Abstract
Objective
Acromegaly is a disease characterized by GH hypersecretion, and is typically caused by a pituitary somatotroph adenoma. The primary mode of therapy is surgery, and radiotherapy is utilized as an adjuvant strategy to treat persistent disease. The aim of this study was to determine the efficacy and tolerability of CyberKnife stereotactic radiosurgery in acromegaly.
Design
A retrospective review of biochemical and imaging data for subjects with acromegaly treated with CyberKnife stereotactic radiosurgery between 1998 and 2005 at Stanford University Hospital.
Patients
Nine patients with active acromegaly were treated with radiosurgery using the CyberKnife (CK).
Measurements
Biochemical response based on serum insulin-like growth factor-1 (IGF-1), anterior pituitary hormone function, and tumor size with MRI scans were analyzed.
Results
After a mean follow up of 25.4 months (range, 6–53 months), CK radiosurgery resulted in complete biochemical remission in 4 (44.4%) subjects, and in biochemical control with the concomitant use of a somatostatin analog in an additional subject. Smaller tumor size was predictive of treatment success: baseline tumor volume was 1.28 cc (± 0.81, SD) vs. 3.93 cc (± 1.54) in subjects with a normal IGF-1 vs. those with persistent, active disease, respectively (P = 0.02). The mean biologically effective dose (BED) was higher in subjects who achieved a normal IGF-1 vs. those with persistent, active disease, 172 Gy3 (±28) vs. 94 Gy3 (±17), respectively (P < 0.01). At least one new anterior pituitary hormone deficiency was observed after CK in 3 (33%) patients: two developed hypogonadism, and one developed panhypopituitarism.
Conclusions
CK radiosurgery may be a valuable adjuvant therapy for the management of acromegaly.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acromegaly is characterized by growth hormone (GH) hypersecretion and is caused, in the vast majority of patients, by a pituitary somatotroph adenoma. Acromegaly is associated with multiple medical co-morbidities which predispose to premature mortality [1]. To prevent the long-term medical consequences and mortality risk of acromegaly, it is critical to normalize the GH secretion and insulin-like growth factor-1 (IGF-1) level [2, 3]. Surgery is the primary therapy for acromegaly. Transsphenoidal surgery provides biochemical cure in up to 30–70% of subjects with pituitary macroadenomas, which occur in the majority of cases [4, 5]. Therefore, active acromegaly persists in a substantial number of patients following surgery. In these cases, additional therapeutic options must be considered.
Radiotherapy (RT) is frequently utilized as an adjuvant treatment of acromegaly; and is indicated for residual disease following surgery and in patients poorly tolerant to or unresponsive to medical therapy [6]. Studies using conventional RT for adjuvant treatment of acromegaly show efficacy rates ranging from 5% [7] to greater than 75% [8–10]. Even if biochemical control is attained, normalization of GH levels may take a decade or more. Moreover, conventional fractionated RT is associated with complications caused by local tissue exposure to radiation, including hypopituitarism, cranial nerve neuritis, visual-field defects, possible cognitive disturbances or increased cerebrovascular disorders, radiation-induced gliomas, and delayed brain necrosis [9, 21–30]. In summary, conventional RT is effective in only a subset of patients, and may cause significant short and long-term complications.
Stereotactic radiosurgery (SRS), delivery of highly focused radiation to stereotactically defined intracranial sites, may be more effective and more rapidly acting than conventional radiotherapy [11]. It is unclear whether radiosurgery reduces the risk of local tissue side effects that have been associated with conventional radiation. The CyberKnife (Accuray, Inc., Sunnyvale, CA), an image-guided frameless robotic radiosurgical device, delivers highly conformal radiation in one or more sessions with an application accuracy equal to that of conventional stereotactic frames [12, 13]. This study reviews the Stanford University experience with the CyberKnife (CK) in the management of acromegaly.
Methods
Patients
We retrospectively reviewed the medical records of patients with acromegaly treated with CK SRS at Stanford University Medical Center between February, 1998 and February, 2005. Study inclusion criteria were active acromegaly, as determined by clinical criteria and an elevated serum IGF-1 value using age and gender normalized ranges, and availability of biochemical and imaging data at least 6 months following radiosurgical treatment. This study was approved by the Institutional Review Board for Human Subjects at Stanford University.
