Abstract
Dry seeds accumulate translatable mRNAs as well as functional proteins for transcription and translation. They are possibly involved in early physiological responses after imbibition, however, their functions remain poorly understood. The aim of this study is to investigate the function of seed stored transcriptional machinery in resumption of gene expression after the onset of imbibition in Arabidopsis thaliana. First, we examined the characters of stored mRNAs in A. thaliana dry seeds using microarray data from non-dormant Columbia (Col) and dormant Cape Verde Islands (Cvi) accessions. Transcriptomes of Col and Cvi dry seeds resembled one another, suggesting that patterns of stored mRNA do not reflect either the degree of dormancy or germination potential, but rather reflect the developmental context, such as seed maturation. Upon imbibition, changes in mRNA abundance of many genes were initiated between 1- and 2-h after the onset of imbibition. RT–PCR expression analysis of imbibition-responsive genes indicates that early induction was not altered by treatment of cycloheximide. This suggests that de novo protein synthesis is not required for gene expression during early imbibition stages. Moreover, controlled deterioration treatment (CDT), which causes artificial damages on dry seeds, disrupted gene expression specifically during the first 3 h after the start of imbibition, suggesting that seed stored transcription factors play a pivotal role in gene expression during early imbibition periods. Furthermore, the negligible effect of CDT on germination indicates that early imbibition response is dispensable and de novo synthesized proteins compensate for the function of stored proteins for germination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stored macro-molecules in dry seeds are required for the resumption of genetic programs after the start of imbibition. Dry seeds accumulate a large amount of translatable mRNAs as well as functional proteins for metabolism, transcription and translation (Marcus and Feeley 1964; Dure and Waters 1965; Bewley and Black 1994). They are produced during seed development, retain their functions during the dry storage, and are used upon imbibition. These stored macro-molecules are damaged gradually during the period of dry storage, which reduces seed vigor and viability (Oracz et al. 2007). It is also known that storage of dry seeds, a period called after-ripening, reduces the degree of dormancy. Therefore, the functions of stored macro-molecules in dry seeds are essential for seed vigor and longevity, as well as maintenance of seed dormancy.
Dry seeds accumulate various mRNAs, called stored mRNA or long-lived RNA. Stored mRNAs were first identified in cotton seeds (Dure and Waters 1965), and are now known to be present in the dry seeds of all angiosperms examined. The stored mRNAs are thought to be translated after the onset of imbibition and to function during the early stage of imbibition (Comai et al. 1989; Hughes and Galau 1989, 1991). Microarray analysis revealed that more than 10,000 different mRNA species were present in Arabidopsis dry seeds of the Columbia (Col) accession, which include the genes for all functional categories (Nakabayashi et al. 2005). Accumulation of stored mRNAs in dry seeds is regulated by endogenous and environmental signals. Abscisic acid (ABA) plays an important role in seed development, maturation and germination (Nambara and Marion-Poll 2005) and is involved in the accumulation of stored mRNA in seeds (Nakabayashi et al. 2005). ACGT-containing ABA-responsive elements (ABREs) are highly over-represented in the promoters of the abundant stored mRNA genes. CE and RY elements are also co-located with ABREs in their promoters. ACGT-containing ABREs, CE, and RY elements are the targets of ABSCISIC ACID-INSENSITIVE5 (ABI5), ABSCISIC ACID-INSENSITIVE4 (ABI4), and ABSCISIC ACID-INSENSITIVE3 (ABI3) transcription factors, respectively (Giraudat et al. 1992; Finkelstein et al. 1998; Finkelstein and Lynch 2000; Lopez-Molina and Chua 2000). This indicates that ABA-mediated transcription plays a key role in determining the patterns of the abundant class of stored mRNAs. Environmental conditions of maternal plants are known to affect the accumulation pattern of a subset of stored mRNAs (Matakiadis et al. 2009).
It was reported that dry seeds contain the functional proteins for translation (Marcus and Feeley 1964). Rajjou et al. (2004) reported that germination of Arabidopsis thaliana seeds was inhibited by the cycloheximide (CHX) treatment, an inhibitor of protein synthesis, but not by α-amanitin that blocks PolII-mediated transcription (Rajjou et al. 2004). The authors concluded that de novo transcription is dispensable, but translatable mRNAs and proteins stored in dry seeds are sufficient for Arabidopsis germination. Recent progresses in proteome analysis have revealed overall components of stored proteins in dry and imbibed seeds (Gallardo et al. 2001, 2002). The major seed proteins in Arabidopsis are 12S globulins (cruciferins), 2S albumin (napins), late embryogenesis abundant (LEA) proteins, and stress inducible proteins, such as heat shock proteins (HSPs). Seed proteins abundantly accumulated in dry seeds also include enzymes for primary metabolism, such as respiration and gluconeogenesis. In rice seeds, similar proteins are also accumulated (Yang et al. 2007). Although it has been studied for a long period, it remains unclear how seed stored proteins are important for processes in seed imbibition, germination and dormancy.
