LL, Han LB et al (2009) Proteomic identification of differentially expressed proteins in the Ligon lintless mutant of upland cotton (Gossypium hirsutum L.). J Proteome Res 9:1076–1087
Zhao LN, Shen LK, Zhang WZ et al (2013) Ca2+-dependent protein kinase 11 and 24 modulate the activity of the inward rectifying K+ channels in Arabidopsis pollen tubes. Plant Cell 25:649–661
Zhou L, Duan J, Wang XM et al (2011) Characterization of a novel annexin gene from cotton (Gossypium hirsutum cv. CRI 35) and antioxidative role of its recombinant protein. J Integr Plant Biol 53:347–357
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 31230056).
Author information
Authors and Affiliations
Corresponding author
Additional information
Wenxin Tang and Yonghui He have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tang, W., He, Y., Tu, L. et al. Down-regulating annexin gene GhAnn2 inhibits cotton fiber elongation and decreases Ca2+ influx at the cell apex. Plant Mol Biol 85, 613–625 (2014). https://doi.org/10.1007/s11103-014-0208-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-014-0208-7