Abstract
A combined chemical, biochemical and molecular study was conducted to understand the differential accumulation of volatile sesquiterpenes in melon fruits. Sesquiterpenes were present mainly in the rinds of climacteric varieties, and a great diversity in their composition was found among varieties. Sesquiterpenes were generally absent in non-climacteric varieties. Two climacteric melon varieties, the green-fleshed ‘Noy Yizre'el’, and the orange-fleshed ‘Dulce’ were further examined. In ‘Noy Yizre'el’ the main sesquiterpenes accumulated are δ-cadinene, γ-cadinene and α-copaene, while α-farnesene is the main sesquiterpene in ‘Dulce’. Sesquiterpene synthase activities, mainly restricted to rinds of mature fruits, were shown to generate different sesquiterpenes in each variety according to the compositions found in rinds. EST melon database mining yielded two novel cDNAs coding for members of the Tps gene family termed CmTpsNY and CmTpsDul respectively, that are 43.2% similar. Heterologous expression in E. coli of CmTpsNY produced mainly δ-copaene, α-copaene, β-caryophyllene, germacrene D, α-muurolene, γ-cadinene, δ-cadinene, and α-cadinene, while CmTpsDul produced α-farnesene only. CmTpsNY was mostly expressed in ‘Noy Yizre'el’ rind while CmTpsDul expression was specific to ’Dulce’ rind. None of these genes was expressed in rinds of the non-climacteric ‘Tam Dew’ cultivar. Our results indicate that different sesquiterpene synthases encoded by different members of the Tps gene family are active in melon varieties and this specificity modulates the accumulation of sesquiterpenes. The genes are differentially transcriptionally regulated during fruit development and according to variety and are likely to be associated with chemical differences responsible for the unique aromas of melon varieties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Melons (Cucumis melo L., Cucurbitaceae) comprise a broad array of wild and cultivated genotypes differing in many traits such as climactericity during fruit ripening, the patterns of sugar and acid accumulation, as well as in the content and composition of secondary metabolites associated with color, taste and aroma (Seymour and McGlasson 1993; Perin et al. 2002; Morales et al. 2004; Burger et al. 2006). The aroma of melons, as is the case with most fruits, consists of complex mixtures of volatile compounds (Beaulieu and Grimm 2001; Jordan et al. 2001; Shalit et al. 2001; Yahyaoui et al. 2002; Aubert and Bourger 2004). Different cultivars display different aromas, and this is reflected in their volatile compositions (Shalit et al. 2001; Aubert and Bourger 2004; Aubert and Pitrat 2006; Beaulieu 2006). Volatile esters, mainly acetate derivatives, are prominent in climacteric melon varieties, together with lower amounts of norisoprenes, short-chain alcohols, aldehydes and sesquiterpenes (Shalit et al. 2000; Beaulieu and Grimm 2001; Shalit et al. 2001; Yahyaoui et al. 2002). Non-climacteric varieties often have lower levels of total volatiles, and especially lack the volatile esters. In contrast, volatile aldehydes and alcohols, albeit in low levels, are most abundant in non-climacteric melons (Shalit et al. 2001; Burger et al. 2006). This metabolic pattern is also prominent in ethylene-repressed transgenic melons (Flores et al. 2002). The variation in sesquiterpenes among cultivars has not been well characterized to date.
The biochemical and molecular events that control the generation of aroma compounds have recently been the focus of increased attention (Goff and Klee 2006; Pichersky et al. 2006). As a result, some genes involved in fruit aroma formation have been described (Aharoni et al. 2000; Sharon-Asa et al. 2003; Beekwilder et al. 2004; Pechous and Whitaker 2004; Simkin et al. 2004; Goff and Klee 2006). In melons, genes that encode acyl-CoA alcohol acetyltransferases (El-Sharkawy et al. 2005) and alcohol dehydrogenases (Manriquez et al. 2006), involved in the formation of volatile alcohols and esters that determine the unique aroma of fruit flesh, have been characterized. Orange-fleshed melons rich in β-carotene contain β-ionone, a key β-carotene-derived norisoprenoid with a profound typical aroma that is either absent or only present in minute quantities, in white and green-fleshed melon varieties. A gene encoding a carotenoid cleavage dioxygenase able to cleave carotenoids to release norisoprenoid compounds has recently been described (Ibdah et al. 2006). However, little is known about genes that control the unique aromas of rind tissues and the sesquiterpene content of these tissues.
Sesquiterpenes are fifteen-carbon compounds found in the majority of plant species and serve diverse roles in communication and defense (McGarvey and Croteau 1995; Trapp and Croteau 2001; Degenhardt et al. 2003; Ro et al. 2006). Sesquiterpenes are derived from farnesyl diphosphate by a group of enzymes termed sesquiterpene synthases, and encoded by members of the Tps gene family (Aubourg et al. 2002). Many sesquiterpene synthase genes have been described in plants. For example, two sesquiterpene synthase genes are induced in cucumber (Cucumis sativus L.) leaves upon spider-mite attacks. Their respective gene products are able to catalyze the conversion of farnesyl diphosphate to (E,E)-α-farnesene and (E)-β-caryophyllene, respectively, when expressed in E. coli, (Mercke et al. 2004). Although sesquiterpenes have been found in the flesh of melon fruits (Horvat and Senter 1987; Homatidou et al. 1992; Beaulieu and Grimm 2001; Shalit et al. 2001; Aubert and Bourger 2004; Beaulieu 2007) little is known about the composition in rinds and the variation within cultivars as well as the factors and biological processes that control their occurrence and accumulation. Here we describe the identification, characterization and molecular regulation of two novel members of the Tps family that are primarily expressed in ripe melon rind tissues: CmTpsNY and CmTpsDul. Following heterologous expression in E. coil we functionally identified them as cadinene synthase and α-farnesene synthase, respectively. This work is another step towards the understanding of the factors that contribute to the formation of aroma compounds in melon fruits as part of a larger program aimed at the molecular and biochemical characterization of traits determining fruit quality and diversity.
Materials and methods
Plant material
Sixteen commercial melon varieties and breeding lines that differ in color, flavor, and aroma, as well as in rind color and texture, were examined (Table 1). Four varieties are related to the ‘Galia’ market type. ‘Galia’ is an Israeli-bred melon F1 hybrid (Karchi 2000). Galia-type varieties are characterized by juicy green flesh and a finely netted rind. ‘Noy Yizre’el’ is a powdery mildew resistant cultivar derived from ‘Ha’Ogen’ which serves as the female parent of ‘Galia’, while ‘Krymka’, a muskmelon, is the male parent. A cross between ‘Krymka’ (female) and Eshkolit Ha’Amaqim’ (male) gave rise to another ‘Galia’ type variety named ‘Arava’ (Karchi 2000; Nuñez-Palenius et al. 2006). The remaining genotypes in this study are open pollinated accessions that represent the major marketing types of melons. Melons were grown in open field conditions at the Newe-Ya’ar Research Center under standard irrigation and fertilization during the summer seasons of 2004–2006. Female flowers were tagged, and fruits were harvested at the following stages of development: (1) young, green fruits (12 days after anthesis—DAA); (2) green fruits (25 DAA); (3) mature fruits (after change of color and the development of abscission zone); and (4) post-harvest fruits (mature fruits that were picked and kept for 7 days at 20°C). Fruit tissues were frozen in liquid nitrogen following the removal of seeds and the separation of the rind from the flesh by a manual vegetable peeler. Tissues from three separate fruits were collected for each developmental stage.
