Abstract
At present, no effective medical treatment exists for recurrent and aggressive craniopharyngiomas that are resistant to conventional therapies, including surgery and adjuvant radiotherapy. Temozolomide is an alkylating chemotherapeutic agent used routinely in the management of high grade gliomas. The response to temozolomide is suggested to be dependent on the tumoral expression of O-6 methylguanine DNA methyltransferase (MGMT). Evidence supports that low MGMT immunoexpression correlates with positive response to temozolomide. Therefore, we aimed to assess MGMT immunoexpression in adamantinomatous craniopharyngiomas, in an effort to predict the likelihood of response to temozolomide. The MGMT immunostaining was performed on 23 adamantinomatous craniofaryngiomas operated at the Sisli Etfal Training and Research Hospital and identified by histological analysis. Paraffin embedded tissue sections were immunostained for MGMT and were evaluated semi-quantitatively. Of the 23 cases evaluated, 22 (96%) demonstrated negative (<10%) and 1 (4%) demonstrated low (10%) MGMT immunoexpression. Data from this study suggest a high proportion of adamantinomatous craniopharyngiomas exhibit negative/low MGMT immunoreactivity and could be treated with temozolomide, if conventional therapy fails.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Craniopharyngiomas are benign epithelial tumors, most of them are located in the sellar/parasellar region and arise from epithelial nests of the Rathke’s pouch. According to World Health Organization (WHO) classification, craniopharyngiomas correspond histologically to tumoral grade I [1]. However, the growth characteristics of craniopharyngiomas show considerable variation and often they behave as aggressive tumors. Histologically, craniopharyngiomas are consisted of two subtypes, the adamantinomatous and the papillary, but a mixture of both types is frequently present. The adamantinomatous subtype is the most common form predominantly affecting young children but also all ages, whereas the papillary subtype is almost entirely seen in adults [2].
The management of craniopharyngioma remains challenging. Gross total resection or subtototal resection in combination with adjuvant conventional radiotherapy (RT) is currently the treatment of choice [2]. However, due to their large size, calcification and adherence to crucial surrounding neurovascular structures, complete resection is difficult. Therefore, they are associated with a high rate of recurrence. Treating recurrent tumors is more difficult compared to primary therapy and is associated with high rates of mortality and morbidity [3]. Currently, aside from repeat surgery and RT, treatment options are few in recurrent and aggressive cases [2]. For craniopharyngiomas that escape standard therapeutic approaches, the establishment of new alternative therapeutic options is important.
Temozolomide (TMZ) is an oral alkylating chemotherapeutic agent that is converted physiologically to 5-(3-methyltriazen-1-yl) imidazole-4-carboximide, has a cytotoxic effect by methylation of guanine at the O-6 position of DNA and inhibits DNA replication [4, 5]. Susceptibility of various tumors, including high grade gliomas and conventional treatment resistant pituitary adenomas and carcinomas, to temozolomide is well established [6–12].
O-6 methylguanine DNA methyltransferase (MGMT) is a DNA repair enzyme that alters the methylation state of DNA and removes the alkyl group adducts at the O-6 position of guanine, thereby counteracts TMZ’s anti-neoplastic effect [12–14]. Immunohistochemical studies suggest that low MGMT immunoexpression correlates with a high likelihood of response to TMZ therapy [7, 8, 11, 14, 15]. Therefore, we aimed to assess MGMT immunoexpression in adamantinomatous craniopharyngiomas in an effort to anticipate the likelihood of response to TMZ. Our data may serve as a ground for considering whether TMZ could be used as an alternative treatment option for the cases of recurrent and aggressive adamantinomatous craniopharyngiomas that are resistant to conventional treatment modalities.
Materials and methods
For the current study, the database of the neurosurgery and pathology clinics of the Sisli Etfal Training and Research Hospital was searched for all cases of adamantinomatous craniopharyngioma. The initial diagnosis was based on clinical and radiological findings confirmed by histological evaluation. The paraffin embedded tissue sections of 23 patients, operated at our center during the interval 1993–2009 and diagnosed as adamantinomatous craniopharyngioma according to histological findings, were eligible for immunohistochemical analysis.
