Abstract
The ATP-dependent Clp protease has been well-characterized in Escherichia coli, but knowledge of its function in higher plants is limited. In bacteria, this two-component protease consists of a Ser-type endopeptidase ClpP, which relies on the ATP-dependent unfolding activity from an Hsp100 molecular chaperone to initiate protein degradation. In the chloroplasts of higher plants, multiple isoforms of the proteolytic subunit exist, with Arabidopsis having five ClpPs and four ClpP-like proteins termed ClpR predicted in its genome. In this work we characterized an Arabidopsis mutant impaired in one subunit of the chloroplast-localized Clp protease core, ClpR1. clpR1-1, a virescent mutant, carries a pre-mature stop codon in the clpR1 gene, resulting in no detectable ClpR1 protein. The accumulation of several chloroplast proteins, as well as most of the chloroplast-localized Clp protease subunits, is inhibited in clpR1-1. Unexpectedly, some plastid-encoded proteins do not accumulate, although their transcripts accumulate to wild-type levels. Maturation of 23S and 4.5S chloroplast ribosomal RNA (cp-rRNA) is delayed in clpR1-1, and both RNAs accumulate as higher molecular weight precursors. Also, chloroplasts in clpR1-1 are smaller than in wild type and have fewer thylakoid membranes with smaller grana stacks. We propose that a ClpR1-containing activity is required for chloroplast development and differentiation and in its absence both are delayed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plastids arose from a single endosymbiotic event between a photosynthetic cyanobacteria and a eukaryotic host (Moreira et al. 2000). As such, it is not surprising that proteases of prokaryotic origin exist within plant plastids. These include the ATP-independent Deg and SppA and the ATP-dependent Lon, FtsH and Clp proteases (Adam et al. 2001, 2006; Adam and Clarke 2002; Sakamoto 2006), all of which have been extensively characterized (Gottesman 1996). The Clp protease from Escherichia coli is a two-component enzyme consisting of an endopeptidase, ClpP that relies on the unfolding activity of a molecular chaperone, either ClpX or ClpA, to initiate protein degradation. (Adam et al. 2006; Gottesman 1996; Sakamoto 2006). The activity of ClpP is restricted due to the structure of the core. Two heptameric rings of ClpP form a barrel-like structure, with a single cavity (50 Å diameter) enclosing the Ser-His-Asp residues that make up the catalytic triad of serine-type proteases (Wang et al. 1997). The narrow axial entrance pores (11 Å) prevent most folded polypeptides from entering the proteolytic chamber. For proteolysis to occur, a single hexameric ring of the chaperone regulatory Clp subunit must associate to one or both ends of the two-tiered ClpP core. The chaperones confer substrate specificity to the protease, which differs between ClpA and ClpX, and thread the substrate protein via ATP-hydrolysis through the pore into the proteolytic chamber of ClpP (Grimaud et al. 1998). The unfolded protein is then quickly degraded into small peptides which can later diffuse out of the core. ClpA and ClpX are also able to function on their own as chaperones and are members of the Hsp100 chaperone family (Schirmer et al. 1996).
Although Clp proteases have been identified in many different organisms, higher plants have the greatest diversity of Clp proteins. The Arabidopsis thaliana genome contains at least 23 genes predicted to encode various Clp proteins (Adam et al. 2006; Sakamoto 2006). All of these are nuclear-encoded, with the exception of ClpP1 which is encoded in the plastid genome (Adam et al. 2006; Sakamoto 2006). Of these proteins, ten are Hsp100 chaperones (four ClpB, two ClpC, one ClpD and three ClpX), six are proteolytic subunits (ClpP), and four are ClpP-like (ClpR). ClpRs are unique to photosynthetic organisms; they are similar in sequence and in size to the ClpP proteins but lack the conserved catalytic triad (Ser-His-Asp) typical of Ser-type proteases (Adam et al. 2006; Clarke 1999; Peltier et al. 2004; Sakamoto 2006). In addition to the aforementioned Clp proteins, two other categories have been identified in recent years. ClpS appears to be specific to higher plants (Peltier et al. 2001), whereas ClpT is the plant version of a bacterial Clp modulator (Peltier et al. 2004). The plant ClpS should not be confused with the bacterial modulator of the same name (Dougan et al. 2002), which in plants has been termed ClpT. ClpB3, the other Hsp100 localized in chloroplasts, does not contain a ClpP recognition site in its sequence and likely acts as a chaperone on its own (Keeler et al. 2000).
Most Clp proteins are chloroplast-localized, with only ClpP2, ClpB1-2, ClpB4, and the three ClpXs found elsewhere in the cell (Halperin et al. 2001b; Peltier et al. 2004). All of the chloroplast Clp proteins are localized primarily in the stroma (Adam and Clarke, 2002; Nielsen et al. 1997; Peltier et al. 2004). A 350 kDa complex containing ClpP/R/S subunits was isolated from chloroplasts. Based on the molecular mass of the complex it appears to contain 14 ClpP/R subunits and two ClpS (Peltier et al. 2001). The stoichiometry of the complex did not change significantly between chloroplasts and non-photosynthetic plastids, suggesting that regulation of proteolysis depends on the Hsp100 chaperone partners that associate with the core (Peltier et al. 2004). Although ClpC/P interaction in the stroma has been identified (Halperin et al. 2001a; Sokolenko et al. 1998), the 350 kDa complex did not contain an Hsp100 chaperone partner, such as ClpC or ClpD.
Very little is known about the role of Clp proteolytic activity in plastids and its possible substrates in higher plants (Adam and Clarke 2002). In Chlamydomonas reinhardtii, the plastid encoded ClpP1 is required for the degradation of the cytochrome b 6 f complex, as well as for the adaptation to elevated CO2 levels (Majeran et al. 2000). Reducing the amounts of ClpP1 in tobacco causes defects in both plastid and whole plant development (Shikanai et al. 2001), and loss of clpP1 function results in a seedling lethal phenotype (Kuroda and Maliga 2003). Arabidopsis thaliana plants lacking ClpC1 are chlorotic and slow growing, with reduced photochemical efficiency and photosystem content. The expression of two nuclear encoded ClpPs was induced in senescing Arabidopsis leaves (Nakabayashi et al. 1999), suggesting that different subunits might constitute proteases that are active during various stages of development or in response to various signals. Surprisingly, a mutation in clpC2 can suppress the variegated phenotype of var2-5, impaired in the thylakoid protease FtsH2 (Park and Rodermel 2004). It was suggested that while FtsH prevents photo-oxidative damage by degrading photo-damaged photosystem II D1 proteins, ClpC might affect thylakoid development in a way that would promote photo-oxidative damage (Park and Rodermel 2004).
