Abstract
Purpose
Data regarding atherosclerosis in acromegaly is controversial in literature. We aimed to investigate the markers of early atherosclerosis, oxidative stress, inflammation and their relationships with each other in acromegaly.
Methods
Thirty-nine patients with acromegaly and 40 control subjects were enrolled. Patients were classified into two groups; active acromegaly (AA) and controlled acromegaly (CA). Controls were matched by age, gender, body mass index and presence of cardiovascular risk factors. Flow mediated dilatation (FMD), carotid intima media thickness (CIMT), epicardial adipose tissue thickness (EAT) were measured and serum levels of oxidative stress parameters, high mobility group box 1 protein (HMGB1) and high sensitive CRP (hs CRP) were evaluated.
Results
Significantly decreased FMD, increased CIMT and EAT were found in patients with acromegaly compared to controls (p < 0.01, p < 0.05, p < 0.001, respectively). EAT correlated negatively with FMD (r = −0.24, p = 0.038) and positively with CIMT (r = 0.37, p < 0.01). Presence of acromegaly, hypertension and age were found to be the predictors of early atherosclerosis (p < 0.05). Hs CRP was decreased in AA compared to controls (p = 0.01). There were no significant differences for HMGB1 and oxidized LDL (ox-LDL) cholesterol levels and total antioxidant capacity (TAC) between AA, CA and controls (p > 0.05).
Conclusion
Early atherosclerosis measured with FMD, CIMT and EAT may exist in acromegaly. However, decreased hs CRP and unchanged HMGB1, ox-LDL and TAC levels suggest that inflammation and oxidative stress do not seem to contribute to the development of atherosclerosis in these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acromegaly is an endocrine disease characterized by increased circulating growth hormone (GH) and insulin-like growth factor (IGF)-1 levels, usually resulting from a pituitary adenoma [1]. Acromegaly has an estimated prevalence of approximately 60 per million and an annual incidence of 3 or 4 per million [2]. Cardiac failure, cardiac valve diseases, cardiomyopathy and arrhythmias are the causes of increased mortality in acromegaly [3]. Due to the effects of increased GH, patients with acromegaly carry increased risks of insulin resistance, diabetes mellitus (DM), hypertension (HT) and hyperlipidemia (HL) [3]. Despite increased cardiovascular risk factors, data in the literature on the development of atherosclerosis in acromegaly is controversial. Some previous reports suggested that patients with acromegaly have a low risk for coronary artery disease (CAD) [4] while others indicate the opposite result [5].
Impaired flow mediated dilatation (FMD), which is a marker of endothelial dysfunction has been demonstrated as an early marker of atherosclerosis [6]. Endothelial dysfunction and carotid intima media thickness (CIMT) have been explored in several studies performed in patients with acromegaly [7–11]. Epicardial adipose tissue is located around the heart, especially around the major coronary vessels, and previous studies have shown that increased epicardial adipose tissue thickness (EAT) is associated with CAD and major adverse cardiac events [12]. It has been suggested in a meta-analysis that EAT may be an early marker of CAD [13]. In the literature, there is only one study investigating EAT in patients with acromegaly [14].
Oxidative stress and inflammation are associated with the development of atherosclerosis and cardiovascular disease (CVD) [15]. In the literature, a few studies have shown increased oxidative stress and reduced antioxidant status in acromegaly [16]. In contrast, the role of subclinical inflammation on atherosclerosis in acromegaly is still contradictory because previous studies have shown that patients with acromegaly primarily have decreased [17] as well as unchanged [18] high sensitive C-reactive protein (hs CRP) levels.
High mobility group box 1 (HMGB1) protein is an intranuclear DNA binding protein and it has an essential role in DNA repair [19]. The accumulation of HMGB1 protein has been shown at sites of oxidative stress and it has been proposed as an early marker of oxidative damage [19]. HMGB1 protein is also released actively and passively in response to cell injury and has recently been implicated in the pathogenesis and the progression of atherosclerosis [20]. Increased expression of HMGB1 has also been found in atherosclerotic lesions in cardiopulmonary bypass surgery patients [21]. To the best of our knowledge, there is no study evaluating HMGB1 in acromegaly as a molecule which may have a role in the pathogenesis of both oxidative stress and inflammation.
In this study; to determine the existence of early atherosclerosis, we investigated the markers of atherosclerosis (FMD, CIMT and EAT), parameters of inflammation (HMGB1 and hs CRP) and indicators of oxidative stress (total antioxidant capacity (TAC) and oxidized LDL (ox-LDL) cholesterol levels) in patients with acromegaly according to their disease activity.
