Abstract
Introduction
Temozolomide (TMZ) is currently considered as a rational therapeutic option for patients with progressively aggressive pituitary adenomas and carcinomas not responding to conventional therapies. Administration of TMZ results in clinical response and improvement in survival of many of these patients depending upon the expression of the DNA repair enzyme O-6 methylguanine DNA transferase (MGMT). Low or negative MGMT immunoreactivity predicts responsiveness to TMZ therapy. Therefore, MGMT serves as a criterion to select candidate patients anticipating response to treatment.
Materials and Methods
The MGMT expression was investigated in 25 pituitary adenomas with Ki-67 labeling index more that 3% and p53 expression, using various antigen retrieval protocols. After direct application of the antibody, only one adenoma yielded positive for MGMT. However, after pretreatment of tissue sections with antigen retrieval protocols, another 3 adenomas, initially negative turned to positive.
Conclusions
These findings could explain lack of response to TMZ treatment in patients with false negative MGMT immunohistochemistry. Evaluation of tumor samples for MGMT expression should carefully be carried-out using the optimum immunohistochemical protocol to obtain consistent and reliable results that help to identify patients that could respond to TMZ therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pituitary adenomas are encountered in 1/1000–2000 inhabitants that can effectively be managed with modern surgical interventions and advances in medical treatment although a subset may require additional radiotherapy. Although the majority follows an indolent course, a subset of mostly macroadenomas, may exhibit an aggressive behavior with early recurrences and progression, whereas approximately 0.2% may become truly malignant with central nervous system and distant metastases [1, 2].
A significant progress has been made during the last two decades in the medical management of pituitary adenomas. The treatment of these clinically refractory aggressive tumors remains challenging, as conventional chemotherapy exhibits low response rates, whereas radiotherapy fails to control tumor growth and may be associated with considerable side effects. Temozolomide (TMZ) is an imidazotetrazine derivative, oral cytotoxic chemotherapeutic agent that inhibits DNA replication. It was initially used to treat highly malignant gliomas and when used in aggressive/malignant pituitary tumors was associated with remarkable improvement rate in the 5-year overall survival and 5-year progression-free survival [3,4,5,6]. This drug has currently become a rational therapeutic option for such neoplasms not responding to conventional treatment modalities [7,8,9,10,11]. The cytotoxic activity of temozolomide depends upon the expression of O-6 methylguanine DNA transferase (MGMT), a DNA repair enzyme as its presence in tumor cells leads to TMZ inactivation. Lack of MGMT immunoreactivity predicts responsiveness to therapy [6, 10, 12, 13]. In addition, decreased or negative MGMT expression is noted in tumors removed from responding patients with initially low MGMT expression, indicating that TMZ is also capable to absorb low MGMT stores [14, 15]. In contrast, no therapeutic effect is expected in patients with tumors showing high MGMT expression, suggesting that TMZ administration should be restricted to selected patients anticipating response to treatment after evaluating tumor samples for MGMT expression by immunohistochemistry.
Despite a large number of studies considering MGMT as marker to treat patients with TMZ, the question whether it represents the best biomarker to predictor of TMZ therapy is still a subject of debate. Although, initial studies reported a fairly good correlation between tumor response and lack of MGMT expression, suggesting a rational approach for TMZ administration to refractory adenomas, a subset of patients with MGMT negative pituitary tumors did not respond [11, 12, 16]. Specific monoclonal antibodies against MGMT formalin-fixed, paraffin-embedded are available for immunohistochemistry [7, 17]. However, negative results cannot always exclude the presence of MGMT. This can be explained by several technical issues that can be overcome, such as cases of initial immunonegativity may turn positive after pretreatment of tissue sections with antigen retrieval protocols. For this reason, various protocols should be carefully tested and standardized, in order to select the most suitable for each pathology laboratory.
This work attempts to explain histochemical and clinical controversies and highlights the key-role of pathologists in the precise selection of candidates for treatment with TMZ.
