Abstract
Background
Cabergoline is a recommended first-line dopamine agonist for prolactinoma treatment, which is withdrawable for some cases. However, the optimal withdrawal strategy and the accurate recurrence rate associated with cabergoline withdrawal remains uncertain.
Objective
To assess the current recurrence rate of hyperprolactinemia and possible favorable factors associated with cabergoline withdrawal in prolactinoma patients.
Method
The databases of PubMed, EMBASE, and Web of Science were searched up to May 2014 to identify studies containing data of recurrent hyperprolactinemia in prolactinoma patients after cabergoline withdrawal. Meta-analysis, including sensitivity analysis, meta-regression analysis, and subgroup analysis were performed.
Results
When the patients who received cabergoline withdrawal were pooled, it was found that the hyperprolactinemia recurrence rate was 65 % by a random effects meta-analysis [95 % confidence interval 55–74 %]. In a random effects meta-regression adjusting for optimal withdrawal strategies, CAB dose reduced to the lowest level before withdrawal was associated with treatment success (p = 0.006), whereas CAB treatment longer than 2 years showed no trend of effect (p = 0.587). Patients who received the lowest CAB dose and presented a significant reduction in tumor size before withdrawal were more likely to achieve the best success (p < 0.001).
Conclusions
Our meta-analysis shows that hyperprolactinemia recurs after cabergoline withdrawal in a majority of patients. The probability of success favors patients who have achieved normoprolactinemia and considerable reduction in tumor size by low dose of cabergoline treatment. In addition, our study further suggests that a beneficial strategy is associated with tapering CAB dose before withdrawal but not with CAB treatment duration longer than 2 years.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dopamine agonists (DA) are used in the first-line treatment for prolactinoma. DAs lower prolactin (PRL) levels, decrease tumor size, and restore gonadal function [1]. Both the 2006 Pituitary Society guidelines [2] and 2011 Endocrine Society guidelines [1] suggest that, with careful clinical and biochemical follow-up, dopamine agonists therapy may be tapered and perhaps discontinued in patients who meet some conditions. Up to 2007, the pooled recurrent rate of hyperprolactinemia after withdrawal of dopamine agonists varies from 25–100 % [3], indicating the conditions for making DA withdrawal decision need to be optimized. Current dopamine agonists for prolactinoma treatment include cabergoline, bromocriptine, quinagolide, dihydroergocriptine, lisuride, and metergoline, in which cabergoline (CAB) is the most commonly used. CAB has higher efficacy in normalizing PRL levels and a better effect on shrinking tumor than the other dopamine agonists [1]. In addition, the long plasma half-life of CAB (about 65 h) allows once or twice-weekly administration, which makes patient compliance easier [4]. Therefore, CAB is recommended for the first-line therapy by the 2011 Endocrine Society guidelines, it needs to be analyzed separately from the other DAs. Since the publication of 2006 Pituitary Society guidelines, several new studies on CAB withdrawal have been reported. There is a need to perform a systemic review and an analysis on the outcomes of CAB withdrawal. We conducted the present meta-analysis with two aims: (1) to gain an updated status of hyperprolactinemia recurrence in prolactinoma patients after CAB withdrawal; (2) to seek indications associated with favorable outcomes in response to CAB withdrawal.
Materials and methods
Search strategy
We searched the PubMed, EMBASE and Web of Science databases for publications that reported the effect of withdrawal of CAB on the recurrence of prolactinoma. Searches were performed in May 2014, using the following key phrases “cabergoline withdraw” “cabergoline withdrawal” “cabergoline discontinue” “cabergoline discontinued”. No restriction was imposed. In addition, the references of relevant papers were also checked. Abstracts of meetings and unpublished results were not included in the analysis.
The assessment of prolactinoma recurrence was based only on PRL levels, irrespective of clinical symptoms or tumor size. Both observational studies and clinical trials were eligible.
Studies are eligible for inclusion in this review if they meet the following criteria:
-
1.
Rate of hyperprolactinemia recurrence in patients after cabergoline withdrawal must be reported or can be calculated.
-
2.
Sort of dopamine agonist was restricted to only cabergoline.
-
3.