Materials and methods
Subjects
This study included patients diagnosed with acromegaly at the Gazi University Endocrinology and Metabolism Department. Acromegaly was diagnosed by failure of suppression of serum GH concentrations below 1 ng/mL after a 75-g oral glucose tolerance test (OGTT) together with fasting serum IGF-1 concentrations above the normal ranges for age and gender with the presence of clinical features of acromegaly [22]. Patients were classified into two groups; active acromegaly (AA; n = 21) and controlled acromegaly (CA; n = 18). Controlled acromegaly was defined as GH below 1.0 ng/mL on a 75-g OGTT or if in patients under somatostatin analogs a random GH was below 1.0 ng/mL and IGF-1 values were in the reference ranges for age and gender [2]. The CA group consisted of patients who were in remission for at least 6 months. Exclusion criteria were as follows: age younger than 18 years of age; untreated hormonal deficiencies, uncontrolled HT DM and HL, coexisting active inflammatory and infectious diseases, known CAD and smoking. None of our patients had hormonal co-production.
Thirty-nine patients with acromegaly and 40 matched controls according to age, gender, body mass index (BMI) and the presence of cardiovascular risk factors including DM, HT and HL were enrolled into this study. Twenty nine of 39 (74.4 %) patients were treated with surgery alone, 1 (2.6 %) with gamma-knife radiosurgery, 2 (5.1 %) with surgery and gamma-knife radiosurgery. Seven (17.9 %) patients who were included in the study before surgery were newly diagnosed. Four (10.3 %) patients, two patients in the AA group and two patients in the CA group, had hypopituitarism and all were adequately treated. The study was approved by the Gazi University Ethics Committee. Written informed consent were also obtained from all patients.
Anthropometric and laboratory measurements
BMI was calculated as weight in kg/(height in m)2, and waist circumference was measured at the midpoint between the lower border of the rib cage and iliac crest. Blood samples were obtained from all patients and controls after an overnight fast for serum lipid profile, fasting blood glucose, and creatinine and immediately stored at −80 °C for subsequent analysis. Samples from all patients for GH and IGF-1 analysis were also obtained early in the morning after an overnight fast. Serum fasting blood glucose and creatinine were assessed by a local laboratory using standard laboratory methods. Serum total cholesterol, HDL cholesterol and triglycerides levels were measured by an autoanalyzer (Beckman Coulter, Olympus AU2700) with enzymatic colorimetric methods using commercial kits (Beckman Coulter). LDL cholesterol levels were calculated using the Friedewald formula [23]. Serum GH levels were measured with an Immulite2000 (Siemens) autoanalyzer using the chemiluminescence method. Serum IGF-1 levels were determined with an immunoradiometric assay using a Beckman-Coulter Immunotech kit. Age and gender matched normal reference ranges were used for evaluation according to the kit guide.
The Bio-Tek ELX800 absorbance microplate reader and washer (USA, Minnesota) were used for the measurements. Ox-LDL (ng/mL) levels were measured using Immune Diagnostic ox-LDL/MDA adduct Elisa kit with 1/10 diluted plasma samples. HMGB1 (pg/mL) levels were measured using the HMGB1 Elisa kit (CUSABIO) with plasma samples. TAC levels were detected with an antioxidant assay kit (Cayman Chemicals) using serum samples and results were given as an mM Trolox equivalent. For hs CRP measurements, the DIA Source hs CRP enzyme immunoassay (DIA Source ImmunoAssays S.A. Belgium) kit was used and measurements (ng/mL) were obtained using 1/20 diluted serum samples.
Flow mediated dilatation measurement
FMD measurement was performed in a quiet, temperature-controlled room (22–24 °C) by the same trained endocrinologist. Patients fasted for at least 8 h before the study and abstained from exercise and caffeine for 24 h and alcohol for 12 h prior to testing. Measures were obtained in the supine position. FMD of the brachial artery was assessed by B-Mode high-resolution ultrasound imaging (Logic 5 Pro, General Electric) according to previous guidelines [24]. The right brachial artery was imaged above the antecubital fossa in the longitudinal plane with a 7.0 MHz linear array transducer. A baseline rest image was acquired. A sphygmomanometric (blood pressure) cuff was placed on the forearm, and arterial occlusion was created by cuff inflation to 200 mmHg systolic pressure for 5 min. Subsequently, the cuff was deflated and the longitudinal image of the artery was recorded continuously from 30 s before to 2 min after cuff deflation. The maximum change in the post-ischemia diameter as a percentage of the baseline diameter was recorded as FMD [24].
