Abstract
Starch is the major component of cereal grains. In rice, starch properties determine the eating and cooking quality. The dull endosperm of rice grains is a classical morphological and agronomical trait that has long been exploited for breeding and genetics study. To understand the molecular mechanism that regulates the starch biosynthesis in rice grains, we characterized a classic rice mutant dull endosperm1 (du1) and isolated Du1 through a map-based cloning approach. Du1, encoding a member of pre-mRNA processing (Prp1) family, is expressed mainly in panicles. Du1 specifically affects the splicing efficiency of Wx b and regulates starch biosynthesis by mediating the expression of starch biosynthesis genes. Analysis of du1wx shows that Du1 acts upstream of Wx b. These results strongly suggest that Du1 may function as a regulator of the starch biosynthesis by affecting the splicing of Wx b and the expression of other genes involved in the rice starch biosynthetic pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Starch comprises ∼90% of the dry weight of rice grain, the staple food for more than half of the world’s population. Starch properties determine various aspects of rice quality, especially eating and cooking quality (Bao et al. 2004). Varieties different in amylose contents (ACs) have long been utilized to improve grain quality in breeding. High AC, in general, lowers rice eating quality, especially the stickiness of cooked rice, because high AC rice becomes dry and fluffy after cooking. Genetic and molecular marker-based QTL analyses have revealed that AC is mainly controlled by a major locus Wx and multi minor loci (Aluko et al. 2004; Bao et al. 2002; Fan et al. 2005; Hirano and Sano 2000; Lanceras et al. 2000; Okuno 1983; Septiningsih et al. 2003; Tan et al. 1999; Yano et al. 1988), including the 11 dull endosperm loci, du1 to du11 (Kinoshita 1987; Koh 1997; Satoh and Omura 1981, 1986). The du1 mutant was first reported in 1981 (Satoh and Omura 1981), which has a lower endosperm AC and intermediate degree of translucence when its grains are dry. Biochemical analysis suggested that the relative low AC in the du1 mutant is caused by a decreased level of Wx protein (Sano et al. 1985).
At least two functional alleles of the Wx gene, Wx a and Wx b, have been identified in Asian cultivated rice (Oryza sativa L.). The abundance of mature Wx transcripts and Wx protein in the Wx a grains are about 10-fold higher than in Wx b seeds. Wx a is widely distributed in O. sativa spp. indica, a subspecies with higher AC, whereas Wx b mainly in japonica, a subspecies with intermediate AC. It has been shown that the low level of the mature transcripts of Wx b is largely caused by a single nucleotide substitution of G-to-T at the splicing donor site of the first intron in Wx b, which results in the inefficient splicing of the Wx b pre-mRNA and the activation of two cryptic splice sites in exon 1 (Cai et al. 1998; Frances et al. 1998; Isshiki et al. 1998).
Although du1 has been extensively exploited in rice breeding (Qian et al. 1996), the understanding of starch biosynthesis in du mutants is still very limited. We report here the map-based cloning of Du1 and the elucidation of the molecular mechanism that Du1 regulates starch biosynthesis through altering the splicing efficiency of Wx bpre-mRNA in rice.
Materials and methods
Plant materials
The du1 mutant line kl704 (spp. japonica) was crossed with a japonica cultivar Xiushui11 (Qian et al. 1991) and an isogenic line carrying the du1 mutation was generated by 12 rounds of backcrosses with Xiushui11. For convenience, this isogenic line was renamed as the du1 mutant in this work and Xiushui11 was regarded as the wild type. To map and clone the Du1 gene, du1 was crossed with an indica variety, Minghui63, to construct an F2 mapping population.
All the rice materials including Xiushui11, Zhefu802 (ZF802), Qiufengnuo (QFN, a Chinese elite glutinous cultivar with a homozygous null mutation of wx), and du1 plants were cultivated in the field either at the China National Rice Research Institute or at Hainan Island in the natural growing seasons. For sampling, panicles were harvested 18 days after pollination (DAP). Leaf blades, sheathes, culms, and roots were sampled at the day of pollination and grains at the mature stage. Each sample was a collection of four independent major tillers, each from a different plant. All samples except the mature grains were immediately frozen in liquid nitrogen and stored at −80°C until use.
Genetic analysis and marker development
The Du1 locus was primarily mapped to Chromosome 10 with two newly developed STS markers, P2 and P3. To fine map Du1, additional five STS and four CAPS makers were developed (Supplemental Table 1) based on the sequence differences between indica var. 93-11 and japonica var. Nipponbare (http://www.ncbi.nlm.nih.gov).