Treatment time was at least 2 months, and normoprolactinemia had to be attained during the treatment.
-
4.
Patient follow-up period was at least 6 months; patients who became pregnant during this period were excluded.
-
5.
Patients who had radiation therapy before CAB treatment were excluded. Patients who had surgery before CAB treatment must have no conspicuous lower PRL and must present clinical symptoms of prolactinoma. Patients who were previously treated with other DAs must have a wash-out time.
-
6.
There should be no duplication of cohort. In studies where partial duplication was present, the largest cohort was chosen.
Data review and data analysis
Whenever possible, each study cohort was stratified by micro- and macroprolactinomas, respectively. The following variables were checked for each study: mean age, percentage of female patients, treatment duration, CAB dose, PRL before treatment, follow-up time, CAB dose reduced to the smallest amount prior to withdrawal (yes/no), significant reduction of tumor size prior to withdrawal (yes/no). In cases where only a subgroup of a cohort was withdrawn from CAB treatment, these parameters were extracted for this subgroup only. If the parameters for the subgroup were not reported separately, the parameters of the total cohort were used as substitutes for the subgroup.
All PRL levels in the present meta-analysis were expressed as nanograms per milliliter. Because it is impossible to acquire the conversion factors for all the assays, the conversion factor for PRL levels from milliunits per liter to nanograms per milliliter used was set as 1:30. For evaluation of recurrence of hyperprolactinemia, the unit used by the authors, not the converted levels, was used. The reported reference range of each individual study was used to determine the presence of hyper and normoprolactinemia. In a few studies, remission was defined as mild hyperprolactinemia without symptoms, which was also adopted in our normoprolactinemia group. For studies reporting outcomes of several intervals, the last phase was chosen for data extraction. For determination of tumor regression during dopamine agonist treatment, results of magnetic resonance imaging (MRI) and computed tomography (CT), but not conventional X-rays, were considered. Patients who became pregnant during follow-up were excluded. Study selection and data extraction were performed by two independent reviewers (J.-T.H. and X.Z.). Disagreements were resolved by discussion.
The weighted average of the proportion of patients with recurrence of hyperprolactinemia after CAB withdrawal was the main outcome of the meta-analysis. All individual studies were weighted according to the inverse of the squared ES. Homogeneity test was calculated with the use of I2 statistic, which is a quantitative measure of heterogeneity across studies. I2 value of 25, 50, and 75 % indicates low, moderate, and high degrees of heterogeneity, respectively [5]. Because of high heterogeneity, we used the random-effects model for summary statistics.
In consideration of the high heterogeneity, sensitivity analysis and random-effects meta-regression analysis were initially performed to explore the sources of heterogeneity. Based on the results of meta-regression, we performed subgroup analysis.
Statistical analyses were performed using Stata (version 12.0; Stata Corporation, College Station, TX).
Results
Literature search (Table 1)
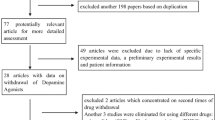
The initial search in the databases resulted in a total of 259 papers. Of these 259, we excluded 232 papers based on title, abstract, and duplication. A total of 27 potentially relevant papers were retrieved for full assessment. Of these studies, nine were excluded from further analysis due to lack of original data of CAB withdrawal associated hyperprolactinemia. In 16 studies, a detailed assessment with respect to the eligibility criteria was performed. One study was excluded because it was written in Japanese [6]. Two studies were excluded from further analysis because of usage of quinagolide and significant abatement in patients’ basal PRL [7, 8]. There are two studies were excluded [9, 10], because they described the same cohorts used by the other studies [11, 12]. Consequently, a total of 11 studies were included in the present review [11–21].
Study characteristics (Table 2)
Details of the 11 included studies are summarized in Table 2. Studies on recurrence of hyperprolactinemia after CAB withdrawal were published between 1992 and 2013. The number of included patients per study ranges from 8 to 194. The total number of patients included in this meta-analysis was 637. Stratified data were available for a total of 492 microprolactinoma patients with and 123 macroprolactinoma patients. The proportion of patients with recurrent hyperprolactinemia after CAB withdrawal ranges from 25 to 93 %.