Dry seeds contain a number of proteins that function after imbibition. The competence of transcription of dry seeds has been reported by several researchers. Nuclei isolated from dry seeds of rapeseed had transcriptional activity (Comai and Harada 1990) and mRNA for the β-1,3-Glucanase gene was increased in tobacco dry seeds during storage periods (Leubner-Metzger 2005). Rajjou et al. (2007) reported that the translational capacity of dry seeds is critical for seed viability and vigor. Recently, we reported a kinetics study on gene expression analysis during early imbibition of non-dormant Col and dormant Cape Verde Islands (Cvi) seeds (Preston et al. 2009). This analysis indicated that resumption of gene expression was initiated 1 h after the start of imbibition regardless of accessions. The aim of this work is to examine the function of seed stored components (i.e., stored mRNA and proteins). To do this, we conducted: (1) a comparison of stored mRNA transcriptomes between Col and Cvi, which was not analyzed in Preston et al. (2009), and (2) gene expression analysis during seed imbibition to examine how the transcriptional capacity of dry seeds contributes to this process. Our dry seed transcriptome data of Col and Cvi were obtained from seeds harvested at the same time from plants grown under the same growth conditions. This data set is suitable for comparative transcriptomes because comparison of multiple transcriptome data often provides a difference of growth conditions rather than experimental treatment (Hirai et al. 2003). We outlined stored mRNAs that are abundantly present in both Col and Cvi dry seeds. Moreover, the function of seed proteins stored in dry seeds on gene expression during early stages of seed imbibition was investigated after the following treatments: seed imbibition in the presence of CHX, an inhibitor of translation, and controlled deterioration treatment (CDT), which causes damage on seed stored components like proteins. These analyses suggest that transcription factors that are stored in dry seeds play a pivotal role in the resumption and maintenance of gene expression during early stages of imbibition.
Materials and methods
Plant materials and growth conditions
Arabidopsis thaliana wild type and tt8 mutants (SALK_030966 and SALK_048673) used in this study were of Col accession (Alonso et al. 2003). The tt8 mutants were donated by Professor Naoto Kawakami (Meiji University, Japan). Surface sterilized seeds were sown on 0.8% agar plates supplemented with 1/2 Murashige and Skoog salt mixture in xx-cm2 Petri dishes. A 2 week-old seedlings were transferred to soil. Plants were grown on soil under a 16 h white light/8 h dark cycle at 22°C. Plants were watered every 2–3 days until they failed to produce new flowers (approximately 2–3 months). Plants were stayed for 1 week without irrigation until harvest. Seeds were harvested and stored in darkness at room temperature.
Controlled Deterioration Test
Controlled deterioration test was performed as described (Delouche and Baskin 1973). Briefly, dry seeds were placed in a sealed plastic box with saturated vapor by adding 100 ml of water at 40°C in darkness for indicated periods. After this treatment, seeds were desiccated on filter paper at room temperature for 1 day.
Microarray data analysis
Triplicate microarray analysis of Col and Cvi dry seeds were performed using Affymetrix ATH1 arrays as described in Preston et al. (2009). The data is available from NASCArrays (http://affy.arabidopsis.info/), reference number: NASCARRAYS-499. Expression data of organelle-coded genes were omitted prior to analysis. Scatterplot was made by Microsoft Excel. Ontological analysis was performed using MIPS Arabidopsis thaliana Database (MAtDB, http://mips.helmholtz-muenchen.de/proj/funcatDB/search_main_frame.html) as described elsewhere (Tatematsu et al. 2008; Yamagishi et al. 2009).
Germination test
Germination tests were performed using 50 seeds per experiment. Triplicate experiments were performed using independent seed batches. A 2–4 week- stored seeds were surface-sterilized, imbibed on agar medium under a continuous white light condition (20 μm/m2/s) at 25 ± 1°C. Visible radicle protrusion was used as the criterion for germination and was scored daily. For CHX treatment, dry seeds were imbibed on 0.8% agar medium supplemented with 100 μM CHX under a continuous white light condition at 25 ± 1°C. One hundred mM of CHX solution in ethanol was added to media at a dilution of 1:1,000 (final concentration was 100 μM), whereas ethanol was added to control media at the same dilution ratio.
RNA isolation and semi-quantitative RT–PCR
Seeds were surface-sterilized and imbibed on filter papers moistened with distilled water under a continuous white light condition at 25°C. Total RNA was prepared from 20 mg of dry and imbibed seeds as described elsewhere (Oñate-Sánchez and Vicente-Carbajosa 2008). cDNA was synthesized using 600 ng of total RNA as a template with First Strand cDNA Synthesis Kit (Fermentas Canada Inc.) following the manufacturer’s protocol. The obtained cDNA were used for PCR analysis. PCR products were subjected to electrophoresis on a 2% agarose gel. The primers used in this study were shown in Table S1.
Results and discussion
Stored mRNA in Arabidopsis dry seeds
Stored mRNA transcriptome in Col and Cvi
Stored mRNAs accumulated in dry seeds of Col and Cvi accessions were analyzed. Triplicate microarray data on dry seeds were used for this analysis. Figure 1 shows a scatterplot of transcript abundance in Col and Cvi dry seeds. These seeds displayed different degrees of dormancy and germination potential, however, the overall feature of dry seed transcriptomes of Col and Cvi resembled one another. Okamoto et al. (2010) reported a comparative transcriptome analysis on Col wild type, ABA-deficient (non-dormant) aba2 and ABA-overaccumulating (hyper dormant) cyp707a1a2a3 triple mutants. Transcriptomes in 24 h-imbibed seeds of wild type and these mutants are largely different from one another, however only subtle differences are observed in dry seeds. Taken together, we conclude that transcriptomes in dry seeds reflect largely on developmental context rather than genetic background or physiological conditions of seeds. Chibani et al. (2006) reported that the dry seed proteomes of Cvi and Landsberg erecta (Ler) showed high conservation.
We also note that a subset of less abundant stored mRNA classes showed the accumulation pattern specific to either Col or Cvi (cut-off value, threefold). Biased gene ontology (GO) categories were searched on the MIPS website (http://mips.helmholtz-muenchen.de/proj/funcatDB/search_main_frame.html).
