Abstract
A reverse-genetics approach was applied to identify genes involved in Tomato yellow leaf curl virus (TYLCV) resistance, taking advantage of two tomato inbred lines from the same breeding program—one susceptible (S), one resistant (R—that used Solanum habrochaites as the source of resistance. cDNA libraries from inoculated and non-inoculated R and S plants were compared, postulating that genes preferentially expressed in the R line may be part of the network sustaining resistance to TYLCV. Further, we assumed that silencing genes located at important nodes of the network would lead to collapse of resistance. Approximately 70 different cDNAs representing genes preferentially expressed in R plants were isolated and their genes identified by comparison with public databases. A Permease I-like protein gene encoding a transmembranal transporter was further studied: it was preferentially expressed in R plants and its expression was enhanced several-fold following TYLCV inoculation. Silencing of the Permease gene of R plants using Tobacco rattle virus-induced gene silencing led to loss of resistance, expressed as development of disease symptoms typical of infected susceptible plants and accumulation of large amounts of virus. Silencing of another membrane protein gene preferentially expressed in R plants, Pectin methylesterase, previously shown to be involved in Tobacco mosaic virus translocation, did not lead to collapse of resistance of R plants. Thus, silencing of a single gene can lead to collapse of resistance, but not every gene preferentially expressed in the R line has the same effect, upon silencing, on resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tomato yellow leaf curl virus (TYLCV) is a geminivirus (genus Begomovirus, family Geminiviridae) transmitted by the whitefly Bemisia tabaci (Czosnek 2007). It possesses a single circular single-stranded DNA genomic component (Navot et al. 1991) encoding two genes on the viral strand and four on the complementary strand (Gronenborn 2007). TYLCV threatens tomato production worldwide. While the domesticated tomato Solanum lycopersicum is susceptible to the virus, natural sources of resistance have been found in several wild tomato species such as S. chilense, S. peruvianum, S. pimpinellifolium and S. habrochaites (Vidavski 2007). Breeding for resistance consists of introgressing these resistant traits in the cultivated tomato (Pilowsky and Cohen 1990). As a result, the resistant tomato contains chromosomal fragments from the wild species on a background of the domesticated tomato, identifiable with polymorphic DNA markers (Ji et al. 2007). Currently five different resistance loci (Ty-1 through Ty-5) have been identified. These loci originate from different wild tomato accessions and possess various modes of TYLCV resistance (Agrama and Scott 2006; Anbinder et al. 2009; Chagué et al. 1997; Ji et al. 2009; Zamir et al. 1994.
Plant defense responses to biotic and abiotic stresses are regulated by different cross-communicating signaling pathways (Baker et al. 1997; Dong 2001; Gilliland et al. 2006; Kunkel and Brooks 2002; McCarty and Chory 2000). These gene and protein networks have been revealed by large-scale protein-protein interaction studies, usually using the yeast two-hybrid system (Giot et al. 2003), and by microarray analyses (Eisen et al. 1998). Likewise, plant resistance to viruses may be described as the outcome of interconnecting gene networks and signaling pathways leading to inhibition of virus replication and/or movement, and in the induction of small molecules that mediate the activation of resistance genes (Culver and Padmanabhan 2007). The establishment of a systemic begomoviral infection is mediated by interactions between viral and host proteins (Castillo et al. 2003, 2004; Hanley-Bowdoin et al. 2004; Selth et al. 2005) which interfere with host-cell proliferation (Gutierrez 2000) and overwhelm host defense mechanisms (Chellappan et al. 2004). The molecular and biochemical mechanisms underlying natural resistance to TYLCV are unknown (Gorovits et al. 2007). None of the genes conferring resistance to this virus have been isolated or characterized.
Describing the networks sustaining resistance to TYLCV and discovering the participating genes are of primary importance to understanding and combating TYLCV disease. To achieve this goal, we took advantage of two inbred tomato lines issued from the same breeding program that used S. habrochaites as a source of resistance: the TYLCV-susceptible line 906-4 and the TYLCV-resistant line 902, respectively, designated S and R hereafter (Vidavski and Czosnek 1998). Upon whitefly-mediated inoculation of TYLCV, the R line remains symptomless and contains barely detectable amounts of virus, while the S line presents the typical disease symptoms of stunting, leaf yellowing and leaf curling, and contains large amounts of virus. None of the polymorphic DNA markers tagging resistance to TYLCV from S. chilense (Ji et al. 2009; Zamir et al. 1994) or to Tomato leaf curl virus (TLCV) from S. habrochaites (Hanson et al. 2000) have been found to be linked with resistance of line 902 (unpublished results).
Postulating that genes involved in resistance will be expressed at higher levels in R vs. S plants, we compared the trancriptomes of R and S tomato lines, before and after inoculation. This approach did not provide any significant results (URLs http://www.tigr.org/tdb/potato/images/_descr/56.pdf; http://www.tigr.org/tigr-scripts/tdb/sol/study/sol_study_hybs.pl?study=56&user=&pass=&sort=id&order=asc). We therefore took a reverse-genetics approach in the current study based on virus-induced gene silencing (VIGS), a technique which has been used to discover gene networks in plants (Briggs and Singer 2005; Robertson 2004; Wellmer and Riechmann 2005) and other organisms (Lee et al. 2008). We compared cDNA libraries from R and S plants, postulating that those genes that are preferentially expressed in the R line may be part of the gene network sustaining resistance to TYLCV. In addition, we assumed that if those genes are located at important nodes in the network, resistance will collapse upon silencing. This approach proved fertile. From the ca. 300,000 clones assayed, 69 transcripts were found to represent genes preferentially expressed in R plants. Silencing of a Permease I-like protein gene, which is preferentially expressed in R plants and upregulated upon TYLCV inoculation, led to the appearance of disease symptoms similar to those in infected S plants, and in the early accumulation of large amounts of virus.
Materials and methods
Plants
Two inbred tomato lines, both issued from a breeding program aimed at introgressing resistance to TYLCV from S. habrochaites, were used (Vidavski and Czosnek 1998): line 902 is resistant to the virus (R) while line 906-4 is susceptible (S). The pedigree of the lines is shown in Fig. 1 of Vidavski and Czosnek (1998). Lines R and S can be distinguished by a single nucleotide polymorphism (SNP) found in a MnuI restriction site in the intron of an hsp70gene (Gorovits and Czosnek 2007): the SNP is contained in a DNA fragment which is PCR-amplified using the primers HSP17F (5′-GTCGCCATGAATCCTATTAACACCG-3′) and HSP678R (5′-CCCAGTTTGATGTCA CTCTGTAC-3′); the PCR products are incubated with MnuI, and the amplicon from the R plants is cut by the enzyme while that of the S plants is not. The two lines present horticultural characteristics of S. lycopersicum and produce 80–120 g of red fruit. Resistance in the R line is not tagged by the DNA polymorphic markers associated with TYLCV/TLCV resistance (Ji et al. 2007) from S. chilense and S. habrochaites (unpublished).
