Abstract
The expression of dopamine receptor subtypes has been reported in corticotroph adenomas, and this finding support the possibility for medical treatment of Cushing’s disease (CD) with dopamine agonists when conventional treatment has failed. The aim of this study was to evaluate the effectiveness of cabergoline (at doses of up 3 mg/week), alone or combined with relatively low doses of ketoconazole (up to 400 mg/day), in 12 patients with CD unsuccessfully treated by transsphenoidal surgery. After 6 months of cabergoline therapy, normalization of 24 h urinary free cortisol (UFC) levels occurred in three patients (25%) at doses ranging from 2–3 mg/week, whereas reductions ranging from 15.0 to 48.4% were found in the remaining. The addition of ketonocazole to the nine patients without an adequate response to cabergoline was able to normalize UFC excretion in six patients (66.7%) at doses of 200 mg/day (three patients), 300 mg/day (two patients) and 400 mg/day (one patient). In the remaining patients UFC levels did not normalize but a significant reduction ranging from to 44.4 to 51.7% was achieved. In two of the six responsive patients to combination therapy, the weekly dose of cabergoline could be later reduced from 3 to 2 mg. Our findings demonstrated that cabergoline monotherapy was able to reverse hypercortisolism in 25% of patients with CD unsuccessfully treated by surgery. Moreover, the addition of relatively low doses of ketoconazole led to normalization of UFC in about two-thirds of patients not achieving a full response to cabergoline.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cushing’s disease (CD), the most common form of endogenous Cushing’s syndrome, is caused by an ACTH-secreting pituitary tumor and is associated with increased morbidity and mortality particularly due to metabolic and cardiovascular complications [1–3]. The treatment of choice for CD is transsphenoidal surgery (TSS) which yields immediate disease remission in about 70% of patients but the late disease remission rate is around 50% [4, 5]. Pituitary irradiation [6] and bilateral adrenalectomy [7] are alternative treatment approaches but they can be associated with serious complications, such as hypopituitarism and Nelson’s syndrome, respectively [4, 5].
There is currently no medical therapy for CD that targets the pituitary adenoma. Pharmacotherapy, particularly ketoconazole (an adrenal blocking drug), is rather used before surgery or as a transient palliative treatment before definitive cure in patients submitted to pituitary radiotherapy [4, 8]. There are also few reports of successful primary therapy with ketoconazole in patients that refused or had contraindications to surgery [9, 10]. In the past successful treatment of CD was also sporadically reported with drugs that modulate pituitary ACTH release, such as the dopamine agonist (DA) bromocriptine, cyproheptadine, and sodium valproate [11–13]. Recently, it was shown that longterm therapy with cabergoline, a DA more potent than bromocriptine, at weekly doses of 1–7 mg normalized 24 h urinary free cortisol (UFC) excretion in eight out 20 (40%) patients with CD not cured by surgery [14]. The effectiveness of cabergoline in normalizing ACTH levels and in inducing tumor shrinkage has also been demonstrated in some cases of Nelson’s syndrome [15, 16]. Also recently, a multicenter phase II trial has shown normalization of UFC concentration in 17% of 29 patients with refractory or persistent CD after 15 days of treatment with pasireotide, a multireceptor ligand somatostatin analog [17].
The aim of this study was to evaluate the effectiveness of relatively low doses of cabergoline (up to 3 mg/week), alone or combined with ketoconazole, in patients with Cushing’s disease unsuccessfully treated by surgery.
Materials and methods
Patients
Twelve patients (eight women and four men; mean age, 42.8 ± 6.2; age range 34–52) with persistent CD after unsuccessful surgery were included in this prospective study. Patients were selected from outpatients of the Division of Endocrinology of Pernambuco Federal University and Pernambuco Center for Diabetes and Endocrinology, Recife, Brazil.
The diagnosis of CD was mainly based on (1) the confirmation of the hypercortisolism by the demonstration of at least two of the following findings: elevation of daily UFC concentration and/or midnight salivary cortisol levels (two or more times the upper limit of normal range) and failure of cortisol suppression to less than 1.8 μg/dl after low-dose dexamethasone suppression tests, (2) plasma ACTH greater than 20 pg/ml, (3) serum cortisol suppression greater than 50% after high-dose oral dexamethasone suppression test and/or ACTH increase greater than 35% after CRH or desmopressin stimulation tests, (4) evidence of a pituitary tumor at magnetic resonance imaging (MRI) of the pituitary gland or at the bilateral inferior petrosal sinus sampling [1–3, 18].