Meta-analysis (Table 3, 4)
The pooled proportion of patients with recurrent hyperprolactinemia after CAB withdrawal was 65 % in a random effects model [95 % CI 55–74 %; I2 83 %].
Stratified analysis
Firstly, we conducted sensitivity analysis to investigate the influence of a single study on the overall risk estimation by omitting one study at each turn, which yielded a narrow range of ESs from 0.62 (55–71 %) to 0.67 (60–75 %).
Secondly, in order to relate differences in effect sizes to study characteristics, we further investigated the potential sources of heterogeneity using meta-regression analysis. Factors examined in both individual and multiple-variable models were: initial tumor size (micro vs. macro); treatment duration (<24 months vs. >24 months), PRL levels before treatment (<200 ng/ml vs. >200 ng/ml); CAB dose reduced to the lowest level before withdrawal (yes/no); significant reduction of tumor size before withdrawal (yes/no). We defined those patients who received the lowest CAB dose and presented a significant reduction in tumor size before withdrawal as the “chosen patients”, and the rest patients were defined as the “unchosen patients”. Results are shown in Table 3. Among these variables, the CAB dose reduced to the lowest level before withdrawal (p = 0.006) and the “chosen patients” (p < 0.001) are associated with lower recurrence rates. In addition, initial tumor size (micro vs. macro) may at least partially contribute to the overall between-study variation (p = 0.085) in multiple-variable models meta-regression.
Thirdly, the differences identified by the meta-regression analysis became more obvious when the analysis was performed between subgroups (Table 4). Lower recurrence rates were found in microprolactinoma patients (60 %; 95 % CI 49–71 %) compared with macroprolactinoma patients (75 %; 95 % CI 54–95 %), in patients with CAB dosage reduced to the smallest amount before withdrawal (50 %; 95 % CI 35–64 %) compared with patients with CAB dose not reduced to the smallest amount before withdrawal (74 %; 95 % CI 65–83 %), in patients with significant reduction in tumor size before withdrawal (61 %; 95 % CI 48–74 %) compared with patients with no significant reduction in tumor size before withdrawal (72 %; 95 % CI 64–79 %), and in the “chosen patients” (44 %; 95 % CI 33–56 %) compared with the “unchosen patients” (74 %; 95 % CI 66–82 %).
Discussion
The debate over whether prolactinoma can be cured by dopamine agonists has existed since the 1980s. The most contentious points were the optimal treatment strategy and the unmanageably high recurrence rates. In the early 1980s, patients with normoprolactinemia and patients presented with no clinical manifestation after treatment of dopamine agonists were considered candidates for treatment withdrawal. At that time, the recurrence rate could be as high as 100 % [7]. This rate was improved slightly in later studies [8, 19, 20], but it was still disappointing. In 2003, a landmark study by Colao [9] demonstrated that cabergoline can be precisely withdrawn in a considerable proportion of patients, provided they fulfill a set of selected clinical criteria, such as significant tumor size reduction and a prolonged period of normoprolactinemia during treatment time. After that, there was at least one relevant study reported each year. In 2006, the Pituitary Society provided guidelines, which suggested that patients with normoprolactinemia for at least 3 years and markedly reduced tumor sizes might safely have their dopamine agonist dosages tapered and treatment discontinued [2]. Then, in 2011, the Endocrine Society also issued guidelines that recommended this withdrawal strategy of dopamine agonist in patients with prolactinoma [1]. These guidelines recommended further reduction of treatment duration from at least 3 years to at least 2 years, and tumor size reduction from “markedly” to “invisible”.
In both sets of guidelines, however, suggestions for withdrawal strategies for dopamine agonists in patients with prolactinoma were based upon a few studies: the 2006 guidelines were mainly based on Colao’s study [9], and the 2011 guidelines were based on only four studies [3, 9, 12, 18]. In a meta-analysis [3] included a total of 19 studies that were published between 1979 and 2007, the researchers concluded that the probability of recurrence was the lowest when cabergoline was used for at least 2 years compared to other dopamine agonists. This meta-analysis, however, included only four studies relevant to cabergoline, with a total of 284 patients, and the most recent study was published in 2007. After that, a few more new studies have been published [13–18]. To renew these data and get new data concentrated on cabergoline, which is the recommended agent for the first-line treatment among currently available dopamine agonists, we made this meta-analysis.