In this work, we describe an A. thaliana mutant that is impaired in the clpR1 gene. A premature stop-codon in clpR1 results in almost complete inhibition of clpR1 expression. The clpR1-1 mutant is virescent (i.e., pale initially but later greens resulting in a yellow/pale green center), and is relatively small and slow growing. The levels of several nuclear and chloroplast-encoded proteins are significantly reduced in the mutant. Transcription of chloroplast genes is unaffected in the mutant but the maturation of the 4.5S and 23S cp-rRNA is inhibited. Chloroplasts in the clpR1-1 mutant are significantly smaller and contain fewer thylakoids with less grana stacks, suggesting that ClpR1-containing complexes are required for early development of plastids and that impaired plastid development in clpR1-1 affects nuclear gene expression.
Results
Identification of an Arabidopsis mutant with impaired leaf development and delayed greening
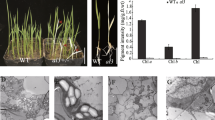
We identified a small, pale mutant in an EMS-mutagenized, M2 population of A. thaliana. Five days after germination, the mutant’s cotyledons contained very little Chl (Fig. 1A and F). Although the cotyledons eventually accumulated Chl, they remained pale compared to the wild type (Fig. 1E). This pattern was repeated in leaf development throughout the life cycle, with leaves pale green upon emergence (Fig. 1C) and becoming greener as the leaf expanded, resulting in a vegetative rosette with a yellow center (Fig. 1E). Even the inflorescence was relatively pale at first and accumulated Chl later in development (data not shown). Mutants were smaller than wild type (Fig. 1E) suggesting that metabolic processes like photosynthesis were significantly reduced. They were also late to flower although they flowered at the same number of leaves as wild type (data not shown). This suggests that the delay in flowering was a result of slow growth rather than an effect on flowering time.
A virescent slow developing mutant. (A) and (C), 6 and 10 days-old mutant seedlings. (B) and (D), 6 and 10 day-old wild type (Col-0) seedlings. (E), 30 day old-seedlings of mutant (left) and wild type (right) (F), wild type and mutant seedlings were grown on plates containing 1/2 MS media supplemented with 2% sucrose. Seedlings were harvested at the indicated age and Chl concentration was measured for wild type (full circles) and mutant (open circles) seedlings
Map based cloning
We used map-based cloning (Li and Chory 1997) to identify the defective gene in the virescent mutant. Based on the analysis of 400 recombinant chromosomes from an F2 mapping population derived from a cross between the mutant in Col-0 background to Ler, the mutation was mapped to the bottom of chromosome I (Fig. 2A) to an interval of 50 kb. We used a candidate gene approach to identify the mutant gene in the interval. Sequencing of the clpR1 gene (At1g49970) identified a G to A substitution resulting in a stop codon instead of Trp299 (Fig. 2A). We obtained a second independent allele, clpR1-2 (originally Salk_088407) in which a T-DNA was inserted in the first intron of At1g49970 (www.signal.salk.edu). The homozygous T-DNA line had reduced amounts of ClpR1 (supp. Fig 1) with only 5% of the wild type level and a similar virescent phenotype (supp. Fig 2) supporting our identification of the mutation in clpR1 as the cause of the virescent phenotype. Transforming clpR1-1 seedlings with ClpR1 cDNA driven by the CaMV 35S promoter rescued the virescent phenotype (Fig. 2B).
Cloning of clpR1–1. (A) clpR1-1 was mapped by analyzing 400 chromosomes from F2 plants of clpR1-1 (in Col-0) crossed to Ler. In the clpR1-1 gene, solid lines represent introns, black boxes represent exons, and the triangle indicates the positions of the T-DNA insertion. (B) 5 week-old clpR1-1 (left), Col-0 (center) and a clpR1-1 seedling over expressing ClpR1 (right). This is a T3 homozygous seedling from 1 out of 6 independent lines checked
Accumulation of other Clp subunits is reduced in clpR1-1
We next measured how the mutation in clpR1-1 affected the amounts of other chloroplast-localized Clp proteins. Unlike clpR2-1 that still contained significant amounts of ClpR2 (Rudella et al. 2006), ClpR1 could not be detected in clpR1-1; a week signal that does not exist in the wild type was detected at a lower molecular weight (Fig. 3). This suggests that the premature stop-codon resulted not only in a non-functional protein but also in a less stable one. Levels of ClpC and ClpD, two potential Hsp100 chaperone partners of the protease, were reduced by 40 and 70%, respectively (Fig. 3) whereas the amount of several Clp core subunits (ClpP1, -P3, -P5, and –R2) were reduced by 30–50%. Accumulation of the remaining proteolytic core proteins, ClpP4, ClpP6, ClpR3 and ClpR4 was unaffected or only slightly reduced.
