Abstract
The genus Selaginella belongs to the family Selaginellaceae, which is well known for its unique structural classes of natural products and their wide range of biological effects. This review provides a comprehensive list of unique secondary metabolites isolated from the genus Selaginella and also provides insight into their important biological activities. For the benefit of the readers, this review is divided into two main sections. The first section elaborates on the natural products isolated from Selaginella, with the emphasis on compounds exclusive to this genus, while the second section provides an in-depth discussion of a number of different pharmacological activities that the compounds and Selaginella extracts exhibited.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selaginella is the only surviving genus in the family Selaginellaceae, and contains approximately 700 species (Yang et al. 2012; Bieski et al. 2015; Nguyen et al. 2015a, b; Woo et al. 2019). Also known as spike moss, it is globally distributed, mostly in tropical and sub-tropical regions (Nguyen et al. 2015a, b). Numerous Selaginella species are used in traditional medicines for the treatment of various diseases including inflammation, dysmenorrhea, chronic hepatitis and hyperglycemia (Nguyen et al. 2015a, b; Le et al. 2017; Zhang et al. 2017). About 50 of them are found in China and of these, 20 species have been reported in Chinese folk medicines (Yang et al. 2012). S. tamariscina (Beauv.) and S. pulvinata are two species reported in the Chinese Pharmacopoeia as Juanbai and are used as traditional herbs (Zhang et al. 2020). S. tamariscina (Beauv) and S. pulvinata Maxim (Hook. et Grev.) have been used in Traditional Chinese Medicine for the treatment of amenorrhea, dysmenorrhea, metrorrhagia, abdominal lumps in women and used in folk medicine as an anticancer, chronic hepatitis, anti-inflammatory, and antidiabetic agent and also to improve blood circulation (Liu et al. 2014; He et al. 2019). This genus has been extensively studied for the discovery of bioactive secondary metabolites, and a variety of molecules unique to Selaginella have been reported (Yang et al. 2012; Woo et al. 2019). Natural products specific to Selaginella include selaginellins and selaginellin analogues, which are pigments with a unique para-quinone methide and alkynylphenol carbon skeleton (Yang et al. 2012; Liu et al. 2018a, b), and selaginpulvilins, which possesses an unprecedented 9,9-diphenyl-1-(phenylethynyl)-9H-fluorene skeleton (Liu et al. 2014). The colour of these compounds changes from red to pink (pH 1.0 to 1.5) and red to purple (pH 7.5 to 8.0) which is attributed to their unique structural features and tautomerization (Zhang et al. 2007; Kim et al. 2015). Other well-known structural classes, such as flavonoids and lignans, have also been commonly isolated from Selaginella.
For the purpose of this review, the chemical structures of compounds that have only been isolated from the genus Selaginella for the first time are shown here. Structures of the other well-known compounds are shown in the Supplementary Information. This review has been divided into two main sections: Firstly, natural products isolated from Selaginella and secondly, biological activities of pure compounds or extracts from Selaginella. The first section has been subdivided into different structural classes, which include: selaginellins, selaginpulvilins, biflavonoids, flavonoids, diterpenes, alkaloids, lignans and neolignans and saponins. Together with the unique secondary metabolites, the biological activities of these compounds are also briefly discussed in this section. The pharmacological activities of compounds or extracts obtained from Selaginella are detailed in the following section. This section has also been further subdivided into various biological activities, including anticancer, antimicrobial, neuroprotective, and immunomodulation activities. Due to a large number of publications relating to anticancer activities, an in-depth overview in this area has been provided. To make the biological activity data concise for our readers, we have summarized this information in various tables. The main goal of this review is to provide a comprehensive analysis of the structural diversity, phytochemistry, and pharmacological activities of the unique secondary metabolites isolated from the genus Selaginella.