Analyses of volatiles
Solid Phase Microextraction (SPME)
Fresh melon tissues (1.5 g) were ground into a uniform powder under liquid nitrogen with a mortar and pestle. The powder was placed in a 20 ml SPME vial containing 3 ml of 20% (w/v) NaCl and 0.3 μg internal standard (2-heptanone), sealed and placed in 4°C until used. Headspace sampling was conducted immediately utilizing a 65 μm fused silica fiber coated with polydimethylsiloxane/divinylbenzene (PDMS/DVB) (Supelco). After 40 min the SPME syringe was introduced into the injector port of the GC-MS apparatus for further analysis (see below).
GC-MS analysis
Volatile compounds were analyzed on a GC-MS apparatus (Agilent Technologies CA, USA) equipped with an Rtx-5SIL MS (30 m*0.25 mm*0.25 μm) fused-silica capillary column. Helium (1 ml/min) was used as a carrier gas. The injector temperature was 250°C, set for splitless injection. The oven was set to 50°C for 1 min, and then the temperature was increased to 220°C at a rate of 5°C/min. Thermal desorption was allowed for 10 min. The detector temperature was 280°C. The mass range was recorded from m/z 41 to 350, with electron energy of 70 eV. Identification of the main components was done by comparison of mass spectra and retention time data with those of authentic samples and supplemented with a Wiley7 N and HP CH1607 GC-MS libraries.
Sesquiterpene synthase enzymatic activity
Cell-free extracts derived from melon
Fruits were cut into small pieces (1–2 g) and frozen at −40°C for up to 1 month until use. The frozen pieces were placed in a chilled mortar and ground with a pestle in the presence of sand and 1 g polyvinylpolypyrrolidone (PVPP) until a uniform powder was obtained. Ice-cold extraction buffer [50 mM bis Tris, pH 6.9, 10% (v/v) glycerol, 10 mM dithiothretiol (DTT), 5 mM Na2S2O5] was added (4:1 v/w) and the suspension was further extracted for an additional 30 s. The slurry was centrifuged at 20,000g for 10 min at 4°C. The supernatant (crude extract) was either used fresh or kept for up to 2 weeks at −40°C until its enzymatic activity determination (Shalit et al. 2000; Shalit et al. 2001).
Preparation of bacterial lysates
Individual bacterial pellets were suspended in 3 ml of lysis buffer containing 50 mM Bis tris pH 6.9, 10% v/v glycerol, 10 mM DTT, 5 mM Na2S2O5 and 30 μg lysozyme (Sigma grade VI from chicken egg, 60,000 units mg−1 protein). The samples were vigorously mixed and incubated in ice water (4°C) for 15 min. After the cells lysed, the suspensions were centrifuged (20,000g for 10 min at 4°C). The supernatants were used for characterization of the enzymatic activity of the gene products.
Enzymatic GC-MS assay
Enzymatic assays were performed by mixing 100 μl of crude extract, 10 mM MgCl2, 10 μM MnCl2, 10 μM FDP and reaction buffer [50 mM bis Tris, pH 6.9, 10% (v/v) glycerol, 10 mM dithiothretiol (DTT), 5 mM Na2S2O5] to a total volume of 300 μl. The reactions were incubated for 3 h at 30°C. After incubation the samples were analyzed by SPME GC-MS for the identification of volatile sesquiterpenes generated during the 30°C incubation.
Isolation and characterization of sesquiterpene synthase genes
Isolation of RNA and construction of melon fruit cDNA libraries
Total RNA was isolated using a modification to the method of LaClaire and Herrin (1997) from: (1) slices of the rind of young and mature fruits of cv. ‘Noy Yizre’el’ or (2) slices of mature fruit containing both rind and flesh tissues of ‘Dulce’ melons. Poly (A)+ mRNA was purified from 1 mg of total RNA by use of the Oligotex® mRNA purification kit according to manufacturer’s recommendations (Qiagen, Hilden, Germany).
Two types of cDNA libraries were created: (1) A SSH library enriched for the mature rind (against the young rind) of ‘Noy Yizre’el’ was constructed using the PCR-SelectTM kit (Clontech Laboratories, USA). The PCR products generated by the SSH were cloned into pGEM®-T Easy vector with the pGEM®-T Easy system kit (Promega, WI, USA). (2) An EST library of mature fruit of ‘Dulce’ was constructed using the ZAP cDNA Synthesis Kit and ZAP Express® cDNA Gigapack® III Gold Cloning Kit according to manufacturer’s recommendations (Stratagene, USA). Phage clones were converted to pBK-CMV phagemid expression-vector following the manufacturer’s instructions (Stratagene, USA).
Isolation of full length clones of CmTpsNY and CmTpsDul
Full length sequence of the CmTpsNY gene (1,716 bp) was obtained by 5′/3′ RACE (Roche, Mannheim, Germany) of the clone SSH9B15 (http://melon.bti.cornell.edu/). The 3′-Race was performed using the primer 5′-GACATCGCTTCCCACAAG-3′ while for the 5′-Race the primer 5′-CGTTTGTTACAATGTGATCACC-3′ was used. Full-length sequence of the second gene, FR15G19 (http://melon.bti.cornell.edu/), CmTpsDul, (1,683 bp) was obtained using a forward primer of (5′-ATGAGTTCAAACATATCAGCAATTCC-3′) and a reverse primer from the homologous gene Cucumis sativus E,E-α-farnesene synthase (5′ TTAGAGTGGTACAGAATCAACAAGC-3′). DNA sequencing was performed by Macrogen Inc. (Seoul, Korea).
