Abstract
Combination with cabergoline may offer additional benefits to acromegalic patients on pegvisomant monotherapy. We evaluated the safety and efficacy profile of this combination and investigated the determinants of response. An observational, retrospective, cross-sectional study. Fourteen acromegalic patients (9 females), who were partially resistant to somatostatin analogs and on pegvisomant monotherapy. Cabergoline was added because of the presence of persistent mildly increased IGF-I. The mean follow-up time was 18.3 ± 10.4 months. The efficacy and safety profile was assessed. The influence of clinical and biochemical characteristics on treatment efficacy was studied. IGF-I levels returned to normal in 4 patients (28%) at the end of the study. In addition, some decline in IGF-I levels was observed in a further 5 patients. The % IGF-I decreased from 158 ± 64% to 124 ± 44% (p = 0.001). The average change in IGF-I was −18 ± 27% (range −67 to +24%). Lower baseline IGF-I (p = 0.007), female gender (p = 0.013), lower body weight (p = 0.031), and higher prolactin (PRL) levels (p = 0.007) were associated with a better response to combination therapy. There were no significant severe adverse events. Significant tumour shrinkage was observed in 1 patient. Combination therapy with pegvisomant and cabergoline could provide better control of IGF-I in some patients with acromegaly. Baseline IGF-I levels, female gender, body weight, and PRL levels affect the response to this combination therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In acromegaly, selective resection of the adenoma by transsphenoidal surgery is still the treatment of choice, although adequate biochemical remission or cure is achieved in only 60% of cases. As the pituitary radiation effect is slow to develop and is accompanied by a high occurrence of hypopituitarism, medical therapy is still needed in a high percentage of acromegalic patients [1].
Long-acting somatostatin receptor ligands (SRL) are currently the first-line medical therapy in acromegaly, as they can normalize insulin-like growth factor (IGF) I levels in approximately 50% of patients [2]. A more recent medical treatment for acromegaly is the growth hormone (GH) receptor antagonist pegvisomant (PEG), which can normalize IGF-I in 60–84% of patients in clinical practice [3, 4]. PEG blocks the GH receptor and thus prevents GH signaling. However, PEG does not suppress but rather enhances GH secretion—thus increasing GH levels—and does not exert any inhibitory effect on tumoral somatotrophs [5]. Although some studies have shown that PEG treatment does not promote tumour growth [6–8], this continues to be the main concern of this treatment modality [9]. A higher risk of tumour growth has been described in patients with a higher GH level during PEG treatment [8] and in those cases with higher tumour GH expression [6]. PEG is expensive [10] and requires daily parenteral administration. To overcome these drawbacks, combination therapy using SRL and PEG (daily, once or twice per week) has been proposed [11–14]. Combined SRL and PEG is not more effective in terms of IGF-I control than PEG monotherapy [3, 14], but it can offer some advantages in control of tumour size, compliance, quality of life, and GH levels [11–13, 15]. In any case, this combination is also expensive and requires parenteral administration of both drugs.
Dopamine receptor agonists (DA) inhibit GH secretion in acromegaly [16] and can exert an antiproliferative and pro-apoptotic effect on pituitary tumour cells [17]. Cabergoline (CAB) is an ergot-derived DA with a longer half-life, better tolerability and higher efficacy than bromocriptine [18, 19]. Whether administered as adjuvant monotherapy or as combined treatment with SRL, CAB can normalize IGF-I in up to 50% of acromegalic patients with baseline IGF-I lower than 150% of the upper limit of normal (ULN) and can induce somatotroph tumour shrinkage in some cases [20]. CAB is less expensive and widely available, it can be administered orally, and it acts directly on the pituitary adenoma. Combined treatment with PEG and CAB is an attractive option, as the antisecretory and antiproliferative effects of CAB could complement the action of PEG [16, 17] and may improve biochemical and tumour control [20]. Experimental data suggest that the response to CAB is preserved in acromegalic patients treated with PEG [21]. Furthermore, this combination could enable the use of lower doses of PEG and, therefore, reduce costs. In this regard, use of the combination PEG and CAB does not appear to be exceptional in clinical practice, as 10% of acromegalic patients included in the ACROSTUDY [3] were on this treatment. However, there are no published studies on PEG and CAB combination therapy. We retrospectively reviewed our experience with this treatment modality to assess whether its effect was similar to that described for the combination of SRL and CAB [20]. We analyzed the safety and efficacy profile of combined PEG and CAB and the determinants of response (such as gender, age, previous pituitary radiotherapy, prolactin (PRL) immunostaining, and baseline levels of GH, IGF-I, and PRL).