Natural products isolated from the genus Selaginella
The structures of all the compounds mentioned below were determined by NMR and MS data analyses, while the absolute configuration was determined by experimental and/or calculated ECD analysis. In cases where crystals were obtained, single-crystal X-ray diffraction data analysis was used to confirm the absolute configuration. Due to the expansion in the chemical and biological investigation of Selaginella, an increasing number of new compounds are isolated and reported frequently, which in some instances has caused “coincidental simultaneous assignment” (Ramabharathi and Schuehly 2014) of the same trivial names to distinct compounds by independent groups. The compounds in question are specifically selaginellins P, Q, T, and U and selaginpulvilins E and J. Therefore, to avoid further confusion, each of these compounds and their biological activities will be discussed separately.
Selaginellins
The first member of this group, selaginellin (1) (Fig. 1), was identified from the acetone extract of Selaginella sinensis after chromatographic purification (Zhang et al. 2007). The methoxy derivative (1a), a racemate of compound 1, was synthesized, crystallized and X-ray crystallographic analysis was utilized for its structure determination (Zhang et al. 2007). After the isolation of selaginellin, a number of selaginellin analogues were isolated, characterized and screened for their biological potential across a range of assays as discussed below.
Two unique pigments, namely selaginellins A (2) and B (3), together with selaginellin, were isolated from the Chinese herb, S. tamariscina (Cheng et al. 2008). The ability of compounds 1–3 to inhibit soluble epoxide hydrolase (sEH) was evaluated for their potential use in the treatment of cardiovascular diseases (Kim et al. 2015), exhibiting low IC50 values of 4.2 ± 0.2, 3.1 ± 0.1, 8.2 ± 2.2 µM respectively. This indicates that these compounds can be developed as potential inhibitors (Kim et al. 2015). Selaginellin C (4) was isolated as a brown powder along with selaginellin and selaginellin A from S. pulvinata (Hook. et Grev.) Maxim (Tan et al. 2009). The biological activity of compound 4 was not reported in the original article. Three new selaginellin derivatives, selaginellin D–F (5–7), were isolated from the ethyl acetate extract of S. pulvinata (Hook. et Grev.) Maxim (Cao et al. 2010a, b). Selaginellin E was obtained as an inseparable mixture with the ratio of 1:0.95 (6a:6b) and was believed to be a chromatographic artefact as this compound was not detected in the original extract. The structural elucidation of selaginellin F (7) proved rather challenging due to the limited amount isolated and an overlap of NMR signals, however following extensive NMR analysis in different deuterated solvents, the structure was confirmed to be (R,S)-4-[(40-hydroxy-2-[(4-hydroxylphenyl)(4-oxocyclo-hexa-2,5-dienylidene)methyl]-3-[(4-hydroxy-phenyl)eth-ynyl)biphenyl-4-yl]methoxy]benzoic acid (Cao et al. 2010a, b).
Selaginellin G (8) and H (9) isolated from S. pulvinata, exhibited good antifungal activity against C. albicans with an IC50 value of 5.3 µg/mL (Cao et al. 2010a, b). Selaginellins I–J (10–11) (Xu et al. 2011a, b) and K–L (12–13) (Xu et al. 2011a, b) were isolated from an ethanolic extract of S. tamariscina (Xu et al. 2011a, b). The biological activities of these compounds were not reported (Xu et al. 2011a, b). Two new selaginellin derivatives, selaginellins M (14) and N (15), together with the known compounds, selaginellin (1), selaginellins A (2) and C (4) were purified from the 50% ethanolic extract of S. tamariscina (Zhang et al. 2012). The cytotoxic activities of compounds 14 and 15 together with selaginellin and selaginellin A were tested in vitro against U251 (human glioma), HeLa (human cervical carcinoma), and MCF-7 (human breast cancer) cells. Compound 14 exhibited inhibitory activity against all the cell lines tested, while compound 15 was only active against the MCF-7 cell line (Zhang et al. 2012).