Real-time PCR analysis
Total RNA was isolated separately from the rind and flesh tissues of ‘Dulce’ and ‘Noy Yizre’el’ fruits at various developmental stages as described above. This RNA was used as template for cDNA synthesis. RNA samples (50 μg) were treated with RNAse-free DNAse I (20 units) (EPICENTRE®, USA) for 15 min at 37°C. First-strand cDNA was synthesized from 0.8 μg of total RNA by Reverse-iTTM MAX 1st Strand Synthesis kit (ABgene®’s Inc., Epsom, UK), using a blend of random hexamers and anchored oligo-dT primers (3:1). The cDNA was then diluted in a total volume of 100 μl of 3 mM Tris–HCl, pH 7.2, 0.2 mM EDTA solution. A 2-μl aliquot of cDNA was used for each real-time PCR performed with the ABI Prism 7000 Sequence Detection System (Applied Biosystems, Foster, CA). Amplifications were conducted using the ABsoluteTM QPCR SYBR® Green Mixes (ABgene®’s Inc., Epsom, UK). The following primer sequences (0.2 μm final concentration) were used: (1) cyclophiline (a house-keeping gene, accessions no. DV632830) forward primer 5′-GATGGAGCTCTACGCCGATGTC-3′ and reverse 5′-CCTCCCTGGCACATGAAATTAG-3′; (2) CmTpsNY: forward primer 5′-CGAGGTTCTTCAATGGCTTTCAA-3′ and reverse primer 5′-GCGATGTCGTCCATGAGTCTACA-3′; (3) CmTpsDul: forward primer 5′-AATGAGCCAAAAGGAAACAACACC-3′ and reverse primer 5′-CCTTACACCATTTGGCCTCTACC-3′.
Thermal cycling was initiated by 15 min at 95°C, followed by 40 cycles of 90°C, 15 s; 60°C, for 1 min. A melting curve analysis was performed for each reaction to confirm the specificity of the amplification. Real-time PCR was performed in duplicate for each primer combination. Three biological samples of each developmental stage were tested. C t values were determined by the ABI Prism 7000 SDS software and exported into an MS Excel workbook (Microsoft Inc., Redmond, WA) for statistical analysis. Real-time efficiencies (E) were calculated from the slopes of standard curves for each gene (E = 10[−1/slope]). The relative expression ratio (R) was calculated according to Pfaffl (2001) while the control was the sample of rind of the respective young fruits (12 DPA).
Northern blot analysis
Twenty-five micrograms of total RNA pooled from three fruits for each developmental stage or tissue, were separated on 1% formaldehyde-agarose gel and transferred to Hybond N+ nylon (Amersham Biosciences) (Sambrook and Russell 2001). DNA fragments of CmTpsNY and CmTpsDul were labeled using the Rediprime TM II Prime Labeling System kit (Amersham Biosciences). Hybridization and washing were carried out as described by Church and Gilbert (1984). The radioactively labeled membranes were exposed to Kodak Biomax MS film and kept at −70°C for several days, according to the intensity of the radioactive signal.
Cloning of sesquiterpene synthase genes
CmTpsNY and CmTpsDul were subcloned into expression vector T7/NT-TOPO (Invitrogen) by PCR with the appropriate primers according to the manufacturer’s instructions.
Expression of CmTpsNY and CmTpsDul in Escherichia coli
Recombinant E. coli BL21 (DE3) Gold (Stratagene, La Jolla, CA) bacteria were plated in Luria-Bertani broth (LB)-agar containing 50 μg ml−1 ampicillin. Individual colonies were grown in 2 ml of LB liquid medium containing 50 μg ml−1 ampicillin overnight to be used as starter cultures. Five hundred microliters of bacterial cell suspensions were transferred into 50 ml of LB liquid medium containing ampicillin and grown at 37°C with shaking (200 rpm) until the OD600 reached 0.6. Isopropylthio-β-galactoside was then added to a final concentration of 0.3 mM, and the cultures were grown for another 4–5 h at room temperature and aliquoted into 1.5-ml aliquots. The cells were harvested by centrifugation at 20,000g for 10 min at 4°C and frozen at −20°C until use (Lavid et al. 2002).
Results and discussion
Sesquiterpene content in melon varieties
Sesquiterpene content and composition greatly differed according to the variety examined (Table 2). The highest levels of sesquiterpenes were found in ‘Eshkolit Ha’Amaqim’ rinds. ‘Eshkolit Ha’Amaqim’ is a climacteric smooth yellow elite breeding line. Indeed, ‘Eshkolit’ is one of the parental lines of ‘Arava’ an important economic line that has a very similar rind sesquiterpene composition as compared to ‘Eshkolit’ (Table 2).
The main sesquiterpene compounds in these varieties are γ-cadinene, δ-cadinene, α-copaene, E-caryophyllene and α-muurolene. Other lines analyzed, including ‘Noy Yizre’el’ and ‘Dudaim’, displayed a great variability in sesquiterpene compositions while ‘Krymka’ rinds contained mainly α-copaene. Interestingly, none of the above varieties contained α-farnesene, the only sesquiterpene component of ‘Dulce’ rinds. All of the non-climacteric varieties examined lacked significant levels of sesquiterpenes, but some of the climacteric varieties such ‘Vedrantais’ and ‘PI414723’ also lacked sesquiterpenes in their rind (Table 2). This indicates that sesquiterpene patterns in melon rinds are highly polymorphic and their levels might be influenced by climacteric traits.
To further investigate the developmental and tissue compartmentalization of sesquiterpene accumulation, two varieties, ‘Noy Yizre’el’ and ‘Dulce’, that differ in sesquiterpene composition were further analyzed. The main sesquiterpenes found in ‘Noy Yizre’el’ rind are α-copaene, β-caryophyllene, germacrene D, α-muurolene, γ-cadinene, δ-cadinene, and α-cadinene. No α-farnesene was detected in ‘Noy Yizre’el’ rinds, but conversely, the only sesquiterpene found in ‘Dulce’ rind was E,E α-farnesene (Tables 2 and 3). In both varieties, the flesh (both ripe and unripe) and the unripe rind tissues lacked noticeable levels of sesquiterpenes.
Low levels of sesquiterpenes have been previously found in the flesh of melon cultivars (Horvat and Senter 1987; Homatidou et al. 1992; Shalit et al. 2001). Sesquiterpenes have also been found in fruit rinds of apple and orange (Sharon-Asa et al. 2003; Pechous and Whitaker 2004). In apple rind, α-farnesene levels increased when the plant was attacked by pests (Pechous and Whitaker 2004). Citrus fruits contain sesquiterpenes (such as the sesquiterpene aldehydes α- and β-sinensal) that might serve defensive roles or greatly contribute to its aroma. Valencene, an important sesquiterpene hydrocarbon present in oranges and mandarins, is a precursor of the oxygenated derivative nootkatone, a compound with a typical grapefruit aroma present in pummelos and grapefruits (Sharon-Asa et al. 2003). Valencene is accumulated in oranges only upon maturation, or after exogenous ethylene applications (Sharon-Asa et al. 2003). Orange rinds also contain the sesquiterpene β-farnesene, which might play a role in plant defence against aphids (Maruyama et al. 2001). The role of the sesquiterpenes in melons, apart from imparting unique notes to melon rind aroma, is presently unknown.