Carotid intima media thickness measurement
CIMT measurement was performed using B-mode ultrasound imaging (Logic 5 Pro, General Electric) by the same endocrinologist. The common carotid, the carotid bifurcation and the internal carotid near and far wall segments were scanned bilaterally with a linear-array transducer (7–12 mHz). Subjects were examined in the supine position with the head turned 20° contra-lateral to the side of imaging. Images were obtained in the longitudinal plane. Measurements from three segments of both the left and right common carotid arteries 1 cm proximal to the origin of the carotid bulb were averaged for each side, and the mean value of a combination of both sides was calculated as CIMT [25].
Epicardial adipose tissue thickness measurement
Echocardiograms were performed with a VIVID 7 (Vingmed-General Electric, Horten, Norway) instrument according to the recommendations of the American Society of Echocardiography [26]. Echocardiographic images were recorded onto a computerized database and videotape. EAT was measured on the free wall of the right ventricle from the parasternal long-axis views. Epicardial adipose tissue was identified as an echo-free space in the pericardial layers on the 2-dimensional echocardiography, and its thickness was measured perpendicularly on the free wall of the right ventricle at end-diastole for three cardiac cycles [27]. The average values of three cardiac cycles from each echocardiographic view were determined. The average value was used for statistical analysis. A single, experienced cardiologist performed all of the EAT assessments.
Statistical analysis
The Statistical Packages for Social Sciences SPSS version 21 was used for data analysis. Continuous variables were presented as the mean ± SD or median (minimum–maximum). Categorical data were presented as numbers and percentages. Differences between groups were detected using the Chi Square test for categorical variables. Normality of distribution for continuous variables was assessed using the Shapiro–Wilks test, and homogeneity of variances was assessed using the Levene test. For continuous variables, differences between groups were determined using one-way variance analysis or the Welch ANOVA test if parametric test assumptions were satisfied. If differences were identified between groups, Tukey’s HSD test or Games Howell test were used to identify the differences between pairs. When parametric test assumptions were not satisfied, the Kruskal–Wallis test was used to determine differences between groups and the group which caused difference was detected using the Siegel-Castellan test. The Mann–Whitney U test was used to compare differences between two groups when parametric test assumptions were not satisfied. The correlation between numerical variables was presented with a Spearman correlation coefficient. Multiple stepwise linear regression analysis which includes forward selection and backward elimination was used to determine factors affecting continuous variables. A p value of <0.05 was considered statistically significant.
Results
Clinical and demographical characteristics of patient and control groups are shown in Table 1. Regarding age, gender, BMI, waist circumference, systolic and diastolic pressure there were no significant differences between the groups (p > 0.05). DM, HT and HL rates were similar between groups (p > 0.05). Also, there was no significant difference between the groups for lipid profile and fasting blood glucose levels (p > 0.05). There was no significant difference between AA and CA groups for waist circumference (p > 0.05). Disease duration and somatostatin analog usage rates were similar between AA and CA groups (p > 0.05). GH and IGF-1 levels were significantly higher in the AA group than CA group (p < 0.001).
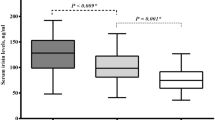
Mean FMD was 9.33 ± 4.62 % in AA, 8.66 ± 2.91 % in CA and 12.39 ± 5.80 % in control group. FMD was found significantly lower in acromegaly group compared to control group (p = 0.008). The difference between AA and CA groups was not statistically significant (p > 0.05). Median CIMT was 0.67 mm (0.55–1.24) in AA group, 0.67 mm (0.46–1.09) in CA group and 0.59 mm (0.49–0.93) in control group. CIMT was found significantly higher in acromegaly group compared to control group (p = 0.038). There was no statististical difference between AA and CA groups (p > 0.05). CIMT was significantly higher in AA and CA compared to controls (p = 0.029, p = 0.047, respectively). Mean EAT was measured 2.64 ± 0.94 mm in AA, 2.48 ± 0.57 mm in CA and 1.45 ± 0.52 mm in control group. EAT was found to be significantly increased both in AA and CA groups compared to control group (p < 0.001, p < 0.001, respectively). EAT was found to be increased in AA group compared to CA group but there was no statistical significance between the groups (p = 0.80). Measurements of FMD, CIMT and EAT are shown in Fig. 1.