Abundance of chloroplast localized Clp proteins in clpr1-1. (A) Cellular proteins were extracted from 5 days old wild type (Col-0) and clpr1-1 seedlings. Equal amounts of proteins were separated by SDS-PAGE. Proteins were detected by immunoblotting using specific polyclonal antibodies. (B) Coomassie blue stained gel. (C) Quantitative results of A; bars represent the average (+/−SD, n = 6) amount of each protein relative to wild type levels, set at 100%
Levels of multiple chloroplast-localized proteins are reduced in clpR1–1
We next studied how the lack of ClpR1 affected plastid development by measuring the amounts of several chloroplast proteins, each representing different photosynthetic complexes. The abundance of most proteins examined was significantly reduced in clpR1-1 (Fig. 4). We used antibodies against a light-harvesting Chl a/b binding protein (Lhcb2), the large (LSU) and small (SSU) subunit of ribulose bisphosphate carboxylase/oxygenase (Rubisco), subunit XI (PsaL) and subunit II (PsaD) of PS I, chaperonin 60 (Cpn60) and CF1 β subunit of ATPase (β-ATPase). Accumulation of both nuclear- and plastid-encoded proteins was significantly reduced in the clpR1-1 mutant. This suggests that the development of functional chloroplasts is compromised in clpR1-1 (Fig. 4). In general, the change in protein abundance is larger in the clpR1-1 mutant compared to wild type than in the clpC1 mutant, which correlates with the more severe phenotype of clpR1-1. Interestingly, a second, higher molecular mass band was observed when anti-Lhcb antibodies were used (Fig. 4), indicating the possible accumulation of an Lhcb2 precursor in the mutant line, accumulation of various LHCII precursors as well as a PsaF precursor is observed in the clpR2-1 mutant (Rudella et al. 2006). clpR1-1 also accumulated significantly lower amounts of Cpn60 (Fig. 4). Cpn60 is the plant ortholog of the bacterial GroEL that can function together with ClpP in vitro in the degradation of the short-lived fusion protein, CRAG, in E. coli (Kandror et al. 1994). In contrast, accumulation of β-ATPase was significantly enhanced in clpR1-1 resulting in a 50% increase compared to wild type (Fig. 4).
Abundance of selected chloroplast proteins in clpr1-1. (A) Cellular proteins were extracted from 5 days old wild type (Col-0) and clpr1-1 seedlings. Equal amounts of proteins were separated by SDS-PAGE. Proteins were detected by immunoblotting using specific polyclonal antibodies, loading control is shown in fig. 3B. (B) Quantitative results of A; bars represent the average (+/−SD, n = 6) amount of each protein relative to wild type levels, set at 100%
Accumulation of several transcripts is impaired in clpR1-1
In order to further study the effect of the lack of ClpR1 on chloroplast development, we examined the accumulation of several transcripts encoding for chloroplast proteins. The accumulation of the nuclear-encoded Lhcb2 mRNA was significantly inhibited in clpR1-1 as was that of RbcS (coding for SSU) (Fig. 5A). Lhcb2 expression was also inhibited by treating seedlings with the herbicide norflurazon (NF) (Fig. 5A). NF is a non-competitive inhibitor of phytoene desaturase and prevents the accumulation of carotenoids. In light-grown seedlings, NF treatment causes photo-oxidative damage to chloroplast constituents, resulting in photobleaching (Puente et al. 1996). Inhibition by NF is a result of retrograde signaling between the chloroplast and nucleus that inhibits expression of photosynthetic genes when chloroplast development is inhibited (Nott et al. 2006). This suggests that the reduced levels of Lhcb2 transcripts in clpR1-1 may also be a result of retrograde signaling. In contrast, no difference was observed for transcripts encoded by chloroplast genes: RbcL (coding for LSU), PsbA (coding for the photosystem II D1 protein) (Fig. 5A) and PsbD (coding for the photosystem II D2 protein) (data not shown). This suggests that the reduced abundance of LSU observed in clpR1-1 seedlings (Fig. 4A) was not a result of reduced transcription but rather an inhibition of protein translation in clpR1-1. Alternatively in the absence of SSU all the unincorporated LSU is rapidly degraded.
Transcription in clpR1-1 plastids is not inhibited. Total RNA was extracted from 5 days old wild type (Col-0) and clpR1-1 seedlings. Five micrograms of total RNA were separated on 1% agarose-formaldehyde gels and analyzed by northern blotting with the indicated probe. (A) Abundance of nuclear (Lhcb, RbcS) and plastid (PsbA, RbcL) encoded transcripts. (B) Accumulation of cp-rRNA variants. Ethidium bromide-stained gels and membranes probed with 25S rRNA are shown as control for both A and B. Col-0+NF indicates RNA isolated from wild type seedlings grown on 5 μM norflurazon. Cp-rRNA is indicated. (C) A schematic representation of the polycistronic cp-rRNA transcript. rRNA (16S, 23S, 4.5S and 4S) and tRNAs (Ile and Ala) are indicated. The lower bars represent the probes used in Fig. 5B and 6C
The Et-Br stained total RNA gel suggested that cp-rRNA is affected in clpR1-1. Instead of three bands representing cp-rRNA, clpR1-1 accumulated only a single band (Fig. 5B). When chloroplasts were photobleached by NF and light, none of the cp-rRNA specific bands were present (Fig. 5B). In clpR1-1, accumulation of the 16S rRNA was unaffected while levels of 23S rRNA were significantly reduced. Furthermore, most of the 23S rRNA, accumulated as a higher molecular weight form, probably a pre-processed 23S precursor (p23S) (Fig. 5B). A similar reduction in 23S cp-rRNA was observed in ClpC1 mutants (data not shown). Cp-rRNA was still visible however; this is in agreement with the weaker phenotype of the ClpC1 mutants or could be a result of ClpC2 still being present (Sjögren et al. 2004).
Maturation of chloroplast 23S rRNA is delayed in clpr1-1
To further investigate cp-rRNA maturation we examined a time course of 23S cp-rRNA accumulation in developing chloroplasts. In 5 d old dark-grown wild-type seedlings, only low levels of 23S cp-rRNA were observed (Fig. 6A). Upon exposure to light, plants started to accumulate 23S cp-rRNA, although even after 24 h of illumination a large portion of the cp-rRNA was still in the p23S form (Fig. 6A). This suggests that maturation of 23S cp-rRNA plays a part in the development and differentiation of chloroplasts. Accumulation of 23S cp-rRNA in response to light was slower for both clpR1-1 and clpR1-2 (Fig. 6A and data not shown respectively) suggesting that ClpR1 is involved in chloroplast development in response to light. We then asked if the fact that clpR1-1 seedlings eventually turn green is also reflected in the maturation of 23S cp-rRNA. We analyzed the levels of 23S cp-rRNA in 7, 14 and 21 d old seedlings grown in short day conditions. While no increase in the amount of 23S cp-rRNA was observed for wild type plants, clpR1-1 seedlings showed an increase in 23S cp-rRNA levels over time (Fig. 6B). A considerable amount of p23S remained even in the 21 d old clpR1-1 seedlings (Fig. 6B), likely reflecting the mixture of older green leaves (containing mostly 23S cp-rRNA) and younger developing leaves that contain mostly p23S. Alternatively this difference could be a result of having, within the same leaf, older and younger cells, the latter having more under-developed chloroplasts with mostly p23S. A similar pattern of 23S cp-rRNA was observed in tobacco leaves during leaf development and maturation, where the younger leaves and younger cells within a leaf had more p23S than the more mature leaves and cells (T. Nakano personal communication).