Immunohistochemistry (IHC) for MGMT was performed using avidin–biotin-peroxidase complex method. 3–5 μm cut formalin-fixed, paraffin embedded tumor tissue specimens underwent heat induced antigen retrieval for 20 min in 0,01 M citrate buffer (PH 6,0) in microwave. Sections were incubated overnight with a mouse monoclonal anti MGMT antibody (Clone MT 3,1: Novus Biologicals, NB 100-692) at 1/50 dilution. Positive control consisted of tonsil with squamous metaplastic mucosa and basaloid cells with positive staining for MGMT. In addition, non-neoplastic endothelial cells and lymphocytes served as internal positive controls. MGMT immunoexpression was evaluated on a multi-head light microscope by to observer (C.T, H.Ö) and defined semi-quantitatively by visual impression according to the fraction of positive nuclear staining.
According to a study performed by Lau et al. the MGMT immunoexpression scored as 1 = negative or limited to <10%, 2 = 10–25%, 3 = 25–50%, and 4 ≥ 50%. Scores 1 and 2 represent low level MGMT expression, whereas scores 3 and 4 represent intermediate and high MGMT expression, respectively [8]. Clinical and radiological data were obtained through retrospective chart review. The age and gender of the patients, number of surgeries and frequency of recurrence was correlated with MGMT immunoexpression. The project was approved by the local ethical board.
Results
The current study included 23 patients (13 male, 10 female), ranging in age between 5 and 61 years old (26,3 ± 16,9 years old). Of the study population, 9 (39%) were children and 14 (61%) were adult patients. Among the 23 tumors,13 were suprasellar, 8 were sellar/suprasellar and 2 were sellar according to radiological findings. The provided tissues for MGMT immunostaining were obtained at initial surgery in 16 (69%), and at surgeries performed following at least one recurrence in remaining 7 (31%) of the patients.
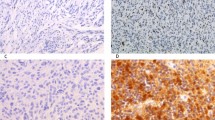
Overall 23 cases of adamantinomatous craniopharyngioma exhibited negative/low MGMT immunoreactivity. Staining for MGMT was limited to endothelium which served as positive internal control (Fig. 1a). Staining for MGMT was <10% (score 1) in 22 (96%) tumors and 10% (score 2) in 1 (4%) tumor (Fig. 1b, c). There is no correlation between age, gender, number of surgeries and frequency of recurrence with MGMT immunoreactivity. Patient characteristics and MGMT immunoexpression levels are shown in Table 1.
MGMT immunohistochemistry. a and b negative MGMT staining (score 1) in adamantinomatous craniopharyngioma (case 23 and 5). The endothelial cells serve as internal positive control (a). c low MGMT staining (score 2) in adamantinomatous craniopharyngioma (case 2), the upper part of photomicrograph displays reactive gliosis with low MGMT immunoexpression. a–c ×200
Discussion
Craniopharyngiomas are rare, mainly sellar/suprasellar benign epithelial tumors arising from remnants of the Rathke’s pouch and diagnosed during childhood and adult life.
Many therapeutic modalities have been tried so far with the goal of eradicating these tumors or to diminish their size. Currently, gross total resection or subtotal resection followed by RT is the treatment of choice. However, according to different studies, recurrence could occur in 0–62% of cases after gross total resection and in 25–100% of cases after subtotal resection [16]. This condition requires further therapy such as repeat surgeries and/or RT. On the other hand, repeat surgeries are associated with a high failure rate of tumor control and carries the high risk of endocrine, hypothalamic and neuro-cognitive dysfunction [17]. Recurrence could also occur in approximately 20% of patients after the use of RT for residual disease [18]. Nevertheless, RT is associated with less early morbidity but in long-term it is associated with serious complications, which is more important in children [19].