Complementation test
An 8,285-bp genomic DNA fragment, containing an entire Du1 coding region, a 3,051-bp upstream region, and a 2,114-bp downstream sequence, was inserted into the binary vector pCAMBIA1300 to generate the transformation plasmid p1300Du1 for complementation. The two plasmids, p1300Du1 and its control pCAMBIA1300, were introduced into Agrobacterium tumefaciens EHA105 by electroporation and rice transformation was carried out as previously described (Li et al. 2003a). To identify the Du1 transgene in the transgenic lines, a CAPS marker dudc was developed based on the nucleotide difference between the mutant and wild type plants (Supplemental Table 1).
Sequence and phylogenetic analyses
The Prp1 family protein sequences were retrieved from SwissProt or GenBank and used for phylogenetic analyses. Multiple sequence alignments were conducted using ClustalX version 8.0 (Thompson et al. 1997). A neighbor-joining tree was built using MEGA version 2.1 adopting Poisson correction distance and the tree was presented using TreeView (Page 1996). Support for the tree obtained was assessed using the bootstrap method with 1,000 replicates. Similar topology was obtained by using the Protpas program in the Phylip package to estimate maximum parsimony and the Proml program in the Phylip package to estimate maximum likelihood (Felsenstein 2000). Alignments over the conserved regions of each family member produced trees of similar topology.
RNA extraction and cDNA preparation
Total RNAs were isolation from seed endosperm and other tissues as described previously (Mou et al. 2000). Total RNA (1 μg) was first treated with 1 unit of RNase-free DNase (Invitrogen) at room temperature for 15 min, denatured at 70°C for 10 min in a 10 μl reaction mixture containing 1 μl 25 mM EDTA, and put on ice immediately. The first-strand cDNA was synthesized according to the manufacture’s protocol of Reverse Transcription System (Promega).
Quantitative real-time RT-PCR
Quantitative assay of transcript abundance was performed with 1 μl of each cDNA diluted with SYBR Green Master mix and assayed with an ABI 7900 sequence detection system according to the manufacture’s protocol (Applied Biosystems). To distinguish the spliced transcripts from the intron-containing transcripts, gene-specific primers were designed and listed in Supplemental Table 2. The amplicon of Actin mRNA was used as an internal control. The relative quantification method (ΔΔCT) was used to evaluate the relative abundance of transcripts (Lan et al. 2004).
Analysis of starch properties
Approximately 40 days after heading, rice grains were harvested, air-dried and stored at room temperature for 3 months before milling. Twelve grains of milled rice were selected for measuring the alkali spreading values (ASV), and 10 g grains were ground to flour and used to measure the AC and gel consistency (GC). AC (%) was measured as described previously with slight modification (Juliano 1971). Briefly, samples were boiled for 10 min in the volumetric flasks to completely disperse the grain powder and the optical density of the amylose-iodine blue was measured at 620 nm using a spectrophotometer. GC was measured using 100 mg of milled rice flour. The flour was first wetted with 0.2 ml of 95% ethanol containing 0.025% (w/v) thymol blue in 11 × 100 mm culture tubes, followed by adding 2 ml of 0.2 N KOH, and mixed vigorously. Tubes were covered with glass marbles, heated in a boiling water bath for 8 min, mixed again, and kept in ice water bath for 20 min. Finally, the tubes were laid horizontally against a ruled graphing paper and gel length was measured after 1 h. ASV was determined by incubating six milled grains in 10 ml of 1.7% KOH at 28°C for 23 h with two replicates. The degree of spreading was rated using the following 7-point semi-quantitative criteria: 1, grain not affected; 2, grain swollen; 3, grain swollen, collar incomplete and narrow; 4, grain swollen, collar complete and wide; 5, grain splitted, collar complete and wide; 6, grain dispersed, merging with collar; 7, grain completely dispersed and intermingled.
Sequence analysis
Bioinformatic analyses were performed using the Lasergene software package (DNASTAR, Inc., Madison, WI). Blast searches were performed using the Tblastn program (http://www.ncbi.nlm. nih.gov/BLAST/) with the default parameters.