Treatment intervention
The Cyberknife Robotic Radiosurgical System (Accuray, Sunnyvale, CA, USA) was used. In this frameless method, patients were immobilized supine on the Cyberknife treatment table with an Aquaplast mask (WFR/Aquaplast Corp., Wyckoff NJ, USA). A thin slice (1.25 mm) high-resolution computed tomogram (CT) was obtained with a GE Light Speed 8i Scanner (Milwaukee, WI, USA) after the administration of 125 cc of Omnipaque intravenous contrast (iohexol, 350 mg I/cc; Nycomed, Inc., Princeton NJ, USA). The acquired scan was transferred by network to the Cyberknife treatment planning workstation. Nonisocentric, interative inverse planning was performed to generate a radiosurgical treatment plan. Digitally Reconstructed Radiograms (DRRs) were constructed to allow real-time patient tracking during treatment. Radiosurgery was delivered as a single session (n = 5), two sessions (n = 3) or three sessions (n = 1) in this study. Given the range of marginal prescribed doses and number of sessions, the Biologically Effective Dose (BED), derived from the linear quadratic formula, was calculated [14–16]. Pituitary adenomas were assumed to react as late responding tissue, therefore an α/β ratio of 3 was used: BED (Gy3) = nd (1 + d/(α/β)), where n is equal to the number of fractions of dose, d.
Hormonal and radiographic evaluation
Clinic notes, medication charts, laboratory studies, and radiographic reports were reviewed. The primary outcome endpoint was normalization of serum IGF-1 levels at least 6 months following CK SRS. “Biochemical remission” was defined as a normal serum IGF-1 level, using a gender and age-standardized normal range without concomitant use of medical therapy, such as a dopamine agonist or somatostatin analog, for at least 12 weeks. Patients were considered “biochemically controlled” if a normal serum IGF-1 was attained only when medical therapy with a somatostatin analog was added. Active, residual disease was considered present if IGF-1 levels remained elevated despite medical therapy. Serum IGF-1 levels and anterior pituitary hormone function were assessed at variable times following CK, depending on local care. Hypopituitarism was defined as follows: gonadotroph deficiency in men by low plasma testosterone in the presence of low or inappropriately normal serum LH and FSH levels or the need for testosterone supplementation; gonadotroph deficiency in premenopausal women by amenorrhea and in menopausal women by low or normal LH and FSH; corticotroph deficiency by low morning cortisol (cortisol level < 5 mcg/ml), a subnormal response to cosyntropin (peak cortisol < 18 mcg/dl), or having initiated glucocorticoid replacement since CK treatment; thyrotroph deficiency by low free plasma T4 with normal or diminished plasma TSH, or having been placed on thyroid hormone since CK treatment; and, somatotroph deficiency by IGF-1 less than age or gender adjusted normal range or having been placed on recombinant human GH since CK treatment. Tumor size was evaluated on contrast MRI performed before and at variable times after CK treatment.
Statistical analysis
Data are shown as the mean ± SD, with P < 0.05 taken as statistically significant. Means were compared using an unpaired Student’s t-test. Logistic regression was used to analyze the effect of independent variables on IGF-1. Maximum likelihood logistic regression was used to avoid the need to assume that the distributions of the independent variables are discrete or continuous. This method instead assumes that the logarithm of the odds of belonging to one population is a linear function of the variables used for classification.
Results
Treatment group
Nine subjects with acromegaly, four men and five women, met the inclusion criteria (see Table 1). Ages ranged from 22 to 60 years (mean, 43.3 ± 11.6 years). Eight were treated with CyberKnife because of residual disease after transsphenoidal surgery. The other received a second CK treatment after failure of an initial CK treatment used as primary therapy. No patients had prior conventional external beam radiation therapy.
Treatment parameters
The prescribed margin dose ranged from 18–24 Gy (mean 21 Gy), and the BED ranged from 72 to 216 Gy3 (mean 137 ± 47 Gy3), administered over a range of one to three sessions depending on several factors, including tumor volume, distance to visual pathways, age, and previous treatment with radiotherapy (see Table 1). Three patients were receiving the somatostatin analog, octreotide, at the time of CK treatment.
Follow-up
The average duration of follow-up after CK therapy was 25.4 ± 14.0 months (range, 6–53 mo); Four patients were followed for less than 2 yrs, and the remainder were followed for 2–5 yrs.
Biochemical response
At last follow-up, four patients (44.4%) achieved biochemical remission (See Table 2). One additional subject achieved biochemical control, based on achievement of a normal serum IGF-1 with administration of the somatostatin analog, octreotide. Four patients (44.4%) had persistent, active disease following CK administration. The mean time to biochemical remission or control was 12 months (range, 3–19 mo), although follow-up IGF-1 levels were drawn at variable time points following CK, depending on local care. The mean duration of follow-up in patients with persistent, active disease was 17.8 months (range, 6–31 mo).