Maturation of 23S cp-rRNA is delayed in clpR1-1. Total RNA was extracted from 5 days old wild type (Col-0) and clpR1-1seedlings. 5 μg of total RNA were separated on 1% agarose-formaldehyde gels and analyzed by northern blotting with the indicated probe. A. RNA was extracted from light grown seedlings and from dark grown seedlings before or after a 4 or 24 h exposure to light. Membranes were probed with 23S cp-rRNA. B RNA was extracted from 7, 14 and 21 days old, light grown seedlings and probed with 23S cp-rRNA. C. RNA from 5 d old light- grown seedlings was analyzed with probes against 23S cp-rRNA, the two tRNAs between the 16S and 23S cp-rRNA (tRNAs), and the 4.5S cp-rRNA. Membranes probed with 25S rRNA are shown as control
We then asked, in which step is the 23S cp-rRNA maturation inhibited in clpR1-1 seedlings? This was addressed by designing probes that span either the region between the 16S and 23S cp-rRNA (i.e. the two tRNAs) or the 3′ end of the polycistronic transcript (i.e. the 4.5S cp-rRNA, Fig. 5C). While the two tRNA molecules accumulated in the mutant to wild type (or even slightly higher) levels, 4.5S cp-rRNA levels decreased significantly. Furthermore, at least two distinct higher molecular weight transcripts were observed with the 4.5S cp-rRNA probe (Fig. 6C).
Chloroplasts in clpR1-1 are small and underdeveloped
We asked whether the defects in maturation of chloroplast ribosomes and subsequently protein translation were reflected in the ultra-structure of chloroplasts. We used light and electron microscopy to examine plastids from the cotyledons of 5 days-old wild-type and clpR1-1 seedlings. There was no significant difference between clpR1-1 and wild type in the size or number of cells in cotyledons (data not shown). Furthermore, cells in clpR1-1 cotyledons appeared to have a similar number of chloroplasts per cell as wild type (Fig. 7A and B) In contrast, chloroplasts from mutant seedlings were significantly smaller, had fewer thylakoid membranes and smaller grana areas than wild-type chloroplasts (Fig. 7C and D). However all the chloroplasts we analyzed had distinct thylakoids with both stroma and grana membranes suggesting that chloroplast development was slowed but not defective. This is consistent with the observed phenotype of the clpR1-1 that is pale at first but later turns green, developing into a small, slow-growing but fully functional plant.
Chloroplast in clpR1-1 cotyledons are smaller than those in wild type. Cotyledons from 5 days old wild type and clpR1-1 seedlings were used and sectioned for light (A and B) or transmission electron microscopy (C and D). Shown are cotyledons and chloroplasts from clpR1-1 (A and C) and wild type (B and D). Scale bars for A and B are 50 μm and 1 μm for TEM
Discussion
The Clp gene family encodes Hsp100 chaperones, proteolytic subunits and adaptor proteins (Adam et al. 2006; Sakamoto 2006). To date several clp mutants have been reported. These include T-DNA lines impaired in the expression of the Hsp100 molecular chaperones ClpC1and ClpC2 (Constan et al. 2004; Park and Rodermel 2004; Sjögren et al. 2004), a mutation in the plastid-encoded clpP1 of tobacco that proved to be lethal (Kuroda and Maliga 2003; Shikanai et al. 2001; Sjögren et al. 2004) and viable antisense lines in which the ClpP4 proteolytic subunit is repressed (Zheng et al. 2006). clpR1-1 and the recently published clpR2 (Rudella et al. 2006) are the first and, to our knowledge, only reported mutant impaired in a nuclear-encoded ClpR subunits. Furthermore, unlike all previously reported viable clp mutants that still accumulate detectable amounts of the affected Clp proteins (Constan et al. 2004; Park and Rodermel 2004; Rudella et al. 2006; Sjögren et al. 2004), no ClpR1 protein was observed in clpR1-1. ClpR1 is one subunit of the Clp proteolytic core complex present in various plastids (Peltier et al. 2001, 2004). The fact that clpR1-1 completes a full life cycle, despite having practically no ClpR1, and produces viable seeds suggests that at least some redundancy might exist between ClpP/R proteins. Even though several core subunits accumulate to wild-type levels with the remaining subunits present at significant levels, it is clear from the phenotype of clpr1-1 that the ClpR1-containing complex is required for normal plastid development. Alternatively, it can suggest that ClpR1 is required for the assembly of the Clp protease and the phenotype of clpR1-1 results from reduced Clp activity. The similar phenotype of clpR2-1 (Rudella et al. 2006) further supports the role of ClpR proteins in plastid development.
