Abstract
Subarachnoid haemorrhage (SAH) is known to be related to pituitary dysfuntion in retrospective and short-term prospective studies. We aimed to investigate pituitary functions in patients with SAH in longer follow-up periods to demonstrate if pituitary hormone deficiencies recover, persist or new hormone deficiencies occur. Twenty patients with SAH, who were followed up for 3 years, were included in the present study. Patients were evaluated with basal hormone levels and glucagon stimulation test (GST). Serum basal cortisol and adrenocorticotropic hormone (ACTH) levels were found to be significantly elevated at 3rd year of SAH compared to 1st year. Other basal hormone levels at 3rd year did not show a significant change from the levels found at 1st year. One of the patients had ACTH deficiency at 1st year of SAH and recovered at 3rd year. Growth hormone (GH) deficiency, according to GST, was diagnosed in 4 patients. One patient with GH deficiency at first year was still deficient, 3 of them recovered and 3 patients were found to have new-onset GH deficiency 3 years after SAH. SAH is associated with anterior pituitary dysfunction and GH is the most frequently found deficient hormone in the patients. Although one year after SAH seems to be an appropriate time for the evaluation of pituitary functions, further follow-up may be required at least in some cases due to recovered and new-onset hormone deficiencies at 3rd year of SAH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Subarachnoid haemorrhage is seen in 6/100,000 patients per year with an age predilection of 4th and 6th decades [1]. SAH has been linked to neuroendocrine dysfuntion due to close anatomical proximity to the circle of Willis. In the studies performed over the last few years, hypopituitarism was found to be increased in patients with SAH with a prevalence of hypopituitarism ranging from 37.5 to 63% [2–6]. Recently Klose et al. [8] have reported that SAH was associated with low prevalence of hypopituitarism which was evaluated with powerful diagnostic stimulation tests with confirmation. The evaluation time of pituitary functions after SAH, different tests and cut-off levels used for the diagnosis of deficient pituitary hormones all affect the prevalence of pituitary insufficiency related to SAH.
Prospective investigation of patients in terms of pituitary insufficiency after SAH at baseline and 12 months, at 3, 6 and 12 months have been carried out previously [6–9]. Although pituitary functions have been evaluated in few studies up to 10 years following SAH, these studies were cross-sectional [3, 4, 10] and lacked prospective follow up.
The present prospective study investigated pituitary functions after SAH with longest follow-up period at least according to our knowledge. We aimed to determine if there was any recovery or deterioration of pituitary functions in further follow-up of patients after 1st year of SAH. Therefore, pituitary functions of 20 patients were prospectively investigated at 1st and 3rd year of SAH.
Materials and method
Twenty patients, who were followed up for 3 years, with SAH were included in the present study. Some of the data regarding baseline and first year evaluation of pituitary functions in these patients were previously published [6]. The study was approved by Local Ethics Committee.
The clinical severity of the patients was assessed according to the grading system of Hunt and Hess, the distribution of the severity of bleeding was assessed according to the grading system of Fisher et al. on admission. For Hunt and Hess grades: grade (1) Asymptomatic or minimal headache and slight nuchal rigidity; (2) Moderate to severe headache, nuchal rigidity and no neurological deficit other than cranial nerve palsy; (3) Drowsiness, confusion or mild focal deficit; (4) Stupor, moderate to severe hemiparesis, possibly early decerebrate rigidity, and vegetative disturbances and (5) Deep coma, decerebrate rigidity, moribund appearance, For Fisher CT score: (1) No blood detected; (2) A diffuse disposition or thin layer with all vertical layers of blood (interhemispheric fissure, insular cistern, ambient cistern) less than 1 mm thick; (3) Localized clots and/or vertical layers of blood 1 mm or greater in thickness; and (4) Diffuse or no subarachnoid blood, but with intracerebral or intraventricular blood [11, 12].
Body mass indeces (BMI) of the patients were calculated at 1st and 3rd year.