Sesquiterpene synthase activity in flesh and rind of ripe ‘Noy Yizre’el’ and ‘Dulce’ melons
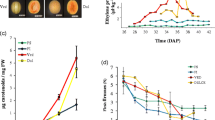
The bulk of sesquiterpenes in melon fruit are present in the rind (Tables 2 and 3). It was therefore of interest to determine if sesquiterpenes are biosynthesized in rind tissues or imported from the adjacent flesh tissues. To address this question, we compared the levels of sesquiterpene synthase activity in cell-free extracts derived from either rind or flesh tissues of ripe ‘Noy Yizre’el’ and ‘Dulce’ melons. Our results clearly indicate that enzymatic activity is measurable only in the rind of each cultivar, while flesh tissues displayed almost no sesquiterpene synthase activity (Fig. 1 and S1). There was a marked difference between the products generated in vitro from farnesyl diphosphate by the enzymatic extracts derived from each variety. The products generated by ‘Noy Yizre’el’ cell-free extracts were the same as those present in ‘Noy Yizre’el’ mature rinds and included α-copaene, caryophyllene, germacrene D, γ-, α- and δ-cadinene. In contrast, α-farnesene (the only sesquiterpene that accumulates in ‘Dulce’ rind) was the major product generated in cell-free extracts derived from the rind tissues of ‘Dulce’ (Fig. 1 and S1).
Sesquiterpene synthase activity in the flesh and rind of ripe ‘Noy Yizre’el’ and ‘Dulce’ melon fruits. Cell-free extracts prepared from the flesh (A and C) and from the rind (B and D) of ‘Noy Yizre’el’ and ‘Dulce’ respectively, were incubated with FDP under the assay conditions. The products formed in rind of ‘Noy Yizre’el’ (left panels) were: 1—a-Copaene, 2—Caryophyllene-E, 3—Germacrene D, 4—α-Muurolene, 5—γ-Cadinene, 6—δ-Cadinene, 7—α Cadinene. The product formed by ‘Dulce’ extracts (right panels) was α-farnesene. See Table 3 for structures and the chemical identification and supplemental material for the mass spectra
Optimization of the levels of sesquiterpene synthase activity was performed using cell-free extracts derived from the rinds of ripe ‘Noy Yizre’el’ melons. The pH optimum was pH 7 (within the pH range of 6.5–9) and the enzyme activity had an absolute requirement for the presence of divalent metal ions for efficient catalysis. Optimal levels of activity were obtained in the presence of either 10 mM MgCl2 or 10 μM MnCl2. These conditions are in accordance with results obtained for other enzymes of the terpene synthase family that also require the presence of divalent metal ions for catalysis (Alonso and Croteau 1993; Croteau and Cane 1985), and thus these conditions were adopted for the rest of the experiments.
Sesquiterpene synthase activity during fruit development
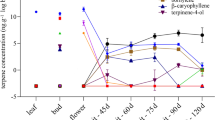
Since both the aroma properties of melons and the levels of sesquiterpenes are enhanced during fruit ripening (Table 3) it was of interest to assess whether sesquiterpene synthase activity limits the accumulation of sesquiterpenes during development. Sesquiterpene synthase activity was measured in protein extracts derived from rind tissues of ‘Noy Yizre’el’ and ‘Dulce’ melons at four different developmental stages. Extracts derived from young green fruits (12 and 25 days after anthesis) showed no detectable sesquiterpene synthase activity. Only the extracts derived from the rind of mature and post-harvest (see material and methods section) fruits produced sesquiterpenes following incubation with farnesyl diphosphate. As before, the sesquiterpene products obtained with the ‘Noy Yizre’el’ extracts were α-copaene, caryophyllene, germacrene D, γ-, α- and δ-cadinene (Fig. 2). Extracts from mature and postharvest ‘Dulce’ rind tissues yielded only E-E-α-farnesene (Fig. 2). Our results further indicate that sesquiterpenes in melon rinds are biosynthesized in situ, and this process is developmentally regulated by the levels of sesquiterpene synthase activity. In citrus fruit, the levels of the sesquiterpene valencene are enhanced upon ripening or upon exogenous ethylene treatments (Sharon-Asa et al. 2003). Although ethylene has been shown to influence the biosynthesis of many volatiles in melons, it is presently unknown if it has any effect on sesquiterpene levels (Flores et al. 2002; Yahyaoui et al. 2002; Manriquez et al. 2006). Non-climacteric melon varieties do not display sesquiterpenes (Table 2 and Shalit et al. 2001), but not all the climacteric varieties display sesquiterpenes in their mature rinds (Table 2).
Sesquiterpene synthase activity of ‘Noy Yizre’el’ and ‘Dulce melons’ during various stages of development. Protein extracts prepared from the rind of young, green fruits (12 DAA), green fruits (25 DAA), mature fruits and post-harvest fruits were incubated with FDP under the conditions described in Materials and Methods. Identification of the products was done by GC-MS according to the retention time and the mass spectrum. The products are: 1—α-Copaene, 2—β-Caryophyllene, 3—α-Muurolene, 4—γ-Cadinene, 5—δ-Cadinene, for ‘Noy Yizre’el’ and only is α-Farnesene for ‘Dulce’. See Table 3 for structures and the chemical identification and supplemental material for the mass spectra
Isolation and characterization of CmTpsNY and CmTpsDul
The melon EST Database (http://melon.bti.cornell.edu/) contains a comprehensive annotation of clones from a collection of EST libraries, most of them from fruit, has proven to be a good source for identifying genes affecting quality characteristics in melon, including aroma traits (Lewinsohn et al. 2004; Yariv et al. 2004; Burger et al. 2006; Ibdah et al. 2006). Data mining of this database yielded two different ESTs with significant similarity to members of the Tps gene family. The first clone, SSH9B15, a 306 bp sequence mined from an SSH library enriched for rind of ripe ‘Noy Yizre’el’ fruit versus the rind of young ‘Noy Yizre’el’ fruit, showed a relatively high similarity (59% at the amino acid level) to a sequence coding for a germacrene D synthase from poplar (Arimura et al. 2004). The full-length clone was obtained using rapid amplification of cDNA ends (RACE). The resulted full length gene, termed CmTpsNY, encoded for 571 aa and displayed a high similarity (72% at the amino acid level) to the β-caryophyllene synthase from Cucumis sativus leaves (Mercke et al. 2004) (Fig. 3). The second clone, FR15G19, consisting 432 bp, was isolated from an EST library of mature ‘Dulce’ fruit and displayed a high similarity (86% at the amino acid level) to the α-farnesene synthase from Cucumis sativus leaves (Mercke et al. 2004). A full-length cDNA clone of this gene was obtained by PCR using a forward primer based on the melon sequence and a reverse primer based on the cucumber sequence. The full-length clone was designated CmTpsDul. The largest open-reading frame of CmTpsDul encoded a predicted protein of 560 aa (Fig. 3).