Material and Methods
We investigated MGMT expression in a series of 25 adenomas, retrieved from the files of the Hellenic Pituitary Tumor Reference Center. All were macroadenomas, previously classified as “atypical adenomas”, based on elevated Ki-67 labeling index (LI), ranging from 3 to 30%, and extensive p53 expression [18]. These included 3 somatotroph, 6 lactotroph, 1 mixed somatotroph—lactotroph, 1 acidophil stem cell, 1 functioning corticotroph, 1 thyrotroph and 12 gonadotroph adenomas. Immunohistochemistry for MGMT was carried-out on 4 sets of sections from formalin-fixed, paraffin-embedded tissues, using a specific mouse monoclonal antibody, clone MT3.1 raised against recombinant MGMT protein (Abnova, Taipei Taiwan, at dilution 1:100). Incubations with primary antibody were carried out overnight at 4°C. The one-step EnVision polymer detection system (Dako A/S, Glostrup Denmark), as a secondary link to nickel chloride—DAB enhancing chromogen (Sigma, St Lewis, MO).
In the first set of sections, the primary antibody was applied directly. In the second set, sections were pretreated with 1 mg/100 ml pronase E (Sigma Chemical Companies, St. Louis, MO, USA) for 20 min at room temperature [19]. In the third set of sections, before application of the primary antibody, sections were pretreated with antigen retrieval system employing pressure-cooking in Tris/EDTA buffer (pH 9.0) for 20 min. A fourth set of sections, where the primary antibody was substituted by phosphate buffer solution was used as negative control. Nuclear immunostaining of endothelial cells served as internal positive control.
Results
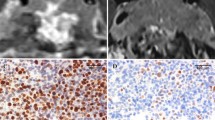
In the first set of sections, only one sparsely granulated somatotroph adenoma in a 29-year-old man with a Ki-67 LI of 4% showed focal immunoreactivity for MGMT (4%), while the remaining were negative (96%). Surprisingly, in the second set of sections pretreated with pronase, the somatotroph adenoma showing focal MGMT expression became strongly and extensively reactive (Fig 1). In addition, another 3 tumors, that were initially negative, turned to positive (Fig 2). These included an acidophil stem cell adenoma in a 40-year-old woman with a Ki-67 LI of 30%, a lactotroph adenoma in a 60-year-old man with Ki-67 LI of 4% and a gonadotroph adenoma in a 74-year-old man with a Ki-67 LI of 5%. Therefore, another 12% of adenomas became positive after enzymatic pretreatment. Similar results were obtained from the third set of sections pretreated with pressure-cooking in Tris/EDTA buffer. The endothelial cells, serving as internal controls, were strongly positive (Fig. 2a). No correlation between Ki-67 LI and p53 with MGMT expression was noted.
a An acidophil stem cell adenoma, originally immunonegative for MGMT. Only endothelial cells are selectively MGMT immunoreactive, serving as internal positive control, confirming the accuracy of the technique (× 20). b The same tumor turned to immunopositive after application of antigen retrieval protocol. Note focal nuclear MGMT expression (× 20)
Discussion
The results of the present study highlight the significance of applying proper immunohistochemical techniques in order to reliably identify patients with aggressive pituitary adenomas that could respond to treatment with TMZ.
The term “aggressive tumors”, used by clinicians, refers to invasive and frequently recurrent pituitary adenomas and carcinomas, which are resistant to a combination of surgical, medical and radiation therapies [11, 20]. In the previous edition of the WHO classification it was assumed that a subset of these tumors belongs to the category of “atypical adenomas”, which were histologically defined on the basis of high Ki-67 LI more than 3% and extensive nuclear p53 immunostaining [18]. However, a lack of correlation between atypical adenomas and tumor aggressiveness is occasionally noted. Despite of more than 10 years of intensive research, the prognostic significance of this marker could not be justified [21]. In addition, the implication of p53 status in aggressive tumors remains questionable. In particular, p53 mutations are extremely rare, exceptionally identified in pituitary carcinomas [22]. Therefore, it remains uncertain whether p53 is an independent prognostic factor [21]. Nevertheless, high proliferation tumor rate, which is conceptually connected to tumor growth, is usually correlated with elevated Ki-67.