Col-specific mRNAs were over-represented for small Heat Shock Proteins (HSP) genes (P = 1.18e-8). Expression of small HSP genes is ABI3-dependent (Kotak et al. 2007) and is associated with the dormant state of Cvi seeds (Cadman et al. 2006). Proteome analysis indicates that these small HSPs were induced specifically by osmo-priming (Gallardo et al. 2001), suggesting that these are not correlated with germination. Another remarkable feature of Col-specific stored mRNAs are the over-representation of those related to reactive oxygen species (ROS). Those include superoxide metabolism (P = 4.16e-4), oxidative stress response (P = 1.56e-3), and glutathione conjugation reaction (P = 3.37e-3). These suggest that Col seeds are more active in either ROS production or signaling than the Cvi.
Cvi-specific mRNAs were highly over-represented for phosphate metabolism (P = 2.39e-07), suggesting that a subset of phosphate metabolism genes is regulated in an accession-specific manner. Also, Cvi-specific stored mRNAs are over-represented for those related to tubulin dependent transport (P = 5.53e-06), microtubule cytoskelton (P = 3.65e-04), actin cytoskelton (P = 3.84e-03), DNA topology (P = 3.53e-02), and lipid, fatty acid and isoprenoid metabolism (P = 4.46e-02). The fatty acid metabolism genes include those for 3-ketoacyl-CoA synthase1 (KCS1) (Todd et al. 1999), fatty acid elongase 1 (FAE1) (James et al. 1995), acyltransferase (FDH) (Yephremov et al. 1999), and acyl-CoA carboxylase (ACC2) (Baud et al. 2003). It is notable that all enriched Cvi-specific stored mRNAs are involved in lipid biosynthesis but none were found for those related to fatty acid breakdown.
Stored mRNAs for amino acid metabolisms are differentially accumulated in accessions. Col-specific stored mRNAs were over-represented for those related to metabolisms of valine, leucine and isoleucine. On the other hand, Cvi-specific stored mRNAs enriched for biosynthesis of glutamate and phenylalanine or for metabolisms of glycine, proline, threonine, and cysteine. It is unknown what do these biased GO categories mean, but these unique patterns of stored mRNAs might be useful fingerprints for characterizing the nature of stored mRNAs.
The abundant class of stored mRNAs
Microarray analysis reveals stored mRNAs from 13,202 Col genes and from 14,096 Cvi genes judged as “Present” by the analytical software MAS 5.0 at least once in triplicate experiments. We selected 100 genes whose mRNA levels were the highest in these stored mRNA genes in either Col or Cvi dry seeds (Table S2 and S3). These genes are similar to one another, and 79 genes are shared. In this study, we define these 79 genes as the abundant class of stored mRNA. This class represents less than 1% of genes in > 10,000 stored mRNA genes. This abundant mRNA class included many known genes for seed proteins.
Stored mRNA for LEA and seed storage proteins
The majority of the abundant class of stored mRNAs are the LEA genes: 17 genes are included in the abundant class of stored mRNAs among 21 genes in Col and 19 genes in Cvi (Table 1). LEA mRNAs accumulate in the late stage of seed development. The Arabidopsis genome contains 51 LEA genes that are structurally classified into nine groups (Wise 2003; Hundertmark and Hincha 2008). A subset of LEA mRNAs accumulate in dry seeds but some are also induced in plants under low temperature, drought, high light and salt stresses (Seki et al. 2002; Kimura et al. 2003; Hundertmark and Hincha 2008). The physiological and biochemical function of LEA proteins have not been fully elucidated, however, they are known to be involved in the stress tolerance response of plant cells (Brini et al. 2007; Dalal et al. 2009). The other major family of abundant class of stored mRNAs is those for seed storage proteins. The mRNAs for three cruciferins (12S globulins) and two napins (2S albumins) accumulate in dry seeds of both Col and Cvi (Table 1). There are three cruciferin genes and five napin genes in the Arabidopsis Col genome (Fujiwara et al. 2002). In addition, another abundant class of stored mRNAs includes those for stress proteins, such as HSPs (3 in Col and 4 in Cvi).
Stored mRNAs related to metabolism
The abundant class of stored mRNA contains those for lipid storage and mobilization (Table 1). Oil seeds including Arabidopsis seeds store triacylglycerol (TAG) in the oil bodies, and TAG is converted to sucrose during and after germination (Graham 2008). Recent proteome analysis on oil bodies revealed a set of protein components for the oil bodies (Jolivet et al. 2004; Katavic et al. 2006). Oleosins are the abundant proteins in oil bodies and are necessary for stabilization of oil bodies (Siloto et al. 2006). Oleosin genes compose a multigene family but only four isoforms are abundantly present in the Arabidopsis seeds (Jolivet et al. 2004). The abundant class of stored mRNA includes three out of four oleosin mRNAs for seeds. In addition, proteome analysis identified several other components for oil bodies, including 11-β-hydroxysteroid dehydrogenase, short-chain dehydrogenase, embryo-specific protein, β-glucosidase, predicted GPI-anchored protein, aquaporin, myrosinase and its associated proteins. The abundant stored mRNAs in Col and Cvi dry seeds included those for three oleosins, 11-β-hydroxysteroid dehydrogenase (At5g50700/At5g50600), β-TIP (aquaporin; At1g17810), and short-chain dehydrogenase (At1g54870; Table S5). Moreover, stored mRNAs for the predicted GPI-anchored protein (At1g54860) and myrosinase-binding protein (At3g21380) are found in the abundant class in Col.