Patients and methods
Seventeen acromegalic patients treated with PEG and CAB between 2006 and 2010 were included in this study, which was conducted at 5 university tertiary hospitals in Spain. The ethics committee of each hospital approved the protocol, and all patients gave their written informed consent before inclusion. All but 1 patient has been previously described [6]. All the patients showed a partial response [22] to maximum doses of long-acting SRL therapy, with IGF-I levels at least 1.2-fold higher than the individual ULN after a minimum of 6 months of treatment. Before the advent of PEG, some patients had received prolonged treatment with SRL despite showing only a partial response. After SRL therapy, all cases had been on PEG monotherapy for a mean of 40 ± 26 months, with a dose ranging between 10 and 30 mg/day.
The main indication for adding CAB was mild but persistent elevated IGF-I on PEG monotherapy. Only 3 cases were treated with PEG at the maximum recommended dose of 30 mg/day; in the remaining cases, CAB was added as empiric treatment to avoid increasing the dose of PEG. We included only those cases in which treatment with PEG + CAB was administered under the following conditions: (1) CAB therapy was initiated after >6 months of treatment with PEG monotherapy and without any change in the PEG dose during the last 3 months; (2) follow-up was >3 months; and (3) there was no change in PEG dose during treatment with PEG + CAB. Only 14 out of 17 patients met these criteria, therefore 3 patients were excluded. None had been treated with long-term SRL for at least 6 months before CAB was added or during combination therapy. Patients attended follow-up visits, and the CAB dose was titrated according to criteria of the attending physician. IGF-I and PRL levels were measured in the hospital laboratories at baseline (precombination) and during follow up. PRL was measured using a chemiluminescence assay (Immulite 2000 Kit, DPC, Los Angeles, California, USA), as was IGF-I (Immulite, Euro/DPC, Gwynedd, UK), with intra- and interassay coefficients of variation of 2.3–3.9% and 3.7–8.1%, respectively. The IGF-I results from the different hospitals were given as total IGF-I levels and were also expressed as % IGF-I related to the individual ULN for IGF-I [(IGF-I ng/ml)/(ULN IGF-I) 100×)] to ensure standardized comparability values between centres.
We recorded clinical, biochemical, and tumour characteristics during PEG treatment, before addition of CAB, and regularly thereafter. Histopathology studies were reviewed to record PRL immunostaining, when available. PRL and IGF-I (expressed in ng/ml and as % of ULN) were recorded at baseline, during, and at the end of follow-up. The relative change in IGF-I level and the rate of IGF-I normalization (IGF-I ≤100%) at any time and at the end of follow-up were also recorded. Echocardiography and pituitary MRI studies before and after combination treatment were reviewed to evaluate changes in cardiac valve morphology and tumour size. Safety was assessed by monitoring the serum concentrations of alkaline phosphatase, alanine aminotransferase (ALT), aspartate aminotransferase (AST), g-glutamyltranspeptidase (g-GT), lactate dehydrogenase, total bilirubin, and creatinine. Serum glucose and glycated haemoglobin A1c (HbA1c) levels were also studied.
Statistical analysis
Data are shown as the mean ± SD (range), as absolute values, or as percentages, where appropriate. For continuous variables, normality was assessed using the Kolmogorov–Smirnov test, and logarithmic transformations were applied as necessary to ensure a normal distribution. The differences between groups in continuous variables (age, weight, months on treatment and biochemical data) were analyzed using Mann-Witney U test. The (two-samples) paired t test was used to compare initial and final biochemical values (when the patients were compared with themselves). For discontinuous variables, the Chi-squared test or Fisher exact test was applied, as appropriate. A multiple linear regression analysis using a stepwise method (probability for entry ≤0.05, probability for removal ≥0.10) for the introduction of independent variables was applied to identify the main determinants of the decrease in IGF-I. All analyses were performed using SPSS 16.0 for Windows (SPSS Inc, Chicago, Illinois, USA), and p < 0.05 was considered statistically significant.