Results
Amylose content of du1 grains
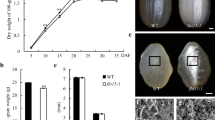
The phenotype of du1 mutant grains was compared with those of indica ZF802, japonica Xiushui11 and glutinous rice QFN grains. As shown in Fig. 1A, the completely dried du1 grain exhibits an intermediate degree of translucence in the cross-section, showing a higher similarity to glutinous rice QFN than to the grains of indica ZF802 or japonica WT. When stained with I2-KI, the du1 grain looks dark blue, distinctive from the brown red of QFN (Fig. 1B). The AC of du1 grains is dramatically decreased compared to those of the wild-type Xiushui11 or ZF802 grains, but was still significantly higher than that of glutinous varieties (Fig. 1C).
Phenotypes and amylose contents of the wild-type and du1 mutant. (A) Cross-sections of QFN, du1, wild-type, and ZF802 grains, showing that the du1 is semi-translucent, different from the opaque QFN and transparent wild-type grains. (B) Cross-sections stained with I2-KI, showing that the du1, wild-type and ZF802 are dark blue, a sharp contrast to the brown color of QFN. (C) The amylose contents of QFN, du1, wild-type, and ZF802. Data are means ± SE from at least three independent measurements
Map-based cloning of Du1
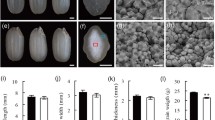
To clone the Du1 gene through a map-based approach, we generated a large F2 mapping population derived from a cross between du1 and Minghui63, in which a total of 1,936 segregants showed the du1 mutant phenotype. Based on the previous mapping result that du1 is located on Chromosome 10 (Eguchi et al. 1998), a set of PCR-based molecular markers were developed and Du1 was placed on the genome segment between the two markers, P2 and P3, with a genetic distance of 1.6 and 1.1 cM, respectively (Fig. 2A, Supplemental Table 1). Between P2 and P3 markers, additional nine molecular markers (P7–P15) (Supplemental Table 1) were developed and Du1 was fine mapped in an interval of ∼65-kb region between markers P9 and P10 (Fig. 2B, C). To find the mutation lesion of du1, the 65-kb genomic DNA segments from wild-type and mutant plants were sequenced and compared. A mutation was identified in the predicted OSJNBa0017E08.20 gene in the du1 genome. Comparison of the OSJNBa0017E08.20 cDNA sequences between du1 and wild-type plants showed that the mutation in du1 results from a substitution of G by A (G1742 → A1742) in exon 1, leading to a non-synonymous change from serine to asparagine (Fig. 2D).
Cloning and confirmation of the Du1 gene. (A) The Du1 locus was mapped to a region between markers P2 and P3 on chromosome 10. (B) A BAC contig covering the Du1 locus. The numerals indicate the number of recombinants identified from 1,936 du1 F2 plants. BAC1, OSJNBa0051D19; BAC2, OSJNBa0041P03; BAC3, OSJNBa0066I08; BAC4, OSJNBa0017E08; BAC5, OSJNBa0078O01; BAC6, OSJNBb0073N24; BAC7, OSJNBa0062C05; BAC8, OSJNBb0015K05. (C) Fine mapping of the Du1 locus. The Du1 locus was narrowed to a ∼65-kb region between markers P9 and P10 and cosegregated with marker P15. (D) Structure of Du1, showing the du1 mutation site. The start (ATG) and stop codon (TAG) are indicated. Closed boxes stand for exons, open boxes for the 5′ and 3′ UTRs, and lines for introns. (E) The complementation construct p1300Du1 containing the entire Du1 gene. (F) The phenotypes of the transgenic line ZD6-3 and its controls. (G) Identification of transgenic plants with the dCAPS marker dudc
To confirm that the mutation of Du1 is responsible for the mutant phenotype, we carried out a genetic complementation experiment. The plasmid p1300Du1 containing the entire wild-type Du1 gene and its regulatory sequences, which consist of a 3,120-bp coding sequence, a 3,051-bp upstream sequence including the regulatory region and untranslated region (UTR), and a 2,114-bp downstream region (Fig. 2E), was introduced into the mutant plants through Agrobacterium-mediated transformation (Li et al. 2003b). All the eight independent transgenic lines carrying the Du1 transgene showed a complete complementation of the du1 phenotype (Fig. 2F), whereas all the six lines transformed by pCAMBIA1300 failed to rescue the mutant phenotype (data not shown). The authentic transgenic plants were identified with a dCAPS marker dudc (Fig. 2G), which could distinguish the transformants from du1 plants by digestion of the amplified transgene DNA with AcuI (Supplemental Table 1).