During a phytochemical investigation, one new selaginellin derivative, named selaginellin O (16) and three known selaginellins; selaginellin (1), selaginellin A (2), and selaginellin M (14), were isolated from S. tamariscina (Beauv.) Spring (Yang et al. 2012). Selaginellin O (16), together with selaginellin M (14) and selaginellin, were screened for their cytotoxic activities against HeLa cells. All the compounds exhibited moderate cytotoxic activity, with selaginellin O (16) exhibiting the most significant inhibitory activity. Its IC50 value of 26.4 μM suggests that the formyl group at C-10 might be important for its activity (Yang et al. 2012). A novel selaginellin-type isoquinoline alkaloid, selaginisoquinoline A (17), together with three ethoxy selaginellins P–R (18–20) were reported from the ethanolic extract of S. pulvinata (Cao et al. 2015). Compound 17 was isolated as a racemic mixture. To obtain biological data, compounds 17–20 were screened for cytotoxicity against human alveolar basal epithelial, gastric and hepatocellular adenocarcinoma cells (BGC-823, SMMC-7721 and A549). All these compounds exhibited moderate cytotoxic activity. Furthermore, the compounds exhibited good anti-fungal activities against C. albicans, Aspergillus fumigatus, Trichophyton rubrum, and T. mentagrophytes, with IC50 values of 4–32 µg/mL (Cao et al. 2015). Another article published in the same year (2015) reported the isolation and characterization of two new selaginellin derivatives from the Chinese herb S. tamariscina (Beauv.) which was coincidentally also named selaginellins P (21) and Q (22) (Xu et al. 2015). However, as can be noted from the chemical structures, these compounds are very distinct from 18 and 19, which were also given the trivial names selaginellin P and Q. No biological data was reported for compounds 21 and 22. Selaginellin S (23) (Fig. 2) was isolated from S. moellendorffii Hieron (Zhu et al. 2016). When applied to a human hepatoblastoma cell line (HepG2.2.15), selaginellin S inhibited the production of hepatitis B surface antigen (HbsAg) (IC50 0.026 µg/mL) and another hepatitis B virus (HBV) protein HBeAg (IC50 0.032 µg/mL) after 48 h. Selaginellin S also significantly reduced the intracellular and released HBV-DNA concentrations (Zhu et al. 2016).
To avoid confusion, it is important to mention that three independent articles were published on the isolation of new natural products that were simultaneously given the trivial names selaginellin T and two of them selaginellin U. Selaginellin T (24) and a novel example of naturally occurring triarylbenzophenone analogue, selagibenzophenone A (25), were isolated from the Chinese plant S. pulvinata (Liu et al. 2018a, b). The isolated compounds were evaluated for phosphodiesterase-4 (PDE4) inhibitory effects, and the results showed that compound 25 possessed good inhibitory activity, with an IC50 value of 1.04 ± 0.07 µM, while compound 24 exhibited moderate activity, with an IC50 value of 9.35 ± 0.60 µM (Liu et al. 2018a, b).
Furthermore, in 2017, Woo et al. described the isolation of four new selaginellins, T–W (26–29) from S. tamariscina (Beauv (Le et al. 2017). Selaginellin T (26), structurally different from 24, exhibited moderate inhibitory activity on protein-tyrosine phosphatase 1B (PTP1B) enzyme, with an IC50 value of 57.9 µM, while selaginellins U–V (27–29) exhibited significantly better activity, with IC50 values ranging from 4.8–15.9 µM, further suggesting that this class of compounds has the potential to inhibit the activity of the PTP1B enzyme (Le et al. 2017). Together with eleven known selaginellin derivatives (selaginpulvilin A–E, selaginellin, selaginellin A, B, G, M and O), two new lactone-containing selaginellins were also coincidentally given the trivial names selaginellins T and U (30) which were isolated from S. tamariscina (Zhu et al. 2017). Interestingly, selaginellin T from this report was structurally the same as compound 24, while selaginellin U (30) was very distinct from compound 27. Selaginellin U (30), which possessed a rare unsaturated δ-lactone ring attached to ring A, was a new structural type among selaginellins. Due to complications during structure elucidation, biomimetic semi-synthesis was carried out from the trimethyl ether of selaginellin not only to determine the structure of selaginellin U (30), but also to establish a plausible biogenetic pathway for both compounds (Zhu et al. 2017). Compound 30 was fully methylated using MeI/K2CO3 to yield 31 and by comparing the NMR data, the structure of 30 was established.