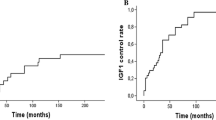
In a regression analysis, no significant differences in biochemical response were observed among patients differing with respect to gender, age, or the use of somatostatin analogs at the time of CK treatment. Of the three subjects receiving octreotide therapy at the time of CK, one was in biochemical remission after CK, and two had persistent, active disease. Achievement of biochemical remission or control was more likely with smaller tumors. The mean tumor size in subjects with IGF-1 normalization was smaller than that of subjects who had persistent, active disease at last follow-up: 1.28 cc (±0.81) vs. 3.93 cc (±1.54) (P = 0.02), respectively. The mean biologically effective dose (BED) was higher in patients achieving biochemical remission or control vs. those with persistent, active disease, 172 Gy3 (± 28) vs. 94 Gy3 (± 17), respectively (P < 0.01). A BED above or below 150 Gy3 separated subjects who attained IGF-1 normalization from those with persistent, active disease, respectively (see Fig. 1).
Radiographic response
MRI scan results were available for all patients at follow-up with a mean duration of 25.4 months (range, 6–53 mo). None showed tumor enlargement.
Adverse effects
At least one new pituitary deficiency was observed after CK in 3 (33%) patients: two developed new hypogonadism, and one developed new panhypopituitarism (See Table 2). Growth hormone deficiency was noted in one of the three subjects. Two of these three patients with a new pituitary deficiency had active disease at follow-up. At last follow-up, there were no reports of cranial nerve neuritis, visual field defects, stroke, a secondary malignancy, or brain necrosis.
Discussion
In acromegaly, radiation therapy (RT) is usually reserved for subjects who have failed surgery and/or medical therapy. Its utility, however, remains unclear and controversial [7, 17]. First, rates of biochemical cure following conventional, fractionated RT range widely from 5% [7] to greater than 75% [8–10]. In a recent study utilizing serum IGF-1 as the definition of biochemical cure, normal IGF-1 was achieved in 44% of 32 subjects who received conventional RT [18]. Several factors may explain the variable response rates to conventional RT: use of different levels of GH or IGF-1 to define biochemical remission, study attrition rates, different durations of follow-up, variation in surgical and medical therapy prior to RT, RT dose, and selection bias [10, 19]. Second, it can take many years (up to a decade or more) to achieve biochemical control following conventional RT [8, 10]. This is problematic, as persistently elevated GH levels may expose patients to a higher risk of morbidity and mortality [20]. Third, conventional RT is associated with significant side effects including hypopituitarism (in approximately 40% of subjects), cranial nerve neuritis, optic neuropathy, cerebrovascular disease, delayed brain necrosis with behavioral or cognitive sequelae, and secondary tumors [9, 21–30].
Stereotactic radiosurgery (SRS) enables precise delivery of large, highly conformal doses of radiation in one or more sessions. The smaller volume of normal brain irradiated and the shorter course of treatment are potential advantages of radiosurgery over conventional, fractionated RT. Radiosurgical devices include the conventional linear accelerator, proton accelerator, stereotactic multiple arc radiation therapy (SMART), Gamma Knife, and Cyber-Knife (CK) systems. Using conventional linear accelerator based radiosurgery with SMART to treat acromegaly not cured by conventional radiation, Swords et al. [31] demonstrated normalization of GH and IGF-1 in 58% of 13 subjects at a median of 25 months after treatment, without loss of anterior pituitary function. In a recent larger series of Gamma Knife SRS in both previously treated and naive acromegaly patients, 40% of 82 subjects were biochemically controlled at a mean follow-up duration of 49.5 months [32]. Factors such as gender, age, initial tumor volume, tumor location, marginal dose, or use of somatostatin analogs at the time of therapy did not influence biochemical outcome. In this study, 14 (17%) patients developed at least one new anterior pituitary deficiency, but the incidence of other side effects was rare [32]. Further studies using SRS (typically Gamma Knife) on smaller numbers of subjects have shown control rates ranging from 23 to 90% [33–37]. In addition, Landolt et al. [11] showed that 16 patients treated with adjuvant Gamma Knife radiosurgery achieved rates of biochemical remission comparable to those in 50 patients who received fractionated RT. Moreover, the mean time to remission was 1.5 years in patients after Gamma Knife therapy compared to 7.5 years in patients after conventional RT. These data suggest that single fraction SRS may be associated with a shorter time to biochemical remission, although other studies have not confirmed this finding [35, 36]. The cumulative conclusion from these studies is that SRS, using Gamma Knife or SMART, has equal or greater efficacy than conventional RT as an adjuvant treatment for GH-producing pituitary tumors.
