Abstract
We employed a comparative genomic approach to understand protein phosphatase 2C (PP2C)-mediated abscisic acid (ABA) signaling in the moss Physcomitrella patens. Ectopic expression of Arabidopsis (Arabidopsis thaliana) abi1-1, a dominant mutant allele of ABI1 encoding a PP2C involved in the negative regulation of ABA signaling, caused ABA insensitivity of P. patens both in gene expression of late embryogenesis abundant (LEA) genes and in ABA-induced protonemal growth inhibition. The transgenic abi1-1 plants showed decreased ABA-induced freezing tolerance, and decreased tolerance to osmotic stress. Analyses of the P. patens genome revealed that only two (PpABI1A and PpABI1B) PP2C genes were related to ABI1. In the ppabi1a null mutants, ABA-induced expression of LEA genes was elevated, and protonemal growth was inhibited with lower ABA concentration compared to the wild type. Moreover, ABA-induced freezing tolerance of the ppabi1a mutants was markedly enhanced. We provide the genetic evidence that PP2C-mediated ABA signaling is evolutionarily conserved between Arabidopsis and P. patens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Abscisic acid (ABA) regulates the maturation, dormancy and germination of seeds (Finkelstein et al. 2002), and postembryonic development, such as lateral root development (Brady et al. 2003; De Smet et al. 2003). ABA also plays an important role in plant adaptation to stresses, such as drought, high salinity and low temperature, and regulates the expression of genes involved in the response to abiotic stresses (Finkelstein et al. 2002).
A genetic approach using the model plant Arabidopsis (Arabidopsis thaliana) contributed to the identification of important factors involved in the ABA signal transduction pathway. ABA-insensitive (abi) mutants were identified by screening for seedlings that could germinate in the presence of ABA (Koornneef et al. 1984). Subsequent cloning identified ABI1 and ABI2, which encode homologous type 2C protein phosphatases (PP2Cs) (Leung et al. 1994, 1997; Meyer et al. 1994), and ABI3, ABI4 and ABI5, which encode transcription factors (Giraudat et al. 1992; Finkelstein et al. 1998; Finkelstein and Lynch 2000). Many other factors involved in ABA signaling were also identified through non-ABA based mutant screenings and reverse genetics (Finkelstein et al. 2002). In spite of these studies, our knowledge of how these gene products function in ABA signaling is still fragmentary.
Among the ABI genes, ABI1 and ABI2 are unique in that they encode PP2Cs, which are ubiquitously found in all eukaryotes and involved in phosphorylation-mediated signaling; in addition, these genes function through seed maturation and germination to vegetative growth. The abi1-1 and abi2-1 mutations, dominant alleles of ABI1 and ABI2, respectively, have the same single amino acid substitution (Gly to Asp) in the highly conserved phosphatase catalytic domain, and are capable of blocking ABA response (Leung et al. 1994, 1997; Meyer et al. 1994). The mutants show a broad range of ABA-related phenotypes, including reduced seed dormancy, ABA-resistant seed germination and seedling growth, abnormal stomatal regulation, and defects in various responses to drought (Koornneef et al. 1984; Finkelstein and Somerville 1990). Isolation of intragenic suppressors of abi1-1 and abi2-1 provided crucial evidence that these proteins act as negative regulators of the ABA signaling pathway (Gosti et al. 1999).
Database analysis of the Arabidopsis genome identified 76 PP2C genes, and phylogenetic analysis separated these genes into 10 groups (A–J) (Schweighofer et al. 2004). Interestingly, Group A contains most of the genes associated with ABA signaling, including ABI1 and ABI2. HAB1 was originally identified based on its sequence homology to ABI1 and ABI2, and the loss-of-function approach provided the concrete genetic evidence of the function of a PP2C as a negative regulator of ABA signaling (Saez et al. 2004). T-DNA or Ds insertion mutants of 8 Group A PP2C genes were analyzed for ABA sensitivity, and insertion mutations in ABI1, ABI2, HAB1, HAB2 and AtPP2CA were found to increase ABA sensitivity during germination (Yoshida et al. 2006b). These and other genetic studies (Sheen 1998; Tähtiharju and Palva 2001; Kuhn et al. 2006; Nishimura et al. 2007) have demonstrated the significant role of Group A PP2Cs in the regulation of ABA-signaling in Arabidopsis, and also revealed the redundant and distinct functions of Group A PP2Cs in the negative regulation of ABA signaling.
In contrast, the role of PP2Cs in ABA signaling in other plant species has not been well characterized. Arabidopsis abi1-1 protein has been shown as a strong negative regulator of ABA signaling in other seed plant species, such as Nicotiana benthamiana (Armstrong et al. 1995), tomato (Carrera and Prat 1998), rice protoplasts (Hagenbeek et al. 2000) and barley aleurone layer (Casaretto and Ho 2003) by gain-of-function analyses. These results suggested the likely existence of a PP2C-mediated ABA signaling pathway in these seed plants. However, no loss-of-function analysis has been carried out in these plants due to the technical difficulties. Thus, the significance of PP2Cs in ABA-signaling regulation in plants other than Arabidopsis has yet to be elucidated.
The nuclear genes of the moss Physcomitrella patens can be efficiently targeted by homologous recombination, therefore this system is useful for loss-of-function analysis of gene function (Schaefer and Zrÿd 2001). P. patens accumulates ABA and responds to ABA in a similar manner as angiosperms (Knight et al. 1995). In fact, our previous report demonstrated that the regulatory factor of seed maturation, ABI3, is conserved in the non-seed plant P. patens and activates ABA responsive promoters of late embryogenesis abundant (LEA) genes in barley as well as P. patens (Marella et al. 2006). As the evolutionary view considers bryophytes to be the basal land plants, comparative studies between seed plants and bryophytes could provide insights into the molecular evolution of ABA function and signaling in land plants. This evolutionary position, coupled with the sequenced genome (Rensing et al. 2008) and advanced tools for studying gene function, such as RNA interference, inducible promoters and gene targeting (Frank et al. 2005a; Quatrano et al. 2007), supports P. patens as a model system for such comparative and functional genomics on ABA studies.
To undertake comparative analysis of PP2Cs in ABA signaling, we generated transgenic P. patens expressing Arabidopsis abi1-1 to evaluate the ABA signaling of P. patens.
Materials and methods
Plant materials
P. patens subspecies patens (Gransden) was the wild-type strain. Protonemal tissue was grown on BCD or BCDAT medium at 25°C under continuous light (50–80 μmol m−2 s−1) (Nishiyama et al. 2000). The gametophores were induced on sterile peat pellets (Jiffy-7; Sakata seed, Japan) in a plant culture box by culturing at 25°C under continuous light for 1–1.5 months. For induction of sporophytes, gametophore cultures were transferred to 15°C under 8 h light/16 h dark conditions. PEG-mediated protoplast transformation was performed as previously described (Schaefer and Zrÿd 1997).