Among inflammatory and oxidative stress parameters, hs CRP levels were found to be lowest in the AA group and highest in control group. Median hs CRP level was 168 ng/mL (76–1,127) in AA, 367 ng/mL (146–1,478) in CA and 512 ng/mL (122–1,611) in control group. Hs CRP was significantly decreased in AA compared to control group (p = 0.01). There were no statistically significant differences for ox-LDL, TAC and HMGB1 levels between the groups (p > 0.05). Biochemical parameters of patient and control groups are shown in Table 2.
Correlation analysis
In correlation analysis, in the whole group, FMD was significantly and inversely corrrelated with CIMT and EAT (r = −0.32, p < 0.01, r = −0.24, p = 0.038, respectively). CIMT and EAT were positively correlated with each other (r = 0.37, p < 0.01). There were no significant correlations between measured oxidative stress and inflammatory parameters and FMD, CIMT, EAT (p > 0.05). Among inflammatory parameters, hs CRP was positively correlated with BMI (r = 0.53, p < 0.01) and inversely correlated with GH and IGF-1 levels (r = −0.54, p < 0.01, r = −0.45, p < 0.01, respectively). The correlation graphics between EAT, FMD and CIMT are shown in Fig. 2.
Regression analysis
To determine the factors affecting FMD, CIMT and EAT, stepwise regression analysis was performed with the variables; BMI, age and presence of acromegaly, DM, HT, HL. The model in which EAT was the dependent variable (R2 = 0.47, p < 0.001) revealed that presence of acromegaly and HT were determinants for increased EAT in patients with acromegaly (β coefficient = 1.11, p < 0.01; β coefficient = 0.46, p < 0.01; respectively). Also, in stepwise regression analysis for FMD as dependent parameter (R2 = 0.30, p < 0.01), presence of acromegaly and age were independent variables leading to decreased FMD in acromegaly (β coefficient = −0.33, p < 0.01, β coefficient = −0.47, p < 0.01, respectively). Moreover, in the model in which CIMT was the dependent variable (R2 = 0.22, p < 0.01), presence of acromegaly and HT were found to be the significant parameters for increased CIMT in acromegaly (β coefficient = 0.26, p = 0.018, β coefficient = 0.41, p < 0.01, respectively). Factors affecting EAT, CIMT and FMD in regression analysis are shown in Table 3.
Discussion
Our results indicated decreased FMD and increased CIMT and EAT as early markers of atherosclerosis in patients with acromegaly. Additionally, we found significant relationships between FMD, CIMT and EAT. Moreover, our data suggested that the markers of atherosclerosis were not different between active and controlled acromegaly groups. In contrast, hs CRP levels were decreased but oxidative stress parameters and HMGB1 levels did not change in our patients with acromegaly.
Excess GH leads to several co-morbidities such as DM, HT, and HL in patients with acromegaly. The presence of these co-morbidities makes the evaluation of the effect of excess GH on atherosclerosis difficult. Most studies on acromegaly in the literature have compared patients with acromegaly to healthy individuals. In our study, to minimize the possible effects of co-morbidities, we matched the controls according to the presence of DM, HT and HL. In doing this, we attempted to investigate the specific role of acromegaly in the development of atherosclerosis in patients with acromegaly. On the other hand, in a recent study, Reyes-Vidal et al. [28] revealed that some cardiovascular risk factors, including body weight and waist circumference, were increased when remission was achieved in acromegaly. In our study, waist circumference in the AA group was higher than the CA group; however, this difference was not statistically significant. Therefore, lower body fat did not seem to influence our results in patients with acromegaly.
In the present study, FMD was found to be significantly decreased in patients with acromegaly compared to the control group. However, there were no significant differences between active and controlled patients. Additionally, the ‘presence of acromegaly’ and ‘age’ were found to be the most important predictors of endothelial dysfunction in our study. Chanson et al. [8] reported increased local resistance and decreased blood flow in the brachial artery compared to matched controls in acromegaly. Similarly, Baykan et al. [9] evaluated patients with acromegaly and reported decreased FMD in acromegaly. Moreover, some studies revealed that patients with active acromegaly had significantly decreased FMD compared to controls [10, 11]. Significant improvements in endothelial dysfunction after transsphenoidal surgery or GH receptor antagonist therapy in patients with acromegaly were found in other previous studies [29, 30]. Akgul et al. [7] found decreased FMD in acromegaly, but they suggested that endothelial dysfunction persists even after treatment with somatostatin analogs. These findings and our data support the presence of endothelial dysfunction, which plays an important role in the development of atherosclerosis. Although the possible mechanisms related to endothelial dysfunction in acromegaly is not fully known, it has been suggested in a study that GH may exert acute vascular effects independent of IGF-1 via GH receptors and endothelial nitric oxide synthase in the vascular endothelium [31].