Basal hormone levels and evaluation of TSH and gonadotropin deficiencies
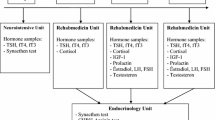
Basal hormone levels including free (f) T3, fT4, TSH, PRL, cortisol, ACTH, FSH, LH, IGF-1, total and free testosterone in males and estradiol in females were measured in the morning. The patients were free of glucocorticoids, dopamine or any other drugs that may affect pituitary functions at the time of assessment.
Gonadotropin deficiency was evaluated by history of sexual functions in males and menstrual cycles in females and the diagnosis was confirmed by low serum total testosterone (<134 ng/dl) and free testosterone (<11.5 pg/ml) in the presence of normal or decreased gonadotropin levels in males. In females, having low serum estradiol (<11 pg/ml) levels in the presence of inappropriately low gonadotropins was accepted as gonadotropin deficiency [13, 14].
TSH deficiency was defined by low serum fT4 level (<8 pg/ml) in the presence of low or normal serum TSH [13, 14].
Evaluation of somatotropic and corticotropic functions by dynamic tests
Patients were evaluated with glucagon stimulation test (GST) for the diagnosis of GH and ACTH deficiencies. 1 mg intramuscular glucagon was administered and serum samples for GH at baseline, 90, 120, 150, 180, 210 and 240 min were obtained. GH deficiency was diagnosed if peak GH response was below 1.18 μg/l. ACTH deficiency was diagnosed if peak cortisol response was less than 10.7 μg/dl. These cut-off levels were derived from cortisol and GH responses of 20 healthy individuals reported previously [6, 15]. Serum GH levels were measured by two-site immunoradiometric assay (IRMA with Active Human Growth Hormone Diagnostic System Laboratories, (DSL)-1900 Webster, Texas, USA) (calibrated to WHO 88/624). The lower detection limit for GH was 0.01 μg/L with intraassay and interassay coefficients of variation (CV)s of 3.1 and 5.9% respectively. Serum IGF-1 levels were measured by immunoradiometric assay (IRMA) after formic acid–ethanol extraction with Diagnostic System Laboratories (Webster, Texas, USA); with intraassay and interassay CVs ranging from 3.9 to 7% and interassay CVs ranging from 3.4 and 8.2%. IGF-1 reference ranges (mean ± 2 SD) for the relevant ages were: (197–476 ng/ml (18–30 years), 100–494 ng/ml (30–40 years) and 101–303 ng/ml (40–70 years).
Serum cortisol levels were measured by radioimmunoassay (RIA) (Active Cortisol RIA, Diagnostic System Laboratories 2100, Webster, Texas, USA). This procedure had intra-assay and inter-assay CVs of 8.4 and 9.1% respectively. Its sensitivity was 8.3 nmol/l (0.3 μg/dl). FSH (ICN Biomedicals, CA, USA; intraassay and interassay CVs 2.4 and 7.3% respectively), LH (ICN Biomedicals, CA, USA; intraassay and interassay CVs 3.6 and 7.8% respectively), PRL (ICN Biomedicals, CA, USA; intraassay and interassay CVs 7 and 8.9% respectively), fT3 (ZenTech, Liege, Belgium; intraassay and interassay CVs 2.7 and 8.3% respectively), fT4 (ZenTech, Liege, Belgium; intraassay and interassay CVs 3.7 and 4.5% respectively), total testosterone (Biosource, Nivelles, Belgium; intraassay and interassay CVs 4.6 and 6.2% respectively) and free testosterone (Diagnostic System Laboratories 2100, Webster, Texas, USA); intraassay and interassay CVs 6.2 and 9.7% respectively) were measured by RIA. Estradiol was measured by Advia centaur system chemiluminescent technology, Bayer, Germany with intraassay and interassay CVs of 5.5 and 5.2% respectively. ACTH (DSL; intraassay and interassay CVs 2.3 and 8% respectively) and TSH (Biosource, Nivelles, Belgium; intraassay and interassay CVs of 6 and 4.1% respectively) were measured by IRMA.