Fatty acids are converted to acetyl-CoA by β-oxidation and then to sucrose by glyoxylate cycle and gluconeogenesis. The abundant stored mRNAs include those for the glyoxylate cycle enzymes, malate synthase and isocitrate lyase, that accumulate abundantly in both Col and Cvi. In addition, PED1/KAT2 is also listed in the abundant class. PED1/KAT2 encodes a 3-keto-acyl-CoA thiolase that catalyzes the conversion of 3-keto-acyl-CoA to acyl-CoA in glyoxysomes (Hayashi et al. 1998; Germain et al. 2001). Other mRNAs for lipid biosynthesis and mobilization did not belong to the abundant class, but their mRNAs were stored moderately in dry seeds. Some of these mRNAs tended to be differentially accumulated between accessions (see below).
Stored mRNAs for transcription factors
The Arabidopsis genome contains at least 1,834 genes for transcription factors, which are divided into 50 families based on the nature of DNA-binding domains (http://arabidopsis.med.ohio-state.edu/AtTFDB/). ATH1 arrays cover expression of 1,467 of 1,834 TF genes. The abundant classes of 79 stored mRNAs contain only one encoding a transcription factor (TF); ATAF1, a membrane-bound NAC domain transcription factor (Kim et al. 2006). ATAF1 has been implicated in the roles of stress response (Lu et al. 2007) and ABA signaling (Jensen et al. 2008). Interestingly, this family was recently shown to be involved in multiple responses to DNA damage (Yoshiyama et al. 2009).
Although ATAF1 is the only TF gene in the abundant class of 79 stored mRNAs, analytical software MAS5.0 indicates 741 Col and 818 Cvi TF mRNAs are called “Present” in the stored mRNA transcriptomes. Table 2 depicts the top 20 TF mRNAs in Col and Cvi dry seeds. Several families of TFs accumulated their mRNAs as the stored mRNA form relatively abundantly: NAC, AP2-EREBP, and zinc-finger proteins. Also, stored mRNAs for TFs that function in the regulation of ABA signaling and stress responses accumulated in dry seeds. RD26 encodes a NAC transcription factor involved in ABA signaling and stress responses (Fujita et al. 2004). The members of RAP2 transcription factors (RAP2.3, and 2.4) as well as DREB2A are the abundant class of AP2 TFs. ABI5 encodes a bZIP-type transcription factor (Finkelstein and Lynch 2000), and is a key regulator for ABA-responsive element (ABRE)-mediated transcription in seeds (Bensmihen et al. 2002; Carles et al. 2002; Kim et al. 2002). ABI5 mRNA was dramatically decreased after imbibition and was induced by drought, salt and ABA (Lopez-Molina et al. 2001; Nakashima et al. 2006). Enrichment of TFs involved in ABA signaling in the stored mRNA may be related to the previous report that ABREs were highly over-represented in the promoters of genes for stored mRNA (Nakabayashi et al. 2005).
Germination of seeds treated with CHX and CDT
Effects of CHX and CDT on germination
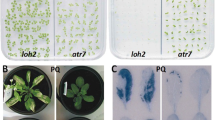
Seeds of some transparent testa (tt) mutants have increased permeability (Debeaujon et al. 2000). We used the tt8 mutants of the Col accession (Gonzalez et al. 2009) to examine the effect of CHX on gene expression during imbibition. The treatment of 100 μM CHX inhibited germination of both Col and tt8 seeds (data not shown). We also tested the effect of CDT on germination of Col and tt8 seeds (Fig. 2). Dry seeds were subjected to saturated vapor at 40°C for various periods (Delouche and Baskin 1973). The tt8 mutant seeds did not alter germination kinetics after the CDT for 1 day, but % germination and velocity of germination were affected after the CDT for 3 days (Fig. 2a). The mutant seeds failed to germinate when the seeds were subjected to the CDT for more than 5 days. The negative effects of CDT on Col germination were enhanced gradually when the period was prolonged to 7 days (Fig. 2b), in contrast to the abrupt decline of the tt8 mutant after 5 days of the treatment. This is in agreement with a previous report that Arabidopsis testa mutants reduced both seed longevity and dormancy (Debeaujon et al. 2000).
Germination of seeds after controlled deterioration treatment Col (A) and tt8 (B) seeds were subjected to controlled deterioration treatment and sown on agar medium. Germination was scored daily and triplicate experiments were performed using independent seed batches. A bar indicates the standard error
Effects of CHX on mRNA abundance during imbibition
We investigated the effect of CHX on these imbibition-regulated genes in tt8 mutant seeds to examine if early induction of these genes requires de novo synthesized proteins after imbibition. Down-regulated and up-regulated genes by imbibition were chosen based on clear changes in mRNA abundance from the gene list obtained from microarray data in Preston et al. (2009).
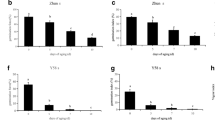
We chose the following five down-regulated genes: RD29B (Nakashima et al. 2006), At2g42560 (Hundertmark and Hincha 2008), ABI2 (Leung et al. 1997), ABI5 (Finkelstein and Lynch 2000; Lopez-Molina and Chua 2000), and CP29 (Andersson et al. 2001). The decline of mRNA abundance of these genes in the control was first observed 2 h after the onset of imbibition (Fig. 3a). The down-regulation of all these genes was partially alleviated by CHX application (Fig. 3a). It is noteworthy that mRNA abundance of most of these genes was higher in CHX-treated seeds relative to the control at 1 h. The increase of mRNA by CHX treatment suggests that inhibition of translation precociously activated transcription possibly using stored transcriptional machinery. This suggests that, under our conditions, resumption of gene expression has a 1-h lag period after the start of imbibition but can be precociously induced by CHX treatment. It is also possible that inhibition of translation enhanced mRNA protection more prominently than in control seeds. Rajjou et al. (2004) reported that α-amanitin-treated seeds enhanced translation of stored mRNA, suggestive of two parallel mechanisms that may be explained the effect of CHX on expression patterns of the down-regulated genes.