The diagnosis of the persistence of CD after surgery was based on the persistent detection of high UFC levels. The inclusion criteria for the study were persistence of CD after surgery and UFC excretion 1.5 times or more the upper limit of normal range. At the beginning of the study all patients included in the study had UFC levels more than 2.0 times higher than the upper limit of the normal range, varying from 226 to 991 μg/24 h (mean ± SD, 592.4 ± 224.6). All patients had normal prolactin (PRL) levels. Pituitary MRI revealed microadenomas in six of the 12 patients (50%) with a median diameter of 4.5 mm (range 3–6 mm) whereas in the remaining patients there was no evidence of tumor. Immunohystochemistry showed positive staining for ACTH only in all patients.
Study protocol and assays
The study protocol included the evaluation of the effectiveness of cabergoline in monotherapy or in association with ketoconazole in normalizing UFC levels. Cabergoline was administered at the initial dose of 1 mg/week (0.5 mg twice weekly), which was progressively increased by 1 mg every month until normalization of UFC levels or a maximal dose of 3 mg/week were reached (1.5 mg twice weekly at bedtime). In cases with persistent elevation of UFC after 6 months of cabergoline treatment, the therapy with ketoconazole was added. This drug was given at initial dose of 100 mg/day that was progressively increased by 100 mg every month until normalization of UFC levels or a maximal dose of 400 mg/day were reached (200 mg twice a day after meals).
Patients were assessed clinically and by hormone and routine chemistry determinations once a month. Patients who achieved UFC normalization were considered full responders to cabergoline, whereas those who achieved a 25% or more decrease without normalization of UFC levels were labeled as partial responders. Patients who achieved less than a 25% reduction of UFC levels were considered resistant to cabergoline.
Plasma ACTH and serum PRL were measured by a solid-phase two-site sequential chemiluminescent immunometric assay, whereas serum cortisol and UFC were measured by a solid-phase competitive chemiluminescent enzyme immunoassay. The normal range for UFC levels was 10–90 μg/day. Midnight salivary cortisol levels were assessed by radioimmunoassay or by an electrochemiluminescent assay.
The radiological study included the evaluation of the sellar region by MRI (Sigma LX GE, Milwaukee, WI), 1.5T, and gradient of 23 mT/m. The slices were axial, coronal, and sagittal in T1, pre- and post-gadolinium, and in T2. Furthermore, all patients underwent an echocardiography at study entry and every 6 months during the entire period of treatment in order to investigate the presence of a cardiac valve disease.
The study was performed in accordance with the declaration of Helsinki and was approved by the local ethics committee. All study participants provided informed consent before enrollment had been reached.
Statistical analysis
In the analysis of qualitative variables, the χ2 test or the Fisher exact test (when necessary) was used. The Student t-test was performed for the comparative analysis of quantitative variables. Results are expressed as percentages and mean values ± SD unless otherwise indicated. P values <0.05 were considered statistically significant.
Results
Responsiveness to treatment with cabergoline
Comparing the last value during cabergoline monotherapy with baseline (Table 1), mean ± SD UFC levels decreased from 637.8 ± 245.7 (range 226–991) μg/day to 434.1 ± 256.8 (range 82–791) μg/day (P < 0.001). Normalization of UFC levels occurred in three patients (25%) at cabergoline doses ranging from 2 to 3 mg/week (mean ± SD, 2.5 ± 0.5), whereas reductions ranging from 15.0 to 48.4% were found in the remaining. Three patients were labeled as resistant to cabergoline (UFC decrease <25%). In two patients, the normalization of UFC excretion was achieved at the dose of 2 mg/week (Table 1) (Fig. 1).
Regardless the UFC normalization, all patients reported improvement of clinical symptoms during the treatment with cabergoline.