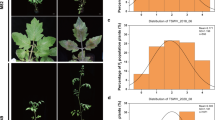
a TYLCV-infected susceptible (S) and resistant (R) tomato plants in the field; typical symptoms in S plants (S’) and symptomless appearance of R plants (R’). b Identification of R and S genotypes using a SNP in the hsp70 intron; the R plants contain a MunI site in the intron amplicon while the S plants do not; actin DNA amplicon was used as a reference. c Isolation of transcripts of genes preferentially expressed in inoculated R (Ri) tomato plants. Replicas of plated phages from a Ri cDNA library were sequentially hybridized with probes consisting of cDNA libraries from infected Ri and Si plants; the autoradiograms were superimposed to identify R-specific clones. Eleven Ri-specific phages were subjected to PCR and sequentially hybridized with probes consisting of Ri cDNA library and Si cDNA library. M: 100-bp ladder molecular weight markers. The red arrow points to the DNA fragment encoding the Permease I-like protein gene
Whitefly-mediated inoculation of R and S plants with TYLCV
TYLCV was maintained in tomato plants (cv. Daniella). Whiteflies, B. tabaci B biotype, were reared on cotton plants as described previously (Zeidan and Czosnek 1991). All experiments were conducted in insect-proof wooden cages kept at 24–27°C in an insect-proof growth chamber. TYLCV was acquired by whiteflies during a 48-h acquisition access period on TYLCV-infected tomato plants at the 7- to 10-leaf stage (6 weeks after whitefly-mediated inoculation). The viruliferous insects were then caged with R and S tomato plants at their 4- to 6-leaf stage for a 3-day-long inoculation access period (approximately 30 insects per plant). Plants were mock-inoculated with a similar number of non-viruliferous whiteflies for similar inoculation-access periods. The insects were treated with Imidacloprid and the plants were grown in an insect-proof greenhouse maintained at 18–24°C, with an 8/16 h dark/light regimen.
Preparation of cDNA libraries from TYLCV-inoculated and non-inoculated R and S plants
Thirty tomato plants from the S and R genotypes were inoculated with TYLCV (and 30 were mock-inoculated). The two youngest true leaves of three randomly chosen plants were collected 1, 3, 5 and 7 days after inoculation and RNA was extracted using Tri-Reagent according to the manufacturer’s directions (Molecular Research Center, USA). Equal amounts of RNA from each time point were pooled. cDNA libraries from R and S plants were prepared using Lambda ZAP-CMV XR library construction kit according to the manufacturer’s instructions (Stratagene, USA). Briefly, mRNA was purified and cDNA was prepared using the supplied primers. The cDNA was ligated to adaptors provided by the kit, digested with XhoI and EcoRI and cloned into the Lambda Zap-CMV XR vector. The recombinant vector was packaged in bacteriophage λ using Gigapack III gold packaging extract (Stratagene). Escherichia coli XL1-Blue MRF cells infected with the phages were plated at high density on NZY-Agar, and incubated for 8 h at 37°C. Replicas of the plaques were made on nitrocellulose Hybond-N filters (Amersham Biosciences, USA) and used for hybridization.
Screening of cDNA libraries, display of cDNA clones preferentially expressed in R plants
Differential screens were performed to identify genes preferentially expressed in R plants. Screens involved libraries from non-infected S plants vs. non-infected R plants (So vs. Ro), infected S plants vs. infected R plants (Si vs. Ri), So vs. Ri, and Si vs. Ro. About 70,000 clones from each cDNA library were plated and screened. For example, to isolate those clones that represented genes preferentially expressed in Ri vs. Si libraries, phages containing cDNAs of the Ri library were plated and the replicas were sequentially hybridized with probes derived from the Ri cDNA library followed by probes derived from the Si cDNA library. The probes were prepared as follows: the cDNA inserts from aliquots of the Ri and Si libraries (with approximately the same titer) were labeled with digoxigenin-11-dUTP (DIG) during PCR according to the manufacturer’s directions (Roche, USA) using the primer pair AS31R/AS51F designed to avoid contamination by vector sequences (AS31R: 5′-CTCGAGTTTTTTTTTTTTTTTTTT-3′; AS51F: 5′-CGGGCT GCAGGAATTC-3′). Plaques that hybridized solely with Ri probes were isolated for further analysis. The screening was repeated three times.
Gene ontology (GO) analysis of genes preferentially expressed in R plants
Gene ontology analysis was based on the DFCI Tomato Gene Index (TIGR) (http://compbio.dfci.harvard.edu/tgi/cgi-bin/tgi/Blast/index.cgi) database, the Solanum Genome Project (www.sgn.cornell.edu/tools/blast/), the European Bioinformatics Institute (EBI) database (www.ebi.ac.uk), and the Gene Ontology website (www.geneontology.org).
Gene silencing using VIGS vectors
Fragments of a Permease I-like protein gene (nt 738–932, TC204704) or of a Pectin methylesterase gene (nucleotides 553–752, TC212221) were T/A cloned into a pDrive vector (Qiagen, USA). The gene fragments were excised from the vector using XbaI and KpnI and ligated to the TRV RNA2 vector (Liu et al. 2002) using the same enzymes, resulting in the silencing vectors TRV-Perm and TRV-Pect, respectively. The plasmid was introduced into Agrobacterium LB4404 cells by electroporation. The agrobacteria containing TRV-Perm or TRV-Pect and TRV RNA1 were cultured in YEB medium for 48 h at 28°C. A mixture of TRV RNA1 and TRV-Perm (or TRV-Pect) was introduced into 30 R and S tomato seedlings at the 6- to 8-leaf stage by agroinoculation. Five days later, 20 agroinfected plants were inoculated with TYLCV for a 3-day inoculation period as described above (ten control plants were caged with a similar number of non-viruliferous insects). Each silencing experiment was repeated at least three times and samples were analyzed in triplicate.
PCR and semi-quantitative PCR analyses of Permease I-like protein and Pectin methylesterase transcripts
A tomato leaflet was taken from the two youngest true leaves of each plant. DNA was extracted from the two pooled leaflets as described (Bernatzky and Tanksley 1986), and RNA was prepared from these samples using the Tri-Reagent method (Sigma–Aldrich, USA). For each PCR and PCR-related experiment, the reactions were conducted in triplicate with three different plants (three technical repeats and three biological repeats). A 398-bp fragment corresponding to nt 530–928 of the TYLCV coat protein gene (GenBank accession number ×15656) was amplified using the primer pair TY530F (5′-ATTGGGCTGTTTCCATAGGGC-3′) and TY928R (5′-CACACGGATGGGAAATAC TT-3′). A 205-bp fragment corresponding to nt 1,450–1,655 of a tomato Permease I-like protein gene (TC204704) was amplified using the primer pair PERMF1450 (5′-TTAACGTGCCATTCTCATCG-3′) and PERMR1655 (5′-CACACGGATGGGAAATAC TT-3′). A 205-bp fragment corresponding to nt 553–752 of a tomato Pectin methylesterase gene (TC212221) was amplified using the primer pair PECTF553 (5′-TGGTGATTTTGCG CTGAAC) and PECTR752. A 180-bp fragment corresponding to nt 771–951 of a tomato β-actin gene (TC178617) was amplified using the primer pair ACTF771 (5′-GGAAAAGCTTGCCTATGTGG-3′) and ACTR951 (5′-CCTGCAGCTTC CATACCAAT-3′). The PCR mix contained (in a total volume of 25 μl): 100 ng tomato genomic DNA, 4 μl of a 25 mM mixture of four dNTPs, 2.5 μl Taq reaction buffer (10×), 1 U Taq DNA polymerase, 2.5 pmol of each primer. Cycling was as follows: initial denaturation for 3 min at 95°C followed by cycles consisting of 30 s at 95°C, 30 s at 55°C and 1 min at 72°C. In the semi-quantitative PCR, the reaction was stopped after various numbers of cycles, and 5 μl of the PCR products was subjected to electrophoresis in a 1% agarose gel in Tris-phosphate-EDTA buffer (TAE) and stained with ethidium bromide. In the semi-quantitative RT-PCR, cDNA was prepared using the EZ-first-strand cDNA synthesis kit (Biological Industries, Israel) according to the manufacturer’s directions. The reaction products were subjected to PCR as described above. Each measure was performed in triplicate for three independent biological repeats.