Intratumoral bleomycin has been proposed as an alternative to surgery or RT for predominatly cystic craniopharyngiomas. It has a limited role in the management of these tumors and just provide short-term control. Furthermore, serious damage to surrounding brain structures has been exhibited after the leakage of bleomycin from inside of the tumor [19]. Progression of disease required re-operation within 1 year of treatment has also been reported [20].
As an alternative to intracystic bleomycin, intracystic interferon-alpha-2a (IFN-α) has been used in the treatment of predominantly cystic craniopharyngiomas. According to a recent study conducted by Cavalheiro et al. tumor control was achieved in 47 patients treated with intracystic IFN-α [21]. However, in 13 patients the tumor continued to grow and 8 patients developed new different pituitary hormone deficiencies after treatment [21]. A rapid increase in the size of cyst has also been reported in 2 patients, after the commencement of intracystic IFN-α [19]. Like intracystic bleomycin, intracystic IFN-α may provide short term control of predominantly cystic craniopharyngiomas, but long term effect is yet unknown. IFN-α has a minimal effect when administered systemically. Morever, tumor enlargement has been observed in most patients after discontinuation of the drug [2].
Systemic chemotherapy has been applied in very small number of patients with aggressive craniopharyngioma. Bremer et al. treated a case of cystic craniopharyngioma with systemic chemotherapy using the combination of vincristine, carmustine and procarbazine, resulting in tumor shrinkage and clinical improvement [22]. Lippens et al. reported four cases of adamantinomatous craniopharyngioma with multiple recurrences after several surgical procedures and RT. These patients were treated with the combination of doxorubicin and lomustin. In follow-up, they observed complete remission in one, stable disease in two, and cystic relapse in one of the patients [23]. However, to date, TMZ has not been administered in the management of conventional treatment resistant craniopharyngioma of any subtype. The advantage of TMZ is that it is not cell-cycle specific, so it can inhibit all stages of tumor cell growth. Thus, it is suitable for slow-growing tumors [24].
In the present study, 23 cases of adamantinomatous craniopharyngioma assessed for MGMT immunoreactivity. Of the 23 tumors evaluated, 22 (96%) demonstrated negative (<10%) and 1 (4%) demonstrated low (10%) MGMT immunoexpression. There was no correlation between age, gender, number of surgeries and frequency of recurrence with MGMT immunoreactivity. Thus, our findings suggest that adamantinomatous craniopharyngiomas may be susceptible to TMZ therapy.
In summary, the present study suggests a high proportion of adamantinomatous craniopharyngiomas express a low level of MGMT. These findings provide a ground for the assessment of TMZ’s efficacy in clinical trials as an alternative agent in this rare tumor subtype, if conventional therapy, including repeat surgeries and RT, fails.
References
Janzer RC, Burger PC, Giangaspero F, Paulus W (2000) Craniopharyngioma. In: Kleihues P, Cavenee WK (eds) World Health Organization classification of tumours of the central nervous system. IARC, Lyon, pp 244–246
Karavitaki N, Cudlip S, Adams C, Wass JAH (2006) Craniopharyngiomas. Endocr Rev 27(4):371–397
Barua KK, Ehara K, Kohmura E, Tamaki N (2003) Treatment of recurrent craniopharyngiomas. Kobe J Med Sci 49(5–6):123–132
Su YB, Sohn S, Krown SE, Livingston PE, Wolchok JD, Quinn C et al (2004) Selective CD4+ lymphopenia in melanoma patients treated with temozolomide: a toxicity with therapeutic implications. J Clin Oncol 22:610–616