Responsiveness to treatment with cabergoline and ketoconazole
The addition of ketonocazole, at doses ranging from 200 to 400 mg/day (mean ± SD, 325 ± 103.5), to the nine patients without a full response to cabergoline yielded a mean ± SD UFC levels decrease from 520.7 ± 156.1 (range 342–791) μg/day to 149.6 ± 124.1 (range 72–385) μg/day (P < 0.001). Normalization of UFC levels were achieved at doses of 200 mg/day in three patients, 300 mg/day in two patients and 400 mg/day in one patient. In the remaining patients UFC levels could not be normalized but a significant reduction ranging from to 44.4 to 51.7% was achieved (Table 2) (Fig. 2). As shown in Table 2, UFC levels in patients that did not respond to combination therapy was significantly higher than those of responsive patients: 746.3 ± 40.5 (range 712–791) μg/day versus 453 ± 109.7 (range 342–582) μg/day (P < 0.001). By contrast, there was no correlation between the response to treatment and PRL levels (normal in all patients) or the expression of PRL by immunohystochemistry (negative in all subjects). In two out of the six responsive patients to combined therapy, the weekly dose of CAB could be later reduced from 3 to 2 mg without elevation UFC levels beyond the upper limit of the normal range.
Overall, normalization of daily UFC excretion could be obtained in nine patients (75%), three (25%) submitted to cabergoline monotherapy and in six (66.7%) undergoing the combination of cabergoline with ketoconazole. The response was not infleuTreatment escape, manifested as relapse of hypercortisolism and elevation of UFC levels during the use of cabergoline alone or combination therapy, was not observed in any patient.
Tolerability
Cabergoline and ketoconazole were well tolerated and none of the patients had to interrupt the treatment. Transient moderate dizziness and/or nausea were reported by three patients treated with cabergoline. After the addition of ketoconazole, mild transient elevation of transaminases was found in one out of nine patients (11.1%). No patients developed symptoms of hypoglycemia while on cabergoline or combined therapy. Moreover, none of the patients experienced any significant symptom or sign related to cardiac disease; particularly, no patients developed cardiac valve regurgitation.
Discussion
Unlike prolactinomas and acromegaly, currently there is not an effective medication to treat Cushing’s disease that acts at the level of the pituitary gland inhibiting ACTH secretion by the corticotroph tumor [1, 2]. Some neuromodulatory drugs were used in the past, however, due to their limited efficacy, they never reached a widespread clinical use for the treatment of CD [4, 8, 10]. The most effective was the DA bromocriptine that was reported to induce a significant inhibition or normalization of cortisol secretion in up 40% of patients after short-term treatment [11, 19–21], but normalization of cortisol secretion was rarely maintained and tumor shrinkage was only sporadically found after long-term treatment [19, 22, 23].
Better results have been reported with the potent DA cabergoline, such as normalization of ACTH and/or cortisol secretion and/or significant tumor shrinkage in two ACTH-secreting pituitary tumors associated with Nelson’s syndrome [15, 16], a silent ACTH [24], an aberrant ACTH-secreting [25], and a mixed ACTH and PRL-secreting [26] pituitary tumors. Moreover, it was recently demonstrated a sustained control of cortisol secretion for 24 months during cabergoline treatment at doses ranging from 1 to 7 mg/week (median 3.5) in eight of 20 patients (40%) with CD unsuccessfully treated by surgery [14]. In this study, after short-term treatment, 15 out of 20 patients (75%) were responsive to cabergoline treatment [14].
The rationale for the use of cabergoline in CD was the demonstration that the dopamine receptor subtype 2 (D2) is expressed in approximately 80% of human corticotroph adenomas and that these adenomas can be responsive to the ACTH-inhibiting actions of D2-agonists in vitro [27, 28]. In other studies, this rate varied between 51 and 75% in initial responders (both complete and partial) observed in vivo with cabergoline monotherapy [14, 27, 29]. In the pituitary, the response to dopamine agonists is related to the activity of the D2 receptor [27]. Compared to bromocriptine, cabergoline has a higher specificity and affinity for the D2 receptor and a longer duration of action, and is much better tolerated [30]. All these factors explain the greater efficacy of cabergoline in the management of pituitary tumors [30].