Phylogenetic analysis
We searched the annotated P. patens genome database ver. 1.0 (http://genome.jgi-psf.org/Phypa1_1/) with “Ser/Thr protein phosphatase” as a keyword and retrieved 75 putative protein phosphatase genes. We analyzed each annotation, and selected 42 putative PP2C genes. We then performed a tblastn search against the database using putative amino acid sequences encoded by the 42 genes as the queries and identified 9 additional putative PP2C genes. The catalytic domains of PP2Cs from 73 (A. thaliana) and 50 (P. patens) PP2C genes identified with PlantsP Motif/Domain Scan (http://plantsp.genomics.purdue.edu/html/) were used for phylogenetic analysis. The multiple sequence alignment was performed by MAFFT ver. 6 (Katoh et al. 2005) using the L-INS-i strategy, and gaps were excluded manually. For the maximum-likelihood (ML) analyses, the ProtML and NJdist programs in MOLPHY ver. 2.3b3 package (Adachi and Hasegawa 1996) were used. The ML distances were calculated using ProtML under the JTT model (Jones et al. 1992), and a Neighbor-Joining (NJ) tree was obtained with NJdist. A local rearrangement search was performed with the NJ tree as the starting tree. The local bootstrap probability of each branch was calculated with re-sampling of estimated log likelihood (RELL) method (Kishino et al. 1990; Hasegawa and Kishino 1994). Bootstrap test was performed with 100 replications.
Cloning of PpABI1A, PpABI1B and pphn39k21
The full-length cDNA clone (pphn8m22) corresponding to PpABI1A gene was obtained from RIKEN Bio Resource Center (BRC), and used as the representative sequence of PpABI1A. Since the full-length cDNA clone for PpABI1B was not in the PHYSCObase, we used information from the predicted transcript model of PpABI1B in the annotated P. patens genome database. We designed primers corresponding to the 5′- and 3′-end of the predicted transcript model, respectively, and obtained the corresponding cDNA fragment by RT-PCR, and subcloned the fragment into the Zero Blunt TOPO vector (Invitrogen, USA). The full-length cDNA clone pphn39k21, corresponding to Phypa1_1 160175 and encoding a PP2C from a different clade, was obtained from RIKEN BRC. The complete sequences of the clones and the cDNA fragment were determined.
DNA constructs
The stable transformation construct Act:abi1-1 and the effector constructs Act::ABI1, Act::PpABI1A and Act::pphn39k21 were generated as follows. The coding sequences of abi1-1, ABI1, PpABI1A and pphn39k21 were amplified by PCR from the cDNAs, and subcloned into the Zero Blunt TOPO vector. The amplified fragments were confirmed by sequencing, and each fragment was placed downstream of the rice Actin 1 promoter (McElroy et al. 1990) with the terminator of the CaMV 35S gene at the 3′ end of the fragment. The cassettes were then transferred to a vector containing the hygromycin resistance cassette (Schaefer and Zrÿd 1997). The Em-GUS and Ubi-LUC constructs were described previously (Marella et al. 2006). To create the PpABI1A targeting construct, the upstream (1,383 bp) and downstream regions (953 bp) of the ORF were amplified by PCR. PCR products were cloned into the pGEM-T Easy vector (Promega, USA), and the fragments were then excised and cloned into the both ends of the resistant cassette Lox-CaMV35S::NptII::NOS-Lox with SfiI (5′ fragment) and AscI (3′ fragment).
Measurement of plant growth
One-week-old protonemal tissues were harvested and the fresh weight was measured after aseptic removal of excess water. A tenth g of fresh tissue was homogenized in 2 ml of 0.1% (w/v) agar solution, and spotted on BCD agar medium (10 spots/plate, 5 μl each spot). After 2 weeks of culture, colony areas were digitally measured using LIA for Win32 image analysis software (freely available from http://www.agr.nagoya-u.ac.jp/%7Eshinkan/LIA32/index.html). To confirm the accuracy of the image analysis, we evaluated the colony growth of wild type protonemata on various concentration of ABA, and the growth of colonies was evaluated by the image analysis as well as chlorophyll contents, which is conventionally used for growth quantification of moss plants (Supplemental Fig. S1). Chlorophyll was extracted from each colony using dimethylformamide. Chlorophyll contents (Chl a + Chl b = 17.67A646.8 + 7.12A663.8) were measured with spectrophotometer, and calculated according to Porra et al. (1989).
Measurement of freezing tolerance
One-week-old protonemal tissues were treated with various concentrations of ABA for 24 h. Then freezing tolerance was determined by the measurement of electrolyte leakage from cells after equilibrium freezing to −5°C, as previously described (Minami et al. 2003).
Transient assay
Particle bombardment was carried out as previously described (Marella et al. 2006). We used 0.8 μg each of the reporter constructs (Em-GUS and Ubi-LUC) and the effector construct to prepare DNA-coated gold particles for four shots. One-week-old protonemal tissue was used, and incubated on BCDAT agar medium with or without 10 μM ABA for 48 h.
RNA analysis
Total RNAs were extracted from protonemal tissues using the RNeasy plant mini Kit (QIAGEN, USA). About 10 μg of total RNA was separated by a formaldehyde-denaturing agarose gel and transferred onto a nylon membrane. The PpABI1A probe was amplified by PCR from full-length cDNA clone. Probes for 19C6 (Minami et al. 2005) and PpLea1 (Kamisugi and Cuming 2005) were amplified by PCR from cDNA prepared from protonemal tissue. PCR fragments were purified using the Gel Extraction kit (QIAGEN, USA). Hybridization with 32P-labeled DNA probes was carried out as described previously (Yamaguchi-Shinozaki and Shinozaki 1994). BAStaion 2500 (Fuji Film, Japan) was used for visualization of the blot.
RT-PCR
For cDNA synthesis, 1 μg of total RNA was first treated with DNaseI (Sigma-Aldrich, USA) for 15 min at room temperature, and the enzyme was inactivated by heating at 70°C for 10 min. Reverse transcription was performed with the ThermoScript RT-PCR system (Invitrogen, USA) according to the manufacturer’s instructions. Synthesized cDNAs were purified using the Gel Extraction kit. Semi-quantitative RT-PCR analysis for PpABI1A and PpABI1B expression was performed using 1 μl of the cDNA, the primer set D and ExTaq polymerase with the supplied buffer and dNTP (Takara-Bio, Japan). The PCR conditions were as follows: 30 (PpABI1A and PpABI1B) or 27 (PpActin5, AY382284) cycles of 95°C for 30 s, 45°C (PpABI1A and PpABI1B) or 55°C (PpActin5) for 30 s and 72°C for 15 s. A 5 μl aliquot of each PCR reaction was separated on an agarose gel. As a negative control for the absence of genomic DNA contamination in the cDNA, we performed an PCR with a primer set that is designed to amplify the 4th intron of PpABI1A, and confirmed the absence of a DNA fragment that is corresponding to genomic DNA. abi1-1 expression in Act::abi1-1 plants was analyzed by quantitative PCR with LightCycler Systems for Real-Time PCR (Roche Applied Science, Japan) using the LightCycler-FastStart DNA Master SYBR Green I kit (Roche Applied Science, Japan) according to the manufacturer’s instructions. The PCR conditions were as follows: 40 cycles of 95°C for 10 s, 55°C for 10 s and 72°C for 10 s. The quantities of cDNA were calculated using the Second Derivative Maximum Method on LightCycler Data Analysis software (Roche Applied Science, Japan). The plastocyanin-like gene (GenBank Accession AW509984) was used to normalize abi1-1 expression.
Analysis of ppabi1a mutants
Genomic DNA was isolated using the Nucleon PhytoPure system (Amersham biosciences, USA). To check homologous recombination at the 5′ and 3′ flanking regions of the PpABI1A genomic locus, PCR was performed using primer set A for the 5′ recombination, and primer set B for the 3′ recombination. The absence of the PpABI1A genomic region was confirmed by PCR with primer set C. The PCR condition was 30 cycles of 95°C for 30 s, 59°C for 30 s, 72°C for 1 min. The expressions of PpABI1A and PpABI1B were analyzed by non-quantitative RT-PCR using the primer set D.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AB369256 (PpABI1A), AB369255 (PpABI1B), and AB369257 (pphn39k21).