The present systematic review and meta-analysis was performed to evaluate the pooled proportion of patients recurred hyperprolactinemia after withdrawal from CAB. The study shows that withdrawal has failed in 65 % (I2 83.2 %, 95 % CI 55–74 %) of all patients. I2 > 75 % indicates high heterogeneity within the studies. To explore sources of heterogeneity, sensitivity analysis and random effects meta-regression analysis were performed first. The results show that success rates were higher in patients (the “Chosen patients”) who presented persist normoprolactinemia by using lowest dose of CAB and a considerable reduction on adenoma size, as compared with those patients who do not meet these criteria (p < 0.001).
Only three studies have the “chosen patients” [11, 14, 18], although these studies all followed guidelines, heterogeneity between studies remain significant (I2 = 61.4). Besides prospective or retrospective, there are no more indexes for us to judge the quality of each study. The same consideration appeared in all subgroups, except for one, in which patients did not achieve significant reduction of tumor sizes (I2 = 0). More prospective studies with larger sample size separating the “chosen patients” and the “unchosen patients” are recommended to resolve the heterogeneity between published studies.
In referencing Dekkers’ analysis [3], treatment duration cannot explain the heterogeneity observed in our study. Some differences between studies included in Dekkers’ retrospective study and ours may provide an explanation. Dekkers’ research comprised eight studies, and most of them were published in the 1980s and did not include CAB. In these studies, the average treatment duration was less than 24 months and the recurrence rates were 100 %. In our research, there are only two studies with treatment durations less than 24 months and there was no significant heterogeneity between treatment time >24 months or <24 months (p = 0.587). In our meta-analysis, there is no significant trend of lower recurrence with longer treatment duration either. Our meta-analysis indicates that treatment for more than 2 years seems to be not a positive indicator for CAB withdrawal.
There was one study examined short-term maintenance treatment [16]. In this study, patients were treated for 2 months with CAB in four different doses (0, 0.125, 0.25, 0.5 mg, biweekly), then maintenance was finalized and patients were followed up for 6 months. The study showed that the short-term treatment in microadenoma-related hyperprolactinemia had the same effect as the long-term treatment. The limitation of this study is that the follow-up time was as short as only 6 months. Except for Dekkers’ meta-analysis [3], there is no support to negate short-term treatment, because randomized controlled studies comparing different withdrawal strategies after successful treatment of hyperprolactinemia are still lacking.
Under the premise of maintaining normoprolactinemia, patients who reduced CAB the lowest level before withdrawal may have lower recurrence rate than patients who didn’t reduce CAB dose (p = 0.006). However, the difference of recurrent rate between micro- and macroprolactinoma patients after cabergoline withdrawal is not statistically significant (p = 0.197). Table 1 shows that initial tumor size (micro vs. macro) or PRL levels prior treatment (<200 ng/ml vs. >200 ng/ml) plays the same role in grouping. Therefore, it is proposed that tapering CAB dose before withdrawal does not seem to be a beneficial strategy; however, initial tumor size nor PRL level can forecast the recurrence of hyperprolactinemia.
It must be noted that, in the present meta-analysis, the two variables (CAB dose reduced to the smallest amount before withdrawal and tumor size had a considerable reduction before withdrawal) were given qualitative judgments with “yes” or “no” according to the original studies. We are unable to quantify these variables because the details of each individual patient were not available.
As previously stated, dopamine agonist can not only reduce secretion of prolactin, but can also decreases prolactin gene expression and proliferation of lactotrophs [22], but why do some patients have recurrent prolactinomas after achieving normoprolactinemia with no clinical symptoms? What is the difference between patients with and without recurrence? Current literature suggests four possible influential factors for the divergent outcomes of CAB withdrawal: (1) there are still residual tumors when CAB withdrawal is conducted [10, 16, 18], which is echoed by the 2011 Endocrine Society Guidelines emphasizing “invisible tumor” before drug withdrawal [1]; (2) PRL levels before treatment [12], PRL levels during DA therapy [15], and PRL levels before withdrawal [11]; (3) expression status of some tumor associated genes [23]; (4) degree of tumor invasion [24]. Our meta-analysis indicates that low recurrent rate is associated with high sensitivity to CAB treatment regardless of minimum dosage or considerable reduction in tumor size. Mechanisms account for tumor sensitivity to CAB warrant further investigations.