It was also suggested in the early 1980s that some ACTH-secreting adenomas may originate not only from the anterior but also from the neurointermediate lobe, which is under hypothalamic dopaminergic control [31]. These patients would be therefore more likely to respond adequately to dopamine agonists. However, in the study by Croughs et al. [32] the response to bromocriptine was associated with hyperplastic anterior pituitary gland and not only with neurointermediate lobe hyperfunction. More recently, it was shown in six normal human pituitary glands that the expression of D2 receptors was more intense and more homogeneous in corticotrophs from the intermediate lobe than in those from the anterior lobe [33].
In the current study, among the 12 patients with CD that underwent treatment with cabergoline monotherapy, three (25%) achieved normalization of 24 h UFC levels whereas a reduction ranging from 44.4 to 85.2% was observed in the remaining. The lack of full response to this drug in 75% of our patients could be rather attributed to low expression or lack of expression of D2 receptors in tumor cells. Although we did not aim at correlating the response to the treatment to the expression of D2 receptors, it was shown in a previous study that the response to cabergoline was strongly correlated to the presence of these receptors in corticotrophs tumors cells. Other likely explanations would be downregulation of D2 receptor expression by high cortisol levels and inadequate dose of cabergoline. Indeed at a maximal dose of 3 mg/week fully responsive patients had an UFC increase of no more than 4.0 times higher than the upper limit of normal range. As mentioned, UFC normalization was found in 40% of patients from the series by Pivonelo et al. [14]. This better response might be perhaps attributed to the fact that 45% of these patients had mild hyperprolactinemia whereas prolactin levels were normal in all our patients. Moreover, the maximal dose of cabergoline used in that study was higher than in ours (7 versus 3 mg/week). It is therefore possible that we might have had better results if we had used higher cabergoline doses. However, in our protocol we decided that that maximal dose would be 3 mg/week as there is some evidence that higher doses may be associated with an increased risk of valve heart abnormalities [34].
We did not assess the effect of cabergoline on the tumor size as only half of the patients had an abnormal pituitary MRI that revealed small microadenomas. However, in the series by Pivonello et al. [14], tumor shrinkage was observed in four of the eight (50%) who were followed for 24 months, whereas a stable tumor volume was found in the remaining responsive patients.
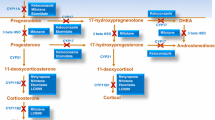
Ketoconazole, an imidazole derivative, is a potent inhibitor of gonadal and adrenal steroidogenesis [35]. It has been used at doses of 200–1,200 mg/day but, in most cases, 600–800 mg/day is required to keep UFC levels within the upper limits of normal, which should be the goal of therapy to avoid the risk of adrenal insufficiency [9, 10, 35]. Compared to lower doses, 600–1,200 mg/day implies a greater risk for endocrine side-effects, such as gynecomastia, impairement of testicular function, and adrenal insufficiency [10, 35].
Some patients undergoing longterm cabergoline or ketoconazole monotherapy may show relapse after having been in full remission [8, 10, 14, 29]. A treatment escape was observed in two of the seven (28.6%) full responders and three of the eight (37.5%) partial responders to cabergoline from the series by Pivonello et al. [14] after 12–18 months of treatment but in none of our patients that were given cabergoline alone or in combination. The mechanisms that cause these escapes from cabergoline monotherapy are not known. Receptor downregulation or various postreceptor desensitization mechanisms may be involved in this process [28]. Regardless of the cause, these treatment escapes may limit the efficacy of cabergoline monotherapy as a long-term treatment option for CD [28]. Adrenal stimulation by high ACTH levels is believed to be the main mechanism of escape during chronic ketoconazole therapy for CD [10, 35].
We also demonstrated that, among the nine unsuccessfully treated by cabergoline, UFC normalization was achieved in six of them (66.7%) after the addition of relatively low doses of ketoconazole (200–400 mg/day). The addition of ketoconazole also enabled reduction of cabergoline dose from 3 to 2 mg/week in two patients who had successfully responded to combination therapy. Similarly the three patients who did not respond to combined therapy had more severe hypercortisolism than patients who achieved UFC normalization.
There are only scant data in the literature regarding the benefits from the combined treatment with cabergoline and ketoconazole for CD [36, 37]. Recently Pivonello and coworkers [37] reported that the addition of ketoconazole at very low doses (50–200 mg/day) led to normalization of UFC excretion in all six patients who were partially responsive to cabergoline (3.5 mg/week). In our series, one-third of patients that were given ketoconazole had been shown to be resistant to cabergoline (UFC reduction <25%). These patients did not benefit from the combination therapy.