Effects of cycloheximide and controlled deterioration treatment on gene expression during imbibition. (A) Expression patterns during imbibition under control (EtOH) and 100 μM cycloheximide (CHX). RT-PCR analysis was carried out by using total RNA extracted from tt8 seeds. Two independent experiments were performed using different tt8 alleles SALK_030966 and SALK_048673 (Figure S2). Consistent results were obtained from these experiments and data from SALK_030966 is shown. The number of PCR cycles is shown on the right. (B) Expression patterns during imbibition in damaged seeds. Seeds were exposed to saturated vapor at 40°C (controlled deterioration treatment) for 1 day, dried on filter paper under normal humidity conditions at room temperature for 1 day, followed by a germination test. Two independent experiments were performed using different tt8 alleles, SALK_030966 and SALK_048673. Consistent results were obtained from these experiments and data from SALK_030966 is shown. The number of PCR cycles is shown on the right
The following five genes were chosen to examine the effect of CHX on imbibition induced gene expression: HSP23.5 (Waters et al. 2008), BME3 (Liu et al. 2005), NPR3 (Zhang et al. 2006), GSTU22 (Edwards et al. 2000; Wagner et al. 2002), and CYP707A2 (Kushiro et al. 2004; Okamoto et al. 2006). The transcript abundance of HSP23.5 was increased 1 h after imbibition and its transcript abundance continued to increase thereafter. Induction of BME3, NRP3, GSTU22 and CYP707A2 were induced either 1 or 2 h after the start of imbibition, but their induction was transient and declined thereafter (Fig. 3a). CHX application did not affect the induction of HSP23.5 at 1 h, but inhibited further induction at 2 h after the onset of imbibition (Fig. 3a). This suggests that induction of this gene after 2 h requires de novo protein synthesis. Moreover, induction of BME3, NPR3, GSTU22 and CYP707A2 at 2 h was also unaffected by CHX (Fig. 3a) suggesting that induction of these genes at this time point does not require de novo protein synthesis. It was recently shown that CYP707A2 mRNA is translated during the first 6 h after the start of imbibition (Liu et al. 2009). We also note that induction of these genes in the CHX treatment was not transient, suggesting that down-regulation at the latter time points require de novo protein synthesis.
Effects of CDT on mRNA abundance during imbibition
Experiments using CHX indicated that stored proteins in dry seeds are involved in gene expression during early imbibition stages. To confirm the importance of stored macro-molecules in dry seeds, we performed gene expression analysis on seeds with CDT (Fig. 3b; Table S7). This treatment is expected to cause damage on stored macro-molecules in dry seeds, including stored components for transcription and translation.
The tt8 mutant seeds were subjected to CDT for 1 day, which did not affect the velocity of germination (Fig. 2a). RT–PCR expression analysis showed gene expression patterns in CDT-treated seeds were largely altered during imbibition even though germination was visibly normal. Down-regulation of five genes was alleviated when compared with that of control seeds (Fig. 3a, EtOH vs. b). This suggests that efficient down-regulation of these genes requires factors stored in dry seeds. On the other hand, up-regulation of four of five genes was severely delayed compared with that of control seeds (Fig. 3a vs. b). This supports the notion that early induction of these genes requires factors stored in dry seeds. The expression pattern of GSTU22 was different from those of four other up-regulated genes. Its mRNA level was high in dry seeds after CDT (Fig. 3b), suggestive of its induction during CDT treatment. Moreover, the abundance of GSTU22 mRNA was maintained high during imbibition. Rajjou et al. (2008) reported that CDT enhanced protein oxidation presumably due to accumulation of reactive oxygen species (ROS). It is possible that GSTU22 was induced by ROS during CDT.
In conclusion, 1-d-CDT had little effect on velocity of germination but did largely affect the expression patterns of genes that are regulated early in seed imbibition. In combination with CHX experiments, we propose that these data reflect the importance of seed stored factors in resumption of transcription and gene expression during seed imbibition. Furthermore, regardless of the severe effect on gene expression during imbibition, 1-d-CDT did not alter the velocity of germination. This suggests transcriptional factors in dry seeds may be dispensable and are probably compensated by neosynthesized proteins, consistent with that translational capacity is correlated with seed vigor and viability (Rajjou et al. 2007).