In conclusion, at current, according to this meta-analysis, the pooled proportion of patients with recurrent hyperprolactinemia after CAB withdrawal was 65 % (95 % CI 55–74 %) in overall effect, and the recurrence rate of hyperprolactinemia is lower in patients who are more sensitive to CAB treatment than those who are less sensitive. In addition, our study further suggests that a beneficial strategy is associated with tapering CAB dose before withdrawal, but not associated with CAB treatment duration longer than 2 years.
References
Melmed S, Casanueva FF, Hoffman AR, Kleinberg DL, Montori VM, Schlechte JA, Wass JAH (2011) Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96(2):273–288. doi:10.1210/jc.2010-1692
Casanueva FF, Molitch ME, Schlechte JA, Abs R, Bonert V, Bronstein MD, Brue T, Cappabianca P, Colao A, Fahlbusch R, Fideleff H, Hadani M, Kelly P, Kleinberg D, Laws E, Marek J, Scanlon M, Sobrinho LG, Wass JAH, Giustina A (2006) Guidelines of the Pituitary Society for the diagnosis and management of prolactinomas. Clin Endocrinol 65(2):265–273. doi:10.1111/j.1365-2265.2006.02562.x
Dekkers OM, Lagro J, Burman P, Jorgensen JO, Romijn JA, Pereira AM (2010) Recurrence of hyperprolactinemia after withdrawal of dopamine agonists: systematic review and meta-analysis. J Clin Endocrinol Metab 95(1):43–51. doi:10.1210/jc.2009-1238
Webster J, Piscitelli G, Polli A, Ferrari CI, Ismail I, Scanlon MF (1994) A comparison of cabergoline and bromocriptine in the treatment of hyperprolactinemic amenorrhea. Cabergoline Comparative Study Group. N Engl J Med 331(14):904–909. doi:10.1056/nejm199410063311403
Higgins JPT, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. Br Med J 327(7414):557–560. doi:10.1136/bmj.327.7414.557
Watanabe S, Takano S, Akutsu H, Sato H, Matsumura A (2011) Prolactinoma treatment status in the cabergoline era. Neurol Surg 39(11):1045–1054
Giusti M, Porcella E, Carraro A, Cuttica M, Valenti S, Giordano G (1994) A cross-over study with the two novel dopaminergic drugs cabergoline and quinagolide in hyperprolactinemic patients. J Endocrinol Invest 17(1):51–57
Di Sarno A, Landi ML, Marzullo P, Di Somma C, Pivonello R, Cerbone G, Lombardi G, Colao A (2000) The effect of quinagolide and cabergoline, two selective dopamine receptor type 2 agonists, in the treatment of prolactinomas. Clin Endocrinol 53(1):53–60. doi:10.1046/j.1365-2265.2000.01016.x
Colao A, Di Sarno A, Cappabianca P, Di Somma C, Pivonello R, Lombardi G (2003) Withdrawal of long-term cabergoline therapy for tumoral and nontumoral hyperprolactinemia. N Engl J Med 349(21):2023–2033. doi:10.1056/NEJMoa022657
Bronstein MD (2006) Potential for long-term remission of microprolactinoma after withdrawal of dopamine-agonist therapy. Nat Clin Pract Endocrinol Metab 2(3):130–131. doi:10.1038/ncpendmet0135
Colao A, Di Sarno A, Guerra E, Pivonello R, Cappabianca P, Caranci F, Elefante A, Cavallo LM, Briganti F, Cirillo S, Lombardi G (2007) Predictors of remission of hyperprolactinaemia after long-term withdrawal of cabergoline therapy. Clin Endocrinol 67(3):426–433. doi:10.1111/j.1365-2265.2007.02905.x
Biswas M, Smith J, Jadon D, McEwan P, Rees DA, Evans LM, Scanlon MF, Davies JS (2005) Long-term remission following withdrawal of dopamine agonist therapy in subjects with microprolactinomas. Clin Endocrinol 63(1):26–31. doi:10.1111/j.1365-2265.2005.02293.x