Statistical analysis
All statistical analyses were performed by the Statistical Package for Social Sciences (SPSS) for Windows version 15 (Chicago, IL, USA). The continous data were presented as mean ± standard deviation. Paired T test and Chi-square tests were used for the comparison of data obtained in the 1st and 3rd years following SAH. P < 0.05 was considered as statistically significant.
Results
Twenty patients were evaluated 1 and 3 years after SAH. The mean age of the patients was 47.6 ± 13 with a range of 15–70. Twelve (60%) patients were male and 8 (40%) patients were female. BMIs of the patients were found to be increased at 3rd year when compared to 1st year.
Basal hormone levels and evaluation of TSH and gonadotropin deficiencies
Individual evaluations of patients are summarized in Table 1. When basal hormone levels 1 and 3 years after SAH were compared, serum basal cortisol and ACTH levels were found to be significantly elevated. Other basal hormone levels at 3rd year did not show a significant change from the levels found at 1st year (Table 2).
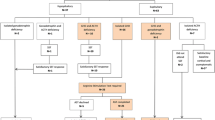
None of the patients had TSH or gonadotropin deficiency at either 1 or 3 years after SAH (Fig. 1). Eight women were included in the study, 6 of them were post-menopausal and 2 of them had regular menstrual cycles. Male patients were free of complaints of sexual dysfunction. None of the patients had hyperprolactinemia 1 year after SAH and only an 18 year-old woman had mild hyperprolactinemia.
The hypothalamo–pituitary–adrenal axis
The HPA axis was evaluated by GST besides basal cortisol and ACTH. Serum cortisol before GST and peak cortisol response following GST were found to be significantly increased 3 years after SAH compared to the levels found at 1st year (Table 2). The HPA axis was found to be insufficient in one of the patients a year after SAH. The patient was carefully observed without replacement therapy and informed about the symptoms of adrenal insufficiency and recommended to have glucocorticoid replacement in case of stress. This patient with ACTH deficiency recovered 3 years after SAH (Fig. 1). The clinical scoring of SAH or presence of vasospasm on admission were not found to affect basal or stimulated cortisol levels.
GH and IGF-1 axis
Although IGF-1 and, basal and glucagon stimulated GH levels were found to be increased 3 years after SAH compared to the levels found at first year, the difference was not statistically significant (Table 2). GH deficiency, according to GST, was diagnosed in 4 of 16 (25%) patients. One patient with GH deficiency at first year was still deficient and 3 of them recovered at 3rd year. 3 patients were found to have new-onset GH deficiency 3 years after SAH (Table 1, Fig. 1). The body mass indeces and IGF-1 levels of the patients did not show significant differences in patients with or without GH deficiency at 1st or 3rd year.
Discussion
The pathophysiology of pituitary dysfunction in patients with SAH is not well-defined. Increased intracranial pressure, changes in local tissue pressure after haemorrhage, ischemia due to vasospasm, microinfarctions of pituitary gland, venous stasis and surgical procedures may damage the pituitary gland [16]. Neuroinflammatory responses, autoimmunity and genetic predisposition as in traumatic brain injury may also be involved in the pathogenesis [17–19]. Deficiency of any pituitary hormone after SAH was reported to vary from none to 68% in previous studies [2, 4–6, 8, 20]. The present study prospectively investigated pituitary functions after SAH with longest follow up period to date. The hypopituitarism prevalence was found to be 25% at first year and 20% at 3rd year of SAH.
The results of present study showed increased cortisol and ACTH levels from 1st to 3rd year of SAH. Hypocortisolism after SAH was reported to be 20.5% in a recent review by Schneider et al. [16]. The different prevalences of ACTH deficiency (ranging between 2.5–56%) reported in various studies is presumably due to different criteria used to diagnose secondary adrenal insufficiency and different evaluation times after SAH [2–6, 20].