Interestingly, inhibiting the expression of the Hsp100 ClpC1 results in increased levels of the proteolytic Clp proteins (Sjögren et al. 2004), possibly in an attempt to compensate for a lack in Clp protease activity. Although it was not found in the 350 kDa complex (Peltier et al. 2004), the Hsp100 ClpC1 is thought to be essential for the Clp proteolytic activity and the increased amounts of core complexes are inactive due to the lack of chaperone activity. It is important to note that this 350 kDa complex was not shown to have proteolytic activity. In contrast, reduced levels of ClpR1 and other ClpP/R proteins in clpR1-1 did not result in increased levels of any Hsp100 protein, but rather a decrease suggesting the ClpC1 levels might, at least in part, be regulated by the amounts of available core complexes. Surprisingly, a significant increase in ClpC HSP100 proteins is observed in clpR2-1 (Rudella et al. 2006) suggesting that not only availability but also the composition of the ClpP/R core might affects accumulation of the chaperones. The observed drop in Hsp100 proteins in clpR1-1 could reflect the proportion of Hsp100 protein involved in Clp proteolysis, with the residual amount functioning in chaperone activities independent of the Clp protease. Induction of Cpn60 in clpC1 could be a way to compensate for the lack of chaperone activity either as a part or independent of the Clp activity (Sjögren et al. 2004). In clpR1-1 no enhancement was observed since the problem is not the lack of chaperone activity.
Although a reduction in chloroplast proteins is common to many pale and virescent mutants, the subset of proteins affected differs (Bellaoui et al. 2003; Kishine et al. 2004). Furthermore, while some mutants show no effect on transcription others show reduced transcription in plastids, the nucleus or both. This suggests that although having the same phenotype, different steps in chloroplast development are affected in these various mutants. It seems that chloroplast differentiation requires balancing many processes and activity of the Clp protease might be involved in one or more of the steps. In the rice virescent2 (v 2 ) mutant accumulation of chloroplast transcripts encoding photosynthetic genes is down-regulated while accumulation of transcripts coding for components of the plastid transcription/translation apparatus is unaffected (Sugimoto et al. 2004). Transcription of clpP1 was induced by v2 suggesting it is involved in chloroplast development. Other proteases that are required for chloroplast development include FtsH (Chen et al. 2000; Takechi et al. 2000) and EGY1, another nuclear-encoded chloroplast-protease induced by light (Chen et al. 2005).
The amount of SSU directly affects the translation initiation of LSU without affecting the translation of other photosynthetic proteins (Rodermel et al. 1996). Although it is possible that accumulation of LSU in clpR1-1 is affected by reduced amounts of SSU, the reduced amounts of other chloroplast encoded proteins such as PsaD (data not shown) suggests that LSU accumulation is probably more affected by the general reduction in plastid protein translation.
The accumulation of almost all chloroplast proteins examined was reduced in clpR1-1. This was not the case in clpR2-1 (Rudella et al. 2006) which, unlike clpR1-1, contains significant amounts of ClpR2 protein and therefore has a less severe phenotype. The affected proteins include a variety of stromal and thylakoid-associated proteins encoded by both plastid and nuclear genes. The only exception is the accumulation of CF1 β-subunit of ATPase. In Brassica rapa CF1-β ATPase is up-regulated under stress conditions to allow for more non-photochemical excitation quenching (NPQ), thereby protecting the photosynthetic machinery (Kanazawa and Kramer 2002; Shunxing et al. 2004). Considering its pale phenotype, small size, and small chloroplasts with fewer thylakoid membranes and grana stacks, it is possible that the up-regulation of CF1-β ATPase in the clpR1-1 mutants might allow the plants to better dissipate excessive excitation energy by NPQ and minimizing damage to the photosystems.
The lower levels of nuclear-encoded proteins in the clpR1-1 mutant are likely due to retrograde signaling, a process that coordinates nuclear gene expression with the functional state of chloroplasts (Nott et al. 2006). It is currently unknown if the retrograde signal is generated by the inhibition of transcription or translation. Since in clpR1-1 plastid transcription is unaffected while translation of some proteins seems to be inhibited, it may suggest that chloroplast protein translation is required for nuclear gene expression.
Lhcb like other nuclear-encoded chloroplast-proteins is imported as a higher molecular mass precursor to the chloroplast, processed, and then routed to its thylakoid location (Jarvis and Robinson 2004). It is possible that the second, higher molecular mass, band in the Lhcb immunoblot is the unprocessed precursor, but it is unlikely that ClpR1 is directly required for its processing, as it has been shown to be processed by a ∼140 kDa metalloendopeptidases (VanderVere et al. 1995). Since ClpC1 is part of the chloroplast-envelope import machinery (Constan et al. 2004) it is possible that reduced ClpC levels in clpR1-1 (Fig. 3) result in a low import efficiency and envelope bound pLhcb accumulate. Alternatively it might suggest that ClpR1, as part of its role in plastid development, might be required for normal development of the protein import machinery.
cp-RNA is transcribed as a highly conserved polycistronic mRNA containing the 16S, 23S, 4.5S, 5S and two tRNAs (Fig. 5C). The polycistronic RNA is then processed by several endo- and exo-nucleases to form the mature tRNAs, 16S and 5S cp-rRNAs and a dicistronic 23S–4.5S cp-rRNA intermediate. The last steps of cp-rRNA formation result in the mature, stabilized 23S and 4.5S cp-rRNA molecules (Bollenbach et al. 2005; Kössel et al. 1982). These final steps are inhibited in clpR1-1 as evident from the reduced levels of 23S and 4.5S cp-rRNA and the shift towards higher molecular weight forms. Maturation of 23S cp-rRNA was not affected in some mutants with impaired plastid development; these include ppi1 (Jarvis et al. 1998), var1-1 (Sakamoto et al. 2002) and var2-2 (Chen et al. 2000) (data not shown). It seems therefore that the differences observed in 23S cp-rRNA in the clpR1-1 mutant were not just a general result of inhibited plastid development. Furthermore, the difference in 23S-rRNA abundance can not be simply explained by slow development, leading to a lack of developmental synchrony between the mutant and wild type, since other transcripts, including 5S and 16S cp-rRNA, accumulate to wild-type levels. It seems unlikely that a ClpR1-containing Clp protease activity is directly involved in cp-rRNA processing. It was suggested that the Clp protease is involved in general protein turnover (Adam and Clarke 2002) and that the Clp proteolytic core is necessary for the transition from proplastids or etioplasts to chloroplasts during early seedling and leaf development (Zheng et al. 2006). It is possible that proteins required for processing of cp-rRNA that occurs during plastid development are regulated by the Clp activity.