In this preliminary study, we used the CK system for radiosurgery for acromegaly. An advantage of the CK system over other SRS methods is that real-time image guidance enables frameless treatment, thus avoiding the painful invasiveness of frame application. It also permits multi-session treatment of tumors whose proximity to the optic nerve precludes the single fraction radiosurgery to which the Gamma Knife is restricted. A treatment plan of limited fractionation (2–5 sessions) can combine the advantages of stereotactic delivery with those of fractionation in preventing damage to normal tissues.
In the only prior study of CK SRS for pituitary adenomas, two patients with acromegaly achieved remission based on normalization of GH [38]. In our study of 9 subjects, CK SRS resulted in biochemical remission in 44% of patients, and in biochemical control using concomitant medical therapy (somatostatin analog) in an additional patient. This suggests that CK SRS is an effective adjuvant for acromegalic patients with persistent, active disease following surgery or medical therapy. The rate of biochemical remission or control achieved is similar to that seen with other forms of SRS [31, 32, 34–36], and as recently well summarized by Sheehan et al. [39]. The mean time to serum IGF-1 normalization was 12 months. This suggests that CK, like other forms of SRS, may produce results more rapidly than conventional RT, though our study did not systematically address this issue. Furthermore, as with conventional radiotherapy, longer follow-up may reveal higher rates of remission or control. Although tumor size was not a primary endpoint of the study, it is noteworthy that no tumors enlarged after CK treatment.
The small number of subjects in this study precludes definitive identification of factors prognostic of outcome. Nonetheless, we found that subjects who achieved biochemical remission or control received higher BED than those with persistent, active disease. In this patient cohort, IGF-1 normalization was achieved in those subjects who received a BED of >150 Gy3. Though preliminary, this finding supports the notion that the highest dose that can be safely delivered should be used in this disorder. Whether or not a radioprotective effect is conferred by the use of somatostatin analogs at the time of RT is controversial [40]. In this limited study, we found no effect of simultaneous use of a somatostatin analog.
Another proposed benefit of SRS over conventional RT is a limited irradiation of surrounding tissues. The incidence of a new anterior pituitary hormone deficiency was 33% (3/9), comparable to that seen after other forms of radiosurgery [32]. With conventional RT, localized nervous tissue damage can occur several years after radiation, and may include cranial nerve neuritis, optic neuropathy, cerebrovascular disease, delayed brain necrosis with behavioral or cognitive sequelae, and secondary tumors [21–30]. In the present study using SRS, no patient reported symptoms that might be attributed to damage to local nerve tissue at a mean follow up of 25.4 months. We recognize that the length of follow-up in this study is insufficient to detect these complications. Although SRS may limit local tissue exposure to radiation, further studies are necessary to determine the long-term risk to localized tissue following radiosurgery.
In summary, CK SRS is an efficacious and well-tolerated adjuvant treatment for acromegaly. Larger prospective studies are necessary to define more clearly the long term efficacy and safety of CK radiosurgery and to identify prognostic factors that may guide its use.