Due to a wide spectrum of clinical behaviors, even though some atypical adenomas cause significant morbidity, the terminology was recently revised. The new WHO edition of pituitary adenomas includes updates regarding the terminology and classification [21]. Given that the prognostic significance of Ki-67 and p53 has never been established, these markers are not recommended for routine diagnosis. However, the Ki-67 can be used for the evaluation of individual adenomas that may have a more clinically aggressive behavior in conjunction to other parameters, such as invasion assessed by MRI and/or intraoperative observation [21, 23]. According to changes made in the 4th edition of WHO, the category “atypical adenomas” has been eliminated and instead, the class of “high-risk” adenomas was introduced. “High-risk” adenomas comprise specific types, which may show rapid growth, radiological invasion, high recurrence rate and resistance to conventional therapy. These include the following types: sparsely granulated somatotroph adenoma, lactortoph adenoma in males, silent Crooke cell corticotroph adenoma, and the Pit-1 positive plurihormonal adenoma, previously termed silent subtype 3 [21]. Recent studies have shown that sparsely granulated somatotroph adenomas demonstrating negative or low MGMT expression could be successfully treated with TMZ, if conventional therapies fail to control [24]. In addition, TMZ has a potential for treating highly aggressive and resistant lactotroph adenomas and carcinomas [10, 20, 25]. However, some patients may develop resistance to TMZ therapy [3, 6, 7, 11, 12, 14].
Clinicopathological controversies
Immunohistochemical evaluation of MGMT expression represents the gold standard criterion to select candidate patients with aggressive adenomas for TMZ treatment although a subset of adenomas showing low expression or negative for MGMT does not respond. Absence or low immunoreactivity for MGMT predicts response to TMZ treatment. However, some of these patients do not respond [6, 10, 13, 15]. This might be a result of false low or false negative MGMT immunohistochemistry, attributed to several technical problems [2, 26]. Because of the inconsistency of MGMT expression, taking into account the paucity of other available treatment modalities, some clinicians, overlooking MGMT status, proceed to treatment with TMZ, based on high Ki-67 LI or strong expression of p53 [2, 15, 26,27,28]. However, high Ki-67 LI or extensive p53 expression do not always predict response to TMZ therapy. According to some reports, the immunohistochemical findings of MGMT as compared with Ki-67 and p53 do not show any significant correlation with TMZ efficacy [24, 28]. Furthermore, TMZ was effective in some patients with aggressive tumors even showing very low Ki-67 LI [15]. This has been attributed to the possible effect of pharmaceutical treatment to lactoroph and somatotroph adenomas with dopamine agonists or somatostatin analogs respectively. Administration of these agents suppresses cell proliferation, resulting in lowering Ki-67 levels [19, 29].
Technical drawbacks
Despite a large number of works using MGMT as a marker to treat patients with TMZ, the question whether it represents the best predictor of response to TMZ therapy is still a subject of debate. Although specific antibodies against MGMT, suitable for formalin-fixed, paraffin-embedded tissues, are available for immunohistochemistry, there are several problems that need to draw attention. Lack of correlation between MGMT and response to TMZ therapy remains controversial and has never been previously conclusively elucidated. In our material, MGMT immunohistochemistry applied directly, without any retrieval protocol, disclosed only one adenoma positive (4%). However, after application of retrieval protocols, another 3 that were initially negative turned to positive (totally 16%) while 21 (84%) remained truly negative. This 4 fold increased MGMT immunoreactivity signifies the implication of technical drawbacks leading to false negative results. It should be noted, that the acidophil stem cell adenoma, a classical aggressive-high risk tumor with Ki-67 of 30%, the highest LI among this series, turned to positive after application of antigen retrieval protocols. This paradigm, could explain the lack of effectiveness of TMZ therapy in some patients with such aggressive tumors.