There is increasing evidence in the literature to suggest that CIMT increases in acromegaly [10]. Brevetti et al. [11] also observed increased CIMT in acromegaly compared to healthy controls; however, no significant difference was found between patients with acromegaly and controls when the control group was matched according to cardiovascular risk factors. CIMT was found to be significantly increased in acromegaly in our study; however, the active and controlled acromegaly groups were similar in terms of CIMT. Our study also revealed that the presence of acromegaly and HT was the most important parameter for increased CIMT in patients with acromegaly. Both GH and IGF-1 may have direct effects on vascular structure and lead to an increase in CIMT. However, the exact mechanism of increased CIMT in acromegaly is not yet clear.
Epicardial adipose tissue is an active endocrine organ and secretes several cytokines that may have physiological and pathological functions [32]. In recent years, several studies have reported an association between epicardial adipose tissue and cardiovascular risk factors, suggesting that EAT may play a role in both the development and progression of atherosclerosis [33, 34]. Mazurek et al. [35] showed that the expression and secretion of some cytokines and chemokines were increased in EAT samples in patients with CAD compared to subcutaneous fat tissue samples. Because there is no fascia separating epicardial adipose tissue from surrounding structures, it is postulated that the inflammatory state observed in epicardial adipose tissue may play a role in atherogenesis via local paracrine and vasocrine mechanisms [32]. To the best of our knowledge, there is only one study evaluating EAT in acromegaly [14]. In that study, EAT was found to be significantly increased in patients with acromegaly and a correlation analysis revealed a positive association between EAT and GH levels. In our study, we evaluated patients with acromegaly with matched controls according to cardiovascular risk factors and, consistent with the study by Topaloglu et al. we found significantly increased EAT in acromegaly compared to controls. Moreover, in a regression analysis for the parameters that were thought to be related to EAT, the presence of acromegaly, not GH or IGF-1 levels, was found to be the most important determinant of an increase in EAT.
In spite of the increased frequency of DM, HT and HL, there is still no clear evidence of increased risk of CAD or carotid artery disease in acromegaly because some previous studies suggested that patients with acromegaly carry low risk for these diseases [4, 36, 37]. However, as we mentioned above, the issue is still controversial. Some investigators speculated that IGF-1 may also have favorable effects on vasculature in some patients with acromegaly [38, 39]. IGF-1 has been suggested to have atheroprotective effects because it has anti-inflammatory and anti-oxidative properties [38]. Additionally, it has been found that IGF-1 may alter vascular smooth muscle cell turnover and phenotype, which results in plaque-stabilization in atherosclerosis [39]. Nevertheless, we found decreased FMD and increased CIMT and EAT, which indicates early atherosclerosis in patients with acromegaly.
Oxidative stress leads to oxidation of LDL and impairs production and activity of nitric oxide, which are the important mechanisms in the atherosclerotic process [40]. Nishizawa et al. [16] showed that increased IGF-1 is associated with increased oxidative stress both in rats and humans. Recently, Anagnostis et al. [41] evaluated fifteen patients with acromegaly with age and sex matched controls and reported increased oxidative stress parameters and reduced antioxidant capacity as well as endothelial dysfunction evaluated with nitric oxide levels in acromegaly. Among oxidative stress parameters, there was a positive correlation between nadir GH levels and total oxidized glutathione [41]. In our study, we evaluated ox-LDL and TAC as oxidative stress parameters and no significant differences were noticed between the groups. Additionally, there was no association between FMD, CIMT, EAT and oxidative stress parameters.
HMGB1 is a chromatin binding protein and accumulates in cells as a response to early oxidative damage. HMGB1 has been shown to cause the production of reactive oxygen species, induction of inflammation and endothelial dysfunction [19]. Previously, HMGB1 protein had not been evaluated in patients with acromegaly. In our study, we could not find any significant difference between acromegaly and control groups for HMGB1 protein levels. Because it is implicated as an early marker of oxidative damage, this result is consistent with the finding of a lack of evidence of oxidative damage in acromegaly.