Alignment of the deduced amino acid sequences of CmTpsNY, CmTpsDul and selected sesquiterpene synthases: Cucumis sativus beta-caryophyllene synthase (accession no. AAU05952), Populus balsamifera (−)-germacrene-D-synthase (accession no. AAR99061), Gossypium arboreum (+)-delta-cadinene synthase (accession no. CAA65289), Cucumis sativus E,E-alpha-farnesene synthase (accession no.AAU05951). The amino acids marked are the conserved motifs: RRx8W; RxR and DDXXD
A comparison of the predicted amino acid sequences of CmTpsNY and CmTpsDul indicates that the level of similarity between them is relatively low (43.2%). The melon genes share three common conserved motifs known to be found in members of the Tps gene family, including (a) RR(x)8W motif previously described to be essential for the initial diphosphate migration of the isomerisation-cyclization reaction of limonene synthase in Mentha. (b) RxR and DDxxD motifs suggested to be involved in catalysis (Croteau and Cane 1985; Alonso and Croteau 1993; Back and Chappell 1995; Steele et al. 1998; Williams et al. 1998; Davis and Croteau 2000; Aubourg et al. 2002). The RR(x)8W motif of CmTpsDul is conserved while that of CmTpsNY has a known variation in which a serine substitutes an arginine RS(x)8W. Based on phylogenetic analysis of the two melon Tps genes with respect to other members of the Tps gene family we conclude that CmTpsNY and CmTpsDul belong to the Tps-a group (class III) (Bohlmann et al. 1998; Trapp and Croteau 2001; Aubourg et al. 2002) (Fig. 4).
Phylogenetic tree of the deduced amino acid sequences of functionally characterized sesquiterpene synthases genes, including CmTpsDul and CmTpsNY. The deduced protein sequences were clustered using simple default parameters of ClustalW program (www.ebi.ac.uk/) and the radial tree representation was realized using the TREEVIEW application (Page 1996). Subdivision of the TPS gene family into six subfamilies, designated TPSa to TPSf, each distinguished by sharing a minimum of 40% identity among members (Bohlmann et al. 1998). Class-I, class-II and class-III designation followed the classification of Aubourg et al. (2002), except for the genes of C. melo, C. sativus and Malus domestica. This classification is based on gene structure (Trapp and Croteau 2001), that is not available for the latter five genes. The accession numbers for the newly described TPS genes are: Cucumis melo—CmTpsNy (EU158098) and Cucumis melo—CmTpsDul (EU158099). The accession numbers for the additional TPS genes represented in the tree are: Abies grandis—4S-limonene synthase (AF006193); Abies grandis—myrcene synthase (U87908); Arabidopsis thaliana—TPS10 (AF178535); Arabidopsis thaliana—TPSGA1 (U11034); Arabidopsis thaliana—TPSGA2 (AF034774); Artemisia annua—epi-cedrol synthase (AF157059); Clarkia breweri—S-linalool synthase (U58314); Cucumus sativus—β-caryophyllene synthase (AAU05952); Cucumus sativus—E,E-α-farnesene synthase (AAU05951); Cucumis sativus—ent-kaurene synthase (AB045310); Cucurbita maxima—copalyl diphosphate synthase (AF049905); Cucurbita maxima—ent-kaurene synthase (U43904); Elaeis oleifera—sesquiterpene synthase (AAC31570); Malus × domestica—E,E-alpha-farnesene synthase (AY787633); Mentha longifolia—4S-limonene synthase (AF175323); Mentha × piperita—E-beta-farnesene synthase (AF024615); Nicotiana tabacum—5-epi-aristolochene synthase (L04680); Pisum sativum—copalyl diphosphate synthase (U63652); Populus balsamifera—germacrene-D-synthase (AAR99061); Salvia officinalis—(+)-sabinene synthase (AF051901); Vitis vinifera—terpene synthase (AAS66357)
Functional expression of CmTpsNY and CmTpsDul in E. coli
In order to functionally determine the biochemical role of CmTpsNY and CmTpsDul the genes were heterologoulsy expressed in E. coli and the recombinant gene products were assayed in vitro for sesquiterpene synthase activity. Bacterial lysates derived from recombinant E. coli expressing CmTpsNY catalyzed the formation of three sesquiterpenes from farnesyl diphosphate: δ-cadinene, γ-cadinene and lower levels of α-copaene (Fig. 5a). In parallel, heterologous expression of CmTpsDul in E. coli yielded a protein extract possessing sesquiterpene synthase activity able to catalyze the conversion of farnesyl diphosphate into α-farnesene (Fig. 5b). These findings further indicate that the database mining yielded genes with biological relevance and probably account for the chemical variation observed within the melon varieties (Table 2, Fig. S1).
GC-MS of the products generated in vitro by recombinant CmTpsNY (A) and CmTpsDul (B) genes. Bacterial lysates derived from E. coli overexpressing recombinant CmTpsNY or CmTpsDul were incubated with FDP under the conditions described in Materials and Methods. Identification of the products was done by GC-MS according to the retention time and by comparing the compound mass spectrum to literature. Recombinant CmTpsNY produced three different sesquiterpenes (A) while recombinant CmTpsDul produced a single product (B). No product was detected in controls without substrate FDP, without cofactors, or construct devoid of the recombinant gene (data not shown)
The cucumber CsαFs gene and the melon CmTpsDul share a relatively high degree of similarity (89% at the aa level and 93.2% at the nucleotide level). Both genes encode for proteins able to catalyze the conversion of farnesyl diphosphate to α-farnesene in vitro when functionally analyzed (Fig. 5b and Mercke et al. 2004), thus they seemingly have identical biochemical functions. Nevertheless they are expressed in different tissues (the cucumber gene is expressed in leaves after spider mite attack while the melon gene in ripe melon rind). These findings possibly indicate the recruitment of similar genes to different biological functions and emphasize the versatility of the terpene pathway in directing sesquiterpene biosynthesis in different physiological situations.
It is well known that some enzymes of the Tps family utilize a single substrate to generate a variety of products (Croteau and Cane 1985; Lewinsohn et al. 1992; Bohlmann et al. 1998; Mercke et al. 1999; Kollner et al. 2004; Deguerry et al. 2006), but others generate single products (Prosser et al. 2002; Sharon-Asa et al. 2003; Iijima et al. 2004; Deguerry et al. 2006; Pichersky et al. 2006). Interestingly, melon cultivars exhibit both of these types of enzymes, CmTpsNY produces several products, and CmTpsDul produces only one sesquiterpene product from farnesyl diphosphate.