The rationale for the combined treatment would be the utilization of drugs with complementary pharmacologic mechanisms. Indeed, while the main action of cabergoline is inhibiting ACTH secretion by the corticotroph adenoma, ketoconazole blocks many steps of adrenal steroidogenesis, including the enzymes 17,20-lyase, 11β-hydroxylase and 17α-hydroxylase [8, 35]. The potential advantages of the combination of cabergoline with ketoconazole compared with either monotherapy would include a higher probability of longterm control of the hypercortisolism at lower doses, a lower incidence of side-effects and, possibly, a lower rate of treatment escapes.
In conclusion, our findings demonstrate that cabergoline may be helpful for patients with Cushing’s disease unsuccessfully treated by surgery. We could also observe that the addition of relatively low doses of ketoconazole (200–400 mg/day) led to normalization of 24 h urinary free cortisol levels in two-thirds of patients that were not full responders to cabergoline. However, further evidence is necessary from studies involving a larger number of patients to confirm the results of the present study.
References
Pivonello R, De Martino MC, De Leo M et al (2008) Cushing’s syndrome. Endocrinol Metab Clin North Am 37:135–149
Nieman LK, Biller BM, Findling JW et al (2008) The diagnosis of Cushing’s syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 93:1526–1540
Vilar L, Freitas MC, Faria M et al (2007) Pitfalls in the diagnosis of Cushing’s syndrome. Arq Brasil Endocrinol Metab 51:1207–1216
Biller BM, Grossman AB, Stewart PM et al (2008) Treatment of adrenocorticotropin-dependent Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab 93:2454–2462
Newell-Price J, Bertagna X, Grossman AB et al (2006) Cushing’s syndrome. Lancet 367:1605–1617
Vance ML (2009) Cushing’s disease: radiation therapy. Pituitary 12:11–14
Smith PW, Turza KC, Carter CO et al (2009) Bilateral adrenalectomy for refractory Cushing disease: a safe and definitive therapy. J Am Coll Surg 208:1059–1064
Dang CN, Trainer P (2007) Pharmacological management of Cushing’s syndrome: an update. Arq Bras Endocrinol Metabol 51:1339–1348
Moncet D, Morando DJ, Pitoia F et al (2007) Ketoconazole therapy: an efficacious alternative to achieve eucortisolism in patients with Cushing’s syndrome. Medicina (B Aires) 67:26–31
Sonino N, Boscaro M (1999) Medical therapy for Cushing’s disease. Endocrinol Metab Clin North Am 28:211–222
Lamberts SW, Klijn JG, De Quijada M et al (1980) The mechanism of the suppressive action of bromocriptine on adrenocorticotropin secretion in patients with Cushing’s disease and Nelson’s syndrome. J Clin Endocrinol Metab 51:307–311
Krieger DT, Amorosa L, Linick F (1975) Cyproheptadine-induced remission of Cushing’s disease. N Engl J Med 293:893–896
Colao A, Pivonello R, Tripodi FS et al (1997) Failure of long-term therapy with sodium valproate in Cushing’s disease. J Endocrinol Invest 20:387–392
Pivonello R, De Martino MC, Cappabianca P et al (2009) The medical treatment of Cushing’s disease: effectiveness of chronic treatment with the dopamine agonist cabergoline in patients unsuccessfully treated by surgery. J Clin Endocrinol Metab 94:223–230
Pivonello R, Faggiano A, Di Salle F et al (1999) Complete remission of Nelson’s syndrome after 1-year treatment with cabergoline. J Endocrinol Invest 22:860–886
Casulari LA, Naves LA, Mello PA et al (2004) Nelson’s syndrome: complete remission with cabergoline but not with bromocriptine or cyproheptadine treatment. Horm Res 62:300–305
Boscaro M, Ludlam WH, Atkinson B et al (2009) Treatment of pituitary-dependent Cushing’s disease with the multireceptor ligand somatostatin analog pasireotide (SOM230): a multicenter, phase II trial. J Clin Endocrinol Metab 94:115–122