Quantitative real-time PCR analysis of Permease I-like protein and Pectin methylesterase transcripts
Quantitative real-time PCR was carried using the LightCycler 480 Real-Time PCR System (Roche). Probe and master PCR mix were provided by Roche after design using the Universal Probe Library (https://www.roche-applied-science.com/sis/rtpcr/upl/acenter.jsp?id=030000). The Universal Probe Library method was used to determine the quantity of desired nucleic acid. The commercial probe #27 (GCTGCCTG) was used to determine the amount of TYLCV and of tomato tubulin (BT012711); probe #48 (ACTGGGA) was used to determine the amount of permease I-like protein transcript. The primers used for TYLCV quantification were TYF2480 (5′-TCCACGTAGGTCTTGACATCTG-3′) and TYR2547 (5′-AGGTCAGCA CATTTCCAT CC-3′), amplifying a 68-nt DNA fragment, from nt 2,480–2,547. The primers used for permease quantification were PERF1236 (5′-TTTGCTG TCTGGCTTGTTTG-3′) and PERR1299 (5′-AAGCCCCACATTTTCAAC AG-3′), amplifying a 64-nt DNA fragment. Tomato β-tubulin (BT012711) was quantified using the primers TUBF344 (5′-TGACGAAGTCAGGACAGGAA-3′) and TUBR417 (5′-CTGCATCTTCTTTGCCACTG-3′) producing a 74-nt-long amplicon. The reactions were performed in the LightCycler 480 system under the following cycling conditions: 10 min at 95°C, 45 cycles consisting of 10 s at 95°C, 60 s at 58°C. The results were analyzed using LightCycler 480 software provided by the manufacturer. All PCR experiments were repeated three times (three biological repeats and three technical repeats).
qrt-PCR and dot-blot hybridization of TRV RNA1 following co-inoculation with TRV RNA2-PDS and TRV RNA2-Perm
First-strand cDNA synthesis was performed using SuperScript III First-Strand Synthesis System for reverse transcription)-PCR (Invitrogen, UK) following the manufacturer’s protocol. TRV gene-specific primer 305 (5′-GGGCGTAATAACGCTTACG-3′) was used to prime the first-strand cDNA synthesis reaction as described in Holeva et al. (2006). TRV RNA1 cDNA was quantified using the primer pair RNA1-F (5′-CATGCTAACAAATTGCGAAAGC-3′) and RNA1-R (5′-CACAGACAAACCATCCAC AATTATTTT-3′) which produces an 87-bp amplicon (Holeva et al. 2006). The time course of TRV RNA1 accumulation was also followed by dot-blot hybridization: 2 μg of total RNA from young leaves of five pooled plants was spotted (2 μl) on nitrocellulose membranes. The membranes were hybridized with a TRV amplicon DIG-labeled by PCR using the TRV RNA1 cDNA as template and the primer pair RNA1-F and RNA1-R.
Results
Identification of genes preferentially expressed in R tomatoes
Upon TYLCV inoculation, R plants remain symptomless while S plants cease to grow and develop typical symptoms of leaf curling and yellowing (Fig. 1a). The rate of TYLCV accumulation is much slower in R than in S plants (Vidavski and Czosnek 1998). All plants used in this study were unequivocally identified as R or S by the presence or absence of a SNP in the MnuI site of a hsp70 gene intron (Fig. 1b).
A differential screen was performed to identify the genes preferentially expressed in R compared to S plants, hypothesizing that they may be involved in the establishment of the resistant phenotype. cDNA libraries from non-inoculated and inoculated R and S plants (Ro and Ri, So and Si, respectively) were obtained using RNA extracted from leaves up to 7 days after whitefly-mediated inoculation (or mock-inoculation). Four screens were performed: So vs. Ro, Si vs. Ri, So vs. Ri, and Si vs. Ro. The clones that hybridized preferentially or exclusively with the R probes (Ro and Ri) were isolated and sequenced. From the ca. 300,000 clones screened, approximately 90 different cDNA clones representing genes preferentially expressed in R plants (Ro and Ri) were isolated. The results were confirmed by PCR-amplification of the inserts of these phages (using vector-specific primers) followed by agarose gel electrophoresis, blotting and sequential hybridization with probes representing the entire R and S cDNA libraries. Figure 1c shows the analysis of 11 phages containing transcripts of genes preferentially expressed in Ri compared to Si plants. More than one PCR product was obtained with each sample because the plaques of interest were isolated together with their close neighbors, owing to the high-density plating. Nonetheless, bands representing the differentially expressed genes were clearly seen. These bands were excised from the gel and the eluted DNA was sequenced.
The cDNAs were identified by sequence homology with the TIGR and Solanum Gene Project databases and annotated accordingly. Table 1 shows a list of the 69 clones representing genes preferentially or exclusively expressed in R plants and their match with sequences in public databases. Only the cDNAs that have a match in the databases and present more than 60% identity with annotated genes were considered. Figure 2 shows the GO analysis of these genes. “Transferase activity” had the highest number of hits in the “Molecular function” category and “membrane” had the highest number of hits in the “Cellular component” category. We were therefore particularly interested in those genes expressed specifically in R plants encoding cytoplasmic membranal proteins which have transporter and signal-transduction activities as defined by the GO analysis, postulating that they were involved in the resistance gene network. To determine whether these genes actually do play a role in the network and whether they are located at critical crossroads, they were silenced in R plants and the phenotype of the treated plants (whether resistant or susceptible) was determined upon whitefly-mediated inoculation of TYLCV.
Gene ontology (GO) analyses of genes preferentially expressed in R vs. S plants. The genes are arranged according to their “cellular component” and “molecular function” attributions. A total of 69 sequences are shown which represent a continuous DNA stretch of more than 50 bp with more than 60% identity with annotated genes in the database. Clones with homologies to “unknown” sequences are omitted
We present here the analysis performed with two genes preferentially expressed in R plants: a Permease I-like protein gene and a Pectin methyltransferase gene. Silencing of the Permease I-like protein gene led to the collapse of resistance, while silencing of the Pectin methyltransferase gene did not.