Results
Patient characteristics
Fourteen patients were analyzed (Table 1). Mean age was 42 ± 8 years (range, 26–55) and the gender distribution was 9 females and 5 males. Most cases had previously undergone surgery (13/14, 93%). Immunohistochemical studies were available in 7 patients, and only 3 showed positive immunostaining for PRL. Eleven patients had been treated with pituitary irradiation (78%). The average interval between pituitary radiotherapy and PEG + CAB was 9.0 ± 6.4 years. All patients had been previously and unsuccessfully treated with long-term SRL therapy for an average of 49 ± 39 months (range, 6–96). After this treatment, PEG monotherapy was administered in all cases with a mean follow up of 40 ± 26 months (9–84). The PEG dose was at least 20 mg/day or higher in 10 cases (30 mg/day in 3 cases); the average PEG dose was 20.7 ± 6.8 mg/day and did not change at least in the 3 months before the addition of CAB or during combination therapy. The % IGF-I level for patients on PEG monotherapy was abnormal in all cases, with a % IGF-I of 158 ± 64% (108–234) (Table 2).
PRL levels before addition of CAB were normal in all cases except 1 (case 7, Table 2), with a 20% increase above the ULN. The initial dose of CAB was 1.16 ± 0.33 (0.75–2) mg/week and the final dose was 1.5 ± 0.7 (1–3) mg/week. The mean follow-up time on combination therapy was 18.3 ± 10.4 months (4–34).
Efficacy
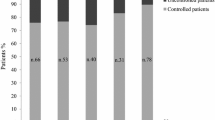
The biochemical response is shown in Tables 1 and 2 and in Fig. 1a/b. Four patients (28%) reached a normal % IGF-I at the end of the study. Four additional patients (28%) normalized % IGF-I at sometime during the follow-up. The % IGF-I level decreased from 158 ± 64% at baseline to 124 ± 44% (p = 0.001) at the end of follow-up. The mean IGF-I change was −18 ± 27.2% (range, −67 to + 24%) at the final visit. As a result of combination therapy, % IGF-I decreased from baseline to the end of follow-up in 9 out of 14 cases (64%) (Table 2). In these 9 patients, the average decrease in % IGF-I was −34 ± 18%. The time to nadir IGF-I was highly variable, from 4 to 25 months (12 ± 7). In patients not controlled with PEG 30 mg/day (cases 4, 6 and 8; Table 2), the decreases in % IGF-I were 38, 32, and 18% respectively, reaching a final % IGF-I of 75, 110, and 118%. The PRL level decreased significantly from 13.3 ± 9.1 at baseline to 1.6 ± 2.7 at the end of the study (p = 0.003).
Factors associated with efficacy: baseline IGF-I, gender, weight, and PRL levels
When we compared groups of patients who normalized and who did not normalize final % IGF-I (Table 1), we did not find any differences in clinical or biochemical characteristics, in previous therapies, in the final dose of PEG or CAB, or in the duration of combination therapy. Pituitary irradiation was not statistically associated with IGF-I normalization (p = 0.505), the percentage change in % IGF-I (p = 0.586), or nadir IGF-I during combination therapy (p = 0.232). All patients whose IGF-I levels returned to normal (cases 1, 2, 4 and 5; Table 2) during PEG + CAB combination therapy had previously been irradiated. The IGF-I values did not return to normal in the 3 non-irradiated patients (cases 3, 6 and 10; Table 2), however, a change in IGF-I of −41.3, −31.9 and +0.28%, respectively, was observed.
Although baseline IGF-I levels were no different between patients with normalized or uncontrolled IGF-I at the end of the study, a positive correlation was observed between baseline and final IGF-I expressed as both ng/ml (p = 0.007) and % IGF-I (p = 0.012). In addition, all patients with normal IGF-I values at the final visit had baseline IGF-I levels lower than 160% of the ULN (Table 2). Four patients (cases 5, 6, 8, and 11, Table 2) normalized % IGF during follow-up, but not at the end of the study. This loss of biochemical control of IGF-I, or escape phenomenon, was not associated with any clinical or biochemical characteristics.