Gerson SL (2004) MGMT: its role in cancer aetiology and cancer therapeutics. Nat Rev Cancer 4(4):296–307
Kovacs K, Horvath E, Syro LV, Uribe H, Penagos LC, Ortiz LD, Fadul CE (2007) Temozolomide therapy in a man with an aggressive prolactin-secreting pituitary neoplasm: morphological findings. Hum Pathol 38:185–189
Kovacs K, Scheithauer BD, Lombardero M, McLendon LE, Syro LV, Uribe H, Ortiz LD, Penagos LD (2008) MGMT immunoexpression predicts responsiveness of pituitary tumours to temozolomide therapy. Acta Neuropathol 115:261–262
Lau Q, Scheithauer B, Kovacs K, Hovarth E, Syro LV, Lloyd R (2010) MGMT immonoexpression in aggressive pituitary adenoma and carcinoma. Pituitary. doi:10.1007/s11102-010-0249-0
Sofietti R, Leoncini B, Ruda R (2007) New devlopments in the treatment of malignant gliomas. Expert Rev Neurother 7(10):1313–1326
Fadul CE, Kominsky AL, Meyer LP, Kingman LS, William B, Kinlaw WB et al (2006) Long-term response of pituitary carcinoma to temozolomide. Report of two cases. J Neurosurg 105:621–626
Takeshita A, Inoshita N, Taguchi M, Okuda C, Fukuhara N, Oyama K et al (2009) High incidence of low O6-methylguanine DNA methyltransferase expression in invasive macroadenomas of Cushing’s disease. Eur J Endocrinol 161:553–559
Abacioglu U, Caglar HB, Yumuk PF, Akgun Z, Atasoy BM, Sengoz M (2010) Efficacy of protracted dose-dense temozolomide in patients with recurrent high-grade glioma. J Neurooncol. doi:10.1007/s11060-010-0423-2
Rodriguez FJ, Thibodeau SN, Jenkins RB, Schowalter KV, Caron BL, O’Neill BP et al (2008) MGMT immunohistochemical expression and promoter methylation in human glioblastoma. Appl Immunohistochem Mol Morphol 16(1):59–65
Pollack IF, Hamilton RL, Sobol RW, Burnham J, Yates AJ, Holmes EJ, Zhou T, Finlay JL (2006) O6-methylguanine-DNA methyltransferase expression strongly correlates with outcome in childhood malignant gliomas: results from the CCG-945 cohort. J Clin Oncol 24:3431–3437
Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M et al (2005) MGMT gene silencing and benifit from temozolomide in glioblastoma. N Eng J Med 352(10):997–1003
Karavitaki N, Wass JAH (2008) Craniopharyngiomas. Endocrinol Metab Clin N Am 37:173–193
Jang WY, Lee KS, Son BC, Jeon SS, Hong YK, Lee SW et al (2009) repeat operations in pediatric patients with reccurent craniopharyngiomas. Pediatr Neurosurg 45:451–455
Garnett MR, Puget S, Grill J, Sainte-Rose C (2007) Craniopharyngioma. Orphanet J Rare Dis 2:18
Steinbok P, Hukin J (2010) Intracystic treatments for craniopharyngioma. Neurosurg Focus 28(4):E13
Frank F, Fabrizi AP, Frank G, Fioravanti A (1995) Stereotactic management of craniopharyngiomas. Stereotactic Funct Neurosurg 65:176–183
Cavalheiro S, Di Rocco C, Valenzuela S, Dastoli PA, Tamburini G, Massimi L et al. (2010) Craniopharyngiomas: intratumoral chemotherapy with interferon-α: a multicenter preliminary study with 60 cases. Neurosurg Focus 28(4):E12
Bremer AM, Nuguyen TQ, Balsys R (1984) Theraputic benifits of combination chemotherapy with vincristine, BCNU and procarbazine on reccurent cystic craniopharyngioma. A case report. J Neurooncol 2:47–51
Lippens RJ, Rotevee JJ, Otten BJ, Merx H (1998) Chemotherapy with adriamycin (doxorubicin) and CCNU (lomustin) in four children with recurrent craniopharyngioma. Eur J paediatr Neurol 2(5):263–268
Lim S, Shahinian H, Maya MM, Yong W, Heaney AP (2006) Temozolomide: a novel treatment for pituitary carcinoma. Lancet Oncol 7:518–520
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zuhur, S.S., Müslüman, A.M., Tanık, C. et al. MGMT immunoexpression in adamantinomatous craniopharyngiomas. Pituitary 14, 323–327 (2011). https://doi.org/10.1007/s11102-011-0297-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-011-0297-0