Abbreviations
- ABA:
-
Abscisic acid
- ABI:
-
Abscisic acid-insensitive
- ABRE:
-
Abscisic acid-responsive element
- BME3:
-
Blue micropylar end 3
- CDT:
-
Controlled deterioration treatment
- CHX:
-
Cycloheximide
- Col:
-
Columbia
- Cvi:
-
Cape Verde Islands
- GSTU22:
-
Glutathione S-transferase tau 22
- GO:
-
Gene ontology
- HSP:
-
Heat shock protein
- LEA:
-
Late embryogenesis abundant
- RT–PCR:
-
Reverse transcription-PCR
- TT:
-
Transparent testa
References
Alonso J, Stepanova A, Leisse T, Kim C, Chen H, Shinn P, Stevenson D, Zimmerman J, Barajas P, Cheuk R, Gadrinab C, Heller C, Jeske A, Koesema E, Meyers C, Parker H, Prednis L, Ansari Y, Choy N, Deen H, Geralt M, Hazari N, Hom E, Karnes M, Mulholland C, Ndubaku R, Schmidt I, Guzman P, Aguilar-Henonin L, Schmid M, Weigel D, Carter D, Marchand T, Risseeuw E, Brogden D, Zeko A, Crosby W, Berry C, Ecker J (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301:653–657
Andersson J, Walters R, Horton P, Jansson S (2001) Antisense inhibition of the photosynthetic antenna proteins CP29 and CP26: implications for the mechanism of protective energy dissipation. Plant Cell 13:1193–1204
Baud S, Guyon V, Kronenberger J, Wuillème S, Miquel M, Caboche M, Lepiniec L, Rochat C (2003) Multifunctional acetyl-CoA carboxylase 1 is essential for very long chain fatty acid elongation and embryo development in Arabidopsis. Plant J 33:75–86
Bensmihen S, Rippa S, Lambert G, Jublot D, Pautot V, Granier F, Giraudat J, Parcy F (2002) The homologous ABI5 and EEL transcription factors function antagonistically to fine-tune gene expression during late embryogenesis. Plant Cell 14:1391–1403
Bewley JD, Black M (1994) Seeds: physiology of development and germination, 2nd edn. Plenum Press, New York
Brini F, Hanin M, Lumbreras V, Amara I, Khoudi H, Hassairi A, Pagès M, Masmoudi K (2007) Overexpression of wheat dehydrin DHN-5 enhances tolerance to salt and osmotic stress in Arabidopsis thaliana. Plant Cell Rep 26:2017–2026
Cadman C, Toorop P, Hilhorst H, Finch-Savage W (2006) Gene expression profiles of Arabidopsis Cvi seeds during dormancy cycling indicate a common underlying dormancy control mechanism. Plant J 46:805–822
Carles C, Bies-Etheve N, Aspart L, Léon-Kloosterziel K, Koornneef M, Echeverria M, Delseny M (2002) Regulation of Arabidopsis thaliana Em genes: role of ABI5. Plant J 30:373–383
Chibani K, Ali-Rachedi S, Job C, Job D, Jullien M, Grappin P (2006) Proteomic analysis of seed dormancy in Arabidopsis. Plant Physiol 142:1493–1510
Comai L, Harada J (1990) Transcriptional activities in dry seed nuclei indicate the timing of the transition from embryogenesis to germination. Proc Natl Acad Sci USA 87:2671–2674
Comai L, Dietrich R, Maslyar D, Baden C, Harada J (1989) Coordinate expression of transcriptionally regulated isocitrate lyase and malate synthase genes in Brassica napus L. Plant Cell 1:293–300
Dalal M, Tayal D, Chinnusamy V, Bansal K (2009) Abiotic stress and ABA-inducible Group 4 LEA from Brassica napus plays a key role in salt and drought tolerance. J Biotechnol 139:137–145
Debeaujon I, Léon-Kloosterziel K, Koornneef M (2000) Influence of the testa on seed dormancy, germination, and longevity in Arabidopsis. Plant Physiol 122:403–414
Delouche JC, Baskin CC (1973) Accelerated aging techniques for predicting the relative storability of seed lots. Seed Sci Technol 1:427–452
Dure L, Waters L (1965) Long-lived messenger RNA: evidence from cotton seed germination. Science 147:410–412
Edwards R, Dixon D, Walbot V (2000) Plant glutathione S-transferases: enzymes with multiple functions in sickness and in health. Trends Plant Sci 5:193–198
Finkelstein R, Lynch T (2000) The Arabidopsis abscisic acid response gene ABI5 encodes a basic leucine zipper transcription factor. Plant Cell 12:599–609
Finkelstein R, Wang M, Lynch T, Rao S, Goodman H (1998) The Arabidopsis abscisic acid response locus ABI4 encodes an APETALA 2 domain protein. Plant Cell 10:1043–1054
Fujita M, Fujita Y, Maruyama K, Seki M, Hiratsu K, Ohme-Takagi M, Tran L, Yamaguchi-Shinozaki K, Shinozaki K (2004) A dehydration-induced NAC protein, RD26, is involved in a novel ABA-dependent stress-signaling pathway. Plant J 39:863–876
Fujiwara T, Nambara E, Yamagishi K, Goto D, Naito S (2002) Storage proteins. The arabidopsis book, pp 1–12
Gallardo K, Job C, Groot S, Puype M, Demol H, Vandekerckhove J, Job D (2001) Proteomic analysis of arabidopsis seed germination and priming. Plant Physiol 126:835–848
Gallardo K, Job C, Groot S, Puype M, Demol H, Vandekerckhove J, Job D (2002) Proteomics of Arabidopsis seed germination. A comparative study of wild-type and gibberellin-deficient seeds. Plant Physiol 129:823–837
Germain V, Rylott E, Larson T, Sherson S, Bechtold N, Carde J, Bryce J, Graham I, Smith S (2001) Requirement for 3-ketoacyl-CoA thiolase-2 in peroxisome development, fatty acid beta-oxidation and breakdown of triacylglycerol in lipid bodies of Arabidopsis seedlings. Plant J 28:1–12
Giraudat J, Hauge B, Valon C, Smalle J, Parcy F, Goodman H (1992) Isolation of the Arabidopsis ABI3 gene by positional cloning. Plant Cell 4:1251–1261