Table 1 shows clinical and biochemical characteristics, previous therapy, and response to PEG + CAB according to gender. Baseline characteristics were similar, apart from a significant difference in body weight between the 2 groups (female 78 ± 10 kg and male 107 ± 19 kg, p = 0.009) and a higher PRL concentration in females (19 ± 10 vs 9 ± 4 ng/ml, p = 0.039). The rate of final normalized IGF-I did not vary according to gender (p = 0.221). However, the average change in IGF-I from baseline to last follow-up visit (Fig. 1) was greater in females than in males (–30.6 ± 21.5 and 4.6 ± 22% respectively, p = 0.014).
The final IGF-I normalization rate was not significantly associated with baseline PRL (p = 0.066), however a lower IGF-I nadir during CAB therapy was significantly associated with higher baseline PRL levels (p = 0.001). The response to treatment was better in those patients with positive PRL immunostaining results showing a more marked decrease in % IGF-I, lower final IGF-I and final normalization of IGF-I. However, given the limited number of available pathological samples, it was not possible to obtain a conclusion in this regard.
In an attempt to clarify the relationships between gender, weight, and PRL levels, we performed a stepwise analysis including decline in IGF-I as the dependent variable and gender, weight, and baseline PRL as independent variables (R2 = 0.414; p = 0.013). Although the stepwise method showed that the main determinant for decline in IGF-I was gender (beta 35.2; p = 0.013), the small sample size of our population precludes us from establishing a definitive conclusion in this regard.
Adverse events and treatment outcome
There were no relevant adverse events associated with combined treatment. Liver function test results remained unchanged except for 2 cases with slight and self-limiting increased g-GT (<2× ULN). Metabolic control in 3 diabetic patients was unchanged. An echocardiographic study performed during combined treatment was normal in 8 out of 12 cases. In the remaining 4 patients, who had previously known heart disease (mild left ventricular hypertrophy, diastolic dysfunction, and established heart valve disease), echocardiography findings remained unchanged. Pituitary magnetic resonance imaging (MRI) was performed at baseline, yearly thereafter, and/or at the end of the study in those cases followed for at least 9 months. There was no change in 9 pituitary MRI studies and, in an additional case, previously reported [6], PEG + CAB and pituitary irradiation were followed by a significant reduction (>20%) in tumour size.
At the end of the study, 8 patients continued with PEG + CAB and the dose of PEG was adjusted in 2 of them; 3 switched to PEG monotherapy with a dose adjustment and 3 switched to the combination of PEG and SRL (Table 2).
Discussion
We studied a small group of patients whose acromegaly was not controlled with various other treatments (including high-dose PEG monotherapy in 3 cases). Our main finding was that PEG + CAB normalized IGF-I levels in 4 out of 14 acromegalic patients (28%) and decreased IGF-I in 9 out of 14 patients (64%). Although IGF-I changes must always be interpreted with caution, we consider this finding clinically valuable. A better response to PEG + CAB was associated with baseline IGF levels (not higher than 160% above the ULN), female gender, lower body weight, and higher baseline PRL concentrations.
Most patients included in this study had been treated with pituitary irradiation; consequently, it is impossible to exclude some effect of radiotherapy in our results. However, radiotherapy was not statistically associated with IGF-I normalization, the change in % IGF-I or nadir IGF-I. The effect of pituitary irradiation is slow, requiring a long latency time to remission [23], and seems unlike to be related with IGF-I changes during the short follow-up of this study. Furthermore, in 2 out of 3 non-irradiated patients, IGF-I showed a decrease higher than 30%. As this was an observational study conducted in a clinical setting, it was unfeasible to perform a trial of CAB discontinuation in order to totally exclude an effect of previous radiotherapy on the results of combined treatment.
DA inhibit GH secretion in acromegaly [16] and exert an antiproliferative and pro-apoptotic effect on pituitary tumour cells [17]. The usefulness of CAB in the adjuvant postsurgical treatment of acromegaly, either alone or in combination with SRL, has been subject to debate [24], although some studies have shown long-term efficacy [19, 20, 25], and even remission of acromegaly [26].