Three new selaginellin derivatives, selariscinin A–C (32–34), together with the known compounds, selaginellin and 10-methoxylated selaginellin M, were isolated from the methanolic extract of S. tamariscina while searching for new antidiabetic agents (Nguyen et al. 2015a, b). These compounds exhibited a significant stimulatory effect on glucose uptake when tested using 2-NBDG (a fluorescent tracer used for monitoring glucose uptake into living cells) in 3T3-L1 adipocyte cells at 5 µM. The inhibitory effect on the PTP1B enzyme of these compounds was also examined. Compound 33 exhibited a comparable inhibitory effect (IC50 value of 4.6 ± 0.1 μM) to the positive control, ursolic acid (IC50 value of 3.5 ± 0.1 μM). The authors suggested that these analogues might have the potential to be developed as an insulin mimetic for antidiabetic agents (Nguyen et al. 2015a, b).
Bioassay-guided isolation of methanol extract of S. tamariscina that exhibited a stimulatory effect on glucose uptake in 3T3-L1 adipocyte cells led to the isolation of two new compounds, selariscinin D (35) and E (36, is a bioflavonoid, Fig. 5), along with five previously reported bioflavonoids (Nguyen et al. 2015a, b). The stimulatory effect of all the isolated compounds were further evaluated on glucose uptake using 2-NBDG in 3T3-L1 adipocyte cells, where the results indicated that compound 35 significantly stimulated 2-NBDG uptake by a 1.24 ± 0.05 fold induction as compared to the DMSO control (Nguyen et al. 2015a, b). Diselaginellins A (37) and B (38) (Fig. 3), an ether-linked dimeric selaginellin derivative, together with selaginellin and selaginellin B, were isolated from the ethanol extract of S. pulvinata (Cao et al. 2017). Diselaginellin B was found to be moderately cytotoxic with an IC50 value of 9.0 µM and antimetastatic towards SMMC-7721cell line (Cao et al. 2017). Selaginones A (39) and B (40) represent the first example of aryl-substituted anthraquinone derivatives isolated from S. tamariscina (Beauv.) Spring (Liu et al. 2018a, b). Selagibenzophenone B (41), a triarylbenzophenone, was also isolated during this study (Liu et al. 2018a, b). Selaginones A, B and selagibenzophenone B were evaluated for their cytotoxic activity against human hepatocellular adenocarcinoma cell lines (HCC, SMMC-7721 and MHCC97-H). Selagibenzophenone B exhibited moderate cytotoxicity with the IC50 values of 39.8 and 51.5 µM, respectively while selaginones A and B were not active (Liu et al. 2018a, b).