Results
Ectopic expression of Arabidopsis abi1-1 reduces ABA sensitivity of P. patens
Previously we demonstrated that transient expression of Arabidopsis abi1-1 represses ABA-dependent transcriptional activation of the wheat Em promoter in P. patens protonemata (Marella et al. 2006). To confirm the effect of abi1-1 on ABA signaling of P. patens at the whole plant level, we established stable transformants expressing HA-tagged abi1-1 under the control of the constitutive rice actin promoter (Perroud and Quatrano 2006). Three independent transformants (Act::abi1-1) A, D and P, which showed different levels of abi1-1 expression as judged by quantitative RT-PCR (Fig. 1a), were used for subsequent analyses.
abi1-1 functions as a negative regulator of ABA signaling in P. patens. a One-week old protonemal tissue from Act::abi1-1 plants line A, D and P were subjected to QRT-PCR analysis for detection of the abi1-1 transgene. Values are average ± SE of triplicate samples from three independent experiments. b Northern blot analysis of ABA-inducible genes in Act::abi1-1 plants treated with 1 μM ABA. Ethidium bromide-stained bands of rRNA (bottom panels) confirmed equal loading. Representative images (c) and quantitative analysis (d) of protonemal growth of Act::abi1-1 plants with and without ABA. Wild type (WT) and Act::abi1-1 plants were grown on ABA media for 2 weeks, and the area of the protonemal colonies was measured by image analysis. The values in (d) are means ± SE (n = 20). Asterisks indicate significant changes between Act::abi1-1 plants and wild type (** P < 0.01, *** P < 0.001). Scale bars: 2 mm
We first analyzed the expression of endogenous ABA-inducible genes in Act::abi1-1 plants (Fig. 1b). PpLea1 and 19C6 encode LEA-like proteins and are induced by both ABA and abiotic stresses, such as hyper-osmolarity and cold temperatures (Minami et al. 2003; Kamisugi and Cuming 2005). Expression levels of these genes were low in the absence of exogenous ABA, and no significant differences in expression levels between the wild type and Act::abi1-1 plants were detected. Notably, treatment of Act::abi1-1 plants with ABA (1 μM) showed reduced gene induction compared to wild-type plants. Down-regulation was most prominent in Act::abi1-1 line P, the highest expressor of abi1-1, suggesting a correlation between the level of abi1-1 expression and the degree of down-regulation.
To further confirm the reduced ABA sensitivity in Act::abi1-1 plants, we measured the growth of protonemata on ABA media. Protonemal growth of transformants on media containing various concentrations of ABA was evaluated by measuring the colony area (Fig. 1c, d). In the presence of ABA, protonemal growth of the wild type was inhibited by 63% (at 10 μM) and 78% (at 100 μM) compared to the untreated control. Although the lowest expressing line A behaved similarly to the wild type in response to ABA, line D showed slightly but significantly better growth than wild type at 10 μM ABA. The highest expressing line P showed clear ABA-resistance compared to wild type with both 10 and 100 μM ABA. These results indicated that Act::abi1-1 plants were less sensitive to ABA than wild type, and that the insensitivity correlated with the level of abi1-1 expression. This demonstrates that abi1-1 functions as a negative regulator of ABA signaling in P. patens.
Arabidopsis abi1-1 reduces tolerance of P. patens to abiotic stresses
Although P. patens protonemata are susceptible to freezing stress, exogenous ABA markedly increases the freezing tolerance (Minami et al. 2003). Thus, we evaluated the tolerance of Act::abi1-1 plants to freezing (Fig. 2a). As reported, ABA treatment of the wild type protonemata enhanced the tolerance to freezing in a dose-dependent manner, and most protenemal cells survived when treated with 10 μM ABA for 24 h. In contrast, all Act::abi1-1 plants treated with 0.1 and 1 μM ABA showed significant reduction of freezing tolerance in proportion to the level of abi1-1 expression. The highest expressing line P exhibited significantly lower freezing tolerance, even at 10 μM ABA, compared to the wild type.
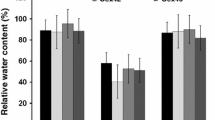
abi1-1 plants exhibit reduced ABA-induced tolerance to freezing and reduced tolerance to osmotic stress. a Wild type (WT) and Act::abi1-1 plants protonemata were treated with various concentrations of ABA for 24 h, and then subjected to freezing stress at −5°C. Cell survival was determined by measurement of electrolyte leakage after freeze-thawing. Values are means ± SE of three different experiments. b Wild type (WT) and Act::abi1-1 plants protonemata were grown on BCD medium containing the indicated concentration of mannitol. The area of protonemal colonies was measured by image analysis. The values shown are means ± SE (n = 20). Asterisks indicate significant changes between Act::abi1-1 plants and wild type (* P < 0.05, ** P < 0.01, *** P < 0.001)
We next investigated the physiological role of ABA signaling regulated by abi1-1 in P. patens. Since mosses accumulate ABA under dry conditions, and exogenously applied ABA enhances drought resistance in Funaria hygrometrica (Cove et al. 2006), endogenous ABA signaling is likely involved in the drought tolerance of P. patens. Therefore, we analyzed their tolerance to osmotic stress (Fig. 2b). Act::abi1-1 protonemata were cultured on media containing various concentrations of mannitol, which causes changes in media osmolality. As the mannitol concentration increased, the growth of the wild type protonemata was inhibited. Growth inhibition caused by mannitol-induced osmotic stress was more pronounced in all Act::abi1-1 plant lines. Together, these results demonstrate that ectopic expression of Arabidopsis abi1-1 reduces tolerance of P. patens protonemata to freezing stress and osmotic stress.
Two ABI1-related genes exist in the genome of P. patens
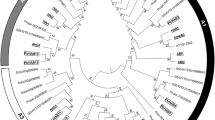
Studies of ectopically expressed Arabidopsis abi1-1 strongly supported the presence of a PP2C-mediated ABA signaling pathway in P. patens. We therefore searched for genes encoding PP2Cs in the P. patens EST database (Nishiyama et al. 2003) as well as in the genome database (http://genome.jgi-psf.org/Phypa1_1/Phypa1_1.home.html) and identified 51 genes containing a highly conserved catalytic domain of PP2C. Among them, two closely related genes showed high similarity to Arabidopsis Group A PP2Cs. We generated a phylogenetic tree based on sequence alignment of the catalytic domains encoded by the P. patens PP2C genes with those of Arabidopsis PP2Cs (Fig. 3). Each cluster was categorized according to the phylogenetic analysis of Arabidopsis PP2Cs (Schweighofer et al. 2004). Almost all groups contained both Arabidopsis PP2Cs and P. patens PP2Cs. However, Group B consisted of only Arabidopsis PP2Cs, suggesting that Group B PP2Cs evolved after the separation of the bryophytes and the vascular plants from a common ancestor, and that these genes are likely involved in physiological events specific to the vascular plants. We also found that some PP2Cs were moss-specific. Only two genes, PpABI1A and PpABI1B, positioned in the same cluster with Arabidopsis Group A PP2Cs with a high bootstrap value. These analyses suggest that the Group A PP2C genes involved in the regulation of Arabidopsis ABA signaling are also conserved in the P. patens genome, although the number of genes is lower than that of Arabidopsis. The exon–intron structures of PpABI1A and PpABI1B were similar to that of Arabidopsis ABI1 (Supplemental Fig. S2), and the coding sequences of PpABI1A and PpABI1B were highly similar to one another (76% amino acid identity and 83% nucleotide identity). In contrast, the sequence similarity between Arabidopsis ABI1 and PpABI1A or PpABI1B was restricted only to the catalytic domains. Two Gly residues in the catalytic domain of ABI1 that correlated with regulation of ABA signaling were also conserved in PpABI1A and PpABI1B (Finkelstein and Rock 2002, Supplemental Fig. S3).