Vilar L, Freitas MC, Naves LA et al (2008) The role of non-invasive dynamic tests in the diagnosis of Cushing’s syndrome. J Endocrinol Invest 31:1008–1013
Miller JW, Crapo L (1993) The medical treatment of Cushing’s syndrome. Endocr Rev 14:443–458
Lamberts SW, Birkenhager JC (1976) Bromocriptine in Nelson’s syndrome and Cushing’s disease. Lancet 2:811
Lamberts SW, Timmermans HA, De Jong FH, Birkenhager JC (1977) The role of dopaminergic depletion in the pathogenesis of Cushing’s disease and the possible consequences for medical therapy. Clin Endocrinol (Oxf) 7:185–193
Mercado-Asis LB, Yasuda K, Murayama M et al (1992) Beneficial effects of high daily dose bromocriptine treatment in Cushing’s disease. Endocrinol Jpn 39:385–395
Bevan JS, Webster J, Burke CW, Scanlon MF (1992) Dopamine agonists and pituitary tumor shrinkage. Endocr Rev 13:220–240
Petrossians P, Ronci N, Valdes-Socin H et al (2001) ACTH silent adenoma shrinking under cabergoline. Eur J Endocrinol 144:51–57
Miyoshi T, Otsuka F, Takeda M et al (2004) Effect of cabergoline treatment on Cushing’s disease caused by aberrant adrenocorticotropin-secreting macroadenoma. J Endocrinol Invest 27:1055–1059
Tsjoen G, Defeyter I, Van De Saffele J, Rubens R, Vandeweghe M (2002) Macroprolactinoma associated with Cushing’s disease, successfully treated with cabergoline. J Endocrinol Invest 25:172–175
Pivonello R, Ferone D, de Herder WW et al (2004) Dopamine receptor expression and function in corticotroph pituitary tumors. J Clin Endocrinol Metab 89:2452–2462
de Bruin C, Pereira AM, Feelders RA et al (2009) Coexpression of dopamine and somatostatin receptor subtypes in corticotroph adenomas. J Clin Endocrinol Metab 94:1118–1124
Godbout A, Manavela M, Danilowicz K et al. (2008) Long-term therapy with cabergoline in Cushing’s disease. Program of the 90th annual meeting of the endocrine society, San Francisco, CA, 2008 (p 2–130)
Colao A, Lombardi G, Annunziato L (2000) Cabergoline. Expert Opin Pharmacother 1:555–574
Lamberts SW, de Lange SA, Stefanko SZ (1982) Adrenocorticotropin-secreting pituitary adenomas originate from the anterior or the intermediate lobe in Cushing’s disease: differences in the regulation of hormone secretion. J Clin Endocrinol Metab 54:286–291
Croughs RJM, Koppeschaar HPF, Van’t Verlaat JW, McNicol AM (1989) Bromocriptine-responsive Cushing’s disease associated with anterior pituitary corticotroph hyperplasia or normal pituitary gland. J Clin Endocrinol Metab 68:495–498
Bricaire L, Brue T (2007) New medical treatments in Cushing’s disease. Ann Endocrinol (Paris) 68(Suppl 1):18–20
Colao A, Galderisi M, Di Sarno A et al (2008) Increased prevalence of tricuspid regurgitation in patients with prolactinomas chronically treated with cabergoline. J Clin Endocr Metab 93:3777–3784
Czepielewski MA, Rollin GAFS, Bruno OD, Vilar L (2009) Treatment of Cushing’s syndrome. In: Vilar L (ed) Clinical Endocrinology, 4th edn. Guanabara Koogan, Rio, pp 459–475
Colao A, Di Sarno A, Marzullo P et al (2000) New medical approaches in pituitary adenomas. Horm Res 53(Suppl 3):76–87
Pivonello R, De Leo M, De Martino MC et al. (2009) Effeectiveness and safety of combined therapy with low dose ketoconazole and cabergoline in patients with Cushing’s disease partially responsive to monotherapy with cabergoline. Program of the 11th international pituitary congress, Washington, DC, pp 36–40
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vilar, L., Naves, L.A., Azevedo, M.F. et al. Effectiveness of cabergoline in monotherapy and combined with ketoconazole in the management of Cushing’s disease. Pituitary 13, 123–129 (2010). https://doi.org/10.1007/s11102-009-0209-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-009-0209-8