TRV RNA accumulation in TRV-treated R plants
To establish the effectiveness of silencing in the tomato genotypes used, R and S plants were treated with a TRV vector that allows silencing of the Phytoene desaturase (PDS) reporter gene (Burch-Smith et al. 2006; Liu et al. 2002). The appearance of bleached areas in leaves of S and R plants about 20 days after treatment indicated that the tomato PDS reporter gene had been silenced (Fig. 3a). TRV treatment by itself did not change the resistance/susceptibility to TYLCV of the R and S tomato lines. S tomato plants inoculated with TYLCV during a period of 7–10 days after PDS silencing presented disease symptoms similar to those characteristic of inoculated untreated S plants, indicating that TRV did not offer cross-protection against TYLCV. Similarly, TYLCV inoculated PDS-silenced R plants lacked the typical TYLCV symptoms (not shown).
a Left leaflets of Ro plants with bleached areas resulting from Phytoene desaturase (PDS) gene silencing (Ro: TRV-PDS); right: Ro plants treated with the Permease I-like protein-silencing construct (Ro: TRV-Perm). b Time course of TRV RNA1 accumulation in Ro tomato plants following co-agroinoculation with the TRV RNA2-PDS (TRV-PDS) and TRV RNA2-Perm (TRV-Perm) constructs. The results were normalized to the RNA1 amounts found in the R plants 5 days after TRV inoculation. Bars represent the average of triplicate measures of five different R plants; the standard error is indicated. Dot-blot hybridization of 2 μg total RNA of these plants with a TRV RNA1 DIG-labeled probe
The Si vs. Ri library screen identified a 453-bp cDNA fragment identical to a tomato Permease I-like protein gene (99% homology with nt 730–1,183 of TC204704), which encodes a transmembranal protein involved in the transport of macromolecules (Swarup et al. 2004). The Permease I-like protein gene was preferentially expressed in Ri plants compared to Si plants (Fig. 1c, arrow). To silence this gene, a 185-bp fragment of the cDNA was inserted into RNA2 of the TRV VIGS vector (termed TRV-Perm) and was agroinoculated together with TRV RNA1 into R (and S) plantlets.
To estimate the effect of silencing of the Permease I-like protein gene on R tomato resistance, the silencing TRV RNAs need to be present before TYLCV inoculation (performed 7 days after TRV treatment) and onwards, during systemic infection. Figure 3b shows qrt-PCR analysis of TRV RNA1 accumulation in R plants following co-inoculation with TRV RNA2-PDS (TRV-PDS) and TRV RNA2-Perm (TRV-Perm). While undetectable 5 days after treatment, the amount of TRV RNA1 gradually increased up to 15 days after TRV-PDS treatment. The amount of TRV RNA1 steadily increased for at least 4 weeks after TRV-Perm inoculation. This analysis was confirmed by dot-blot hybridization. These results indicated that at the time of TYLCV inoculation of TRV-Perm-treated R plants, 7–10 days after TRV treatment, the silencing process was already in the expanding phase. Moreover, the silencing signal did not decrease with plant aging. As observed in Fig. 3b, levels of TRV on TRV-Perm-treated plants did not exceed those detected in TRV-PDS-treated plants. This result can support that TYLCV resistance breaking in TRV-Perm-treated plants (see below) cannot be associated with an indirect effect of altered (increased) accumulation of TRV owe to Permease I silencing, as TRV-PDS treated R-plants retained TYLCV-resistance.
Silencing of a Permease I-like protein gene in TYLCV-resistant plants leads to the collapse of resistance
A semi-quantitative RT-PCR analysis performed 4 weeks after sowing (4- to 6-leaf stage) indicated that the Permease I-like protein gene is also preferentially expressed in Ro plants compared to So plants (Fig. 4a). While it could not be detected in So plants, even after 35 cycles, a product was readily detected in Ro plants after 29 cycles. The 18S rRNA PCR product used as an internal control in the very same samples was conspicuous after 29 cycles in both tomato genotypes.
Semi-quantative (a, b) and quantitative (c) RT-PCR analyses of Permease I-like protein gene expression in Permease-silenced (TRV-Perm) R tomato lines. a Preferential expression of Permease I-like protein gene in untreated R plants (Ro: 0) compared to untreated So plants (So: 0); 18S ribosomal RNA was used as an internal control in the same samples. b Permease I-like protein gene expression in TRV-treated R plants (Ro: TRV-Perm) 10 days after treatment, compared to non-treated plants (Ro: 0); 18S ribosomal RNA was used as an internal control. c Relative amounts of Permease I-like protein gene transcripts in TRV-Perm-treated and non-treated Ro plants; quantitative RT-PCR was performed immediately before TRV treatment, 5 days after treatment, 10 days after treatment (3 days after TYLCV inoculation), 20 and 35 days after treatment. The amount of Permease RNA is relative to that of Ro plants immediately before TRV-Perm treatment (taken as 1). Tubulin RNA was used as a reference gene transcript for each of the plants analyzed. Bars represent the average of triplicate measures of three different plants; the standard error is indicated. d Susceptible-like phenotype of infected Permease I-like protein-silenced R tomato plants, 2 months after TYLCV inoculation; Ri: 0, leaflets of non-silenced TYLCV-infected R plants; Ri: TRV-Perm, leaflets of silenced TYLCV-infected R plants (see typical disease symptoms in silenced plant while the non-silenced plant remains symptomless; the magnitude of the disease symptoms are typical of infected S plants)
Seven days after TRV treatment (at the time of TYLCV inoculation), the relative amounts of Permease I-like protein transcript in TRV-Perm-treated and untreated Ro plants were compared by semi-quantitative RT-PCR (Fig. 4b). While Permease PCR products were conspicuous after 27 cycles in untreated Ro plants, four more cycles were necessary to detect products in the TRV-Perm-treated Ro plants. The 18S rRNA PCR product, used as control with these samples was already conspicuous after 21 amplification cycles in both treated and non-treated Ro plants. Hence, 7 days after TRV-Perm treatment, the amount of Permease I-like protein transcript was reduced by approximately 16-fold (taking into account that under our conditions, the amount of PCR product doubles at every cycle).
The amounts of Permease I-like protein transcript were estimated by qrt-PCR in TRV-Perm-treated and non-treated R plants, before and after TYLCV inoculation (performed 7 days after the silencing treatment) (Fig. 4c). The relative amount of Permease transcript immediately before TRV treatment (7 days before onset of virus inoculation) was considered as 1 and all values were related to this standard. At the end of the TYLCV inoculation period, the amount of Permease transcript in untreated Ri plants had increased approximately six fold, indicating that expression of the Permease I-like protein gene was induced by TYLCV infection. At this time, the relative amount of Permease RNA in silenced Ri plants was approximately 10% of that present in non-silenced Ri plants (approximately half the value before silencing). Twenty and 35 days after TRV-Perm treatment, a decrease in the amounts of Permease transcript in non-treated Ri plants was observed (relative amounts of 5 and 2, respectively), while the relative amounts of Permease RNA in treated Ri plants did not change significantly.
Silencing of the Permease I-like protein gene in Ri tomatoes was correlated with loss of TYLCV resistance expressed by the appearance of strong disease symptoms. Typical TYLCV symptoms appeared in all infected TRV-Perm-treated R plants, approximately 21 days after the end of the inoculation period, at about the same time as in Si plants (Fig. 4d). The magnitude of the symptoms (leaf yellowing, curling, plant stunting) in TRV-Perm-treated R plants increased with time and was as severe as that in Si plants (Fig. 1a). Concomitantly, the non-treated inoculated Ri plants remained symptomless. Treatment of S plants with TRV-Perm did not modify the timing or extent of symptom appearance (not shown).