Selaginpulvilins
Selaginpulvilins (Fig. 4) are a class of natural products unique to the genus Selaginella which possess an unprecedented 9,9-diphenyl-1-(phenylethynyl)-9H-fluorene skeleton (Liu et al. 2014). To date, approximately 20 selaginpulvilins have been isolated (Liu et al. 2014; Huang et al. 2017; Yao et al. 2017a, b; Zhang et al. 2017; Woo et al. 2019). First four members of the selaginpulvilin series, selaginpulvilins A–D (42–45), together with four known selaginellins (selaginellin, selaginellin A, N and H) were isolated from S. pulvinata. Spectroscopic analysis was utilized to elucidate the structures of the new compounds while single-crystal X-ray diffraction was used to confirm the structure and absolute configuration of selaginpulvilin A (Liu et al. 2014). All the compounds were evaluated for their ability to inhibit phosphodiesterase-4 (PDE4D2) and exhibited noteworthy activity, with IC50 values ranging from 0.11 to 5.13 µM. Rolipram was used as a positive control, with an IC50 value of 0.54 µM. Selaginpulvilin B (43) exhibited the strongest activity, which was fivefold higher than the positive control. The unique skeleton of selaginpulvilins makes them a potential candidate for the development of PDE4 inhibitors (Liu et al. 2014). Selaginpulvilins E–J (46–51) were isolated during a large-scale chemical investigation from S. pulvinata (Zhang et al. 2017). Selaginpulvilin E possessed a unique 6-(4-hydroxyphenyl)-2H-pyran-2-one moiety (Zhang et al. 2017). Selaginpulvilins E–J were also evaluated for their ability to inhibit PDE4D2 and exhibited significant inhibitory activities, with IC50 values ranging from 0.22 to 1.38 µM (Zhang et al. 2017). Total synthesis of selaginpulvilins A–J (42–51) was also reported in this study (Zhang et al. 2017).
The issue of trivial names appeared yet again with regards to the structures of selaginpulvilin E and J (Yao et al. 2017a, b; Zhang et al. 2017). Zhang and co-workers described six new analogues of selaginpulvilins (E–J) (46–51) (Zhang et al. 2017), while in a simultaneous publication, Yao and co-workers reported a new derivative that was also named selaginpulvilin E (52) along with selagintamarlin A (53) (Yao et al. 2017a, b). However, selaginpulvin E (52) from Yao’s study was structurally identical to compound 51, which was given the trivial name selaginpulvin J (Zhang et al. 2017). Compound 53 was described as a novel selaginellin derivative with a unique 1H-2-benzopyran unit. Along with selaginpulvilin E (52) (again structurally different but given the same sequential trivial name as compound 46), eight known selaginellin (selaginpulvilins A–D, selaginellin A, B, E and O) analogues were also isolated from S. tamariscina (Yao et al. 2017a, b). A plausible biosynthetic pathway for selagintamarlin A was also proposed with selaginellin O being the most likely biosynthetic precursor. Selaginellin O would undergo oxidation and hydration followed by ring closure to form a pyran ring, thus giving selagintamarlin A. All the isolates were tested for their inhibitory activity against PDE4D2 and exhibited potent inhibitory activity (IC50 values of 40–1680 nM), with selagintamarlin A exhibiting the strongest activity (IC50 value of 40 nM), which was 20-fold higher than the positive control, rolipram (IC50 = 850 nM) (Yao et al. 2017a, b).
Selaginpulvilins K (54) and L (55), fluorene derivative, were isolated from the ethanolic extract of S. pulvinata (Yao et al. 2017a, b). Compounds 54 and 55 exhibited high inhibitory activity against PDE4D2 with IC50 values of 11 and 90 nM, respectively. To identify the binding site, selaginpulvilin K (54) was crystallized with the catalytic domain of PDE4D2 and the crystal structure of the complex was determined by the X-ray diffraction method. The co-crystal structure identified an unusual binding mode and showed that selaginpulvilin K bound weakly to the active site and also provided 54 with the ability to interact in multi-sub pockets in the catalytic domain, resulting in high inhibitory potency (Yao et al. 2017a, b). MS/MS molecular networking technique was utilized to prioritize unknown selaginellin analogues from extracts and fractions of S. tamariscina. This molecular networking technique led to the isolation of eight new diarylfluorene derivatives: selaginpulvilins M–T (56–63) (Woo et al. 2019). Two unusual 1H,3H-dibenzo[de,h]isochromene derivatives were also isolated, selariscins A (64) and B (65). Their PDE4 inhibitory activities were studied, together with the binding modes of the analogues. Of the compounds tested, 56–64 exhibited an inhibitory effect, with IC50 values ranging from 2.8 to 33.8 µM, while the positive control, rolipram, exhibited an IC50 of 0.2 µM. Molecular docking studies were also conducted to determine the interactions between the selaginellin derivatives and PDE4D2 (Woo et al. 2019). A selaginellin analogue designated Isoselagintamarlin A (66), which possessed a rare benzofuran unit, was isolated from a 70% ethanol extract of S. tamariscina. Isoselagintamarlin A was also biomimetically synthesized via sequential oxidations and the intramolecular cyclization of selaginpulvilin A (Zhu et al. 2019).