The P. patens genome contains two ABI1-related genes. The maximum-likelihood tree of plant PP2C genes, including 73 A. thaliana PP2C genes (black) and 50 P. patens PP2C genes (blue). The local bootstrap probabilities are shown on the branches. The horizontal branch lengths are proportional to the estimated evolutionary distance. Each cluster is categorized according to the phylogenetic analysis of Arabidopsis PP2C genes (Schweighofer et al. 2004)
Semi-quantitative RT-PCR analysis, using a primer set that is able to distinguish the PCR products of PpABI1A from that of PpABI1B by the size, revealed that PpABI1A and PpABI1B were expressed ubiquitously during the moss life cycle although PpABI1A exhibited higher expression than PpABI1B (Fig. 4a, b). Arabidopsis Group A PP2C genes are transcriptionally induced in response to exogenous ABA. Similarly, PpABI1A and PpABI1B quickly responded to exogenously applied ABA, and sustained high levels of expression even after 12 h of ABA treatment, eventually decreasing (Fig. 4c). PpABI1A and PpABI1B were also responsive to osmotic stress. Interestingly, PpABI1A showed enhanced transcriptional induction in response to cold treatment. These data suggest that while PpABI1A and PpABI1B are ubiquitously expressed and responsive to various stresses, PpABI1A is predominantly expressed and strongly induced by low temperature stress. Therefore, we focused on PpABI1A in subsequent experiments.
PpABI1A and PpABI1B are ubiquitously expressed through the life cycle, and are induced by ABA and abiotic stresses. a Schematic of the corresponding regions of the primers in PpABI1A and PpABI1B for RT-PCR analysis. b Semi-quantitative RT-PCR of PpABI1A and PpABI1B was performed using total RNA isolated at different stages of development. Expression of PpAct5 was used as an internal control. c Northern blot analysis of PpABI1A and PpABI1B expression. Total RNA was isolated from protonemata treated with 10 μM ABA, 0.4 M mannitol, or cold stress (on ice) at indicated times after treatment. Ethidium bromide-stained bands of rRNA confirm equal loading
Transient expression of PpABI1A in P. patens protonemata blocks the ABA-induced expression of Em-GUS
We previously developed a useful transient assay to assess the ABA sensitivity of P. patens, in which the wheat Em gene promoter fused to the β-glucuronidase (GUS) gene was introduced into protonemal cells and the Em promoter activity was assessed by measuring GUS activity (Marella et al. 2006). We used this system to analyze the function of PpABI1A in ABA signaling (Fig. 5). The entire PpABI1A coding sequence was inserted downstream of the rice actin 1 promoter, and this construct was introduced into protonemal cells along with the Em-GUS construct via particle bombardment. Arabidopsis ABI1 and a PP2C gene from P. patens (pphn39k21) that is distinct from group A PP2Cs (Fig. 3) were used as a positive and negative control, respectively. PpABI1A completely suppressed the ABA-dependent activation of the Em promoter, and the degree of repression was comparable to that of Arabidopsis ABI1. In contrast, pphn39k21 hardly affected the Em promoter activity irrespective of ABA treatment. These data suggest that PpABI1A specifically functions as a negative regulator of ABA signaling in P. patens.
Transient expression of PpABI1A blocked ABA-induced transcription of Em-GUS in P. patens protonemata. The Em-GUS reporter construct and each of the effector constructs were introduced into P. patens protonemal tissue by particle bombardment. After bombardment, the protonemal tissue was incubated with (black bars) or without (white bars) 10 μM ABA for 48 h. Protein lysates were extracted from the treated tissues for GUS and LUC assays. Bars indicate the relative GUS activities ± SE (n = 4)
Targeted disruption of PpABI1A causes ABA hypersensitive phenotypes
To confirm the function of PpABI1A in ABA signaling, we performed a loss-of-function analysis and disrupted PpABI1A by gene targeting via homologous recombination (Fig. 6a). Two independent disruptants (lines 3 and 7) were selected after PCR analysis confirmed the complete deletion of the PpABI1A coding sequence (Fig. 6b). RT-PCR analysis confirmed that both transgenic lines lost the expression of PpABI1A while retaining PpABI1B expression (Fig. 6c). We next evaluated ABA sensitivities of the disruptants by analyzing temporal expression patterns of ABA-inducible genes, PpLea1 and 19C6, in protonemata (Fig. 7a). ABA treatment enhanced induction of PpLea1 and 19C6 in the ppabi1a plant (line 7) compared to wild type plants, suggesting a negative regulatory role of PpABI1A in ABA-regulated gene expression in P. patens.
Generation of PpABI1A disruptant by gene targeting. a Schematic of the PpABI1A disruption by homologous recombination. The locations of the primer sets used in (b) and (c) are shown by arrowheads. The 5′ and 3′ untranslated regions and the coding region of PpABI1A are shown by open boxes and gray boxes, respectively. The dark gray box represents the nptII cassette. b Disruption of PpABI1A is confirmed by PCR analysis with the primer set A and the primer set B, respectively. Deletion of PpABI1A sequence is confirmed with the primer set C. c Non-quantitative RT-PCR analysis of the expression of PpABI1A and PpABI1B in the wild type, the ppabi1a mutant line 3 and line 7 using the primer set D. Expression of PpAct5 was used as an internal control
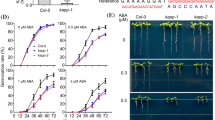
Disruption of PpABI1A resulted in an ABA-hypersensitive phenotype. a RNA analysis of ABA-inducible genes in the ppabi1a mutant line 7. Protonemata were treated with 1 μM ABA for 0, 3, 6, 12, and 24 h, and isolated total RNA (10 μg) was subjected to RNA blot analysis for the indicated genes. b Protonemal growth of the ppabi1a mutants on ABA medium. Wild type, ppabi1a mutant line 3, and mutant line 7 were grown on ABA medium with the indicated concentration of ABA for 2 weeks. Areas of protonemal colonies were measured by image analysis. The values are mean ± SE (n = 10). c Phenotypes of protonemal cells of the wild type and the ppabi1a mutant line 7. Protonemata were cultured on BCDAT agar plates for 2 weeks with or without 1 μM ABA treatment. The brood cells are indicated by arrowheads. Scale bars: 100 μm. d Freezing tolerance of wild type and the ppabi1a mutants treated with various concentrations of ABA for 24 h. The values are mean ± SE (n = 3). Asterisks indicate significant changes between ppabi1a mutant lines and wild type (* P < 0.05, ** P < 0.01, *** P < 0.001)
Growth of the ppabi1a plant protonemata on media with or without 0.01 μM ABA was similar to that of wild type. Statistically significant and reproducible growth inhibition of the ppabi1a plants in response to 0.1 μM ABA was detected, although the growth of wild type plant was unaffected at this concentration (Fig. 7b). Protonemal wild type cells can differentiate into spherical brood cells in response to stresses or after long periods of culture (Goode et al. 1993), which are also induced by relatively high concentrations of ABA (>10 μM). Therefore we examined brood cell formation of the ppabi1a plants in response to exogenous ABA. To obtain better images of protonemal filaments under a microscope, we examined protonemata cultured between two cellophane sheets. Under these culture conditions, the effect of ABA was weakened due to the presence of a cellophane sheet on the medium. We observed brood cell formation on many side branches of the ppabi1a plants cultured on media containing 1 μM ABA (Fig. 7c), although no brood cells in the wild type plants were detected. We also measured the freezing tolerance (Fig. 7d) and found that even in the absence of ABA treatment, the ppabi1a plants showed slightly higher survival rates than the wild type plants. In the presence of ABA, the survival of the ppabi1a plants markedly increased even with treatment as low as 0.01 μM ABA, whereas wild type plants were unaffected at this concentration. Upon treatment with 0.1 μM ABA, 88.7% (line 3) and 95.3% (line 7) of the ppabi1a plant protonemata survived, while in contrast only 23.3% of wild type survived. These data demonstrate that disruption of PpABI1A leads to ABA-hypersensitive phenotypes, providing concrete evidence for the role of PpABI1A as a negative regulator of ABA signaling in the moss. These data also suggest that the acquisition of freezing tolerance of P. patens involves PpABI1A-mediated ABA signaling.