Although HPA axis is the 2nd most affected axis following SAH in most studies, Klose et al. has recently reported that hypopituitarism was uncommon after SAH and ACTH deficiency was found to be only 2% which could not be demonstrated by confirmatory testing 14 months after SAH. They have used insulin tolerance test (ITT) or standard ACTH stimulation test (in patients with contraindication to ITT) for HPA axis evaluation and cortisol response equal or higher than 18 μg/dl was accepted to be sufficient [8]. Kreitschman et al. have also used ITT with the same cut-off level for cortisol and found ACTH deficiency in 40% of patients with SAH [4]. Besides clinical studies, population-based studies have also revealed increased prevalence of pituitary hormone deficiency in patients with SAH and traumatic brain injury [21, 22]. Although it is difficult to standardize collected data in the epidemiological studies due to variable clinical practices, high number of patients involved should not be underestimated. In the present study, ACTH deficiency was diagnosed in a patient who recovered in the 3rd year of SAH. GST was used to assess HPA axis functions and the cut-off level of sufficient cortisol response was derived from responses of healthy individuals [6]. Recently, we compared glucagon and low and standard dose ACTH stimulation tests in healthy individuals and defined cortisol response of higher than 9.1 μg/dl as sufficient according to cortisol response of 18 μg/dl after standard dose ACTH stimulation [23]. When we reanalysed the results according to peak cortisol response of 9.1 μg/dl no ACTH deficiency was detected in either first or third year.
There are few prospective studies in the literature investigating pituitary functions after SAH (Table 3) [4, 6–8, 24]. Bendel et al. found increased total and free cortisol levels in patients with SAH due to aneurysm when compared to in patients with aneurysm without SAH. The follow up period was only 3 months and the authors explained the reason for increased basal and ACTH stimulated cortisol levels in the very acute phase by acute stress response. The other pituitary axes were not evaluated in the study [24]. The common finding in other prospective studies [6, 8] is the recovery of HPA axis in the follow up of SAH in some patients at 1st year. In a recent study, diurnal cortisol rhythm was found to be disturbed after SAH [25]. Although the number of patients with HPA axis insufficiency was found to be unchanged in the present study, mean basal cortisol and ACTH, and stimulated cortisol levels were found to be increased at 3rd year compared to the levels at 1st year. Therefore, subtle changes may occur in HPA axis functions in long-term follow up of patients with SAH, but the clinical consequences of these changes may not be noteworthy.
GH-IGF-1 axis is reported to be the most commonly affected axis after SAH in most studies [2, 3, 6, 8]. Basal IGF-1, ITT or GHRH-arginine stimulation test have been used to assess GH-IGF-1 axis. In the present study, we found GH deficiency in 25% (4/16, 50% were isolated) and 20% (4/20, all were isolated) of the patients at 1st and 3rd year of SAH respectively. One of the patients with recovered GH deficiency had high IGF-1 levels at 3rd year, but the patient was free of symptoms of acromegaly and in the follow-up IGF-1 was found to be 127 ng/ml within normal references. In one of our previous studies, we found that persistant GH deficiency in follow-up was associated with lower IGF-1 levels [6]. The discrepancies between GH and IGF-1 may be related to relatively dynamic changes during follow-up e.g: recovered, new-onset or persistant hormone deficiencies. GH deficiency following SAH was reported to vary between 20 and 37% in different studies. Although variable methods have been used and evaluation times were different in these studies, the reported rates of GH deficiency did not show considerable variations [2–4, 6, 8, 10]. In some prospective studies with varying follow-up, GH deficiency was not demonstrated after SAH [8]. In the study by Lammert et al., ITT was performed only in patients with clinical suspicion. They did not perform ITT in patients with normal IGF-1 levels unless an associated pituitary hormone deficiency was present [9]. This could be an explanation for the low incidence of GH deficiency reported. However GH deficiency may be suspected in patients with low IGF-1 levels, but one cannot rule out GH deficiency in a patient with normal IGF-1 if the patient has a risk for hypopituitarism. So, a glucagon stimulation test was used in the present study and the results were evaluated with a highly specific cut-off level of GH derived from healthy people.