In this study, the response to CAB therapy when combined with PEG was similar to, although somewhat less effective than, that reported in combination with SRL [20]: in patients not controlled with SRL, combining CAB with SRL led to a mean decrease of 30% in IGF-I levels, with normalization of IGF-I in 52% of cases [20].
To our knowledge, no previously published studies have analyzed PEG + CAB combination therapy in acromegaly patients. A cross-sectional study performed in a small group of PEG-treated acromegalic patients showed a decrease in GH level of >30% in 4 out of 9 patients after a single acute dose of CAB, suggesting that response to CAB is preserved under PEG therapy [21]. Only an unpublished study [27] has shown a higher IGF-I normalization rate with PEG + CAB (68%) than with PEG (10 mg/day fixed-dose) in monotherapy (26%).
We found that baseline IGF levels not higher than 160% above the ULN were associated with a better response to therapy. In this regard, a better response to CAB monotherapy and the combination of CAB and SRL has previously been associated with baseline IGF-I levels lower than 150% ULN [20]. We also found that female gender, lower body weight, and higher baseline PRL concentrations were associated with a better response to PEG + CAB. Although multiple regression analysis identified gender as the main determinant of the decrease in IGF-I level, the small sample size prevents us from considering this result as definitive. Gender and weight are probably closely related. To our knowledge, there are no previous reports of a better response to CAB in females, although this association has not been specifically evaluated [19, 20, 25, 28, 29]. Hypothetically, sexual dimorphism in GH secretion and in somatotroph axis regulation [30] could be associated with this better response to PEG + CAB observed in females. This is particularly interesting, as women have a worse response to PEG [4] and therefore require higher PEG doses to achieve the same effect on IGF-I control [31].
Finally, the predictive value of PRL cosecretion on the efficacy of DA in patients with acromegaly has been widely studied, with frequently discordant results supporting this association [19, 32, 33] or not [24, 28, 29, 34]. We observed a relationship between baseline PRL levels and IGF-I response, as patients with normal-high PRL levels had a lower IGF-I nadir during combined treatment. Although a positive PRL immunostaining result was associated with better response to treatment, it was not possible to draw definitive conclusions, because only 7 immunohistopathology studies were available.
PEG + CAB was generally well tolerated. Most adverse effects were self-limiting and did not require specific treatment. Abnormal liver function test results were clinically irrelevant and their prevalence was not higher than that reported for PEG monotherapy, in contrast to the higher risk described during combined treatment with PEG and SRL [11, 12]. Echocardiographic studies were unchanged and pituitary MRI did not reveal tumour growth in any case, showing a significant shrinkage in one case.
Although our study has some limitations, as the design is retrospective and observational and the sample is small, the results are very interesting in the clinical practice setting. We show that PEG + CAB is an effective option that allows better control of IGF-I hypersecretion in >50% of acromegalic patients on PEG monotherapy, including patients not controlled with maximum PEG doses. Further prospective and larger-scale studies will be necessary to confirm the efficacy of this treatment modality and to assess its potential economic benefit. This combination could make it possible to use lower doses of PEG—thus reducing cost—and provide better tumour control.