Kwancharoen R, Auriemma RS, Yenokyan G, Wand GS, Colao A, Salvatori R (2013) Second attempt to withdraw cabergoline in prolactinomas: a pilot study. Pituitary. doi:10.1007/s11102-013-0525-x
Anagnostis P, Adamidou F, Polyzos SA, Efstathiadou Z, Karathanassi E, Kita M (2012) Long term follow-up of patients with prolactinomas and outcome of dopamine agonist withdrawal: a single center experience. Pituitary 15(1):25–29. doi:10.1007/s11102-011-0303-6
Barber TM, Kenkre J, Garnett C, Scott RV, Byrne JV, Wass JAH (2011) Recurrence of hyperprolactinaemia following discontinuation of dopamine agonist therapy in patients with prolactinoma occurs commonly especially in macroprolactinoma. Clin Endocrinol 75(6):819–824. doi:10.1111/j.1365-2265.2011.04136.x
Huda MSB, Athauda NB, Teh MM, Carroll PV, Powrie JK (2010) Factors determining the remission of microprolactinomas after dopamine agonist withdrawal. Clin Endocrinol 72(4):507–511. doi:10.1111/j.1365-2265.2009.03657.x
Buyukbayrak EE, Karsidag AYK, Kars B, Balcik O, Pirimoglu M, Unal O, Turan C (2010) Effectiveness of short-term maintenance treatment with cabergoline in microadenoma-related and idiopathic hyperprolactinemia. Arch Gynecol Obstet 282(5):561–566. doi:10.1007/s00404-010-1562-6
Kharlip J, Salvatori R, Yenokyan G, Wand GS (2009) Recurrence of hyperprolactinemia after withdrawal of long-term cabergoline therapy. J Clin Endocrinol Metab 94(7):2428–2436. doi:10.1210/jc.2008-2103
Cannavo S, Curto L, Squadrito S, Almoto B, Vieni A, Trimarchi F (1999) Cabergoline: a first-choice treatment in patients with previously untreated prolactin-secreting pituitary adenoma. J Endocrinol Invest 22(5):354–359
Muratori M, Arosio M, Gambino G, Romano C, Biella O, Faglia G (1997) Use of cabergoline in the long-term treatment of hyperprolactinemic and acromegalic patients. J Endocrinol Invest 20(9):537–546
Ferrari C, Paracchi A, Mattei AM, de Vincentiis S, D’Alberton A, Crosignani P (1992) Cabergoline in the long-term therapy of hyperprolactinemic disorders. Acta Endocrinol 126(6):489–494
Ben-Jonathan N, Hnasko R (2001) Dopamine as a prolactin (PRL) inhibitor. Endocr Rev 22(6):724–763. doi:10.1210/er.22.6.724
Wang F, Gao H, Li C, Bai J, Lu R, Cao L, Wu Y, Hong L, Wu Y, Lan X, Zhang Y (2014) Low levels of PRB3 mRNA are associated with dopamine-agonist resistance and tumor recurrence in prolactinomas. J Neurooncol 116(1):83–88. doi:10.1007/s11060-013-1276-2
Popescu MN, Ionescu E, Iovanescu LC, Cotoi BV, Popescu AI, Ganescu AE, Glodeanu A, Geormaneanu C, Moraru A, Patrascu A (2013) Clinical aggression of prolactinomas: correlations with invasion and recurrence. Rom J Morphol Embryol 54(4):1075–1080
Acknowledgments
The authors sincerely thank Prof. Rufu Xu (Evidence Based Medicine and Epidemiology Center, Third Military University, Chongqing, China) for helpful comments on the statistical methods.
Conflict of interest
No conflict of interest exits in the submission of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hu, J., Zheng, X., Zhang, W. et al. Current drug withdrawal strategy in prolactinoma patients treated with cabergoline: a systematic review and meta-analysis. Pituitary 18, 745–751 (2015). https://doi.org/10.1007/s11102-014-0617-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-014-0617-2