Du1 encodes a Prp1 protein
Sequence analysis of 5′- and 3′-RACE cDNA products indicated that the Du1 full-length cDNA consists of two exons, including a 3,120-bp coding sequence, a 71-bp 5′ UTR, and a 479-bp 3′ UTR (Fig. 2D). Alignment analysis of the Du1 sequences from four indica and four japonica varieties showed no variation at the amino acid level between indica and japonica, though four synonymous substitutes of bases were found (Supplemental Fig. 1), suggesting that Du1 may play a very conserved role in rice.
BlastP search against SwissProt and GenBank databases found two Du1 homologs with 44.7% identity to Prp1p/Zer1p of Schizosaccharomyces pombe (Urushiyama et al. 1997) and 54.8% to Homo sapiens U5-102kD (Makarov et al. 2000). Previous studies have shown that the Prp1p/Zer1p protein involves in pre-mRNA splicing and the U5-102kD protein is a component of the U4/U6 snRNP required for spliceosome assembly. Therefore, it is likely that Du1 may function in mRNA maturation. Structural analysis revealed that Du1 has all the conserved features of the Prp1 family (Lamb et al. 1995), including a highly conserved N-terminal domain and a 19-repeat of tetratric peptide repeats (TPR) at the C-terminal (Fig. 3A). Since the TPR domain functions in protein–protein interactions (Chung et al. 1999; Lockhart and Rymond 1994; Makarov et al. 2000), the substitution of serine by asparagines (S581 → N581) in the du1 TPR7 motif may affect its interaction with other proteins, leading to the functional deficiency.
Structural features and phylogenetic analysis of Du1. (A) The predicted Du1 amino acid sequence and its 19 conserved TPR motifs, which are aligned with the consensus sequence of the S. pombe Prp1p/Zer1p and listed on the right. Bold letters indicate the specific N-terminal domain. (B) Phylogenetic relationship of Du1 and Du1L to other Prp1 proteins. Accessions included in the list are maize (Zea mays L., CD_439474), barley (Hordeum vulgare L., TC150206), rice Du1 (Oryza sativa L., NP_922169) and Du1L (NP_912872), AtPrp1 (Arabidopsis thaliana, NP_192252), M. truncatula (ABE92650), fruit fly CG6841-PA (Drosophila melanogaster, NP_649073), zebrafish (Danio rerio, AHH_56710), mouse U5-102 (Mus musculus, NP_598462), human U5-102 (O94906), and S. pombe Prp1 (CAA_17050). The number at each node represents the bootstrap support (percentage). The scale bar is an indicator of genetic distance based on the branch length
BlastP analysis identified a Du1-like (Du1L) gene located in Chromosome 1, which shares 73.9% identity to Du1 (Supplemental Fig. 2). Phylogenetic analysis showed that both Du1 and Du1L belong to a higher plant subfamily, but Du1 has a closer relationship to barley and maize than Du1L (Fig. 3B). The Prp1 family in cereal and dicots forms a monophyletic clade with 99% bootstrap support, suggests that the genes in monocots and dicots diverged from a common ancestral species. Phylogenetic analysis also demonstrated that Prp1 may serve as a molecular marker to determine the phylogenetic relation.
Expression patterns of Du1 and Du1L
The expression patterns of Du1 and Du1L (Fig. 4A) were assayed with gene specific primers, pdu1 and pdu1L (Supplemental Table 1). As shown in Fig. 4B, Du1 is expressed in all the five examined organs including panicles, blades, sheathes, culms and roots, with a highest expression in panicles (4-fold higher than in roots), showing a well-correlation to the starch biosynthesis in the organ (Fig. 4B). In contrast, Du1L has lower but relatively constant expression levels in all five organs, just ∼10% of that in the Du1 panicles (Fig. 4B). However, the expression of Du1 or Du1L displayed no significant difference between the du1 and wild-type plants (Fig. 4B).