An association between hs CRP levels and CVD has been described in many studies [42]. We also evaluated hs CRP levels with other atherosclerosis markers, such as FMD, CIMT and EAT. Interestingly, although these three parameters were found to be impaired in our patients, hs CRP levels were significantly decreased in the acromegaly group. Hs CRP levels were lowest in the active acromegaly group and highest in the control group. Consistent with our result, Vilar et al. [43] reported decreased hs CRP levels in patients with acromegaly. Andreassen et al. [44] evaluated hs CRP levels in patients with acromegaly and reported that serum levels of hs CRP were reduced in acromegaly compared to the control group and increased with treatment. In another study, active acromegaly, GH sufficient, and GH deficient groups were compared and it was found that hs CRP serum levels were lowest in the active acromegaly group and highest in the GH deficient group [45]. However, others studies have reported no significant difference in hs CRP levels between acromegaly and control groups [14, 18, 46]. Taken together, subclinical inflammation does not appear to contribute to the development of atherosclerosis in patients with acromegaly. With respect to the negative association between acromegaly and CRP, data from a population based study recently revealed an inverse correlation between hs CRP and IGF-1 levels [47]. Further studies are needed to define the underlying pathogenetic mechanisms of decreased subclinical inflammation in acromegaly.
In conclusion, the results of our study suggest that acromegaly is associated with decreased FMD and increased CIMT and EAT as the early markers of atherosclerosis. However, decreased hs CRP and unchanged HMGB1, ox-LDL and TAC levels indicate that inflammation and oxidative stress do not seem to contribute to the atherogenesis in acromegaly. Beyond these pathways, there may be unknown mechanisms related to the development of atherosclerosis in acromegaly. The possible effects of GH and IGF-1 on the components of vessels, such as smooth muscle cells or fibroblasts, may play a role in the pathogenesis of atherosclerosis in acromegaly. Studies investigating the direct effects of GH and IGF-1 on vascular system especially at tissue level may help to elucidate the underlying pathogenetic mechanisms of atherosclerosis in acromegaly.
References
Colao A, Merola B, Ferone D, Lombardi G (1997) Acromegaly. J Clin Endocrinol Metab 82:2777–2781
Katznelson L, Atkinson JL, Cook DM, Ezzat SZ, Hamrahian AH, Miller KK (2011) American Association of Clinical Endocrinologists Medical Guidelines for clinical practice for the diagnosis and treatment of acromegaly–2011 update: executive summary. Endocr Pract 17:636–646
Colao A, Ferone D, Marzullo P, Lombardi G (2004) Systemic complications of acromegaly: epidemiology, pathogenesis and management. Endocr Rev 25:102–152
Akutsu H, Kreutzer J, Wasmeier G, Ropers D, Rost C, Mohlig M, Wallaschofski H, Buchfelder M, Schofl C (2010) Acromegaly per se does not increase the risk for coronary artery disease. Eur J Endocrinol 162:879–886
Mosca S, Paolillo S, Colao A, Bossone E, Cittadini A, Iudice FL, Parente A, Conte S, Rengo G, Leosco D, Trimarco B, Filardi PP (2013) Cardiovascular involvement in patients affected by acromegaly: an appraisal. Int J Cardiol 167:1712–1718
Anderson TJ (1999) Assessment and treatment of endothelial dysfunction in humans. J Am Coll Cardiol 34:631–638
Akgul E, Tokgozoglu SL, Erbas T, Kabakci G, Aytemir K, Haznedaroglu I, Oto A, Kes SS (2010) Evaluation of the impact of treatment on endothelial function and cardiac performance in acromegaly. Echocardiography 27:990–996
Chanson P, Megnien JL, del Pino M, Coirault C, Merli I, Houdouin L, Harris AG, Levenson J, Lecarpentier Y, Simon A, Chemla D (1998) Decreased regional blood flow in patients with acromegaly. Clin Endocrinol (Oxf) 49:725–731
Baykan M, Erem C, Gedikli O, Hacihasanoglu A, Erdogan T, Kocak M, Kaplan S, Korkmaz L, Celik S (2009) Impairment in flow-mediated vasodilatation of the brachial artery in acromegaly. Medical principles and practice : international journal of the Kuwait University. Health Sci Cent 18:228–232