The structural classes mentioned below were reported for the first time from the genus Selaginella however, they are not unique to Selaginella. These are secondary metabolites commonly found in the plant kingdom.
Biflavonoids
A new compound with 3′,8″-linked biflavonoids, (2″S)-2″,3″-dihydroamentoflavone-4′-methyl ether (67) (Zheng et al. 2011), together with six known compounds ((2S)-2,3 dihydroamentoflavone-4′-methyl ether, flavone-4′-methyl ether, (2S,2″S)-tetrahydroamentoflavone, (2S,2″S)-2,3,2″,3″-tetrahydroamento(2S)-2,3-dihydroamentoflavone, (2″S)-2″,3″-dihydroamentoflavone (68) and amentoflavone) (Fig. 5) were isolated from the ethanolic extract of S. uncinata (Desv.) Spring. All the compounds isolated were evaluated for their protective effect against anoxia by anoxic pheochromocytoma (PC12) cells assay. Compound 68, tested at 45 µM, exhibited the most potent protective effect, with a promoted survival rate of 19.35 ± 1.53% compared to the positive control, baicalin 21.53 ± 1.17% (Zheng et al. 2011). From the ethanolic extract of S. moellendorffii Hieron, three new flavones, 5-carboxymethyl-4′,7-dihydroxyflavone (69), its ethyl ester (70), and butyl ester (71) were isolated (Cao et al. 2010a, b). Isolated natural products were evaluated for their anti-HBV activity using a HepG2.215 cell line transfected with the HBV genome. The ethyl ester (70) and butyl ester (71) exhibited anti-HBV activity at non-toxic concentrations, with SI values of ≥ 2 for HBsAg and 2 for HBeAg (Cao et al. 2010a, b).
A phytochemical investigation of an ethanolic extract of S. uncinata that exhibited a potent anti-anoxic effect in the anoxic PC12 cell assay resulted in the isolation of four new biflavonoids (72–75) (Zheng et al. 2008). The structures of the new compounds and their absolute configurations were determined with the aid of NMR and CD spectroscopy. Compound 75 exhibited a potent anti-anoxic effect in the anoxic PC12 cell assay (Zheng et al. 2008). From the methanolic extract of S. doederleinii Hieron, a new compound, 2,2″,3,3″-tetrahydrorobustaflavone 7,4′,7″-trimethyl ether (76) and previously reported robustaflavone 7,4′,7″-trimethyl ether (77) were isolated. Compound 77 was reported from the family Sellaginellaceae for the first time. Both the compounds were evaluated for their cytotoxicity against human colorectal and lung carcinoma, and erythroleukemia cell line (HCT116, NCI-H358 and K562) and exhibited moderate cytotoxicity towards the human cancer cell lines (Lee et al. 2008a, b). The EC50 values were 19.1 and 15.6 (HCT116), 23.5 and 20.1 (NCI-H358), and 28.8 and 22.5 µM for compounds 76 and 77, respectively. A number of biflavonoids and sterols have been reported from S. delicatula (Lin et al. 2000). For this reason, the whole plant was re-examined for potential new bioactive compounds. The phytochemical investigation of this plant led to the isolation of four new biflavonoids (78–81) from the ethanolic extract of S. delicatula (Lin et al. 2000). The isolated compounds were evaluated for their cytotoxicity against a number of human tumour cell lines, which included adenocarcinoma of the cervix, lung, B cells, erythroleukemic cell line, and kidney (Raji, Calu-1, K562, HeLa, Vero, and Wish). Compounds 78 and 80 significantly inhibited Raji (77, IC50 = 34.2 ± 5.7 and 79, IC50 = 17.9 ± 2.4 µM) and Calu-1 (77, IC50 = 42.6 ± 2.4 and 79, IC50 = 15.8 ± 2.1 µM) cell growth (Lin et al. 2000).