Several mutants impaired in 23S and 4.5S cp-rRNA maturation have been described. These include the Arabidopsis dal (Bisanz et al. 2003) and rnr1 (Bollenbach et al. 2005; Kishine et al. 2004), and the tomato defective chloroplast and leaves-mutable (dcl-m) which has variegated leaves (Bellaoui et al. 2003). All these mutants show a reduction in plastid-encoded proteins. However, despite the impaired processing of 23S–4.5S cp-rRNA none of these mutants analyzed have reduced levels of 23S cp-rRNA suggesting that the product(s) of ClpR1 containing activity might affect not only primary processing of cp-rRNA, but also its 3′ processing required for stability.
Stable mutations in dcl-m (dcl-s) are embryo lethal and embryo development is arrested at the globular stage (Bellaoui et al. 2003). Interestingly, although clpR1-1 sets viable seeds, embryos isolated from ClpR1/clpR1 plants show a wide range of developmental stages (data not shown). While young embryos isolated from the same silique on a wild type plants are all at approximately the same stage, embryos from a silique grown on heterozygous plants can range between globular and heart stages (data not shown) suggesting that the clpR1/clpR1 embryos are slow to develop. The fact that clpR1-1 seeds are viable suggests that although ClpR1 is required for embryo development there is probably some redundancy either between ClpP/R proteins or between Clp and other plastid proteases. This is also true for chloroplast development since rnr1, dal and dcl mutants all have a more severe phenotype than clpR1-1.
In conclusion, it seems that the phenotype of the clpR1-1 mutant results from a role of a ClpR1-containing protease in regulating plastid development. As a result of the mutation chloroplasts in clpR1-1 are smaller and have fewer thylakoid membranes, and expression of nuclear genes such as lhcb and rbcS is down-regulated because of the inhibition of chloroplast gene expression. The role of ClpR1 in mature plants and in the response of plants to environmental stresses is currently being studied.
Experimental procedures
Plant material and growth conditions
clpR1-1 was identified as a virescent seedling in an EMS mutagenized, M2 population obtained from Lehle seeds (Round Rock, TX, USA). clpR1-2 seeds were obtained from the Arabidopsis Biological Resource Centre (ABRC) at Ohio State University (Columbus, OH, USA). Wild type (Col-0) and mutant Arabidopsis thaliana seeds were surface sterilized with 70% ethanol and 0.01% Triton X100 and plated on 1/2 MS medium (Murashige Skoog, Caisson laboratories; Rexburg, ID, USA) containing 1% sucrose. Where indicated, plates were supplemented with 5 μM norflurazon (Sandoz Pharmaceuticals, Vienna, Austria). Plants were grown under ∼150 μmol m-2sec−1 and a 12/12 light/dark cycle. For longer periods plants were transplanted in commercial potting mix and grown in long (16/8 light/dark) or, if stated, short (8/16 light/dark) day.
Complementation of clpR1-1
A clpR1 cDNA clone (U13007) was ordered from ABRC. Full length cDNA was amplified and cloned into pCHF3 using the Gateway system (Invitrogen, Carlsbad, CA, USA). clpR1-1 seedlings were transformed by floral dipping and transformants were selected on kanamycin containing MS plates.
Protein extraction and immunoblotting
Total proteins were extracted from 5 days old Arabidopsis seedlings ground in liquid N2 using a pre-cooled mortar and pestle. The powder was then quickly transferred to a pre-cooled microfuge tube and weighed. NuPAGE® LDS sample buffer (Invitrogen) was then added to the frozen powder to yield a final concentration of 0.3 mg ml−1. Samples were mixed, heated to 75°C for 5 min, cooled to 4°C and centrifuged at 20,000 × g for 10 min at 4°C. The supernatants from each sample were kept on ice until SDS-PAGE was performed. The concentration of protein was determined using the BCA Protein Assay Kit— Reducing Agent Compatible method (Pierce; Rockford, IL, USA) according to the manufacturer’s protocol.
Protein samples were separated based on protein concentration using SDS-PAGE and different pre-cast gel types (Invitrogen) were used depending on the protein size range being analyzed: 12% Bis-Tris gels using MES buffer for ClpP1, P3-6, ClpR1-4, PsaD, PsaL, and Lhcb2, MOPS buffer for Cpn60, LSU Rubisco, and the CF1 β-subunit of ATPase, and 3–8% Tris-Acetate gels for ClpC and ClpD detection. Proteins were then electrophoretically transferred, incubated with a range of antibodies, imaged and quantified as previously described (Sjögren et al. 2004).
RNA extraction and northern blots
Five days old seedlings were frozen in liquid N2 and stored at −70°C. RNA was extracted with the RNeasy kit (Qiagen; Hilden, Germany). Five micrograms of total RNA were separated on denaturing agarose-formaldehyde gels, blotted onto Hybond N+ (Amersham; Buckinghamshire, UK) membranes and probed as previously described (Muchizuki et al. 2001).
Chlorophyll quantification
Chl was extracted from whole seedlings of wild type (Col-0) and clpR1-1, grown on plates containing 1/2 MS media supplemented with 2% sucrose, and measured as described previously (Sjögren et al. 2004).
Electron microscopy
Samples were fixed in 2% paraformaldehyde, 2.5% glutaraldehyde in 0.1 M sodium phosphate buffer, for 24 h at 4°C, post-fixed in 1% osmium tetroxide, 1.5% potassium ferricyanide for 2 h at 40°C, dehydrated in ethanol and propylene oxide, and embedded in Spurr resin. One-micrometer sections were stained with toluidine blue. One hundred-nanometer sections were stained with uranyl acetate and lead citrate. Micrographs were taken on a JEOL 100CX II TEM, with a Soft Imaging System MegaView III digital camera.