References
Katznelson L (2005) Diagnosis and treatment of acromegaly. Growth Horm IGF Res 15(Suppl A):S31–35
Abosch A, Tyrrell JB, Lamborn KR, Hannegan LT, Applebury CB, Wilson CB (1998) Transsphenoidal microsurgery for growth hormone-secreting pituitary adenomas: initial outcome and long-term results. J Clin Endocrinol Metab 83:3411–3418
Swearingen B, Barker FG 2nd, Katznelson L, Biller BM, Grinspoon S, Klibanski A, Moayeri N, Black PM, Zervas NT (1998) Long-term mortality after transsphenoidal surgery and adjunctive therapy for acromegaly. J Clin Endocrinol Metab 83:3419–3426
Ahmed S, Elsheikh M, Stratton IM, Page RC, Adams CB, Wass JA (1999) Outcome of transphenoidal surgery for acromegaly and its relationship to surgical experience. Clin Endocrinol (Oxf) 50:561–567
Shimon I, Cohen ZR, Ram Z, Hadani M (2001) Transsphenoidal surgery for acromegaly: endocrinological follow-up of 98 patients. Neurosurgery 48:1239–1243; discussion 1244–1245
AACE Medical Guidelines for Clinical Practice for the diagnosis and treatment of acromegaly. Endocr Pract (2004) 10:213–225
Barkan AL, Halasz I, Dornfeld KJ, Jaffe CA, Friberg RD, Chandler WF, Sandler HM (1997) Pituitary irradiation is ineffective in normalizing plasma insulin-like growth factor I in patients with acromegaly. J Clin Endocrinol Metab 82:3187–3191
Barkan AL (2003) Radiotherapy in acromegaly: the argument against. Clin Endocrinol (Oxf) 58:132–135
Barrande G, Pittino-Lungo M, Coste J, Ponvert D, Bertagna X, Luton JP, Bertherat J (2000) Hormonal and metabolic effects of radiotherapy in acromegaly: long-term results in 128 patients followed in a single center. J Clin Endocrinol Metab 85:3779–3785
Biermasz NR, van Dulken H, Roelfsema F (2000) Long-term follow-up results of postoperative radiotherapy in 36 patients with acromegaly. J Clin Endocrinol Metab 85:2476–2482
Landolt AM, Haller D, Lomax N, Scheib S, Schubiger O, Siegfried J, Wellis G (1998) Stereotactic radiosurgery for recurrent surgically treated acromegaly: comparison with fractionated radiotherapy. J Neurosurg 88:1002–1008
Adler JR Jr, Murphy MJ, Chang SD, Hancock SL (1999) Image-guided robotic radiosurgery. Neurosurgery 44:1299–1306; discussion 1306–1307
Chang SD, Main W, Martin DP, Gibbs IC, Heilbrun MP (2003) An analysis of the accuracy of the CyberKnife: a robotic frameless stereotactic radiosurgical system. Neurosurgery 52:140–146; discussion 146–147
Fowler JF (1992) Brief summary of radiobiological principles in fractionated radiotherapy. Semin Radiat Oncol 2:16–21
Hall EJ, Brenner DJ (1993) The radiobiology of radiosurgery: rationale for different treatment regimes for AVMs and malignancies. Int J Radiat Oncol Biol Phys 25:381–385
Shrieve DC, Klish M, Wendland MM, Watson GA (2004) Basic principles of radiobiology, radiotherapy, and radiosurgery. Neurosurg Clin N Am 15:467–479
Cozzi R, Barausse M, Asnaghi D, Dallabonzana D, Lodrini S, Attanasio R (2001) Failure of radiotherapy in acromegaly. Eur J Endocrinol 145:717–726
Powell JS, Wardlaw SL, Post KD, Freda PU (2000) Outcome of radiotherapy for acromegaly using normalization of insulin-like growth factor I to define cure. J Clin Endocrinol Metab 85:2068–2071
Melmed S, Ho K, Klibanski A, Reichlin S, Thorner M (1995) Clinical review 75: recent advances in pathogenesis, diagnosis, and management of acromegaly. J Clin Endocrinol Metab 80:3395–3402
Rajasoorya C, Holdaway IM, Wrightson P, Scott DJ, Ibbertson HK (1994) Determinants of clinical outcome and survival in acromegaly. Clin Endocrinol (Oxf) 41:95–102
Atkinson AB, Allen IV, Gordon DS, Hadden DR, Maguire CJ, Trimble ER, Lyons AR (1979) Progressive visual failure in acromegaly following external pituitary irradiation. Clin Endocrinol (Oxf) 10:469–479
Brada M, Ford D, Ashley S, Bliss JM, Crowley S, Mason M, Rajan B, Traish D (1992) Risk of second brain tumour after conservative surgery and radiotherapy for pituitary adenoma. BMJ 304:1343–1346