Since TRV-Perm-treated plants developed TYLCV disease symptoms, the question of whether viral amounts in the treated R plants were similar to those found in the S plants was addressed. A semi-quantitative PCR analysis (Fig. 5a) conducted 20 days after the end of the inoculation period (30 days after TRV-Perm treatment) clearly showed that while virus-related PCR products were detected in untreated Ri plants after 35 cycles, viral DNA was readily detected in the TRV-Perm-treated Ri plants after 23 cycles. By comparison, viral DNA products were detected after 17 cycles in Si plants. Actin DNA, used as an internal reference, was detected in all samples (untreated Ri and Si, and treated Ri) after 26 cycles. Therefore, the TRV-Perm-treated Ri plants contained approximately 4,000 times more virus than the untreated Ri plants, but about 60 times less that the Si plants. These results confirmed that TRV-Perm treatment had overwhelmed the resistance of R plants.
Comparison of TYLCV amounts in virus-inoculated silenced (Ri: TRV-Perm) and untreated (Ri: 0) R plants, as estimated by semi-quantitative (a) and quantitative (b) PCR. a Left: TYLCV in untreated and TRV-Perm-treated Ri tomato, and in untreated S plant (Si: 0), 28 days post-virus inoculation; right: β-actin in the same plants. b Relative virus amounts in Si and Ri plants, and in three TRV-Perm-treated tomato plants (Ri1, Ri2, Ri3), 3 and 28 days post-TYLCV inoculation (dpi), i.e., 10 and 35 days after TRV-Perm treatment. The amounts of virus are relative to inoculated untreated R plants and were calculated using a standard curve of cloned TYLCV genome
These results were further confirmed by qPCR. The amounts of virus were determined 10 and 35 days after TRV-Perm treatment (3 and 28 days after the start of TYLCV inoculation, before and after the appearance of disease symptoms) and compared with non-treated Ri and Si plants. Figure 5b shows the analysis obtained with three representative TRV-Perm-treated, symptomatic plants (Ri1, Ri2 and Ri3). Three days after TYLCV inoculation, the three TRV-Perm-treated Ri plants contained 8–10 times more virus than the untreated Ri plants, and than the infected Si plants. Twenty-eight days after TYLCV inoculation, the Ri1 TRV-Perm-treated plant contained approximately 500 times more virus than non-treated Ri plants (approximately half the amount in Si plants), while the Ri2 plant contained approximately 15 times more virus than non-treated Ri plants. Interestingly, the symptomatic treated plant Ri3 contained approximated the same amount of virus as the non-treated symptomless Ri plant. By comparison, 3 days after the start of virus inoculation, Si plants contained approximately the same amount of virus as the Ri plants, but far less than the TRV-Perm-treated Ri plants. The fact that large amounts of viral DNA could be detected in TRV-Perm-treated Ri plants as early as 3 days after virus inoculation implies that Permease silencing abolished resistance before or at the onset of infection.
Silencing of a Pectin methylesterase gene preferentially expressed in R plants does not bring about collapse of resistance
In the So vs. Ro screen, several cDNAs representing genes encoding membrane proteins were identified. Among them, a 546-bp cDNA fragment was identified as a tomato Pectin methylesterase gene (97% homology with nt 473–1,017 of TC212221). Pectin methylesterase is a cell-wall protein involved in plant growth and development as well as in wound response (Micheli 2001). In addition, the protein is a host-cell receptor for Tobacco mosaic virus (TMV) (Dorokhov et al. 1999); it interacts with the TMV movement protein during infection (Chen et al. 2000), an essential step for TMV cell-to-cell movement (Chen and Citovsky 2003).
To determine whether silencing the Pectin methylesterase gene influences tomato resistance to TYLCV, a 199-bp fragment of its cDNA was inserted into RNA 2 of the TRV VIGS vector (producing TRV-Pect) and was agroinoculated together with TRV RNA1 into Ro and So plantlets. Seven days after TRV inoculation, the relative amounts of Pectin methylesterase transcript in TRV-Pect-treated and untreated Ro plants were compared by semi-quantitative RT-PCR (Fig. 6a). Pectin PCR products were detected after 29 cycles in untreated Ro plants, and after 33 cycles in TRV-Pect-treated Ro plants. Actin, used as an internal control, was detected after 27 cycles in both treated and non-treated Ro plants. These results indicated that 7 days after TRV-Pect treatment, expression of Pectin methylesterase was reduced by approximately 16-fold. At this time, the plants were inoculated with TYLCV. Twenty days after the end of inoculation, semi-quantitative PCR indicated similar amounts of TYLCV in TRV-Pect-treated and non-treated Ri plants (Fig. 6b). In both samples, TYLCV DNA was only detected after 35 cycles, while actin DNA in the same samples was detected after 29 cycles. The TRV-Pect-treated Ri plants did not develop any symptoms for up to 2 months after virus inoculation and behaved similarly to non-treated Ri plants (Fig. 6c). These results indicated that contrary to silencing of the Permease I-like protein gene, which led to a drastic decrease in the level of TYLCV resistance (Figs. 4, 5), silencing of Pectin methylesterase has no effect on resistance to TYLCV infection. These results also indicated that silencing a gene encoding a membrane protein does not necessarily abolish the resistance of R plants.
Resistant plants in which the Pectin methylesterase gene has been silenced remain resistant to TYLCV inoculation. a Semi-quantitative RT-PCR-based comparison between untreated R plants (Ro: 0) and R plants following Pectin methylesterase gene silencing (Ro: TRV-Pect), 7 days after treatment; β-actin was used as an internal control. b Comparison of TYLCV amounts in untreated inoculated R plants (Ri: 0) and R plants following Pectin methylesterase gene silencing (Ri: TRV-Pect), 20 days after TYLCV inoculation (30 days after TRV treatment); β-actin was used as a control. c Leaves of R and S tomato plants 2 months after TYLCV inoculation; Si: 0 and Ri: 0 are susceptible and resistant plants, respectively; Ri: TRV-Pect is a R plant in which the Pectin methylesterase gene has been silenced
Discussion
Five major TYLCV resistance loci (Ty-1 through Ty-5) have been mapped using polymorphic DNA markers. Two semi-dominant loci from S. chilense (Ty-1 and Ty-3) mapped to the short arm of chromosome 6; resistance was achieved by the addition of several minor loci (Agrama and Scott 2006; Zamir et al. 1994). Another minor resistance locus from S. chilense (Ty-4) was mapped to the long arm of chromosome 3 (Ji et al. 2009). Ty-2, which originated from S. habrochaites, has a partly dominant effect; it was mapped to the long arm of chromosome 11 (Hanson et al. 2000). A locus linked to resistance from S. pimpinellifolium has been localized on the short arm of tomato chromosome 6 (Chagué et al. 1997). Recently a major locus linked to resistance from S. peruvianum (Ty-5) was localized to chromosome 4; four additional loci were mapped to chromosomes 1, 7, 9 and 11 (Anbinder et al. 2009; Friedmann et al. 1998). Resistance of line 902 (R) used in our studies was shown to be under the control of a major dominant locus and several modifiers (Vidavski and Czosnek 1998; Vidavski et al. 2008). None of the markers linked to the known Ty loci could account for resistance of line 902 (unpublished). TYLCV resistance genes have not been identified so far. The number of loci discovered so far and their specific contribution to resistance led us to postulate that, as in the case of many viruses (Goldbach et al. 2003; Murphy et al. 2001), resistance to TYLCV is the result of gene networks that respond to biochemical triggers induced by virus inoculation (Genoud and Métraux 1999).