Discussion
Evolutionarily conserved function of PP2Cs in ABA signaling
The abi1-1 dominant mutation allele of ABI1 functions as a strong negative regulator of ABA signaling in angiosperms. Here we showed that the negative regulatory function of abi1-1 is intact even in the evolutionarily distant plant species P. patens, suggesting that PP2C-mediated ABA signaling is evolutionarily conserved between angiosperms and bryophytes.
The mechanism by which the abi1-1 dominant mutation exerts its negative regulation of ABA signaling is still unclear. The abi1-1 mutation reduced the phosphatase activity of ABI1 in vitro (Bertauche et al. 1996), however, recent studies suggested that the abi1-1 mutation is not a dominant negative mutation but rather a hypermorphic mutation (Saez et al. 2006; Yoshida et al. 2006b; Moes et al. 2008). In this scenario, the abi1-1 protein might irreversibly bind to its substrate(s), a potential master positive regulator(s) of ABA signaling, thus interrupting the positive signaling cascade. If this scenario is accurate, this master positive regulator(s) will likely also be conserved in P. patens. Several ABI1-related PP2C interacting proteins have been identified thus far. The PKS3 protein kinase interacts with ABI2 and, to a lesser extent, with ABI1, and negatively regulates ABA signaling in Arabidopsis (Guo et al. 2002). Another PKS protein kinase, SOS2, interacts with ABI2 but not ABI1 (Ohta et al. 2003). More recently, the SNF1-related protein kinase SnRK2E (or OST1) was found to specifically interact with ABI1, but not with ABI2 or abi1-1 (Yoshida et al. 2006a). Molecules other than protein kinases also interact with Group A PP2Cs (Cherel et al. 2002; Himmelbach et al. 2002; Zhang et al. 2004). None of these interactions, however, provide a mechanism for the dominant effect of abi1-1 in ABA signaling. The moss protonemata consist of only chloronema and caulonema cells, and the large-scale culture is possible in fermenters (Cove et al. 1997). Thus, the protonemata of Act::abi1-1 plants may be a good source for the biochemical identification of the putative master positive regulator in ABA signaling.
Complete genome sequencing of P. patens revealed at least 51 genes encoding putative PP2Cs, enabling us to construct a comparative phylogenetic tree of P. patens PP2Cs with Arabidopsis PP2Cs. We found that Group B consisted of only Arabidopsis PP2Cs, suggesting that this class of PP2Cs are likely involved in physiological events specific to the vascular plants. Although few reports have been made on Group B PP2Cs, it is noteworthy that AP2C1 has been shown recently to play a role in regulating stress responsive mitogen-activated protein kinases (MPK4 and MPK6) and jasmonic acid (JA) levels to modulate innate immunity in Arabidopsis (Schweighofer et al. 2007). The presence or function of JA in P. patens is yet to be investigated (Decker et al. 2006).
Only two genes (PpABI1A and PpABI1B) were closely related to Arabidopsis Group A PP2Cs. Although the two genes encoded proteins highly homologous to one another and were expressed through the moss life cycle, the expression levels and responses to abiotic stresses varied between the genes. Notably, the PpABI1A single disruption was sufficient to alter ABA sensitivity to freezing tolerance as well as gene expression in P. patens protonemata, providing concrete evidence for a conserved PP2C-mediated ABA signaling pathway in the moss. Although the disruption phenotype of PpABI1B is yet to be examined, these data may indicate that PpABI1A and PpABI1B are functionally distinct from each other, possibly due to different expression profiles. A similar idea was also proposed in regards to the function of Arabidopsis group A PP2Cs (Yoshida et al. 2006b). ABI1, ABI2, HAB1, HAB2 and AtPP2CA/AHG3 encode PP2Cs closely related to one another within the catalytic domain, and loss-of-function mutations of each gene resulted in ABA-hypersensitivity in seed germination with varying intensities. Among the mutants, the AtPP2CA/AHG3 mutant, which was expressed the highest in seeds compared to others, showed the strongest ABA-hypersensitivity. The authors concluded that the level of gene expression may be the primary factor for their distinct functions. Other factors, however, may also determine functional specificity. Arabidopsis PP2Cs are highly similar within their catalytic domains, but divergent in the N-terminal regions, suggesting that other post-transcriptional mechanisms, such as substrate specificity or subcellular localization, could also play a role in the functional specificities of PP2Cs. However, the functions of PpABI1A and PpABI1B are distinct especially in tolerance to low temperature stress, despite the high homology throughout the coding sequences of these two genes. Together this strongly supports the idea that different expression levels of the PP2Cs are a critical contributor to the regulation of their distinct functions.
Our study suggests that the Group A PP2C clade evolved before the separation of the bryophytes and vascular plants from a common ancestor, and that angiosperms increased the number of PP2C genes with differential expression profiles during evolution to enable tissue- and organ-specific tuning of ABA signaling.
PP2C-mediated regulation of ABA-inducible genes in P. patens
PpLea1 encodes a Group I LEA protein, which is expressed in a seed-specific manner in angiosperms and is activated by exogenous ABA (Kamisugi and Cuming 2005). We previously demonstrated that PpLea1 is also activated by, thus one of the target genes of, PpABI3A (Marella et al. 2006). Ectopic expression of abi1-1 suppressed the ABA-induced expression of PpLea1 in proportion to the abi1-1 expression level. Accordingly, disruption of PpABI1A activated the ABA-responsive expression of PpLea1. These results suggest that PpLea1 is involved downstream of ABA signaling mediated by PpABI1A and PpABI3A. The ABA signaling pathway in protonemata closely resembled that of ABA signaling in the seeds of Arabidopsis, suggesting that ABA signaling in the vegetative tissue of the ancestor land plants has been functionally diverted specifically to seeds during the evolution of land plants.