Expression of CmTpsNY and CmTpsDul in melon rind and flesh during fruit development
In both varieties, sesquiterpene compounds (Table 3) and the sesquiterpene synthase activities (Fig. 1) are mainly restricted to the rind tissues of the ripe fruit. Northern blots and real-time PCR analyses were performed to explore for possible transcriptional tissue and varietal specific regulation of sesquiterpene accumulation. The results indicate that the levels of expression of CmTpsNY are negligible in ‘Noy Yizre’el’ during the early stages (days 12 and 25 after anthesis) of development but dramatically rise during ripening (Fig. 6a and b), in accordance with the levels of the extractable sesquiterpene synthase activity. Moreover, expression is found exclusively in rind tissues. Similarly, the expression patterns of CmTpsDul in ‘Dulce’ melons follow a pattern parallel to sesquiterpene synthase activity in this variety. The highest levels of expression were found in mature rind tissues, though lower but significant levels of expression were also found in the rind of post-harvest fruits as well as in flesh of mature and post-harvest fruits (Fig. 6c and d).
Expression of Tps genes in melon tissues during development Real-time PCR analysis of CmTpsNY (a) and CmTpsDul (c). RNA was extracted separately from the rind and flesh at various stages of fruit development (12 DAA, 25 DAA, mature and post-harvest). Expression levels were normalized with respect to the internal control cyclophilin and are plotted relative to the expression of the 12 DAA samples. Values presented represent the mean ± SE derived from three biological replicates. (b) and (d) Northern blot analysis of sesquiterpene synthase genes in melon fruit. Each lane was loaded with 25 μg of total RNA extracted from the rind and flesh of ‘Noy Yizre’el’ and ‘Dulce’ at the stages of fruit development listed above. Blots were hybridized to CmTpsNY (b) or to CmTpsDul (d) probes. Ribosomal RNA was visualized by ethidium bromide staining and served as a loading reference
Interestingly, CmTpsDul was not expressed in either the rind or flesh of ‘Noy Yizre’el’ melons at any developmental stage and, reciprocally, CmTpsNY was not expressed in ‘Dulce’ tissues.
Two other melon cultivars: ‘Tam-Dew’ (C. melo subspecies melo group inodorus) and ‘PI 414723’ (C. melo subspecies agrestis group momordica) that do not contain sesquiterpenes in their mature rinds (Table 2) were further examined for possible expression of CmTpsNY or CmTpsDul. In both of these varieties, gene expression was negligible in the mature flesh or rind tissues (not shown). This further indicates that sesquiterpene biosynthesis in melon rinds is regulated at the transcriptional level, in accordance to most of the cases tested, including in elicited tobacco cell cultures (Chappell 1995), potato and tomato leaves (Yoshioka et al. 1999; Kant et al. 2004), basil glandular trichomes (Iijima et al. 2004) cucumber leaves (Mercke et al. 2004), rose flowers (Guterman et al. 2002), orange fruit (Sharon-Asa et al. 2003) and Arabidopsis (Tholl et al. 2005).
Our results not only further corroborate that sesquiterpene formation is transcriptionally regulated during fruit development, but we have also demonstrated that each variety examined expresses unique sesquiterpene synthases that are not expressed in other varieties examined. The unique expression patterns of each gene in a particular variety leads to the formation of unique sesquiterpenes that may contribute to the diversity in the characteristic aromas of melon cultivars. Rind aroma carries a great economical value affecting consumers’ choice of melons. Therefore, our understanding of the underlying biosynthetic pathways to sesquiterpenes and their molecular regulation will enable the development of tools for obtaining novel crop varieties with improved quality and aroma traits without compromising other agronomically important characteristics. The understanding of the diverse ecological roles played by the sesquiterpene family, as defence compounds, pollinator and seed-disperser attractants (McGarvey and Croteau 1995; Trapp and Croteau 2001; Degenhardt et al. 2003), will also benefit from our knowledge of the molecular control of their synthesis.
References
Aharoni A, Keizer LCP, Bouwmeester HJ, Sun ZK, Alvarez-Huerta M, Verhoeven HA, Blaas J, van Houwelingen AMML, De Vos RCH, van der Voet H, Jansen RC, Guis M, Mol J, Davis RW, Schena M, van Tunen AJ, O’Connell AP (2000) Identification of the SAAT gene involved in strawberry flavor biogenesis by use of DNA microarrays. Plant Cell 12:647–661
Alonso WR, Croteau R (1993) Prenyltransferases and cyclases. Methods Plant Biochem 9:239–260
Arimura G, Huber DPW, Bohlmann J (2004) Forest tent caterpillars (Malacosoma disstria) induce local and systemic diurnal emissions of terpenoid volatiles in hybrid poplar (Populus trichocarpa x deltoides): cDNA cloning, functional characterization, and patterns of gene expression of (-)-germacrene D synthase, PtdTPS1. Plant J 37:603–616
Aubert C, Bourger N (2004) Investigation of volatiles in charentais cantaloupe melons (Cucumis melo Var. Cantalupensis). Characterization of aroma constituents in some cultivars. J Agric Food Chem 52:4522–4528
Aubert C, Pitrat M (2006) Volatile compounds in the skin and pulp of Queen Anne’s pocket melon. J Agric Food Chem 54:8177–8182
Aubourg S, Lecharny A, Bohlmann J (2002) Genomic analysis of the terpenoid synthase (AtTPS) gene family of Arabidopsis thaliana. Mol Genet Genom 267:730–745
Back K, Chappell J (1995) Cloning and bacterial expression of a sesquiterpene cyclase from Hyoscyamus-muticus and its molecular comparison to related terpene cyclases. J Biol Chem 270:7375–7381
Beaulieu JC (2006) Volatile changes in cantaloupe during growth, maturation, and in stored fresh-cuts prepared from fruit harvested at various maturities. J Am Soc Hortic Sci 131:127–139
Beaulieu JC (2007) Effect of UV irradiation on cut cantaloupe: Terpenoids and esters. J Food Sci 72:S272–S281
Beaulieu JC, Grimm CC (2001) Identification of volatile compounds in cantaloupe at various developmental stages using solid phase microextraction. J Agric Food Chem 49:1345–1352
Beekwilder J, Alvarez-Huerta M, Neef E, Verstappen FWA, Bouwmeester HJ, Aharoni A (2004) Functional characterization of enzymes forming volatile esters from strawberry and banana. Plant Physiol 135:1865–1878