Although, formaldehyde is widely used for tissue fixation, it might cause technical problems in the application of immunohistochemistry [12, 15, 16, 30]. To overcome the problem of hidden antigen sites, caused by formaldehyde, several pretreatment techniques exist. Nevertheless, optimal working dilution of the antibody should be determined by the end user. Furthermore, inappropriate tissue fixation, including delayed or prolonged fixation of adenoma tissue samples in formaldehyde and the storage duration of paraffin tissue blocks could also contribute to technical failures. Other problems imply low sensitivity of the antibody or the detection system used [26]. In addition, lack of a standard scoring system or semiquantitative counting of MGMT positive nuclei and also interobserver or intraobserver variations contribute to difficulties in determining the accurate level of MGMT expression [24, 26]. Most authors suggest that patients with adenomas showing negative or low MGMT expression are candidates for TMZ therapy, whereas, others consider a percentage lower than 20% of MGMT counting as predictor to start treatment [6, 10, 28]. All the above mentioned pre-analytical and post-analytical issues discourage some clinicians to recommend MGMT immunohistochemical detection and they rely on elevated Ki-67 alone.
Herein, we present the drawbacks of MGMT immunohistochemistry that may lead to false positive results. Our findings elucidate the technical problems involved, trying to explain the possible lack of response to TMZ treatment in some patients with adenomas, initially showing negative or low expression for MGMT, which may turn positive employing antigen retrieval protocols.
We conclude that, for precise selection of patients to receive TMZ therapy, evaluation of MGMT expression by immunohistochemistry using the appropriate antigen retrieval protocol, is strongly recommended.
References
Kaltsas GA, Nomikos P, Kontogeorgos G, Buchfelder M, Grossman AB (2005) Diagnosis and management of pituitary carcinomas. J. Clin. Endocrinol. Metab. 90:3089–3099. https://doi.org/10.1210/jc.2004-2231
Raverot G, Castinetti F, Jouanneau E, Morange I, Figarella-Branger D, Dufour H, Trouillas J, Brue T (2012) Pituitary carcinomas and aggressive pituitary tumours: merits and pitfalls of temozolomide treatment. Clin. Endocrinol. (Oxf.) 76:769–775. https://doi.org/10.1111/j.1365-2265.2012.04381.x
O’Reilly SM, Newlands ES, Glaser MG, Brampton M, Rice-Edwards JM, Illingworth RD, Richards PG, Kennard C, Colquhoun IR, Lewis P, Stevens MFG (1993) Temozolomide: a new oral cytotoxic chemotherapeutic agent with promising activity against primary brain tumours. Eur J Cancer 29A:940–942. Erratum in: Eur J Cancer 29A:1500 (1993)
Gilbert MR, Friedman HS, Kuttesch JF, Prados MD, Olson JJ, Reaman GH, Zaknoen SL (2002) A phase II study of temozolomide in patients with newly diagnosed supratentorial malignant glioma before radiation therapy. Neuro. Oncol. 4:261–267. https://doi.org/10.1093/neuonc/4.4.261
Stupp R, Dietrich PY, Ostermann Kraljevic S, Pica A, Maillard I, Maeder P (2002) Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomide. J. Clin. Oncol. 20:1375–1382. https://doi.org/10.1200/JCO.2002.20.5.1375
Syro LV, Rotondo F, Camargo M, Ortiz LD, Serna CA, Kovacs K (2018) Temozolomide and Pituitary Tumors: Current Understanding, Unresolved Issues, and Future Directions. Front. Endocrinol. (Lausanne) 9:1–14. https://doi.org/10.3389/fendo.2018.00318