Gonzalez A, Mendenhall J, Huo Y, Lloyd A (2009) TTG1 complex MYBs, MYB5 and TT2, control outer seed coat differentiation. Dev Biol 325:412–421
Graham I (2008) Seed storage oil mobilization. Annu Rev Plant Biol 59:115–142
Hayashi H, De Bellis L, Yamaguchi K, Kato A, Hayashi M, Nishimura M (1998) Molecular characterization of a glyoxysomal long chain acyl-CoA oxidase that is synthesized as a precursor of higher molecular mass in pumpkin. J Biol Chem 273:8301–8307
Hirai YM, Fujiwara T, Awazuhara M, Kimura T, Noji M, Saito K (2003) Global expression profiling of sulfur-starved Arabidopsis by DNA microarray reveals the role of O-acetyl-L-serine as a general regulator of gene expression in response to sulfur nutrition. Plant J 33:651–663
Hughes D, Galau G (1989) Temporally modular gene expression during cotyledon development. Genes Dev 3:358–369
Hughes D, Galau G (1991) Developmental and environmental induction of Lea and LeaA mRNAs and the postabscission program during embryo culture. Plant Cell 3:605–618
Hundertmark M, Hincha D (2008) LEA (late embryogenesis abundant) proteins and their encoding genes in Arabidopsis thaliana. BMC Genomics 9:118
James DJ, Lim E, Keller J, Plooy I, Ralston E, Dooner H (1995) Directed tagging of the Arabidopsis FATTY ACID ELONGATION1 (FAE1) gene with the maize transposon activator. Plant Cell 7:309–319
Jensen M, Hagedorn P, de Torres-Zabala M, Grant M, Rung J, Collinge D, Lyngkjaer M (2008) Transcriptional regulation by an NAC (NAM-ATAF1, 2-CUC2) transcription factor attenuates ABA signalling for efficient basal defence towards Blumeria graminis f. sp. hordei in Arabidopsis. Plant J 56:867–880
Jolivet P, Roux E, D’Andrea S, Davanture M, Negroni L, Zivy M, Chardot T (2004) Protein composition of oil bodies in Arabidopsis thaliana ecotype WS. Plant Physiol Biochem 42:501–509
Katavic V, Agrawal G, Hajduch M, Harris S, Thelen J (2006) Protein and lipid composition analysis of oil bodies from two Brassica napus cultivars. Proteomics 6:4586–4598
Kim S, Ma J, Perret P, Li Z, Thomas T (2002) Arabidopsis ABI5 subfamily members have distinct DNA-binding and transcriptional activities. Plant Physiol 130:688–697
Kim Y, Kim S, Park J, Park H, Lim M, Chua N, Park C (2006) A membrane-bound NAC transcription factor regulates cell division in Arabidopsis. Plant Cell 18:3132–3144
Kimura M, Yamamoto Y, Seki M, Sakurai T, Sato M, Abe T, Yoshida S, Manabe K, Shinozaki K, Matsui M (2003) Identification of Arabidopsis genes regulated by high light-stress using cDNA microarray. Photochem Photobiol 77:226–233
Kotak S, Vierling E, Bäumlein H, von Koskull-Döring P (2007) A novel transcriptional cascade regulating expression of heat stress proteins during seed development of Arabidopsis. Plant Cell 19:182–195
Kushiro T, Okamoto M, Nakabayashi K, Yamagishi K, Kitamura S, Asami T, Hirai N, Koshiba T, Kamiya Y, Nambara E (2004) The Arabidopsis cytochrome P450 CYP707A encodes ABA 8’-hydroxylases: key enzymes in ABA catabolism. EMBO J 23:1647–1656
Leubner-Metzger G (2005) Beta-1, 3-Glucanase gene expression in low-hydrated seeds as a mechanism for dormancy release during tobacco after-ripening. Plant J 41:133–145
Leung J, Merlot S, Giraudat J (1997) The Arabidopsis ABSCISIC ACID-INSENSITIVE2 (ABI2) and ABI1 genes encode homologous protein phosphatases 2C involved in abscisic acid signal transduction. Plant Cell 9:759–771
Liu P, Koizuka N, Martin R, Nonogaki H (2005) The BME3 (Blue Micropylar End 3) GATA zinc finger transcription factor is a positive regulator of Arabidopsis seed germination. Plant J 44:960–971
Liu Y, Shi L, Ye N, Liu R, Jia W, Zhang J (2009) Nitric oxide-induced rapid decrease of abscisic acid concentration is required in breaking seed dormancy in Arabidopsis. New Phytol 183:1030–1042
Lopez-Molina L, Chua N (2000) A null mutation in a bZIP factor confers ABA-insensitivity in Arabidopsis thaliana. Plant Cell Physiol 41:541–547
Lopez-Molina L, Mongrand S, Chua N (2001) A postgermination developmental arrest checkpoint is mediated by abscisic acid and requires the ABI5 transcription factor in Arabidopsis. Proc Natl Acad Sci USA 98:4782–4787
Lu P, Chen N, An R, Su Z, Qi B, Ren F, Chen J, Wang X (2007) A novel drought-inducible gene, ATAF1, encodes a NAC family protein that negatively regulates the expression of stress-responsive genes in Arabidopsis. Plant Mol Biol 63:289–305
Marcus A, Feeley J (1964) Activation of protein synthesis in the imbibition phase of seed germination. Proc Natl Acad Sci USA 51:1075–1079
Matakiadis T, Alboresi A, Jikumaru Y, Tatematsu K, Pichon O, Renou J, Kamiya Y, Nambara E, Truong H (2009) The Arabidopsis abscisic acid catabolic gene CYP707A2 plays a key role in nitrate control of seed dormancy. Plant Physiol 149:949–960
Nakabayashi K, Okamoto M, Koshiba T, Kamiya Y, Nambara E (2005) Genome-wide profiling of stored mRNA in Arabidopsis thaliana seed germination: epigenetic and genetic regulation of transcription in seed. Plant J 41:697–709
Nakashima K, Fujita Y, Katsura K, Maruyama K, Narusaka Y, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2006) Transcriptional regulation of ABI3- and ABA-responsive genes including RD29B and RD29A in seeds, germinating embryos, and seedlings of Arabidopsis. Plant Mol Biol 60:51–68