To date very little is known about the cis-elements in ABA- or stress-inducible promoters from P. patens, except PpLea1. The PpLea1 promoter contains one ABA-response element (ABRE)-like motif, and mutation of this element abolished the ability for ABA induction in P. patens, however, the ABRE-like motif was not active in barley aleurone protoplasts (Kamisugi and Cuming 2005). Saez et al. (2006) reported the differential up-regulation of ABA-inducible genes in loss-of-function Arabidopsis PP2C mutants associated with the presence of the ABRE, drought-responsive element (DRE), or a combination of the two. These observations suggested that PP2Cs are involved in at least two independent pathways for the regulation of ABA-inducible genes in Arabidopsis, possibly through different trans-acting factors. Cuming et al. (2007) reported transcriptomic analysis of P. patens treated with ABA, or subjected to osmotic, salt and drought stress. Many ABA- and drought responsive genes are homologues of angiosperm genes expressed during drought stress and seed development, including LEA genes. They also showed that ABREs and DREs are significantly over-represented in the 5′-proximal sequences of the ABA- and stress-induced gene set. In the present study, we demonstrated that ABA induction of a Group I LEA PpLea1 and a Group II LEA 19C6 is under the control of PpABI1A. We found that cis-element databases for angiosperms also predicted ABREs and DREs in the 5′-upstream region of 19C6 (data not shown). Although true cis-elements for the ABA induction of 19C6 have yet to be elucidated experimentally, these facts suggest that cis-elements required for PP2C-mediated regulation of ABA-inducible genes in P. patens is comparable to Arabidopsis.
Physiological role of PP2C-mediated ABA signaling in P. patens
Bryophytes are considered as a modern representation of the first land plants, which were subjected to stresses novel to these plants, such as drought and freezing. Many land plants including bryophytes have been shown to accumulate ABA, and bryophytes also respond to exogenous ABA, suggesting that ABA evolved to protect the land plants from such stresses. However, the role of endogenous ABA or the signaling pathways in response to environmental stresses has yet to be elucidated. P. patens displays a high degree of tolerance against osmotic stress (Frank et al. 2005b), and ABA levels in protonemata increase upon 0.5 M mannitol treatment (Minami et al. 2005). Our results demonstrated that ectopic expression of abi1-1 significantly reduced the ABA sensitivity and tolerance against osmotic stress of protonemata, strongly indicating that endogenous ABA signaling is involved in the water stress response of bryophytes. PpABI1A and PpABI1B levels were ubiquitously detectable even under normal conditions; however, these genes were quickly activated by exogenous ABA and mannitol treatment, indicating that PP2C-mediated ABA signaling is involved in the osmotic tolerance of P. patens protonemata. Thus far, no significant difference in the osmotic tolerance between the protonemata of wild type and the ppabi1a mutants was detectable. Disruption of both the PP2Cs will confirm the role of PP2C-mediated ABA signaling in the osmotic stress response.
ABA levels in seed plant vegetative tissues increase in response to low temperatures (Xiong and Zhu 2003). Antisense inhibition of AtPP2CA also enhanced cold acclimation in Arabidopsis (Tähtiharju and Palva 2001). Thus ABA signaling is considered as one of the important factors involved in the freezing tolerance of Arabidopsis. Exogenous ABA also dramatically increases the freezing tolerance of P. patens protonemata in a dose-dependent manner (Minami et al. 2003). Interestingly, treatment with low temperature increased the freezing tolerance of P. patens, but did not change ABA accumulation, suggesting that cold acclimation in P. patens does not involve ABA biosynthesis (Minami et al. 2005). Our study demonstrated that ectopic expression of abi1-1 or disruption of PpABI1A dramatically affected the ABA-induced freezing tolerance, clearly indicating the indispensable role of PP2C-mediated ABA signaling for the acquisition of freezing tolerance. The expression of LEA genes that are thought to play a role in freezing tolerance was upregulated in the moss upon cold treatment (Minami et al. 2005), suggesting a role for these genes in enhancing the freezing tolerance of the moss. We showed that PpABI1A negatively regulates these LEA genes, and that PpABI1A is upregulated by cold treatment. This suggests that ABA signaling is indeed activated during the cold acclimation without an apparent change of ABA accumulation, and that negative regulation of ABA signaling through PpABI1A is also activated to fine tune the process. Another molecule(s) generated during low temperature treatment might activate ABA signaling to enhance the freezing tolerance in P. patens.
Conclusion
We provided the solid molecular evidence that PP2C-regulated ABA signaling is not specific to the model plant Arabidopsis but is also present in the basal land plant P. patens, suggesting that the function of ABI1-related PP2Cs in the negative regulation of ABA signaling is evolutionarily conserved in land plants. Compared to the number and partially redundant function of Group A PP2Cs in Arabidopsis, P. patens, with only two Group A PP2Cs, is a promising model to understand the function of Group A PP2Cs in ABA signaling.
Abbreviations
- ABA:
-
Abscisic acid
- ABI:
-
ABA-insensitive
- ABRE:
-
ABA-response element
- DRE:
-
Drought-responsive element
- GUS:
-
β-Glucuronidase
- JA:
-
Jasmonic acid
- LEA:
-
Late embryogenesis abundant
- MPK:
-
Mitogen-activated protein kinase
- PP2C:
-
Protein phosphatase 2C
References
Adachi J, Hasegawa M (1996) MOLPHY version 2.3: programs for molecular phylogenetics based on maximum likelihood. The Institute of Statistical Mathematics, Tokyo
Armstrong F, Leung J, Grabov A, Brearley J, Giraudat J, Blatt MR (1995) Sensitivity to abscisic acid of guard-cell K+ channels is suppressed by abi1-1, a mutant Arabidopsis gene encoding a putative protein phosphatase. Proc Natl Acad Sci USA 92:9520–9524. doi:10.1073/pnas.92.21.9520
Bertauche N, Leung J, Giraudat J (1996) Protein phosphatase activity of abscisic acid insensitive 1 (ABI1) protein from Arabidopsis thaliana. Eur J Biochem 241:193–200. doi:10.1111/j.1432-1033.1996.0193t.x
Brady SM, Sarkar SF, Bonetta D, McCourt P (2003) The ABSCISIC ACID INSENSITIVE 3 (ABI3) gene is modulated by farnesylation and is involved in auxin signaling and lateral root development in Arabidopsis. Plant J 34:67–75. doi:10.1046/j.1365-313X.2003.01707.x
Carrera E, Prat S (1998) Expression of the Arabidopsis abi1-1 mutant allele inhibits proteinase inhibitor wound-induction in tomato. Plant J 15:765–771. doi:10.1046/j.1365-313X.1998.00261.x