Expression analysis of Du1 and Du1L. (A) Du1 and Du1L are specifically amplified from the total RNA. Lane 1, DNA size markers; Lanes 2, Du1; Lanes 3, Du1L; Lane 4, Du1 and Du1L. (B) The quantitative real-time RT-PCR analysis of Du1 and Du1L expressed in different organs of panicles (P), blades (B), sheaths (S), culms (C) and roots (R) of du1 and wild-type plants. The relative amounts of transcripts were expressed by the ratio of Du1 and Du1L to Actin, respectively. Data are means ± SE from at least four independent measurements
Du1 affects maturation of the Wx b pre-mRNA
The findings that Du1 encodes a protein highly homologous to pre-mRNA splicing proteins and that the AC of du1 grains is significantly decreased compared to that of the wild-type suggest that Du1 may function in splicing of pre-mRNAs involved in the grain starch biosynthesis. We therefore systematically examined the splicing efficiencies of the pre-mRNA transcripts of Du1 and other 18 genes encoding starch biosynthesis enzymes including Wx, three AGPases, GBSSII, eight SSSs, three SBEs and two DBEs. As shown in Fig. 5A, the splicing efficiency of Wx b pre-mRNA transcripts in du1 was remarkably decreased to ∼30% of the wild-type, but the mRNA maturation of all the other starch biosynthesis genes examined was unaffected. These results strongly suggest that Du1 may play a specific role in the maturation of Wx b pre-mRNAs.
Effects of the mutation in Du1 on the splicing of Wx b pre-mRNAs and the grain AC. (A) Effect on the splicing of Wx b pre-mRNAs in panicle. The value is the ratios of splicing efficiency of du1 to wild type, showing that splicing efficiency of Wx b in dul is only ∼30% of that in wild type. (B) The cross-sections of du1Wx b, Du1wx and du1wx. (C) The cross-sections stained with I2/KI in du1Wx b, Du1wx and du1wx. (D) The ACs of du1Wx b, Du1wx and du1wx
The regulation of Du1 on Wx was further confirmed by phenotypic comparison among du1 (a homozygous Wx b), QFN (a homozygous wx), and their double mutant du1wx. The grains of du1Wx b, Du1wx and du1wx were all opaque (Fig. 5B), but their I2-KI stained grain colors were apparently different: du1wx was brown red, similar to Du1wx but different from the dark blue of du1Wx b (Fig. 5C). Moreover, the amylose content of du1dx is almost the same as that of Du1wx, which is much lower than that of du1Wx b (Fig. 5D). These findings demonstrated that the phenotype of the double mutant du1wx was the same as that of the Du1wx mutant, indicating that Du1 acts upstream of Wx in the starch biosynthetic pathway.
Effect of du1 on the expression of other starch biosynthetic pathway genes
The expression levels of many starch biosynthesis genes were also affected by the mutation of Du1 (Fig. 6). Compared to the wild type, the transcripts of all the three genes encoding AGPase (OsAGPiso, OsAGPL1 and OsAGPS1) were decreased by 26, 26 and 35%, respectively, but those of ALK, OsSSIIIa and OsSSIVb were increased in the mutant. As for genes encoding starch branching enzymes, the expression level of OsSBEIIb was increased in the du1 mutant grains, but that of OsSBEIIa decreased. In addition, we also detected a significant increase in OsPUL transcripts in du1 (Fig. 6). These results suggest that Du1 may regulate the expression of the starch biosynthesis genes through an unidentified mechanism.
To understand whether the expressional alteration of the starch biosynthesis genes in the du1 plant affects other grain properties besides AC, we compared the grain weight, alkali digestibility and gel consistency between the wild-type and du1 grains. As shown in Table 1, the grain weight of brown rice was decreased significantly in du1. The remarkable decrease in ASV (Supplemental Fig. 3) of the du1 grains may reflect the significant increase in the expression of ALK (Fig. 6) that encodes OsSSIIa and controls the ASV in rice (Gao et al. 2003). In addition, the GC of du1 grains is also increased compared to that of the wild type.
Discussion
In this work, we report the genetic and molecular characterization of the classical rice mutant du1. Our results showed that Du1 encodes a protein that is highly homologous to S. pombe Prp1 family members and involves in the splicing of the Wx b pre-mRNAs and the regulation of the expression of rice starch biosynthesis genes.
Pre-mRNA splicing in higher plants is believed to be regulated by mechanisms similar to those in mammals and Drosophila (Simpson and Filipowicz 1996). In mammals, the U5-102kD Prp1 protein interacts within the tri-snRNP with U4/U6 snRNPs and bridges the two particles through the contained TPR elements (Makarov et al. 2000). Structural features of Du1 suggest that it is a member of the Prp1 family and may function in the splicing of pre-mRNA processing. Based on the fact that the du1 mutant grain has a lower AC, we therefore systematically compared the splicing efficiencies of the starch biosynthesis genes between du1 and wild-type plants. The results clearly demonstrated that the mutation in du1 specifically affects the splicing of Wx b pre-mRNAs (Fig. 5A), resulting in a low level of accumulation of mature Wx b transcripts, which in turn causes a dramatic decrease of AC in the du1 mutant grains (Fig. 1C). This is consistent with the previous finding that an inefficient processing of pre-mRNA transcripts of Wx b will result in a lower AC grains in rice (Hirano et al. 1998; Wang et al. 1995). However, the question why the mutation of Du1 specifically affects the splicing of the Wx b pre-mRNA remains to be answered.