Abbreviations
- cp-rRNA:
-
Chloroplast ribosomal RNA
- Lhcb2:
-
Light-harvesting Chl a/b binding protein
- RbcL/LSU and RbcS/SSU:
-
Transcript and protein of large and Small subunits of ribulose bisphosphate carboxylase/oxygenase (Rubisco)
- PsaL, PsaD and PsaF:
-
Subunits XI, II and III of photosystem I (PS I)
- Cpn60:
-
Chaperonin 60
- β-ATPase:
-
CF1 β subunit of ATPase
- PsbA and PsbD :
-
Transcripts encoding for D1 and D2 proteins of photosystem II (PSII)
- NF:
-
Norflurazon
- NPQ:
-
Non-photochemical excitation quenching
References
Adam Z, Adamska I, Nakabayashi K, Ostersetzer O, Haussuhl K, Manuell A, Zheng B, Vallon O, Rodermel SR, Shinozaki K, Clarke AK (2001) Chloroplast and mitochondrial proteases in Arabidopsis. A proposed nomenclature. Plant Physiol 125:1912–1918
Adam Z, Clarke AK (2002) Cutting edge of chloroplast proteolysis. Trends Plant Sci 7:451–456
Adam Z, Rudella A, van Wijk KJ (2006) Recent advances in the study of Clp, FtsH and other proteases located in chloroplasts. Curr Opin Plant Biol 9:234–240
Bellaoui M, Keddie JS, Gruissem W (2003) DCL is a plant-specific protein required for plastid ribosomal RNA processing and embryo development. Plant Mol Biol 53:531–543
Bisanz C, Begot L, Carol P, Perez P, Bligny M, Pesey H, Gallois JL, Lerbs-Mache S, Mache R (2003) The Arabidopsis nuclear DAL gene encodes a chloroplast protein which is required for the maturation of the plastid ribosomal RNAs and is essential for chloroplast differentiation. Plant Mol Biol 51:651–663
Bollenbach TJ, Lange H, Gutierrez R, Erhardt M, Stern DB, Gagliardi D (2005) RNR1, a 3′-5′ exoribonuclease belonging to the RNR superfamily, catalyzes 3′ maturation of chloroplast ribosomal RNAs in Arabidopsis thaliana. Nucleic Acids Res 33:2751–2763
Chen G, Bi YR, Li N (2005) EGY1 encodes a membrane-associated and ATP-independent metalloprotease that is required for chloroplast development. Plant J 41:364–375
Chen M, Choi Y, Voytas DF, Rodermel S (2000) Mutations in the Arabidopsis VAR2 locus cause leaf variegation due to the loss of a chloroplast FtsH protease. Plant J 22:303–313
Clarke AK (1999) ATP-dependent Clp proteases in phtosynthetic organisms—A cut above the rest! Ann Bot 83:593–599
Constan D, Froehlich JE, Rangarajan S, Keegstra K (2004) A stromal Hsp100 protein is required for normal chloroplast development and function in Arabidopsis. Plant Physiol 136:3605–3615
Dougan DA, Reid BG, Horwich AL, Bukau B (2002) ClpS, a substrate modulator of the ClpAP machine. Mol Cell 9:673–683
Gottesman S (1996) Proteases and their targets in Escherichia coli. Annu Rev Genet 30:465–506
Grimaud R, Kessel M, Beuron F, Steven AC, Maurizi MR (1998) Enzymatic and structural similarities between the Escherichia coli ATP-dependent proteases, ClpXP and ClpAP. J Biol Chem 273:12476–12481
Halperin T, Ostersetzer O, Adam Z (2001a) ATP-dependent association between subunits of Clp protease in pea chloroplasts. Planta 213:614–619
Halperin T, Zheng B, Itzhaki H, Clarke AK, Adam Z (2001b) Plant mitochondria contain proteolytic and regulatory subunits of the ATP-dependent Clp protease. Plant Mol Biol 45:461–468
Jarvis P, Chen LJ, Li H, Peto CA, Fankhauser C, Chory J (1998) An Arabidopsis mutant defective in the plastid general protein import apparatus. Science 282:100–103
Jarvis P, Robinson C (2004) Mechanisms of protein import and routing in chloroplasts. Curr Biol 14:R1064–1077
Kanazawa A, Kramer DM (2002) In vivo modulation of nonphotochemical exciton quenching (NPQ) by regulation of the chloroplast ATP synthesis. Proc Natl Acad Sci USA 99:12789–12794
Kandror O, Busconi L, Sherman M, Goldberg AL (1994) Rapid degradation of an abnormal protein in Escherichia coli involves the chaperones GroEL and GroES. J Biol Chem 269:23575–23582
Keeler SJ, Boettger CM, Haynes JG, Kuches KA, Johnson MM, Thureen DL, Keeler CL, Jr., Kitto SL (2000) Acquired thermotolerance and expression of the HSP100/ClpB genes of lima bean. Plant Physiol 123:1121–1132
Kishine M, Takabayashi A, Munekage Y, Shikanai T, Endo T, Sato F (2004) Ribosomal RNA processing and an RNase R family member in chloroplasts of Arabidopsis. Plant Mol Biol 55:595–606
Kössel H, Edwards K, Koch W, Langridge P, Schiefermayr E, Schwarz Z, Strittmatter G, Zenke G (1982) Structural and functional analysis of an rRNA operon and its flanking tRNA genes from Zea mays chloroplasts. Nucleic Acids Symp Ser:117–120
Kuroda H, Maliga P (2003) The plastid clpP1 protease gene is essential for plant development. Nature 425:86–89
Li J, Chory J (1997) A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell 90:929–938
Majeran W, Wollman FA, Vallon O (2000) Evidence for a role of ClpP in the degradation of the chloroplast cytochrome b (6) f complex. Plant Cell 12:137–150
Moreira D, Le Guyader H, Philippe H (2000) The origin of red algae and the evolution of chloroplasts. Nature 405:69–72
Muchizuki N, Brusslan JA, Larkin RM, Nagatani A, Chory J (2001) Arabidopsis genomes uncoupled 5 (gun5) mutant reveals the involvment of Mg-chelatase H subunit in plastid-to nucleus signal transduction. Proc Natl Acad Sci USA 98:2053–2058
Nakabayashi K, Ito M, Kiyosue T, Shinozaki K, Watanabe A (1999) Identification of clp genes expressed in senescing Arabidopsis leaves. Plant Cell Physiol 40:504–514