Long-term response of pituitary carcinoma to temozolomide (2006) Fadul, C.E., Kominsky, A.L., Meyer, L.P. Report of two cases. J. Neurosurg. 105:621. https://doi.org/10.3171/jns.2006.105.4.621
Lim S, Shahinian H, Maya MM, Yong W, Heaney AP (2006) Temozolomide: a novel treatment for pituitary carcinoma. Lancet Oncol. 7:518–520. https://doi.org/10.1016/S1470-2045(06)70728-8
Syro LV, Scheithauer BW, Ortiz LD, Fadul CE, Horvath E, Rotondo F, Kovacs K (2009) Effect of temozolomide in a patient with recurring oncocytic gonadotrophic pituitary adenoma. Hormones 8:303–306. https://doi.org/10.14310/horm.2002.1247
Kovacs K, Horvath E, Syro LV, Uribe H, Penagos LC, Ortiz LD, Fadul CE (2007) Temozolomide therapy in a man with an aggressive prolactin-secreting pituitary neoplasm: Morphological findings. Hum Pathol 38:185–189. Erratum in: Hum Pathol 38:526 (2007)
Hagen C, Schroeder HD, Hansen S, Hagen C, Andersen M (2009) Temozolomide treatment of a pituitary carcinoma and two pituitary macroadenomas resistant to conventional therapy. Eur. J. Endocrinol. 161:631–637. https://doi.org/10.1530/EJE-09-0389
Kovacs K, Scheithauer BW, Lombardero M, McLendon RE, Syro LV, Uribe H, Fadul CE (2008) MGMT immunoexpression predicts responsiveness of pituitary tumors to temozolomide therapy. Acta Neuropathol. 115:261–262. https://doi.org/10.1007/s00401-007-0279-5
Syro LV, Ortiz LD, Scheithauer BW, Lloyd RV, Lau Q, Gonzalez R, Uribe H, Cusimano M, Kovacs K, Horvath E (2011) Treatment of pituitary neoplasms with temozolomide: a review. Cancer 117:454–462. https://doi.org/10.1002/cncr.25413
McCormack AI, Wass JA, Grossman AB (2011) Aggressive pituitary tumours: the role of temozolomide and the assessment of MGMT status. Eur. J. Clin. Invest. 41:1133–1148. https://doi.org/10.1111/j.1365-2362.2011.02520.x
Moshkin O, Syro LV, Scheithauer BW, Ortiz LD, Fadul CE, Uribe H, Gonzalez R, Cusimano M, Horvath E, Rotondo F, Kovacs K (2011) Aggressive silent corticotroph adenoma progressing to pituitary carcinoma: the role of temozolomide therapy. Hormones (Athens) 10:162–167. https://doi.org/10.14310/horm.2002.1307
McCormack AI, McDonald KL, Gill AJ, Clark SJ, Burt MG, Campbell KA (2009) Low 06-methylguanine-DNA methyltransferase [p1] (mgmt) expression and response to temozolomide in aggressive pituitary tumors. Clin. Endocrinol. (Oxf.) 71:226–233. https://doi.org/10.1111/j.1365-2265.2008.03487.x
McLendon RE, Cleveland L, Pegram C, Bigner SH, Bigner DD, Friedman HS (1998) Immunohistochemical detection of the DNA repair enzyme O6-methylguanine-DNA methyltransferase in formalin-fixed, paraffin-embedded astrocytomas. Lab. Invest. 78:643–644
Lloyd RV, Kovacs K, Young WF Jr, Farell WE, Asa SL, Touillas J, Kontogeorgos G, Sano T, Scheithauer BW, Horvath E (2004) Pituitary Tumours: Introduction. In: DeLellis RA, Heitz P, Lloyd RV, Eng C (eds) WHO Classification of Tumours, Pathology and Genetics, Tumours of Endocrine Organs. IARC Press, Lyon, pp 10–13
Kontogeorgos G, Horvath E, Kovacs K, Coire C, Lloyd RV, Scheithauer BW, Smyth HS (2006) Morphologic changes of prolactin-producing pituitary adenomas after short treatment with dopamine agonists. Acta Neuropathol. 111:46–52. https://doi.org/10.1007/s00401-005-1111-8