In the present study, we could not perform ITT because of the underlying cranial pathology or GHRH-GHRP-6 test due to unavailability of GHRP-6. This is an involuntary limitation of the study. But since we have used strict criteria derived from healthy subjects for the diagnosis of both GH and ACTH deficiencies, we think that false positivities are probably very low.
The gonadal and thyroidal axes were found to be unaffected in this study. In previous studies, acute phase of SAH was found to be associated with increased gonadotropin deficiency as 31, 8, 33 and 88% [5, 6, 8]. All patients were found to recover their gonadotroph functions in the follow up [6, 8]. Aimeretti et al. found gonadotropin deficiency in 12.5% of the patients in their study at 3rd month of SAH [7]. In later phases of SAH with longer than a year of follow up, frequency of gonadotropin deficiency was reported to be 0–13% [3, 4, 6, 8, 10]. The deficiency detected in the acute phase of SAH is probably due to critical illness and gonadotropin deficiency seems to be rare. TSH deficiency was also reported to be low varying from 0 to 7.5% [2, 4–6, 8, 9].
Most of the patients had favourable neurological outcome at 3rd year of SAH. None of them had malnourishment, general well-being of all the patients were good. One of the patients had subdural effusion in the early postoperative period which was drained succesfully, but the patient had persistant left sided-hemiparesis. This patient had no deficient pituitary hormone. One of the patients had motor and sensory deficit a year after SAH, but it was shown to be caused by lumbal disc herniation which was corrected after surgery.
In conclusion, SAH is associated with anterior pituitary dysfunction and GH is the most frequently found deficient hormone in the patients. Thyroidal and gonadal functions are almost always preserved and secondary adrenal insufficiency is not common. Although pituitary functions do not show further clinically notable changes after 1st year of SAH, there may be subtle changes especially in HPA axis functions. When the clinical insult is considered, even though one year after SAH seems to be an appropriate time for the evaluation of pituitary functions, further follow up may be required at least in some cases due to recovered and new-onset hormone deficiencies at 3rd year of SAH.
References
vanRinkel GJ GJ, Rinkel GJ (2001) Subarachnoid haemorrhage: diagnosis, causes and management. Brain 124(Pt 2):249–278
Aimaretti G, Ambrosio MR, Di SC et al (2004) Traumatic brain injury and subarachnoid haemorrhage are conditions at high risk for hypopituitarism: screening study at 3 months after the brain injury. Clin Endocrinol (Oxf) 61(3):320–326
Jovanovic V, Pekic S, Stojanovic M et al (2010) Neuroendocrine dysfunction in patients recovering from subarachnoid hemorrhage. Hormones (Athens) 9(3):235–244
Kreitschmann-Andermahr I, Hoff C, Saller B et al (2004) Prevalence of pituitary deficiency in patients after aneurysmal subarachnoid hemorrhage. J Clin Endocrinol Metab 89(10):4986–4992
Parenti G, Cecchi PC, Ragghianti B et al (2011) Evaluation of the anterior pituitary function in the acute phase after spontaneous subarachnoid hemorrhage. J Endocrinol Invest 34(5):361–365
Tanriverdi F, Dagli AT, Karaca Z et al (2007) High risk of pituitary dysfunction due to aneurysmal subarachnoid haemorrhage: a prospective investigation of anterior pituitary function in the acute phase and 12 months after the event. Clin Endocrinol (Oxf) 67(6):931–937
Aimaretti G, Ambrosio MR, Di SC et al (2005) Residual pituitary function after brain injury-induced hypopituitarism: a prospective 12-month study. J Clin Endocrinol Metab 90(11):6085–6092