Nielsen E, Akita M, Davila-Aponte J, Keegstra K (1997) Stable association of chloroplastic precursors with protein translocation complexes that contain proteins from both envelope membranes and a stromal Hsp100 molecular chaperone. Embo J 16:935–946
Nott A, Jung HS, Koussevitzky S, Chory J (2006) Plastid-to-nucleus retrograde signaling. Annu Rev Plant Biol 57:739–759
Park S, Rodermel SR (2004) Mutations in ClpC2/Hsp100 suppress the requirement for FtsH in thylakoid membrane biogenesis. Proc Natl Acad Sci USA 101:12765–12770
Peltier JB, Ripoll DR, Friso G, Rudella A, Cai Y, Ytterberg J, Giacomelli L, Pillardy J, van Wijk KJ (2004) Clp protease complexes from photosynthetic and non-photosynthetic plastids and mitochondria of plants, their predicted three-dimensional structures, and functional implications. J Biol Chem 279:4768–4781
Peltier JB, Ytterberg J, Liberles DA, Roepstorff P, van Wijk KJ (2001) Identification of a 350-kDa ClpP protease complex with 10 different Clp isoforms in chloroplasts of Arabidopsis thaliana. J Biol Chem 276:16318–16327
Puente P, Wei N, Deng XW (1996) Combinatorial interplay of promoter elements constitutes the minimal determinants for light and developmental control of gene expression in Arabidopsis. EMBO J 15:3732–3743
Rodermel S, Haley J, Jiang CZ, Tsai CH, Bogorad L (1996) A mechanism for intergenomic integration: abundance of ribulose bisphosphate carboxylase small-subunit protein influences the translation of the large-subunit mRNA. Proc Natl Acad Sci USA 93:3881–3885
Rudella A, Friso G, Alonso JM, Ecker JR, van Wijk KJ (2006) Downregulation of ClpR2 Leads to Reduced Accumulation of the ClpPRS Protease Complex and Defects in Chloroplast Biogenesis in Arabidopsis. Plant Cell 18:1704–1721
Sakamoto W (2006) Protein degradation machineries in plastids. Annu Rev Plant Biol 57:599–621
Sakamoto W, Tamura T, Hanba-Tomita Y, Murata M (2002) The VAR1 locus of Arabidopsis encodes a chloroplastic FtsH and is responsible for leaf variegation in the mutant alleles. Genes Cells 7:769–780
Schirmer EC, Glover JR, Singer MA, Lindquist S (1996) HSP100/Clp proteins: a common mechanism explains diverse functions. Trends Biochem Sci 21:289–296
Shikanai T, Shimizu K, Ueda K, Nishimura Y, Kuroiwa T, Hashimoto T (2001) The chloroplast clpP gene, encoding a proteolytic subunit of ATP-dependent protease, is indispensable for chloroplast development in tobacco. Plant Cell Physiol 42:264–273
Shunxing J, Hilaire E, Guikema JA (2004) Identification and differential accumulation of two isoforms of the CF1-β subunit under high light stress in Brassica rapa. Plant Physiol Biochem 42:883–890
Sjögren LL, MacDonald TM, Sutinen S, Clarke AK (2004) Inactivation of the clpC1 gene encoding a chloroplast Hsp100 molecular chaperone causes growth retardation, leaf chlorosis, lower photosynthetic activity, and a specific reduction in photosystem content. Plant Physiol 136:4114–4126
Sokolenko A, Lerbs-Mache S, Altschmied L, Herrmann RG (1998) Clp protease complexes and their diversity in chloroplasts. Planta 207:286–295
Sugimoto H, Kusumi K, Tozawa Y, Yazaki J, Kishimoto N, Kikuchi S, Iba K (2004) The virescent-2 mutation inhibits translation of plastid transcripts for the plastid genetic system at an early stage of chloroplast differentiation. Plant Cell Physiol 45:985–996
Takechi K, Sodmergen, Murata M, Motoyoshi F, Sakamoto W (2000) The YELLOW VARIEGATED (VAR2) locus encodes a homologue of FtsH, an ATP-dependent protease in Arabidopsis. Plant Cell Physiol 41:1334–1346
VanderVere PS, Bennett TM, Oblong JE, Lamppa GK (1995) A chloroplast processing enzyme involved in precursor maturation shares a zinc-binding motif with a recently recognized family of metalloendopeptidases. Proc Natl Acad Sci USA 92:7177–7181
Wang J, Hartling JA, Flanagan JM (1997) The structure of ClpP at 2.3 Å resolution suggests a model for ATP-dependent proteolysis. Cell 91:447–456
Zheng B, MacDonald TM, Sutinen S, Hurry V, Clarke AK (2006) A nuclear-encoded ClpP subunit of the chloroplast ATP-dependent Clp protease is essential foe early development in Arabidopsis thaliana. Planta (in press)
Acknowledgement
This work was supported by the Howard Hughes Medical Institute and a grant from the Department of Energy to J.C., the Swedish Research Council for Environmental, Agricultural Sciences and Spatial Planning (Formas) to A.K.C., and an overseas postgraduate scholarship from the Natural Sciences and Engineering Research Council of Canada to T.M.S. S.K. was an EMBO long term fellow (ALTF 118–2000). J.C. is a Howard Hughes Medical Institute Investigator. We thank Takeshi Nakano (Riken Japan) for his help in analyzing chloroplast transcripts, Jason Lim for technical assistance and Olivier Loudet, Chris Schwartz and Jennifer Nemhauser for assisting in the map based cloning of clpR1-1. We thank Paul Sawchenko and the CCMI at Salk Institute for access to microscopy facilities. clpR1-2 seeds and the full length clpR1 cDNA clone were provided by The Arabidopsis Biological Resource Centre (ABRC), Ohio State University (Columbus, Ohio).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Koussevitzky , S., Stanne, T.M., Peto, C.A. et al. An Arabidopsis thaliana virescent mutant reveals a role for ClpR1 in plastid development. Plant Mol Biol 63, 85–96 (2007). https://doi.org/10.1007/s11103-006-9074-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-006-9074-2