Du1 may also play a regulatory role in the starch biosynthetic pathways. Comparison of the expression profiles of all the genes required for the grain starch biosynthesis between du1 and the wild type strongly suggests that Du1 may regulate the starch biosynthesis by affecting the expression of some key genes in the pathway, for example ALK and OsPUL (Fig. 6). ALK controls the gelatilization temperature or AVS of rice grains (Gao et al. 2003), OsPUL facilitates the assembly of amylopectin (Fujita et al. 2003; Kubo et al. 1999), and OsDBEs contribute to the accumulation of amylopectin by clearing the improper branches in starch synthesis (Nakamura 2002). Therefore, it appears that Du1 pleiotropically affects rice grain quality through regulating the gene expression of the starch biosynthetic pathways by two molecular mechanisms: post-transcriptional regulation of Wx b pre-mRNA transcripts and transcriptional regulation of some other key genes.
The yield of rice has been increased dramatically in the last century, but the poor cooking and eating quality of high-yielding cultivars and hybrid rice represents a major problem for rice production (Liu et al. 2003). The improvement of rice quality is increasingly demanded by consumers and has become a priority for rice breeders and genetists. Since AC is a pivotal factor in rice quality, many researchers have been trying to modify it by manipulating the expression levels of Wx transcripts via RNA inference or overexpression, and promising results have been reported (Itoh et al. 2003; Liu et al. 2003; Terada et al. 2000). Therefore, the cloning and molecular characterization of Du1 will facilitate the molecular genetic engineering of rice.
Abbreviations
- AC:
-
Amylose content
- AGPase:
-
ADP-glucose pyrophosphorylase
- ASV:
-
Alkali spreading values
- BAC:
-
Bacterial artificial chromosome
- CAPS:
-
Cleaved amplified polymorphic sequences
- DAP:
-
Days after pollinating
- DBE:
-
Starch debraching enzyme
- du1 :
-
Dull endosperm1
- Du1L:
-
Du1-like
- GBSS:
-
Granule-bound starch synthase
- GC:
-
Gel consistency
- Prp:
-
Pre-mRNA processing
- QTL:
-
Quantitative trait locus
- SBE:
-
Starch branching enzyme
- SR:
-
Serine–arginine-rich
- SSS:
-
Soluble starch synthase
- STS:
-
Sequence tagged site
- TPR:
-
Tetratric peptide repeats
- UTR:
-
Untranslated region
References
Aluko G, Martinez C, Tohme J, Castano C, Bergman C, Oard JH (2004) QTL mapping of grain quality traits from the interspecific cross Oryza sativa x O. glaberrima. Theor Appl Genet 109:630–639
Bao JS, Sun M, Corke H (2002) Analysis of the genetic behavior of some starch properties in indica rice (Oryza sativa L.): thermal properties, gel texture, swelling volume. Theor Appl Genet 104:408–413
Bao J, Kong X, Xie J, Xu L (2004) Analysis of genotypic and environmental effects on rice starch. 1. Apparent amylose content, pasting viscosity, and gel texture. J Agric Food Chem 52:6010–6016
Cai XL, Wang ZY, Xing YY, Zhang JL, Hong MM (1998) Aberrant splicing of intron 1 leads to the heterogeneous 5’ UTR and decreased expression of waxy gene in rice cultivars of intermediate amylose content. Plant J 14:459–465
Chung S, McLean MR, Rymond BC (1999) Yeast ortholog of the Drosophila crooked neck protein promotes spliceosome assembly through stable U4/U6.U5 snRNP addition. RNA 5:1042–1054
Eguchi T, Ashikari M, Yoshimura A, Iwata N (1998) Loci of physiological and morphological characters on RFLP linkage map in rice. Breeding Sci 48:80
Fan CC, Yu XQ, Xing YZ, Xu CG, Luo LJ, Zhang Q (2005) The main effects, epistatic effects and environmental interactions of QTLs on the cooking and eating quality of rice in a doubled-haploid line population. Theor Appl Genet 110:1445–1452
Felsenstein J (2000) PHYLIP: Phylogeny Inference Package, v3.6. Seattle, WA