Kartal I, Oflaz H, Pamukcu B, Meric M, Aral F, Ozbey N, Alagol F (2010) Investigation of early atherosclerotic changes in acromegalic patients. Int J Clin Pract 64:39–44
Brevetti G, Marzullo P, Silvestro A, Pivonello R, Oliva G, di Somma C, Lombardi G, Colao A (2002) Early vascular alterations in acromegaly. J Clin Endocrinol Metab 87:3174–3179
Iacobellis G, Corradi D, Sharma AM (2005) Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med 2:536–543
Xu Y, Cheng X, Hong K, Huang C, Wan L (2012) How to interpret epicardial adipose tissue as a cause of coronary artery disease: a meta-analysis. Coron Artery Dis 23:227–233
Topaloglu O, Sayki Arslan M, Turak O, Ginis Z, Sahin M, Cebeci M, Ucan B, Cakir E, Karbek B, Ozbek M, Cakal E, Delibasi T (2014) Three noninvasive methods in the evaluation of subclinical cardiovascular disease in patients with acromegaly: epicardial fat thickness, aortic stiffness and serum cell adhesion molecules. Clin Endocrinol (Oxf) 80:726–734
Kondo T, Hirose M, Kageyama K (2009) Roles of oxidative stress and redox regulation in atherosclerosis. J Atheroscler Thromb 16:532–538
Nishizawa H, Handayaningsih AE, Iguchi G, Cho Y, Takahashi M, Yamamoto M, Suda K, Kasahara K, Hakuno F, Yamanouchi K, Nishihara M, Seino S, Takahashi S (2012) Enhanced oxidative stress in GH-transgenic rat and acromegaly in humans. Growth Horm IGF Res 22:64–68
Verhelst J, Velkeniers B, Maiter D, Haentjens P, T’Sjoen G, Rietzschel E, Corvilain B, Abrams P, Nobels F, Abs R, Bex M (2013) Active acromegaly is associated with decreased hs-CRP and NT-proBNP serum levels: insights from the Belgian registry of acromegaly. Eur J Endocrinol 168:177–184
Boero L, Manavela M, Gomez Rosso L, Insua C, Berardi V, Fornari MC, Brites F (2009) Alterations in biomarkers of cardiovascular disease (CVD) in active acromegaly. Clin Endocrinol (Oxf) 70:88–95
Tang D, Kang R, Zeh HJ 3rd, Lotze MT (2011) High-mobility group box 1, oxidative stress, and disease. Antioxid Redox Signal 14:1315–1335
Inoue K, Kawahara K, Biswas KK, Ando K, Mitsudo K, Nobuyoshi M, Maruyama I (2007) HMGB1 expression by activated vascular smooth muscle cells in advanced human atherosclerosis plaques. Cardiovasc Pathol 16:136–143
Zhang Z, Wu Y, Zhao Y, Xiao X, Liu J, Zhou X (2013) Dynamic changes in HMGB1 levels correlate with inflammatory responses during cardiopulmonary bypass. Exp Ther Med 5:1523–1527
Giustina A, Chanson P, Bronstein MD, Klibanski A, Lamberts S, Casanueva FF, Trainer P, Ghigo E, Ho K, Melmed S (2010) A consensus on criteria for cure of acromegaly. J Clin Endocrinol Metab 95:3141–3148
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502
Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R (2002) Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 39:257–265
Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, Najjar SS, Rembold CM, Post WS (2008) Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr 21:93–111
Lang RM, Badano LP, Tsang W, Adams DH, Agricola E, Buck T, Faletra FF, Franke A, Hung J, de Isla LP, Kamp O, Kasprzak JD, Lancellotti P, Marwick TH, McCulloch ML, Monaghan MJ, Nihoyannopoulos P, Pandian NG, Pellikka PA, Pepi M, Roberson DA, Shernan SK, Shirali GS, Sugeng L, Ten Cate FJ, Vannan MA, Zamorano JL, Zoghbi WA (2012) EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. Eur Heart J Cardiovasc Imaging 13:1–46
Iacobellis G, Assael F, Ribaudo MC, Zappaterreno A, Alessi G, Di Mario U, Leonetti F (2003) Epicardial fat from echocardiography: a new method for visceral adipose tissue prediction. Obes Res 11:304–310
Reyes-Vidal C, Fernandez JC, Bruce JN, Crisman C, Conwell IM, Kostadinov J, Geer EB, Post KD, Freda PU (2014) Prospective study of surgical treatment of acromegaly: effects on ghrelin, weight, adiposity, and markers of CV risk. J Clin Endocrinol Metab 99:4124–4132
Sakai H, Tsuchiya K, Nakayama C, Iwashima F, Izumiyama H, Doi M, Yoshimoto T, Tsujino M, Yamada S, Hirata Y (2008) Improvement of endothelial dysfunction in acromegaly after transsphenoidal surgery. Endocr J 55:853–859