Domestication of tomato from the wild and selection for high yield and good fruit quality has resulted in the loss of part of these networks, including many of the alleles conferring resistance to TYLCV. Breeding for resistance has therefore consisted of reconstituting at least part of the network sustaining resistance. To decipher the mechanisms establishing resistance to TYLCV in tomato, and to identify the genes involved, we took advantage of two inbred tomato lines: one susceptible (S), the other resistant (R) to the virus (Vidavski and Czosnek 1998). Resistance to stress involves many transcripts and gene networks (Cooper et al. 2003). Therefore we postulated that genes that are preferentially expressed in R plants upon TYLCV inoculation may be involved in the networks sustaining resistance. We also postulated that if these genes are directly or indirectly involved in resistance, we might be able to weaken the resistance of the R tomato genotype by silencing them. Accordingly, we screened cDNA libraries from S and R plants, before and after inoculation. The libraries were made up of RNA collected 1, 3, 5 and 7 days post-inoculation to represent gene expression at the early stages of infection and to avoid the secondary effects of infection-related cell damage that may occur with the appearance of symptoms (Michelson et al. 1997).
Gene ontology analysis (“cellular component” and “molecular function” attributions) of many of the genes preferentially expressed in R tomato plants annotated them as membranal, suggesting that the mechanism of resistance might involve virus entry in the cell and/or cell-to-cell trafficking. Indeed membranes are associated with distinct steps of the life cycle of animal DNA viruses (Miller and Krijnse-Locker 2008). Moreover, membrane proteins are involved in pathogenesis and RNA-silencing suppression. For example, the AC4 gene product of the East African cassava mosaic Cameroon virus (EACMCV), a bipartite whitefly-transmitted geminivirus, has been shown to be involved in pathogenesis and RNA-silencing suppression, and to bind preferentially to the plasma membrane as well as to cytosolic membranes, including the perinucleus (Fondong et al. 2007). Therefore, we were interested in identifying tomato genes encoding proteins that are associated with membranes.
The most attractive gene in this collection was a Permease I-like protein gene. Members of the permease family have ten predicted transmembrane helices (http://compbio.dfci.harvard.edu/tgi/cgi-bin/tgi/Domain_sum.pl). This gene is present in R as well as S plants. The sequences of the ca. 400-bp DNA amplicons obtained using R and S genomic DNA and primers derived from the cDNA clone presented 100% homology (not shown). The Permease I-like protein gene was preferentially expressed in inoculated as well as non-inoculated R plants (Figs. 1c, 4a), while it was undetectable in S plants. Moreover its expression was enhanced upon TYLCV inoculation (Fig. 4c). A literature search did not reveal any possible role of this gene in plant-virus interactions or plant resistance to pathogens. Our results suggest that this Permease I-like protein gene may be involved in the entry/exit of macromolecules (which may not be viral particles) or small signaling metabolites, as one of the components of the network underlying resistance.
To verify this hypothesis, we silenced the expression of the Permease I-like protein gene in R plants using the TRV system. TRV-based vectors allow systemic spread of viral transcripts harboring the targeted gene sequence and allow various levels of systemic silencing of the targeted gene (Benedito et al. 2004; Liu et al. 2002). The time course of silencing-signal accumulation (Fig. 3b) indicated that the amount of Permease transcript was already depleted at the time of TYLCV inoculation (Fig. 4c). Silencing of the Permease I-like protein gene led to a susceptible phenotype (Fig. 4d) that contained significantly higher amounts of viral DNA than the untreated plants (Fig. 5). Hence, silencing a single gene in R plants can lead to abolition of resistance.
A comparison of the amount of virus in S plants and in susceptible-like TRV-Perm-treated Ri plants revealed seemingly large amounts of TYLCV in the latter as early as 3 days postinoculation (Fig. 5b) at a time when the virus is barely detectable in S plants. These results indicated that Permease I-like protein silencing facilitated TYLCV invasion of the resistant tomato. At this time, the virus was able to multiply and spread, which it would be unable to do otherwise, and to establish itself in the Ri plant. Twenty-eight days after virus inoculation, all TRV-Perm-treated R tomato plants presented typical disease symptoms. In most, but not all plants, the amount of virus was higher than that present 3 days after inoculation (Fig. 5b). In some plants the high virus amounts present 3 days after inoculation decreased dramatically 25 days later (see plant Ri3, Fig. 5b). Nonetheless, these plants expressed full disease symptoms.
It is difficult to provide a simple model describing the role of the Permease I-like protein gene in the gene network and signaling pathways involved in resistance to TYLCV. The gene is preferentially expressed in R plants and its level of expression does not significantly change during plant aging. Inoculation of R plants with TYLCV led to a sixfold increase in the level of Permease expression already observed after the 3-days inoculation period, suggesting that the virus is involved in triggering Permease over-expression. While the levels of Permease transcripts remain high in the infected plant (Fig. 4c), the amounts of virus stay low (Fig. 5b) and the plant is symptomless (Fig. 4d). In Permease I-like protein silenced R plants, TYLCV inoculation did not trigger permease over-expression. Permease silencing was accompanied by accumulation of large amounts of virus detected immediately after the onset of inoculation (Fig. 5). By comparison, the amount of TYLCV in infected S plants and in non-silenced R plants was barely detectable at this time (Fig. 5). Early infection of Permease-silenced R plants led to the appearance of disease symptoms similar to those conspicuous in infected S plants (Figs. 1a, 4d). Therefore, we suggest that the Permease I-like protein genes acts at an early step of the cascade of events leading to resistance, perhaps inhibiting virus entry and replication in the permissive cell. Once this barrier is abolished, virus accumulates in large amounts and symptoms develop, suggesting that the defenses of the resistant plant have been overwhelmed.
We screened other cDNA libraries (e.g. So vs. Ro) and found other sets of genes that differentiate the two genotypes. Among them, we found a Pectin methylesterase gene preferentially expressed in R plants. Contrary to the Permease I-like protein product, the Pectin methylesterase gene product has a clear role in plant-virus interactions: it has been shown to be essential for TMV translocation (Chen and Citovsky 2003; Chen et al. 2000). Pectin methylesterase also plays a role in the silencing of foreign nucleic acid entering the cell (Dorokhov et al. 2006). Silencing the expression of the Pectin methylesterase gene in R plants did not bring about the collapse of TYLCV resistance (Fig. 6). The amounts of virus in the TRV-Pect-treated plants did not increase, remaining as low as those in untreated plants. Moreover, the TRV-Pect-treated R plants remained symptomless, even 2 months after TYLCV inoculation. These results indicate that the Pectin methylesterase gene is not directly involved in the establishment of the resistance phenotype. They also stress the specific, albeit largely unknown, role of the Permease I-like protein gene.
In ongoing research, we are silencing other genes preferentially expressed in R plants (infected or not) relative to their S plant counterparts. This wide-range screening and silencing of the detected genes may help delineate and understand the gene network involved in establishing resistance to TYLCV in R tomato, and may help determine whether these proteins interact with permease and whether they belong to the same gene cascade leading to resistance.