Casaretto J, Ho TH (2003) The transcription factors HvABI5 and HvVP1 are required for the abscisic acid induction of gene expression in barley aleurone cells. Plant Cell 15:271–284. doi:10.1105/tpc.007096
Cherel I, Michard E, Platet N, Mouline K, Alcon C, Sentenac H et al (2002) Physical and functional interaction of the Arabidopsis K(+) channel AKT2 and phosphatase AtPP2CA. Plant Cell 14:1133–1146. doi:10.1105/tpc.000943
Cove D, Knight CD, Lamparter T (1997) Mosses as model systems. Trends Plant Sci 2:99–105. doi:10.1016/S1360-1385(96)10056-X
Cove D, Benzanilla M, Harries P, Quatrano R (2006) Mosses as model systems for the study of metabolism and development. Annu Rev Plant Biol 57:497–520
Cuming AC, Cho SH, Kamisugi Y, Graham H, Quatrano RS (2007) Microarray analysis of transcriptional responses to abscisic acid and osmotic, salt, and drought stress in the moss, Physcomitrella patens. New Phytol 176:275–287. doi:10.1111/j.1469-8137.2007.02187.x
De Smet I, Signora L, Beeckman T, Inzé D, Foyer CH, Zhang H (2003) An abscisic acid-sensitive checkpoint in lateral root development of Arabidopsis. Plant J 33:543–555. doi:10.1046/j.1365-313X.2003.01652.x
Decker EL, Frank W, Sarnighausen E, Reski R (2006) Moss systems biology en route: phytohormones in Physcomitrella development. Plant Biol Stuttg 8:397–405. doi:10.1055/s-2006-923952
Finkelstein RR, Lynch TJ (2000) The Arabidopsis abscisic acid response gene ABI5 encodes a basic leucine zipper transcription factor. Plant Cell 12:599–609
Finkelstein RR, Rock CD (2002) Abscisic acid biosynthesis and response. The Arabidopsis book. American Society of Plant Biologists, Rockville. doi:10.1199/tab.0058. www.aspb.org/publications/arabidopsis/
Finkelstein RR, Somerville CR (1990) Three classes of abscisic acid (ABA)-insensitive mutations of Arabidopsis define genes that control overlapping subsets of ABA responses. Plant Physiol 94:1172–1179. doi:10.1104/pp.94.3.1172
Finkelstein RR, Wang ML, Lynch TJ, Rao S, Goodman HM (1998) The Arabidopsis abscisic acid response locus ABI4 encodes an APETALA 2 domain protein. Plant Cell 10:1043–1054
Finkelstein RR, Gampala SS, Rock CD (2002) Abscisic acid signaling in seeds and seedlings. Plant Cell 14(Suppl):S15–S45
Frank W, Holtorf H, Reski R (2005a) Functional genomics in Physcomitrella. In: Leister D (ed) Plant functional genomics. The Haworth Press, New York, pp 203–234
Frank W, Ratnadewi D, Reski R (2005b) Physcomitrella patens is highly tolerant against drought, salt and osmotic stress. Planta 220:384–394. doi:10.1007/s00425-004-1351-1
Giraudat J, Hauge BM, Valon C, Smalle J, Parcy F, Goodman HM (1992) Isolation of the Arabidopsis ABI3 gene by positional cloning. Plant Cell 4:1251–1261
Goode JA, Stead AD, Duckett JG (1993) Redifferentiation of moss protonemata: an experimental and immunofluorescence study of brood cell formation. Can J Bot 71:1510–1519
Gosti F, Beaudoin N, Serizet C, Webb AA, Vartanian N, Giraudat J (1999) ABI1 protein phosphatase 2C is a negative regulator of abscisic acid signaling. Plant Cell 11:1897–1910
Guo Y, Xiong L, Song CP, Gong D, Halfter U, Zhu JK (2002) A calcium sensor and its interacting protein kinase are global regulators of abscisic acid signaling in Arabidopsis. Dev Cell 3:233–244. doi:10.1016/S1534-5807(02)00229-0
Hagenbeek D, Quatrano RS, Rock CD (2000) Trivalent ions activate abscisic acid-inducible promoters through an ABI1-dependent pathway in rice protoplasts. Plant Physiol 123:1553–1560. doi:10.1104/pp.123.4.1553
Hasegawa M, Kishino H (1994) Accuracies of the simple methods for estimating the bootstrap probability of a maximum-likelihood tree. Mol Biol Evol 11:142–145
Himmelbach A, Hoffmann T, Leube M, Hohener B, Grill E (2002) Homeodomain protein ATHB6 is a target of the protein phosphatase ABI1 and regulates hormone responses in Arabidopsis. EMBO J 21:3029–3038. doi:10.1093/emboj/cdf316
Jones DT, Taylor WR, Thornton JM (1992) The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci 275(8):275–282
Kamisugi Y, Cuming AC (2005) The evolution of the abscisic acid-response in land plants: comparative analysis of group 1 LEA gene expression in moss and cereals. Plant Mol Biol 59:723–737. doi:10.1007/s11103-005-0909-z
Katoh K, Kuma K, Toh H, Miyata T (2005) MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Res 33:511–518. doi:10.1093/nar/gki198
Kishino H, Miyata T, Hasegawa M (1990) Maximum likelihood inference of protein phylogeny and the origin of chloroplasts. J Mol Evol 31:151. doi:10.1007/BF02109483
Knight CD, Sehgal A, Atwal K, Wallace JC, Cove DJ, Coates D et al (1995) Molecular responses to abscisic acid and stress are conserved between moss and cereals. Plant Cell 7:499–506
Koornneef M, Reuling G, Karssen CM (1984) The isolation and characterization of abscisic acid-insensitive mutants of Arabidopsis thaliana. Physiol Plant 61:377–383. doi:10.1111/j.1399-3054.1984.tb06343.x
Kuhn JM, Boisson-Dernier A, Dizon MB, Maktabi MH, Schroeder JI (2006) The protein phosphatase AtPP2CA negatively regulates abscisic acid signal transduction in Arabidopsis, and effects of abh1 on AtPP2CA mRNA. Plant Physiol 140:127–139. doi:10.1104/pp.105.070318
Leung J, Bouvier-Durand M, Morris PC, Guerrier D, Chefdor F, Giraudat J (1994) Arabidopsis ABA response gene ABI1: features of a calcium-modulated protein phosphatase. Science 264:1448–1452. doi:10.1126/science.7910981
Leung J, Merlot S, Giraudat J (1997) The Arabidopsis ABSCISIC ACID-INSENSITIVE2 (ABI2) and ABI1 genes encode homologous protein phosphatases 2C involved in abscisic acid signal transduction. Plant Cell 9:759–771
Marella HH, Sakata Y, Quatrano RS (2006) Characterization and functional analysis of ABSCISIC ACID INSENSITIVE3-like genes from Physcomitrella patens. Plant J 46:1032–1044. doi:10.1111/j.1365-313X.2006.02764.x
McElroy D, Zhang W, Cao J, Wu R (1990) Isolation of an efficient actin promoter for use in rice transformation. Plant Cell 2:163–171
Meyer K, Leube MP, Grill E (1994) A protein phosphatase 2C involved in ABA signal transduction in Arabidopsis thaliana. Science 264:1452–1455. doi:10.1126/science.8197457
Minami A, Nagao M, Arakawa K, Fujikawa S, Takezawa D (2003) Abscisic acid-induced freezing tolerance in the moss Physcomitrella patens is accompanied by increased expression of stress-related genes. J Plant Physiol 160:475–483. doi:10.1078/0176-1617-00888
Minami A, Nagao M, Ikegami K, Koshiba T, Arakawa K, Fujikawa S et al (2005) Cold acclimation in bryophytes: low-temperature-induced freezing tolerance in Physcomitrella patens is associated with increases in expression levels of stress-related genes but not with increase in level of endogenous abscisic acid. Planta 220:414–423. doi:10.1007/s00425-004-1361-z
Moes D, Himmelbach A, Korte A, Haberer G, Grill E (2008) Nuclear localization of the mutant protein phosphatase abi1 is required for insensitivity towards ABA responses in Arabidopsis. Plant J 54:806–819. doi:10.1111/j.1365-313X.2008.03454.x