De Martino MC, Auriemma RS, Brevetti G, Vitale G, Schiano V, Galdiero M, Grasso L, Lombardi G, Colao A, Pivonello R (2010) The treatment with growth hormone receptor antagonist in acromegaly: effect on vascular structure and function in patients resistant to somatostatin analogues. J Endocrinol Invest 33:663–670
Li G, Del Rincon JP, Jahn LA, Wu Y, Gaylinn B, Thorner MO, Liu Z (2008) Growth hormone exerts acute vascular effects independent of systemic or muscle insulin-like growth factor I. J Clin Endocrinol Metab 93:1379–1385
Iacobellis G, Malavazos AE, Corsi MM (2011) Epicardial fat: from the biomolecular aspects to the clinical practice. Int J Biochem Cell Biol 43:1651–1654
Whayne TF Jr (2013) Epicardial fat thickness in heart failure and other clinical conditions. Angiology 64:169–172
Sacks HS, Fain JN (2007) Human epicardial adipose tissue: a review. Am Heart J 153:907–917
Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, O’Brien S, Keiper EA, Johnson AG, Martin J, Goldstein BJ, Shi Y (2003) Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 108:2460–2466
Bogazzi F, Battolla L, Spinelli C, Rossi G, Gavioli S, Di Bello V, Cosci C, Sardella C, Volterrani D, Talini E, Pepe P, Falaschi F, Mariani G, Martino E (2007) Risk factors for development of coronary heart disease in patients with acromegaly: a five-year prospective study. J Clin Endocrinol Metab 92:4271–4277
Otsuki M, Kasayama S, Yamamoto H, Saito H, Sumitani S, Kouhara H, Saitoh Y, Ohnishi T, Arita N (2001) Characterization of premature atherosclerosis of carotid arteries in acromegalic patients. Clin Endocrinol (Oxf) 54:791–796
Higashi Y, Sukhanov S, Anwar A, Shai SY, Delafontaine P (2010) IGF-1, oxidative stress and atheroprotection. Trends Endocrinol Metab 21:245–254
von der Thusen JH, Borensztajn KS, Moimas S, van Heiningen S, Teeling P, van Berkel TJ, Biessen EA (2011) IGF-1 has plaque-stabilizing effects in atherosclerosis by altering vascular smooth muscle cell phenotype. Am J Pathol 178:924–934
Steinberg D, Witztum JL (2002) Is the oxidative modification hypothesis relevant to human atherosclerosis? Do the antioxidant trials conducted to date refute the hypothesis? Circulation 105:2107–2111
Anagnostis P, Efstathiadou ZA, Gougoura S, Polyzos SA, Karathanasi E, Dritsa P, Kita M, Koukoulis GN (2013) Oxidative stress and reduced antioxidative status, along with endothelial dysfunction in acromegaly. Horm Metab Res 45:314–318
Yousuf O, Mohanty BD, Martin SS, Joshi PH, Blaha MJ, Nasir K, Blumenthal RS, Budoff MJ (2013) High-sensitivity C-reactive protein and cardiovascular disease: a resolute belief or an elusive link? J Am Coll Cardiol 62:397–408
Vilar L, Naves LA, Costa SS, Abdalla LF, Coelho CE, Casulari LA (2007) Increase of classic and nonclassic cardiovascular risk factors in patients with acromegaly. Endocr Pract 13:363–372
Andreassen M, Vestergaard H, Kristensen LO (2007) Concentrations of the acute phase reactants high-sensitive C-reactive protein and YKL-40 and of interleukin-6 before and after treatment in patients with acromegaly and growth hormone deficiency. Clin Endocrinol (Oxf) 67:909–916
Lin E, Wexler TL, Nachtigall L, Tritos N, Swearingen B, Hemphill L, Loeffler J, Biller BM, Klibanski A, Miller KK (2012) Effects of growth hormone deficiency on body composition and biomarkers of cardiovascular risk after definitive therapy for acromegaly. Clin Endocrinol (Oxf) 77:430–438
Arikan S, Bahceci M, Tuzcu A, Gokalp D (2009) Serum tumour necrosis factor-alpha and interleukin-8 levels in acromegalic patients: acromegaly may be associated with moderate inflammation. Clin Endocrinol (Oxf) 70:498–499
Lohr J, Grotevendt A, Nauck M, Volzke H, Wallaschofski H, Friedrich N (2014) Relation of insulin-like growth factor-I and IGF binding protein 3 with markers of inflammation: results of a population-based study. Clin Endocrinol (Oxf) 80:148–154
Acknowledgments
We gratefully acknowledge Sevilay Karahan for her help in statistical analysis.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ozkan, C., Altinova, A.E., Cerit, E.T. et al. Markers of early atherosclerosis, oxidative stress and inflammation in patients with acromegaly. Pituitary 18, 621–629 (2015). https://doi.org/10.1007/s11102-014-0621-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-014-0621-6