Interactions between genes and proteins underlie all biochemical processes in living organisms (Gavin et al. 2006; Giot et al. 2003; Ma et al. 2007). These interactions help describe functional cell signaling networks, with their positive and negative regulatory signals governing the rate of transcription of the different genes in the network (Brazhnik et al. 2002; Krishnamoorthy 2008), and can even help us understand plant responses to stress (Shinozaki and Yamaguchi-Shinozaki 2007) as well as animal pathological states (Jonsson et al. 2006). These networks may be quite extensive: in Arabidopsis for example, a network of at least 152 genes has been shown to be involved in managing reactive oxygen species (Mittler et al. 2004).
The various types of interactions between virus and host proteins in susceptible plants have been recently reviewed (Culver and Padmanabhan 2007). Such interactions occur during the many steps that lead to systemic infection and symptom development—the virus’s entry into permissive plant cells, replication, cell-to-cell and long-distance movement, induction and suppression of defense responses, appropriation of host physiology, and development of disease symptoms. The upstream (entry of virus into permissive cells) and downstream (following establishment of the virus) events leading to plant disease are unknown. Our knowledge of the interactions between geminiviruses and host proteins is limited to virus entry into host-cell nuclei (Krichevsky et al. 2006) and induction of host-cell division mediated by the interaction between the virus Rep protein and a plant retinoblastoma-related protein (Kong et al. 2000). The interaction between plant gene products and viral proteins in naturally resistant plants has been described for a limited number of systems (Kang et al. 2005).
References
Agrama HA, Scott JW (2006) Quantitative trait loci for Tomato yellow leaf curl virus and Tomato mottle virus resistance in tomato. J Am Soc Hortic Sci 131:267–272
Anbinder I, Reuveni M, Azari R, Paran I, Nahon S, Shlomo H, Chen L, Lapidot M, Levin I (2009) Molecular dissection of Tomato yellow leaf curl virus (TYLCV) resistance in tomato line TY172 derived from Solanum peruvianum. Theor Appl Gen (in press)
Baker B, Zambryski P, Staskawicz B, Dinesh-Kumar SP (1997) Signaling in plant-microbe interactions. Science 276:726–733
Benedito VA, Visser PB, Angenent GC, Krens FA (2004) The potential of virus-induced gene silencing for speeding up functional characterization of plant genes. Genet Mol Res 3:323–341
Bernatzky R, Tanksley SD (1986) Towards a saturated linkage map in tomato based on isozymes and random cDNA sequences. Genetics 112:887–898
Brazhnik PA, de la Fuente PM, Mendes P (2002) Gene networks: how to put the function in genomics. Trends Biotechnol 20:467–472
Briggs SP, Singer T (2005) Genetic networks. Plant Physiol 138:542–544
Burch-Smith T, Schiff M, Liu Y, Dinesh-Kumar SP (2006) Efficient virus-induced silencing in Arabidopsis. Plant Physiol 142:21–27
Castillo AG, Collinet D, Deret S, Kashoggi A, Bejarano ER (2003) Dual interaction of plant PCNA with geminivirus replication accessory protein (Ren) and viral replication protein (Rep). Virology 312:381–394
Castillo AG, Kong LJ, Hanley-Bowdoin L, Bejarano ER (2004) Interaction between a geminivirus replication protein and the plant sumoylation system. J Virol 78:2758–2769
Chagué V, Mercier JC, Guenard M, de Courcel A, Vedel F (1997) Identification of RAPD markers linked to a locus involved in quantitative resistance to TYLCV in tomato by bulked segregant analysis. Theor Appl Genet 95:671–677
Chellappan P, Vanitharani R, Fauquet CM (2004) Short interfering RNA accumulation correlates with host recovery in DNA virus-infected hosts, and gene silencing targets specific viral sequences. J Virol 78:7465–7477
Chen M-H, Citovsky V (2003) Systemic movement of a tobamovirus requires host cell pectin methylesterase. Plant J 35:386–392
Chen M-H, Sheng J, Hind G, Handa AK, Citovsky V (2000) Interaction between the tobacco mosaic virus movement protein and host cell pectin methylesterases is required for viral cell-to-cell movement. EMBO J 19:913–920
Cooper B, Clarke JD, Budworth P, Kreps J, Hutchison D, Park S, Guimil S, Dunn M, Luginbühl P, Ellero C, Goff SA, Glazebrook J (2003) A network of rice genes associated with stress response and seed development. Proc Natl Acad Sci USA 100:4945–4950
Culver JN, Padmanabhan MS (2007) Virus-induced disease: altering host physiology one interaction at a time. Annu Rev Phytopathol 45:221–243
Czosnek H (2007) Tomato yellow leaf curl virus disease: management, molecular biology, breeding for resistance. Springer, Dordrecht
Dong X (2001) Genetic dissection of systemic acquired resistance. Curr Opin Plant Biol 4:309–314
Dorokhov YL, Makinen KM, Frolova OY, Merits A, Kalkkinen N, Saarinen J, Atabekov JG, Saarma M (1999) A novel function for a ubiquitous plant enzyme pectin methylesterase: the host-cell receptor for the tobacco mosaic virus movement protein. FEBS Lett 461:223–228
Dorokhov YL, Frolova OY, Skurat EV, Ivanov PA, Gasanova TV, Sheveleva AS, Ravin NV, Makinen K, Klimyuk V, Skryabin KG, Gleba YY, Atabekov JG (2006) A novel function for a ubiquitous plant enzyme pectin methylesterase: the enhancer of RNA silencing. FEBS Lett 580:3872–3878
Eisen MB, Spellman PT, Brown PO, Botstein D (1998) Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA 95:14863–14868
Fondong VN, Reddy CRV, Lu C, Hankoua B, Felton C, Czymmek K, Achenjang F (2007) The consensus N-myristoylation motif of a geminivirus AC4 protein is required for membrane binding and pathogenicity. Mol Plant Microbe Interact 20:380–391
Friedmann M, Lapidot M, Cohen S, Pilowsky M (1998) A novel source of resistance to tomato yellow leaf curl virus exhibiting a symptomless reaction to viral infection. J Am Soc Hortic Sci 123:1004–1007
Gavin A-C, Aloy P, Grandi P et al (2006) Proteome survey reveals modularity of the yeast cell machinery. Nature 440:631–636
Genoud T, Métraux JP (1999) Crosstalk in plant cell signaling: structure and function of the genetic network. Trends Plant Sci 4:503–507
Gilliland A, Murphy AM, Wong CE, Carson RAJ, Carr JP (2006) Mechanisms involved in induced resistance to plant viruses. In: Tuzun S, Bent E (eds) Multigenic and induced systemic resistance in plants. Springer, Dordrecht, pp 335–359
Giot L, Bader JS, Brouwer C et al (2003) A protein interaction map of Drosophila melanogaster. Science 302:1727–1736
Goldbach R, Bucher E, Prins M (2003) Resistance mechanisms to plant viruses: an overview. Virus Res 92:207–212
Gorovits R, Czosnek H (2007) Biotic and abiotic stress responses in breeding tomato lines resistant and susceptible to Tomato yellow leaf curl virus. In: Czosnek H (ed) Tomato yellow leaf curl virus disease: management, molecular biology and breeding for resistance. Springer, Dordrecht, pp 223–237