Bohlmann J, Meyer-Gauen G, Croteau R (1998) Plant terpenoid synthases: molecular biology and phylogenetic analysis. Proc Natl Acad Sci USA 95:4126–4133
Burger Y, Sa’ar U, Paris HS, Lewinsohn E, Katzir N, Tadmor Y, Schaffer AA (2006) Genetic variability for valuable fruit quality traits in Cucumis melo. Isr J Plant Sci 54:233–242
Chappell J (1995) Biochemistry and molecular-biology of the isoprenoid biosynthetic-pathway in plants. Annu Rev Plant Physiol Plant Mol Biol 46:521–547
Church GM, Gilbert W (1984) Genomic sequencing. Proc Natl Acad Sci USA 81:1991–1995
Croteau R, Cane DE (1985) Monoterpene and sesquiterpene cyclases. Methods Enzymol 110:383–405
Davis EM, Croteau R (2000) Cyclization enzymes in the biosynthesis of monoterpenes, sesquiterpenes, and diterpenes Topics Curr Chem 209:53–95
Degenhardt J, Gershenzon J, Baldwin IT, Kessler A (2003) Attracting friends to feast on foes: engineering terpene emission to make crop plants more attractive to herbivore enemies. Curr Opin Biotechnol 14:169–176
Deguerry F, Pastore L, Wu SQ, Clark A, Chappell J, Schalk M (2006) The diverse sesquiterpene profile of patchouli, Pogostemon cablin, is correlated with a limited number of sesquiterpene synthases. Arch Biochem Biophys 454:123–136
El-Sharkawy I, Manriquez D, Flores F, Regad F, Bouzayen M, Latche A, Pech J (2005) Functional characterization of a melon alcohol acyl-transferase gene family involved in the biosynthesis of ester volatiles. Identification of the crucial role of a threonine residue for enzyme activity. Plant Mol Biol 59:345–362
Flores F, El Yahyaoui F, de Billerbeck G, Romojaro F, Latche A, Bouzayen M, Pech JC, Ambid C (2002) Role of ethylene in the biosynthetic pathway of aliphatic ester aroma volatiles in Charentais Cantaloupe melons. J Exp Bot 53:201–206
Goff SA, Klee HJ (2006) Plant volatile compounds: sensory cues for health and nutritional value? Science 311:815–819
Guterman I, Shalit M, Menda N, Piestun D, Dafny-Yelin M, Shalev G, Bar E, Davydov O, Ovadis M, Emanuel M, Wang J, Adam Z, Pichersky E, Lewinsohn E, Zamir D, Vainstein A, Weiss D (2002) Rose scent: genomics approach to discovering novel floral fragrance-related genes. Plant Cell 14:2325–2338
Homatidou VI, Karvouni SS, Dourtoglou VG, Poulos CN (1992) Determination of total volatile components of Cucumis melo L. variety cantaloupensis. J Agric Food Chem 40:1385–1388
Horvat RJ, Senter SD (1987) Identification of additional volatile compounds from cantaloupe. J Food Sci 52:1097–1098
Ibdah M, Azulay Y, Portnoy V, Wasserman B, Bar E, Meir A, Burger Y, Hirschberg J, Schaffer AA, Katzir N, Tadmor Y, Lewinsohn E (2006) Functional characterization of CmCCD1, a carotenoid cleavage dioxygenase from melon. Phytochemistry 67:1579–1589
Iijima Y, Gang DR, Fridman E, Lewinsohn E, Pichersky E (2004) Characterization of geraniol synthase from the peltate glands of sweet basil. Plant Physiol 134:370–379
Jordan MJ, Shaw PE, Goodner KL (2001) Volatile components in aqueous essence and fresh fruit of Cucumis melo cv. Athena (muskmelon) by GC-MS and GC-O. J Agric Food Chem 49:5929–5933
Kant MR, Ament K, Sabelis MW, Haring MA, Schuurink RC (2004) Differential timing of spider mite-induced direct and indirect defenses in tomato plants. Plant Physiol 135:483–495
Karchi Z (2000) Development of melon culture and breeding in Israel. In: Katzir N, Paris HS (eds) Proceedings of Cucurbitaceae 2000. Acta Hort 510:13–17
Kollner TG, Schnee C, Gershenzon J, Degenhardt J (2004) The Variability of sesquiterpenes emitted from two Zea mays cultivars is controlled by allelic variation of two terpene synthase genes encoding stereoselective multiple product enzymes. Plant Cell 16:1115–1131
LaClaire JW, Herrin DL (1997) Co-isolation of high-quality DNA and RNA from coenocytic green algae. Plant Mol Biol Rep 15:263–272
Lavid N, Wang JH, Shalit M, Guterman I, Bar E, Beuerle T, Menda N, Shafir S, Zamir D, Adam Z, Vainstein A, Weiss D, Pichersky E, Lewinsohn E (2002) O-methyltransferases involved in the biosynthesis of volatile phenolic derivatives in rose petals. Plant Physiol 129:1899–1907
Lewinsohn E, Gijzen M, Croteau R (1992) Wound-inducible pinene cyclase from grand fir – purification, characterization, and renaturation after SDS-PAGE. Arch Biochem Biophys 293:167–173
Lewinsohn E, Benyamini Y, Yariv Y, Portnoy V, Falah M, Larkov O, Bar E, Ravid U, Tadmor Y, Burger Y, Schaffer AA, Katzir N (2004) Multidisciplinary genomic approaches for the identification and characterization of genes involved in the formation of melon aroma. In: Kanlayanarat S, McGlasson WB (eds) Proceedings of the APEC symposium on quality management in postharvest systems. Bangkok, Thailand, pp 57–63
Manriquez D, El-Sharkawy I, Flores FB, El-Yahyaoui F, Regad F, Bouzayen M, Latche A, Pech JC (2006) Two highly divergent alcohol dehydrogenases of melon exhibit fruit ripening-specific expression and distinct biochemical characteristics. Plant Mol Biol 61:675–685
Maruyama T, Ito M, Honda G (2001) Molecular cloning, functional expression and characterization of (e)-beta-farnesene synthase from citrus junos. Biol Pharm Bull 24:1171–1175
McGarvey DJ, Croteau R (1995) Terpenoid metabolism. Plant Cell 7:1015–1026
Mercke P, Crock J, Croteau R, Brodelius PE (1999) Cloning, expression, and characterization of epi-cedrol synthase, a sesquiterpene cyclase from Artemisia annua L. Arch Biochem Biophys 369:213–222
Mercke P, Kappers IF, Verstappen FWA, Vorst O, Dicke M, Bouwmeester HJ (2004) Combined transcript and metabolite analysis reveals genes involved in spider mite induced volatile formation in cucumber plants. Plant Physiol 135:2012–2024
Morales M, Roig E, Monforte AJ, Arus P, Garcia-Mas J (2004) Single-nucleotide polymorphisms detected in expressed sequence tags of melon (Cucumis melo L.). Genome 47:352–360
Nuñez-Palenius H, Cantliffe DJ, Huber DJ, Ciardi J, Klee HJ (2006) Transformation of a muskmelon ‘Galia’ hybrid parental line (Cucumis melo L. var. reticulatus Ser.) with an antisense ACC oxidase gene. Plant Cell Rep 25:198–205
Page RDM (1996) Tree view: an application to display phylogenetic trees on personal computers. Bioinformatics 12:357–358
Pechous SW, Whitaker BD (2004) Cloning and functional expression of an (e,e)-alpha-farnesene synthase cDNA from peel tissue of apple fruit. Planta 219:84–94