References
Chanson P, Salenave S, Kamenicky P, Cazabat L, Young J (2009) Pituitary tumours: acromegaly. Best Pract Res Clin Endocrinol Metab 23(5):555–574
Murray RD, Melmed S (2008) A critical analysis of clinically available somatostatin analog formulations for therapy of acromegaly. J Clin Endocrinol Metab 93(8):2957–2968
Trainer PJ (2009) ACROSTUDY: the first 5 years. Eur J Endocrinol 161(1):S19–S24
Marazuela M, Lucas T, Alvarez-Escola C, Puig-Domingo M, de la Torre NG, de Miguel-Novoa P, Duran-Hervada A, Manzanares R, Luque-Ramirez M, Halperin I, Casanueva FF, Bernabeu I (2009) Long-term treatment of acromegalic patients resistant to somatostatin analogues with the GH receptor antagonist pegvisomant: its efficacy in relation to gender and previous radiotherapy. Eur J Endocrinol 160(4):535–542
Kopchick JJ, Parkinson C, Stevens EC, Trainer PJ (2002) Growth hormone receptor antagonists: discovery, development, and use in patients with acromegaly. Endocr Rev 23(5):623–646
Marazuela M, Paniagua AE, Gahete MD, Lucas T, Alvarez-Escola C, Manzanares R, Cameselle-Teijeiro J, Luque-Ramirez M, Luque RM, Fernandez-Rodriguez E, Castano JP, Bernabeu I (2011) Somatotroph tumor progression during pegvisomant therapy: a clinical and molecular study. J Clin Endocrinol Metab 96(2):E251–E259
Jimenez C, Burman P, Abs R, Clemmons DR, Drake WM, Hutson KR, Messig M, Thorner MO, Trainer PJ, Gagel RF (2008) Follow-up of pituitary tumor volume in patients with acromegaly treated with pegvisomant in clinical trials. Eur J Endocrinol 159(5):517–523
Buchfelder M, Weigel D, Droste M, Mann K, Saller B, Brubach K, Stalla GK, Bidlingmaier M, Strasburger CJ (2009) Pituitary tumor size in acromegaly during pegvisomant treatment: experience from MR re-evaluations of the German Pegvisomant Observational Study. Eur J Endocrinol 161(1):27–35
Hodish I, Barkan A (2008) Long-term effects of pegvisomant in patients with acromegaly. Nat Clin Pract Endocrinol Metab 4(6):324–332
Moore DJ, Adi Y, Connock MJ, Bayliss S (2009) Clinical effectiveness and cost-effectiveness of pegvisomant for the treatment of acromegaly: a systematic review and economic evaluation. BMC Endocr Disord 9:20
Neggers SJ, van Aken MO, Janssen JA, Feelders RA, de Herder WW, van der Lely AJ (2007) Long-term efficacy and safety of combined treatment of somatostatin analogs and pegvisomant in acromegaly. J Clin Endocrinol Metab 92(12):4598–4601
Neggers SJ, de Herder WW, Janssen JA, Feelders RA, van der Lely AJ (2009) Combined treatment for acromegaly with long-acting somatostatin analogs and pegvisomant: long-term safety for up to 4.5 years (median 2.2 years) of follow-up in 86 patients. Eur J Endocrinol 160(4):529–533
Feenstra J, de Herder WW, ten Have SM, van den Beld AW, Feelders RA, Janssen JA, van der Lely AJ (2005) Combined therapy with somatostatin analogues and weekly pegvisomant in active acromegaly. Lancet 365(9471):1644–1646
Trainer PJ, Ezzat S, D’Souza GA, Layton G, Strasburger CJ (2009) A randomized, controlled, multicentre trial comparing pegvisomant alone with combination therapy of pegvisomant and long-acting octreotide in patients with acromegaly. Clin Endocrinol (Oxf) 71(4):549–557
Neggers SJ, van Aken MO, de Herder WW, Feelders RA, Janssen JA, Badia X, Webb SM, van der Lely AJ (2008) Quality of life in acromegalic patients during long-term somatostatin analog treatment with and without pegvisomant. J Clin Endocrinol Metab 93(10):3853–3859
Liuzzi A, Chiodini PG, Botalla L, Cremascoli G, Muller EE, Silvestrini F (1974) Decreased plasma growth hormone (GH) levels in acromegalics following CB 154(2-Br-alpha ergocryptine) administration. J Clin Endocrinol Metab 38(5):910–912
An JJ, Cho SR, Jeong DW, Park KW, Ahn YS, Baik JH (2003) Anti-proliferative effects and cell death mediated by two isoforms of dopamine D2 receptors in pituitary tumor cells. Mol Cell Endocrinol 206(1–2):49–62
Webster J, Piscitelli G, Polli A, Ferrari CI, Ismail I, Scanlon MF (1994) A comparison of cabergoline and bromocriptine in the treatment of hyperprolactinemic amenorrhea. Cabergoline Comparative Study Group. N Engl J Med 331(14):904–909