Gorovits R, Akad F, Beery H, Vidavski F, Mahadav A, Czosnek H (2007) Expression of stress-response proteins upon whitefly-mediated inoculation of Tomato yellow leaf curl virus (TYLCV) in susceptible and resistant tomato plants. Mol Plant Microbe Interact 20:1376–1383
Gronenborn B (2007) The tomato yellow leaf curl virus: genome and function of its proteins. In: Czosnek H (ed) Tomato yellow leaf curl virus disease: management, molecular biology and breeding for resistance. Springer, Dordrecht, pp 67–84
Gutierrez C (2000) DNA replication and cell cycle in plants: learning from geminiviruses. EMBO J 19:792–799
Hanley-Bowdoin L, Settlage SB, Robertson D (2004) Reprogramming plant gene expression: a prerequisite to geminivirus DNA replication. Mol Plant Pathol 5:149–156
Hanson PM, Bernacchi D, Green S, Tanksley SD, Muniyappa V, Padmaja S, Chen HM, Kuo G, Fang D, Chen JT (2000) Mapping a wild tomato introgression associated with tomato yellow leaf curl virus resistance in a cultivated tomato line. J Am Soc Hortic Sci 125:15–20
Holeva R, Phillips MS, Neilson R, Brown DJF, Young V, Boutsika K, Blok VC (2006) Real-time PCR detection and quantification of vector trichodorid nematodes and Tobacco rattle virus. Mol Cell Probes 20:203–211
Ji Y, Scott JW, Hanson P, Graham E, Maxwell DP (2007) Sources of resistance, inheritance, and location of genetic loci conferring resistance to members of the tomato-infecting begomoviruses. In: Czosnek H (ed) Tomato yellow leaf curl virus disease: management, molecular biology and breeding for resistance. Springer, Dordrecht, pp 343–362
Ji Y, Scott JW, Maxwell DP (2009) Molecular mapping of Ty-4, a new Tomato yellow leaf curl virus resistance locus on chromosome 3 of tomato. J Amer Soc Hort Sci 134:281–288
Jonsson PF, Cavanna T, Zicha D, Bates PA (2006) Cluster analysis of networks generated through homology: automatic identification of important protein communities involved in cancer metastasis. BMC Bioinformatics 7:2
Kang B-C, Yeam I, Jahn MM (2005) Genetics of plant virus resistance. Annu Rev Phytopathol 43:581–621
Kong LJ, Orozco BM, Roe JL, Nagar S, Ou S, Feiler HS, Durfee T, Miller AB, Gruissem W, Robertson D, Hanley-Bowdoin L (2000) A geminivirus replication protein interacts with retinoblastoma through a novel domain to determine symptoms and tissue-specificity of infection in plants. EMBO J 19:3485–3495
Krichevsky A, Kozlovsky SV, Gafni Y, Citovsky V (2006) Nuclear import of plant viral proteins and genomes. Mol Plant Pathol 7:131–146
Krishnamoorthy S (2008) Receptor tyrosine kinase (RTK) mediated tyrosine phosphor-proteome from Drosophila S2 (ErbB1) cells reveals novel signaling networks. PLoS ONE 3:e2877
Kunkel BN, Brooks DM (2002) Cross talk between signaling pathways in pathogen defense. Curr Opin Plant Biol 5:325–331
Lee I, Lehner B, Crombie C, Wong W, Fraser AG, Marcotte EM (2008) A single gene network accurately predicts phenotypic effects of gene perturbation in Caenorhabditis elegans. Nat Genet 40:181–188
Liu Y, Schiff M, Dinesh-Kumar SP (2002) Virus-induced gene silencing in tomato. Plant J 31:777–786
Ma S, Gong Q, Bohnert HJ (2007) An Arabidopsis gene network based on the graphical Gaussian model. Genome Res 17:1614–1625
McCarty DR, Chory J (2000) Conservation and innovation in plant signaling pathways. Cell 103:201–209
Micheli F (2001) Pectin methylesterases: cell wall enzymes with important roles in plant physiology. Trends Plant Sci 6:414–419
Michelson I, Zeidan M, Zamski E, Zamir D, Czosnek H (1997) Localization of tomato yellow leaf curl virus (TYLCV) in susceptible and tolerant nearly isogenic tomato lines. Acta Hortic 447:407–414
Miller S, Krijnse-Locker J (2008) Modification of intracellular membrane structures for virus replication. Nat Rev Microbiol 6:363–374
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498
Murphy AM, Gilliland A, Wong CE, West J, Singh DP, Carr JP (2001) Signal transduction in resistance to plant viruses. Eur J Plant Pathol 107:121–128
Navot N, Pichersky E, Zeidan M, Zamir D, Czosnek H (1991) Tomato yellow leaf curl virus: a whitefly-transmitted geminivirus with a single genomic molecule. Virology 185:151–161
Pilowsky M, Cohen S (1990) Tolerance to tomato yellow leaf curl virus derived from Lycopersicon peruvianum. Plant Dis 74:248–250
Robertson D (2004) VIGS vectors for gene silencing: many targets, many tools. Annu Rev Plant Biol 55:495–519
Selth LA, Dogra SC, Rasheed MS, Healy H, Randles JW, Rezaian MA (2005) A NAC domain protein interacts with tomato leaf curl virus replication accessory protein and enhances viral replication. Plant Cell 17:311–325
Shinozaki K, Yamaguchi-Shinozaki K (2007) Gene networks involved in drought stress response and tolerance. J Exp Bot 58:221–227
Swarup R, Kargul J, Marchant A, Zadik D, Rahman A, Mills R, Yemm A, May S, Williams L, Millner P, Tsurumi S, Moore I, Napier R, Kerr ID, Bennett MJ (2004) Structure-function analysis of the presumptive Arabidopsis auxin permease AUX1. Plant Cell 16:3069–3083
Vidavski F (2007) Exploitation of resistance genes found in wild tomato species to produce resistant cultivars; pile up of resistance genes. In: Czosnek H (ed) Tomato yellow leaf curl virus disease: management, molecular biology, breeding for resistance. Springer, Dordrecht, pp 363–372
Vidavski F, Czosnek H (1998) Tomato breeding lines immune and tolerant to tomato yellow leaf curl virus (TYLCV) issued from Lycopersicon hirsutum. Phytopathology 88:910–914
Vidavski F, Czosnek H, Gazit S, Levy D, Lapidot M (2008) Pyramiding of genes conferring resistance to Tomato yellow leaf curl virus from different wild tomato species. Plant Breed 127:625–631
Wellmer F, Riechmann JL (2005) Gene network analysis in plant development by genomic technologies. Int J Dev Biol 49:745–759
Zamir D, Ekstein-Michelson I, Zakay Y, Navot N, Zeidan M, Sarffati M, Eshed Y, Harel E, Pleban T, Vanoss H, Kedar N, Rabinowitch HD, Czosnek H (1994) Mapping and introgression of a tomato Yellow leaf curl virus tolerance gene, TY-1. Theor Appl Genet 88:141–146
Zeidan M, Czosnek H (1991) Acquisition of Tomato yellow leaf curl virus by the whitefly Bemisia tabaci. J Genet Virol 72:2607–2614
Acknowledgments
This research was supported by a grant from the US Agency for International Development, Middle East Research and Cooperation (MERC) program to H C. (GEG-G-00-02-00003-00), Project M21-037.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eybishtz, A., Peretz, Y., Sade, D. et al. Silencing of a single gene in tomato plants resistant to Tomato yellow leaf curl virus renders them susceptible to the virus. Plant Mol Biol 71, 157–171 (2009). https://doi.org/10.1007/s11103-009-9515-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-009-9515-9