Jaffrain-Rea ML (2014) From resistant to aggressive and malignant prolactinomas: a translational approach. J Endocr Disord 1:1–10
Osamura RY, Lopes MBS, Grossman A, Kontogeorgos G, Trouillas J (2017) Tumours of the pituitary gland. Introduction. In: Lloyd RV, Osamura RV, Klöppel G (eds) WHO classification of tumours of endocrine organs. IARC Press, Lyon
Tanizaki Y, Jin L, Scheithauer BW, Kovacs K, Roncaroli F, Lloyd RV (2007) P53 gene mutations in pituitary carcinomas. Endocr. Pathol. 18:217–222. https://doi.org/10.1007/s12022-007-9006-y
Hasanov R, Aydoğan B, Kiremitçi S, Erden E, Güllü S (2019) The Prognostic Roles of the Ki-67 Proliferation Index, P53 Expression, Mitotic Index, and Radiological Tumor Invasion in Pituitary Adenomas. Endocr. Pathol. 30:49–55. https://doi.org/10.1007/s12022-018-9563-2
Zuhur SS, Tanik C, Karaman Ö, Velet S, Çil E, Öztürk FY, Özkayalar H, Müslüman AM, Altuntaş Y (2011) MGMT immunoexpression in growth hormone-secreting pituitary adenomas and its correlation with labeling index and cytokeratin distribution pattern. Endocrine 40:222–227
Almalki MH, Aljoaib NN, Alotaibi MJ, Aldabas BS, Wahedi TS, Ahmad MM, Alshahrani F (2017) Temozolomide therapy for resistant prolactin-secreting pituitary adenomas and carcinomas: a systematic review. Hormones (Athens) 16:139–149. https://doi.org/10.14310/horm.2002.1729
Micko ASG, Wöhrer A, Höftberger R, Vila G, Marosi C, Knosp E, Wolfsberger S (2017) MGMT and MSH6 immunoexpression for functioning pituitary macroadenomas. Pituitary 20:643–653. https://doi.org/10.1007/s11102-017-0829-3
Asimakopoulou A, Tzanela M, Koletti A, Kontogeorgos G, Tsagarakis S (2014) Long-term remission in an aggressive Crooke cell adenoma of the pituitary, 18 months after discontinuation of treatment with temozolomide. Clin. Case Rep. 2:1–3. https://doi.org/10.1002/ccr3.39
Bengtsson D, Schrøder HD, Andersen M, Maiter D, Berinder K, Feldt Rasmussen U, Krogh Rasmussen Å, van der Lely A, Petersson M, Johannsson G, Andersen M, Burman P (2015) Long-term outcome and MGMT as a predictive marker in 24 patients with atypical pituitary adenomas and pituitary carcinomas given treatment with temozolomide. J. Clin. Endocrinol. Metab. 100:1689–1698. https://doi.org/10.1210/jc.2014-4350
Selek A, Cetinarslan B, Canturk Z (2019) The effect of somatostatin analogues on Ki-67 levels in GH-secreting adenomas. Growth Horm. IGF Res. 45:1–45. https://doi.org/10.1016/j.ghir.2019.01.001
Murakami M, Mizutani A, Asano S, Katakami H, Ozawa Y, Yamazaki K, Ishida Y, Takano K, Okinaga H, Matsuno A (2011) A mechanism of acquiring temozolomide resistance during transformation of atypical prolactinoma into prolactin-producing pituitary carcinoma: case report. Neurosurg. 68:E1761–E1767. https://doi.org/10.1227/NEU.0b013e318217161a
Acknowledgements
No funding from official organization was received. We thank Mrs. Magda Pateraki and Soula Roumelioti for excellent technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors certify that they have NO involvement in any organization or entity with any financial interest in the context or materials discussed in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kontogeorgos, G., Thodou, E., Koutourousiou, M. et al. MGMT immunohistochemistry in pituitary tumors: controversies with clinical implications. Pituitary 22, 614–619 (2019). https://doi.org/10.1007/s11102-019-00993-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-019-00993-5