Brada M, Rajan B, Traish D, Ashley S, Holmes-Sellors PJ, Nussey S, Uttley D (1993) The long-term efficacy of conservative surgery and radiotherapy in the control of pituitary adenomas. Clin Endocrinol (Oxf) 38:571–578
Gittoes NJ, Bates AS, Tse W, Bullivant B, Sheppard MC, Clayton RN, Stewart PM (1998) Radiotherapy for non-function pituitary tumours. Clin Endocrinol (Oxf) 48:331–337
Littley MD, Shalet SM, Beardwell CG, Ahmed SR, Applegate G, Sutton ML (1989) Hypopituitarism following external radiotherapy for pituitary tumours in adults. Q J Med 70:145–160
McCollough WM, Marcus RB Jr, Rhoton AL Jr, Ballinger WE, Million RR (1991) Long-term follow-up of radiotherapy for pituitary adenoma:the absence of late recurrence after greater than or equal to 4500 cGy. Int J Radiat Oncol Biol Phys 21:607–614
McCord MW, Buatti JM, Fennell EM, Mendenhall WM, Marcus RB Jr, Rhoton AL, Grant MB, Friedman WA (1997) Radiotherapy for pituitary adenoma: long-term outcome and sequelae. Int J Radiat Oncol Biol Phys 39:437–444
Simmons NE, Laws ER Jr (1998) Glioma occurrence after sellar irradiation: case report and review. Neurosurgery 42:172–178
Tsang RW, Brierley JD, Panzarella T, Gospodarowicz MK, Sutcliffe SB, Simpson WJ (1994) Radiation therapy for pituitary adenoma: treatment outcome and prognostic factors. Int J Radiat Oncol Biol Phys 30:557–565
Zierhut D, Flentje M, Adolph J, Erdmann J, Raue F, Wannenmacher M (1995) External radiotherapy of pituitary adenomas. Int J Radiat Oncol Biol Phys 33:307–314
Swords FM, Allan CA, Plowman PN, Sibtain A, Evanson J, Chew SL, Grossman AB, Besser GM, Monson JP (2003) Stereotactic radiosurgery XVI: a treatment for previously irradiated pituitary adenomas. J Clin Endocrinol Metab 88:5334–5340
Castinetti F, Taieb D, Kuhn JM, Chanson P, Tamura M, Jaquet P, Conte-Devolx B, Regis J, Dufour H, Brue T (2005) Outcome of gamma knife radiosurgery in 82 patients with acromegaly:correlation with initial hypersecretion. J Clin Endocrinol Metab 90:4483–4488
Attanasio R, Epaminonda P, Motti E, Giugni E, Ventrella L, Cozzi R, Farabola M, Loli P, Beck-Peccoz P, Arosio M (2003) Gamma-knife radiosurgery in acromegaly: a 4-year follow-up study. J Clin Endocrinol Metab 88:3105–3112
Gutt B, Wowra B, Alexandrov R, Uhl E, Schaaf L, Stalla GK, Schopohl J (2005) Gamma-knife surgery is effective in normalising plasma insulin-like growth factor I in patients with acromegaly. Exp Clin Endocrinol Diabetes 113:219–224
Motti ED, Losa M, Pieralli S, Zecchinelli A, Longobardi B, Giugni E, Ventrella L (1996) Stereotactic radiosurgery of pituitary adenomas. Metabolism 45:111–114
Pollock BE, Kondziolka D, Lunsford LD, Flickinger JC (1994) Stereotactic radiosurgery for pituitary adenomas: imaging, visual and endocrine results. Acta Neurochir. Suppl 62:33–38
Zhang N, Pan L, Wang EM, Dai JZ, Wang BJ, Cai PW (2000) Radiosurgery for growth hormone-producing pituitary adenomas. J Neurosurg 93(Suppl 3):6–9
Kajiwara K, Saito K, Yoshikawa K, Kato S, Akimura T, Nomura S, Ishihara H, Suzuki M (2005) Image-guided stereotactic radiosurgery with the CyberKnife for pituitary adenomas. Minim Invasive Neurosurg 48:91–96
Sheehan JP, Niranjan A, Sheehan JM, Jane JA Jr, Laws ER, Kondziolka D, Flickinger J, Landolt AM, Loeffler JS, Lunsford LD (2005) Stereotactic radiosurgery for pituitary adenomas: an intermediate review of its safety, efficacy, and role in the neurosurgical treatment armamentarium. J Neurosurg 102:678–691
Landolt AM, Haller D, Lomax N, Scheib S, Schubiger O, Siegfried J, Wellis G (2000) Octreotide may act as a radioprotective agent in acromegaly. J Clin Endocrinol Metab 85:1287–1289
Acknowledgements
We thank Lynn Remedios for her assistance with the statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s11102-007-0024-z.
Rights and permissions
About this article
Cite this article
Roberts, B.K., Ouyang, D.L., Lad, S.P. et al. Efficacy and safety of CyberKnife radiosurgery for acromegaly. Pituitary 10, 19–25 (2007). https://doi.org/10.1007/s11102-007-0004-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-007-0004-3