Nambara E, Marion-Poll A (2005) Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol 56:165–185
Okamoto M, Kuwahara A, Seo M, Kushiro T, Asami T, Hirai N, Kamiya Y, Koshiba T, Nambara E (2006) CYP707A1 and CYP707A2, which encode ABA 8’-hydroxylases, are indispensable for a proper control of seed dormancy and germination in Arabidopsis. Plant Physiol 141:97–107
Okamoto M, Tatematsu K, Matsui A, Morosawa T, Ishida J, Tanaka M, Endo TA, Mochizuki Y, Toyoda T, Kamiya Y, Shinozaki S, Nambara E, Seki M (2010) Genome-wide analysis of endogenous abscisic acid-mediated transcription in dry and imbibed seeds of Arabidopsis using tiling arrays. Plant J (in press)
Oñate-Sánchez L, Vicente-Carbajosa J (2008) DNA-free RNA isolation protocols for Arabidopsis thaliana, including seeds and siliques. BMC Res Notes 1:93
Oracz K, El-Maarouf Bouteau H, Farrant J, Cooper K, Belghazi M, Job C, Job D, Corbineau F, Bailly C (2007) ROS production and protein oxidation as a novel mechanism for seed dormancy alleviation. Plant J 50:452–465
Preston J, Tatematsu K, Kanno Y, Hobo T, Kimura M, Jikumaru Y, Yano R, Kamiya Y, Nambara E (2009) Temporal expression patterns of hormone metabolism genes during imbibition of Arabidopsis thaliana seeds: A comparative study on dormant and non-dormant accessions. Plant Cell Physiol 50:1786–1800
Rajjou L, Gallardo K, Debeaujon I, Vandekerckhove J, Job C, Job D (2004) The effect of alpha-amanitin on the Arabidopsis seed proteome highlights the distinct roles of stored and neosynthesized mRNAs during germination. Plant Physiol 134:1598–1613
Rajjou L, Lovigny Y, Job C, Belghazi M, Groot S, Job D (2007) Seed quality and germination. Seeds: biology, development and ecology. pp (324–332). In Adkins SW, Ashmore SE, Navie SC (eds) CABI book, Recent advance in seed biology, 8th international workshop on seeds
Rajjou L, Lovigny Y, Groot S, Belghazi M, Job C, Job D (2008) Proteome-wide characterization of seed aging in Arabidopsis: a comparison between artificial and natural aging protocols. Plant Physiol 148:620–641
Seki M, Narusaka M, Ishida J, Nanjo T, Fujita M, Oono Y, Kamiya A, Nakajima M, Enju A, Sakurai T, Satou M, Akiyama K, Taji T, Yamaguchi-Shinozaki K, Carninci P, Kawai J, Hayashizaki Y, Shinozaki K (2002) Monitoring the expression profiles of 7000 Arabidopsis genes under drought, cold and high-salinity stresses using a full-length cDNA microarray. Plant J 31:279–292
Siloto R, Findlay K, Lopez-Villalobos A, Yeung E, Nykiforuk C, Moloney M (2006) The accumulation of oleosins determines the size of seed oilbodies in Arabidopsis. Plant Cell 18:1961–1974
Tatematsu K, Nakabayashi K, Kamiya Y, Nambara E (2008) Transcription factor AtTCP14 regulates embryonic growth potential in Arabidopsis thaliana. Plant J 53:42–52
Todd J, Post-Beittenmiller D, Jaworski J (1999) KCS1 encodes a fatty acid elongase 3-ketoacyl-CoA synthase affecting wax biosynthesis in Arabidopsis thaliana. Plant J 17:119–130
Wagner U, Edwards R, Dixon D, Mauch F (2002) Probing the diversity of the Arabidopsis glutathione S-transferase gene family. Plant Mol Biol 49:515–532
Waters E, Nguyen S, Eskandar R, Behan J, Sanders-Reed Z (2008) The recent evolution of a pseudogene: diversity and divergence of a mitochondria-localized small heat shock protein in Arabidopsis thaliana. Genome 51:177–186
Wise M (2003) LEAping to conclusions: a computational reanalysis of late embryogenesis abundant proteins and their possible roles. BMC Bioinformatics 4:52
Yamagishi K, Tatematsu K, Yano R, Preston J, Kitamura S, Takahashi H, McCourt P, Kamiya Y, Nambara E (2009) CHOTTO1, a double AP2 domain protein of Arabidopsis thaliana, regulates germination and seedling growth under excess supply of glucose and nitrate. Plant Cell Physiol 50:330–340
Yang P, Li X, Wang X, Chen H, Chen F, Shen S (2007) Proteomic analysis of rice (Oryza sativa) seeds during germination. Proteomics 7:3358–3368
Yephremov A, Wisman E, Huijser P, Huijser C, Wellesen K, Saedler H (1999) Characterization of the FIDDLEHEAD gene of Arabidopsis reveals a link between adhesion response and cell differentiation in the epidermis. Plant Cell 11:2187–2201
Yoshiyama K, Conklin P, Huefner N, Britt A (2009) Suppressor of gamma response 1 (SOG1) encodes a putative transcription factor governing multiple responses to DNA damage. Proc Natl Acad Sci U S A (in press)
Zhang Y, Cheng Y, Qu N, Zhao Q, Bi D, Li X (2006) Negative regulation of defense responses in Arabidopsis by two NPR1 paralogs. Plant J 48:647–656
Acknowledgments
Authors acknowledge Drs. George Stamatiou and Danielle Vidaurre (University of Toronto) for their critical reading of this manuscript and Professor Naoto Kawakami (Meiji University) for providing the tt8 mutants. This work is supported by NSERC Discovery grant (to E.N.).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Kimura, M., Nambara, E. Stored and neosynthesized mRNA in Arabidopsis seeds: effects of cycloheximide and controlled deterioration treatment on the resumption of transcription during imbibition. Plant Mol Biol 73, 119–129 (2010). https://doi.org/10.1007/s11103-010-9603-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-010-9603-x