Frances H, Bligh J, Larkin PD, Roach PS, Jones CA, Fu H, Park WD (1998) Use of alternate splice sites in granule-bound starch synthase mRNA from low-amylose rice varieties. Plant Mol Biol 38:407–415
Fujita N, Kubo A, Suh DS, Wong KS, Jane JL, Ozawa K, Takaiwa F, Inaba Y, Nakamura Y (2003) Antisense inhibition of isoamylase alters the structure of amylopectin and the physicochemical properties of starch in rice endosperm. Plant Cell Physiol 44:607–618
Gao Z, Zeng D, Cui X, Zhou Y, Yan M, Huang D, Li J, Qian Q (2003) Map-based cloning of the ALK gene, which controls the gelatinization temperature of rice. Sci China Ser C 46:661–668
Hirano HY, Sano Y (2000) Comparison of Waxy gene regulation in the endosperm and pollen in Oryza sativa L. Genes Genet Syst 75:245–249
Hirano HY, Eiguchi M, Sano Y (1998) A single base change altered the regulation of the Waxy gene at the posttranscriptional level during the domestication of rice. Mol Biol Evol 15:978–987
Isshiki M, Morino K, Nakajima M, Okagaki RJ, Wessler SR, Izawa T, Shimamoto K (1998) A naturally occurring functional allele of the rice waxy locus has a GT to TT mutation at the 5’ splice site of the first intron. Plant J 15:133–138
Itoh K, Ozaki H, Okada K, Hori H, Takeda Y, Mitsui T (2003) Introduction of Wx transgene into rice wx mutants leads to both high- and low-amylose rice. Plant Cell Physiol 44:473–480
Juliano BO (1971) A simplified assay for milled rice amylose. Cereal Sci Today 16:334–360
Kinoshita T (1987) Report of the committee on gene symbolization, nomenclature and linkage groups. II. Registration of new gene symbols. Rice Genet Newslett 4:3–7
Koh HJ, Cha KW, Heu MH (1997) Inheritance and some physicochemical properties of newly induced “low-amylose endosperm” mutants in rice. Korean J Breed 29:368–375
Kubo A, Fujita N, Harada K, Matsuda T, Satoh H, Nakamura Y (1999) The starch-debranching enzymes isoamylase and pullulanase are both involved in amylopectin biosynthesis in rice endosperm. Plant Physiol 121:399–410
Lamb JR, Tugendreich S, Hieter P (1995) Tetratrico peptide repeat interactions: to TPR or not to TPR? Trends Biochem Sci 20:257–259
Lan L, Chen W, Lai Y, Suo J, Kong Z, Li C, Lu Y, Zhang Y, Zhao X, Zhang X, Han B, Cheng J, Xue Y (2004) Monitoring of gene expression profiles and isolation of candidate genes involved in pollination and fertilization in rice (Oryza sativa L.) with a 10K cDNA microarray. Plant Mol Biol 54:471–487
Lanceras JC, Huang ZL, Naivikul O, Vanavichit A, Ruanjaichon V, Tragoonrung S (2000) Mapping of genes for cooking and eating qualities in Thai jasmine rice (KDML105). DNA Res 7:93–101
Li X, Qian Q, Fu Z, Wang Y, Xiong G, Zeng D, Wang X, Liu X, Teng S, Hiroshi F, Yuan M, Luo D, Han B, Li J (2003a) Control of tillering in rice. Nature 422:618–621
Li Y, Qian Q, Zhou Y, Yan M, Sun L, Zhang M, Fu Z, Wang Y, Han B, Pang X, Chen M, Li J (2003b) BRITTLE CULM1, which encodes a COBRA-like protein, affects the mechanical properties of rice plants. Plant Cell 15:2020–2031
Liu Q, Wang Z, Chen X, Cai X, Tang S, Yu H, Zhang J, Hong M, Gu M (2003) Stable inheritance of the antisense Waxy gene in transgenic rice with reduced amylose level and improved quality. Transgenic Res 12:71–82
Lockhart SR, Rymond BC (1994) Commitment of yeast pre-mRNA to the splicing pathway requires a novel U1 small nuclear ribonucleoprotein polypeptide, Prp39p. Mol Cell Biol 14:3623–3633
Makarov EM, Makarova OV, Achsel T, Luhrmann R (2000) The human homologue of the yeast splicing factor prp6p contains multiple TPR elements and is stably associated with the U5 snRNP via protein-protein interactions. J Mol Biol 298:567–575
Mou Z, He Y, Dai Y, Liu X, Li J (2000) Deficiency in fatty acid synthase leads to premature cell death and dramatic alterations in plant morphology. Plant Cell 12:405–418
Nakamura Y (2002) Towards a better understanding of the metabolic system for amylopectin biosynthesis in plants: rice endosperm as a model tissue. Plant Cell Physiol 43:718–725