Abs R, Verhelst J, Maiter D, Van Acker K, Nobels F, Coolens JL, Mahler C, Beckers A (1998) Cabergoline in the treatment of acromegaly: a study in 64 patients. J Clin Endocrinol Metab 83(2):374–378
Sandret L, Maison P, Chanson P (2011) Place of cabergoline in acromegaly: a meta-analysis. J Clin Endocrinol Metab 96(5):1327–1335
Roemmler J, Steffin B, Gutt B, Schneider HJ, Sievers C, Bidlingmaier M, Schopohl J (2010) The acute effect of a single application of cabergoline on endogenous GH levels in patients with acromegaly on pegvisomant treatment. Growth Horm IGF Res 20(5):338–344
Colao A, Auriemma RS, Lombardi G, Pivonello R (2011) Resistance to somatostatin analogs in acromegaly. Endocr Rev 32(2):247–271
Castinetti F, Morange I, Dufour H, Regis J, Brue T (2009) Radiotherapy and radiosurgery in acromegaly. Pituitary 12(1):3–10
Freda PU, Reyes CM, Nuruzzaman AT, Sundeen RE, Khandji AG, Post KD (2004) Cabergoline therapy of growth hormone & growth hormone/prolactin secreting pituitary tumors. Pituitary 7(1):21–30
Moyes VJ, Metcalfe KA, Drake WM (2008) Clinical use of cabergoline as primary and adjunctive treatment for acromegaly. Eur J Endocrinol 159(5):541–545
Verhelst JA, Abrams PJ, Abs R (2008) Remission of acromegaly following long-term therapy with cabergoline: report of two cases. Pituitary 11(1):103–107
Higham CE, Atkinson AB, Alywin S, Martin NM, Moyes VJ, Newell-Price J, Trainer PJ (2009) A prospective clinical trial of combined cabergoline and pegvisomant treatment in patients with active acromegaly (Abstract). 91st annual meeting of the endocrine society, 10–13 June 2009, Washington
Cozzi R, Attanasio R, Lodrini S, Lasio G (2004) Cabergoline addition to depot somatostatin analogues in resistant acromegalic patients: efficacy and lack of predictive value of prolactin status. Clin Endocrinol (Oxf) 61(2):209–215
Cozzi R, Attanasio R, Barausse M, Dallabonzana D, Orlandi P, Da Re N, Branca V, Oppizzi G, Gelli D (1998) Cabergoline in acromegaly: a renewed role for dopamine agonist treatment? Eur J Endocrinol 139(5):516–521
Goldenberg N, Barkan A (2007) Factors regulating growth hormone secretion in humans. Endocrinol Metab Clin North Am 36(1):37–55
Parkinson C, Burman P, Messig M, Trainer PJ (2007) Gender, body weight, disease activity, and previous radiotherapy influence the response to pegvisomant. J Clin Endocrinol Metab 92(1):190–195
Lamberts SW, Klijn JG, van Vroonhoven CC, Stefanko SZ, Liuzzi A (1983) The role of prolactin in the inhibitory action of bromocriptine on growth hormone secretion in acromegaly. Acta Endocrinol (Copenh) 103(4):446–450
Lamberts SW, Liuzzi A, Chiodini PG, Verde S, Klijn JG, Birkenhager JC (1982) The value of plasma prolactin levels in the prediction of the responsiveness of growth hormone secretion to bromocriptine and TRH in acromegaly. Eur J Clin Invest 12(2):151–155
Sherlock M, Fernandez-Rodriguez E, Alonso AA, Reulen RC, Ayuk J, Clayton RN, Holder G, Sheppard MC, Bates A, Stewart PM (2009) Medical therapy in patients with acromegaly: predictors of response and comparison of efficacy of dopamine agonists and somatostatin analogues. J Clin Endocrinol Metab 94(4):1255–1263
Acknowledgments
We are grateful to Angel Salgado Barreira, biomedical research technician, for his invaluable assistance with the statistical analysis. This work was supported by Grants FISS 07/1119 (to M.M.) and 11/00161 (to IB).
Conflict of interest
I.B., T.L. and M.M. hold research grants from Pfizer and have received lecture fees from Novartis, Ipsen, and Pfizer. F.F.C. holds research grants from Pfizer and Novartis has and received lecture fees from Pfizer and Novartis. The remaining authors do not have any conflicts of interest that could be perceived as prejudicing the impartiality of the research reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bernabeu, I., Alvarez-Escolá, C., Paniagua, A.E. et al. Pegvisomant and cabergoline combination therapy in acromegaly. Pituitary 16, 101–108 (2013). https://doi.org/10.1007/s11102-012-0382-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-012-0382-z