Klose M, Brennum J, Poulsgaard L, Kosteljanetz M, Wagner A, Feldt-Rasmussen U (2010) Hypopituitarism is uncommon after aneurysmal subarachnoid haemorrhage. Clin Endocrinol (Oxf) 73(1):95–101
Lammert A, Bode H, Hammes HP et al (2011) Neuro-endocrine and neuropsychological outcome after aneurysmal subarachnoid hemorrhage (aSAH): a prospective cohort study. Exp Clin Endocrinol Diabetes 119(2):111–116
Dimopoulou I, Kouyialis AT, Tzanella M et al (2004) High incidence of neuroendocrine dysfunction in long-term survivors of aneurysmal subarachnoid hemorrhage. Stroke 35(12):2884–2889
Fisher CM, Kistler JP, Davis JM (1980) Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery 6(1):1–9
Hunt WE, Hess RM (1968) Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg 28(1):14–20
Burke CW (1992) The pituitary megatest: outdated? Clin Endocrinol (Oxf) 36(2):133–134
Lamberts SW, de Herder WW, van der Lely AJ (1998) Pituitary insufficiency. Lancet 352(9122):127–134
Tanriverdi F, Unluhizarci K, Coksevim B, Selcuklu A, Casanueva FF, Kelestimur F (2007) Kickboxing sport as a new cause of traumatic brain injury-mediated hypopituitarism. Clin Endocrinol (Oxf) 66(3):360–366
Schneider HJ, Kreitschmann-Andermahr I, Ghigo E, Stalla GK, Agha A (2007) Hypothalamopituitary dysfunction following traumatic brain injury and aneurysmal subarachnoid hemorrhage: a systematic review. JAMA 298(12):1429–1438
Tanriverdi F, Taheri S, Ulutabanca H et al (2008) Apolipoprotein E3/E3 genotype decreases the risk of pituitary dysfunction after traumatic brain injury due to various causes: preliminary data. J Neurotrauma 25(9):1071–1077
Tanriverdi F, De BA, Bizzarro A et al (2008) Antipituitary antibodies after traumatic brain injury: is head trauma-induced pituitary dysfunction associated with autoimmunity? Eur J Endocrinol 159(1):7–13
Tanriverdi F, Unluhizarci K, Kelestrimur F (2010) Persistent neuroinflammation may be involved in the pathogenesis of traumatic brain injury (TBI)-induced hypopituitarism: potential genetic and autoimmune factors. J Neurotrauma 27(2):301–302
Weant KA, Sasaki-Adams D, Dziedzic K, Ewend M (2008) Acute relative adrenal insufficiency after aneurysmal subarachnoid hemorrhage. Neurosurgery 63(4):645–649
Kreitschmann-Andermahr I, Hartmann Y, Poll E, Schneider HJ, Buchfelder M, Stalla GK (2011) The German database on hypopituitarism after traumatic brain injury and aneurysmal subarachnoid hemorrhage—description, objectives and design. Exp Clin Endocrinol Diabetes 119(1):15–20
Schneider HJ, Schneider M, Kreitschmannandermahr I et al. (2011) Structured assessment of hypopituitarism following traumatic brain injury and aneurysmal subarachnoid hemorrhage in 1242 patients—the German Interdisciplinary Database. J Neurotrauma 28(9):1693–1698
Karaca Z, Lale A, Tanriverdi F, Kula M, Unluhizarci K, Kelestimur F (2010) The comparison of low and standard dose ACTH and glucagon stimulation tests in the evaluation of hypothalamo-pituitary-adrenal axis in healthy adults. Pituitary 14(2):134–140
Bendel S, Koivisto T, Ruokonen E et al (2008) Pituitary-adrenal function in patients with acute subarachnoid haemorrhage: a prospective cohort study. Crit Care 12(5):R126
Shin IY, Joo HM, Chung YG, Kim MS, Park JW, Ahn RS (2011) Abnormal diurnal pattern of cortisol secretion in patients after aneurysmal subarachnoid hemorrhage. Stress 14(2):156–165
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karaca, Z., Tanriverdi, F., Dagli, A.T. et al. Three years prospective investigation of pituitary functions following subarachnoid haemorrhage. Pituitary 16, 76–82 (2013). https://doi.org/10.1007/s11102-012-0377-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-012-0377-9