Perin C, Gomez-Jimenez M, Hagen L, Dogimont C, Pech JC, Latche A, Pitrat M, Lelievre JM (2002) Molecular and genetic characterization of a non-climacteric phenotype in melon reveals two loci conferring altered ethylene response in fruit. Plant Physiol 129:300–309
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:2002–2007
Pichersky E, Noel JP, Dudareva N (2006) Biosynthesis of plant volatiles: nature’s diversity and ingenuity. Science 311:808–811
Pitrat M, Hanelt H, Hammer K (2000) Some comments on infraspecific classification of cultivars of melon. In: Katzir N, Paris HS (eds) Proceedings of Cucurbitaceae 2000. Acta Hort 510:29–36
Prosser I, Phillips A, Gittings S, Lewis MG, Hooper AM, Pickett JA, Beale MH (2002) (+)-(10R)-Germacrene A synthase from goldenrod, Solidago canadensis; cDNA isolation, bacterial expression and functional analysis. Phytochemistry 60:691–702
Ro DK, Ehlting J, Keeling CI, Lin R, Mattheus N, Bohlmann J (2006) Microarray expression profiling and functional characterization of AtTPS genes: duplicated Arabidopsis thaliana sesquiterpene synthase genes At4g13280 and At4g13300 encode root-specific and wound-inducible (Z)-gamma-bisabolene synthases. Arch Biochem Biophys 448:104–116
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New York
Seymour GB, McGlasson WB (1993) Melons. In: Seymour GB, Taylor J, Tucker G (eds) Biochemistry of fruit ripening. Chapman and Hall, London, pp 273–290
Shalit M, Katzir N, Larkov O, Burger Y, Shalechet F, Lastochkin E, Ravid U, Amar O, Edelstein M, Lewinsohn E (2000) Aroma formation in muskmelons. Volatile acetates in ripening fruits. In: Katzir N, Paris HS (eds) Proceedings of Cucurbitaceae 2000, 7th Eucarpia Meeting on Cucurbit Genetics and Breeding. Acta Hort 510:455–461
Shalit M, Katzir N, Tadmor Y, Larkov O, Burger Y, Shalekhet F, Lastochkin E, Ravid U, Amar O, Edelstein M, Karchi Z, Lewinsohn E (2001) Acetyl-Coa: alcohol acetyltransferase activity and aroma formation in ripening melon fruits. J Agric Food Chem 49:794–799
Sharon-Asa L, Shalit M, Frydman A, Bar E, Holland D, Or E, Lavi U, Lewinsohn E, Eyal Y (2003) Citrus fruit flavor and aroma biosynthesis: isolation, functional characterization, and developmental regulation of Cstps1, a key gene in the production of the sesquiterpene aroma compound valencene. Plant J 36:664–674
Simkin AJ, Schwartz SH, Auldridge M, Taylor MG, Klee HJ (2004) The tomato carotenoid cleavage dioxygenase 1 genes contribute to the formation of the flavor volatiles beta-ionone, pseudoionone, and geranylacetone. Plant J 40:882–892
Steele CL, Crock J, Bohlmann J, Croteau R (1998) Sesquiterpene synthases from grand fir (Abies grandis) – comparison of constitutive and wound-induced activities, and cDNA isolation, characterization and bacterial expression of delta-selinene synthase and gamma-humulene synthase. J Biol Chem 273:2078–2089
Tholl D, Chen F, Petri J, Gershenzon J, Pichersky E (2005) Two sesquiterpene synthases are responsible for the complex mixture of sesquiterpenes emitted from Arabidopsis flowers. Plant J 42:757–771
Trapp SC, Croteau RB (2001) Genomic organization of plant terpene synthases and molecular evolutionary implications. Genetics 158:811–832
Williams DC, McGarvey DJ, Katahira EJ, Croteau R (1998) Truncation of limonene synthase preprotein provides a fully active ‘pseudomature’ form of this monoterpene cyclase and reveals the function of the amino-terminal arginine pair. Biochemistry 37:12213–12220
Yahyaoui FEL, Wongs-Aree C, Latche A, Hackett R, Grierson D, Pech JC (2002) Molecular and biochemical characteristics of a gene encoding an alcohol acyl-transferase involved in the generation of aroma volatile esters during melon ripening. Eur J Biochem 269:2359–2366
Yariv Y, Portnoy V, Burger Y, Benyamini Y, Lewinsohn E, Tadmor Y, Ravid U, White R, Giovannoni J, Schaffer AA, Katzir N (2004) Isolation and characterization of fruit-related genes in melons (Cucumis melo) using SSH and macroarray techniques. In: Lebeda A, Paris HS (eds) Proceedings of Cucurbitaceae 2004, the 8th EACARPIA meeting on cucurbit genetics and breeding. pp 491–497
Yoshioka H, Yamada N, Doke N (1999) cDNA cloning of sesquiterpene cyclase and squalene synthase, and expression of the genes in potato tuber infected with Phytophthora infestans. Plant Cell Physiol 40:993–998
Acknowledgements
This work was partially supported by the ‘ARO Center for the Improvement of Cucurbit Fruit Quality’, by the Israel Ministry of Science Grant and by grant Nos. IS-3333-02 and IS-3877-06 of BARD, the United States-Israel Binational Agricultural Research and Development Fund and by the EU project MetaPhor Food-CT-2006-03622. Publication No. 122/2007 of the Agricultural Research Organization, Bet Dagan Israel. We thank Uzi Ravid, Olga Larkov, Uzi Sa’ar and Fabian Baumkoler and Eti Braindes for assistance with sample preparation and GC-MS analysis. We also thank Eyal Koren, Maya Lotan-Pompan, Tamar Zakai and Rachel Ofir for assistance with figure preparation and scientific writing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Vitaly Portnoy, Yael Benyamini and Einat Bar have equally contributed to the work.
The nucleotide sequence data reported here appears in the GenBank nucleotide database under the accession numbers EU158098 and EU158099.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11103_2008_9296_MOESM1_ESM.tif
Figure S1: Mass spectra of products formed by the expressed sesquiterpene synthase CmTpsNY and CmTpsDul with FDP as substrate. Cell-free extracts prepared from the flesh and rind of 'Noy Yizre’el' and 'Dulce' respectively were incubated with FDP under the assay conditions. Product identification was done by GC-MS according to the retention time and compared to their the mass spectrum (upper chromatogram, A–D, E–H) to literature (lower chromatogram, A–D, E–H). . The products are: A—α-Copaene, B—β-Caryophyllene, C—Germacrene D, D—α-Muurolene, E—g-Cadinene, F—d-Cadinene, G—a Cadinene. The product formed by 'Dulce' extracts (left panels) was α-farnesene (H). (TIF 59 kb)
Rights and permissions
About this article
Cite this article
Portnoy, V., Benyamini, Y., Bar, E. et al. The molecular and biochemical basis for varietal variation in sesquiterpene content in melon (Cucumis melo L.) rinds. Plant Mol Biol 66, 647–661 (2008). https://doi.org/10.1007/s11103-008-9296-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-008-9296-6