Okuno K, Fuwa H., Yano M (1983) A new mutant gene lowering amylose content in endosperm starch of rice, Oryza Sativa L. Jpn J Breed 33:387–394
Page RD (1996) TreeView: an application to display phylogenetic trees on personal computers. Comput Appl Biosci 12:357–358
Qian Q, Lin J, Song J, Yang C (1991) Genetic analysis of dull and its effect on kernal traits. Annual Report China National Rice Research Institute, p 3
Qian Q, Zhu X, Zeng D, Xiong Z, Min S (1996) The improvement of three new-type rice with special embryo and endosperm (in Chinese). Zhejiang Agricult Sci 4:155–156
Sano Y, Maekawa M, Kikuchi H (1985) Temperature effects on the Wx protein level an amylose content in the endosperm of rice. J Hered 76:221–223
Satoh H, Omura T (1981) New endosperm mutations induced by chemical mutagen in rice, Oryza sativa L. Japan J Breed 31:316–326
Satoh H, Omura T (1986) Mutagenesis in rice by treating fertilized egg cells with nitroso compounds. Rice Genet, p 707–717
Septiningsih EM, Trijatmiko KR, Moeljopawiro S, McCouch SR (2003) Identification of quantitative trait loci for grain quality in an advanced backcross population derived from the Oryza sativa variety IR64 and the wild relative O. rufipogon. Theor Appl Genet 107:1433–1441
Simpson GG, Filipowicz W (1996) Splicing of precursors to mRNA in higher plants: mechanism, regulation and sub-nuclear organisation of the spliceosomal machinery. Plant Mol Biol 32:1–41
Tan YF, Li JX, Yu SB, Xing YZ, Xu CG, Zhang Q (1999) The three important traits for cooking and eating quality of rice grains are controlled by a single locus in an elite rice hybrid, Shanyou 63. Theor Appl Genet 99:642–648
Terada R, Nakajima M, Isshiki M, Okagaki RJ, Wessler SR, Shimamoto K (2000) Antisense waxy genes with highly active promoters effectively suppress waxy gene expression in transgenic rice. Plant Cell Physiol 41:881–888
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Urushiyama S, Tani T, Ohshima Y (1997) The prp1 gene required for pre-mRNA splicing in Schizosaccharomyces pombe encodes a protein that contains TPR motifs and is similar to Prp6p of budding yeast. Genetics 147:101–115
Wang ZY, Zheng FQ, Shen GZ, Gao JP, Snustad DP, Li MG, Zhang JL, Hong MM (1995) The amylose content in rice endosperm is related to the post-transcriptional regulation of the waxy gene. Plant J 7:613–622
Yano M, Okuno K, Satoh H, Omura T (1988) Chromosomal location of genes conditioning low amylose content of endosperm starches in rice, Oryza sativa L. Theor Appl Genet 76:183–189
Acknowledgements
We thank Zhixi Tian for the assistance in the analyses of starch properties and his critical comments on the manuscript, Kyushu University for providing the original du1 seeds, Bin Han (National Center for Gene Research, Chinese Academy of Sciences) for providing BAC and cDNA clones. This work was supported by grants from the State Key Basic Research Program (2005CB1208), the National Natural Science Foundation of China (30530470, 30425034), Natural Science Foundation of Zhejiang Province (Y304442).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below are the electronic supplementary materials
11103_2007_9186_MOESM1_ESM.tif
Structural features of the Du1 gene. Numbers at left refer to the position of nucleotides. Red stands for the mutation in du1, blue for polymorphic bases between indica and japonica subspecies, pink for the N terminal domain, and green or orange for the TPR motifs (TIF 2,497 KB)
Rights and permissions
About this article
Cite this article
Zeng, D., Yan, M., Wang, Y. et al. Du1, encoding a novel Prp1 protein, regulates starch biosynthesis through affecting the splicing of Wx b pre-mRNAs in rice (Oryza sativa L.). Plant Mol Biol 65, 501–509 (2007). https://doi.org/10.1007/s11103-007-9186-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-007-9186-3