Nishimura N, Yoshida T, Kitahata N, Asami T, Shinozaki K, Hirayama T (2007) ABA-Hypersensitive Germination1 encodes a protein phosphatase 2C, an essential component of abscisic acid signaling in Arabidopsis seed. Plant J 50(6):935–949
Nishiyama T, Hiwatashi Y, Sakakibara I, Kato M, Hasebe M (2000) Tagged mutagenesis and gene-trap in the moss, Physcomitrella patens by shuttle mutagenesis. DNA Res 7:9–17. doi:10.1093/dnares/7.1.9
Nishiyama T, Fujita T, Shin IT, Seki M, Nishide H, Uchiyama I et al (2003) Comparative genomics of Physcomitrella patens gametophytic transcriptome and Arabidopsis thaliana: implication for land plant evolution. Proc Natl Acad Sci USA 100:8007–8012. doi:10.1073/pnas.0932694100
Ohta M, Guo Y, Halfter U, Zhu JK (2003) A novel domain in the protein kinase SOS2 mediates interaction with the protein phosphatase 2C ABI2. Proc Natl Acad Sci USA 100:11771–11776. doi:10.1073/pnas.2034853100
Perroud PF, Quatrano RS (2006) The role of ARPC4 in tip growth and alignment of the polar axis in filaments of Physcomitrella patens. Cell Motil Cytoskeleton 63:162–171. doi:10.1002/cm.20114
Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochimica et Biophysica Acta 975:384–394
Quatrano RS, McDaniel SF, Khandelwal A, Perroud P-F, Cove DJ (2007) Physcomitrella patens: mosses enter the genomic age. Curr Opin Plant Biol 10:182. doi:10.1016/j.pbi.2007.01.005
Rensing SA, Lang D, Zimmer AD, Terry A, Salamov A, Shapiro H et al (2008) The Physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science 319:64–69. doi:10.1126/science.1150646
Saez A, Apostolova N, Gonzalez-Guzman M, Gonzalez-Garcia MP, Nicolas C, Lorenzo O et al (2004) Gain-of-function and loss-of-function phenotypes of the protein phosphatase 2C HAB1 reveal its role as a negative regulator of abscisic acid signalling. Plant J 37:354–369. doi:10.1046/j.1365-313X.2003.01966.x
Saez A, Robert N, Maktabi MH, Schroeder JI, Serrano R, Rodriguez PL (2006) Enhancement of abscisic acid sensitivity and reduction of water consumption in Arabidopsis by combined inactivation of the protein phosphatases type 2C ABI1 and HAB1. Plant Physiol 141:1389–1399. doi:10.1104/pp.106.081018
Schaefer DG, Zrÿd JP (1997) Efficient gene targeting in the moss Physcomitrella patens. Plant J 11:1195–1206. doi:10.1046/j.1365-313X.1997.11061195.x
Schaefer DG, Zrÿd JP (2001) The moss Physcomitrella patens, now and then. Plant Physiol 127:1430–1438. doi:10.1104/pp.010786
Schweighofer A, Hirt H, Meskiene I (2004) Plant PP2C phosphatases: emerging functions in stress signaling. Trends Plant Sci 9:236. doi:10.1016/j.tplants.2004.03.007
Schweighofer A, Kazanaviciute V, Scheikl E, Teige M, Doczi R, Hirt H et al (2007) The PP2C-type phosphatase AP2C1, which negatively regulates MPK4 and MPK6, modulates innate immunity, jasmonic acid, and ethylene levels in Arabidopsis. Plant Cell 19:2213–2224. doi:10.1105/tpc.106.049585
Sheen J (1998) Mutational analysis of protein phosphatase 2C involved in abscisic acid signal transduction in higher plants. Proc Natl Acad Sci USA 95:975–980. doi:10.1073/pnas.95.3.975
Tähtiharju S, Palva T (2001) Antisense inhibition of protein phosphatase 2C accelerates cold acclimation in Arabidopsis thaliana. Plant J 26:461–470. doi:10.1046/j.1365-313X.2001.01048.x
Xiong L, Zhu JK (2003) Regulation of abscisic acid biosynthesis. Plant Physiol 133:29–36. doi:10.1104/pp.103.025395
Yamaguchi-Shinozaki K, Shinozaki K (1994) A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell 6:251–264
Yoshida R, Umezawa T, Mizoguchi T, Takahashi S, Takahashi F, Shinozaki K (2006a) The regulatory domain of SRK2E/OST1/SnRK2.6 interacts with ABI1 and integrates abscisic acid (ABA) and osmotic stress signals controlling stomatal closure in Arabidopsis. J Biol Chem 281:5310–5318. doi:10.1074/jbc.M509820200
Yoshida T, Nishimura N, Kitahata N, Kuromori T, Ito T, Asami T et al (2006b) ABA-hypersensitive germination3 encodes a protein phosphatase 2C (AtPP2CA) that strongly regulates abscisic acid signaling during germination among Arabidopsis protein phosphatase 2Cs. Plant Physiol 140:115–126. doi:10.1104/pp.105.070128
Zhang W, Qin C, Zhao J, Wang X (2004) Phospholipase D alpha 1-derived phosphatidic acid interacts with ABI1 phosphatase 2C and regulates abscisic acid signaling. Proc Natl Acad Sci USA 101:9508–9513. doi:10.1073/pnas.0402112101
Acknowledgments
We thank Dr. Tuan-hua David Ho and Dr. Jose Casaretto for providing DNA constructs of 35S-abi1-1 and Ubi-LUC, and Dr. Daisuke Takezawa for technical assistance with the freezing experiments and for his valuable advice. This work was supported by a JSPS Postdoctoral Fellowship for Research Abroad to Y.S., the Ministry of Education, Science, Sports and Culture, Grant-in-Aid for Young Scientists (B) to Y.S., and the Science Research Promotion Fund (2007) by the Promotion and Mutual Aid Corporation for Private Schools of Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Accession Numbers: PpABI1A-AB369256, PpABI1B-AB369255, pphn39k21-AB369257.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
Primers used. Supplementary material 1 (DOC 53 kb)
Fig. S1
Comparison of methods for measurement of protonemata growth. a Wild type protonemata were grown for 2 weeks on BCDAT medium, which contained various concentration of ABA. b Growth of protonemal colonies shown in (a) was determined by the image analysis software (black bars), or chlorophyll contents (white bars). Values are means ± SE (n = 21). Scale bars: 10 mm. Supplementary material 2 (EPS 7582 kb)
Fig. S2
The exon–intron structures of the ABI1, PpABI1A and PpABI1B genes. Untranslated regions, coding regions, and introns are represented by open boxes, filled boxes and horizontal thick lines, respectively. The arrowhead indicates the position of the Gly residue mutated in the abi1-1 allele. Numbers show the nucleotide positions from the 5′ end of the cDNAs. Supplementary material 3 (EPS 565 kb)
Fig. S3
Comparison of the amino acid sequences of ABI1, PpABI1A and PpABI1B. Amino acid sequences of ABI1, PpABI1A and PpABI1B were aligned using the T-COFFEE program (http://igs-server.cnrs-mrs.fr/Tcoffee/tcoffee_cgi/index.cgi). Identical amino acids are marked with asterisks (*), strongly similar amino acids are marked with two dots (:), and weakly similar amino acids are marked with one dot (·). Colors indicate alignment quality in a regional context. The PP2C domain is underlined in black. The arrowheads indicate two Gly residues in the catalytic domain of ABI1 that correlated with regulation of ABA signaling. Supplementary material 4 (EPS 3126 kb)
Rights and permissions
About this article
Cite this article
Komatsu, K., Nishikawa, Y., Ohtsuka, T. et al. Functional analyses of the ABI1-related protein phosphatase type 2C reveal evolutionarily conserved regulation of abscisic acid signaling between Arabidopsis and the moss Physcomitrella patens . Plant Mol Biol 70, 327–340 (2009). https://doi.org/10.1007/s11103-009-9476-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-009-9476-z