In search for new cytotoxic compounds, more than 1,000 Formosan plants were screened for cytotoxicity and S. delicatula exhibited promising cytotoxic results. Due to this, it was selected for further chemical investigation (Chen et al. 2005). The phytochemical examination of the chloroform and n-butanol fractions of S. delicatula led to the isolation of five new biflavonoids, 7,4′,4‴-trimethyl ether (82), robustaflavone 4′,4‴-dimethyl ether (83), 2,3-dihydroamentoflavone 7,4′,7″-trimethyl ether (84), 2,3-dihydroamentoflavone 7,4′-dimethyl ether (85), and 2″,3″-dihydroisocryptomerin 7-methyl ether (86). The cytotoxicity of the compounds were evaluated against human colon adenocarcinomic and mouse leukemic (P-388 and HT-29) cell lines (Chen et al. 2005). Compounds 83 and 85 exhibited significant cytotoxicity, with ED50 values of 1.44 and 2.30 µg/mL against a P-388 cell line while compound 83 also exhibited significant cytotoxicity against HT-29 cell line with the ED50 value of 1.59 µg/mL (Chen et al. 2005).
A new compound, uncinatabiflavone C 7-methyl ether (87) and a compound with new configuration, (2R) 2, 3-dihydroamentoflavone (88) was isolated from the ethanolic extract of S. uncinata (Desv.). These compounds exhibited inhibitory activities against protein tyrosine phosphatase 1B (PTP1B) in an enzyme assay with IC50 values ranging from 4.6 to 16.1 μM (Xu et al. 2019).
Flavonoids
In search for novel bioactive secondary metabolites from S. doederleinii, an extensive phytochemical investigation was undertaken (Zou et al. 2017a, b). This chemical profiling resulted in the isolation of eight new flavonoids (Fig. 6), selagintriflavonoids A–H (89–96) which had a unique trimeric skeleton (Zou et al. 2017a, b). Selagintriflavonoids A–C included three naringenin units while selagintriflavonoids D–H contained apigenin and two naringenin units. The new compounds were evaluated for their β-secretase (BACE1) inhibitory properties and displayed BACE1 inhibition with the IC50 values ranging from 0.75 to 46.99 µM. Selagintriflavonoid A, which exhibited the most significant inhibitory effect with the IC50 of 0.75 µM, was identified as a potential compound for the treatment of Alzheimer’s disease (Zou et al. 2017a, b). Chromatographic purification of a 75% ethanolic extract of the herb S. doederleinii yielded six new flavonoids (97–103) (Zou et al. 2017a, b). The new compounds had an aryl substitution at C-3′ position of naringenin or apigenin skeleton with compounds 97 and 103 possessing R configurations (Zou et al. 2017a, b). Compounds 101–103 also exhibited moderate cytotoxicity against human alveolar and lung adenocarcinoma, and an erythroleukemia cell line (NCI-H460, A549, and K562) with IC50 values ranging from 8.17 to 18.66 µM (Zou et al. 2017a, b).
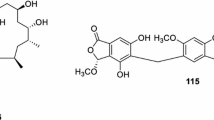
In an effort to search for novel bioactive secondary metabolites, the plant S. moellendorffii Hieron was analysed. The 70% ethanolic extract of S. moellendorffii Hieron was subjected to chromatographic purification, which resulted in the isolation of five new carboxymethyl flavonoids (Fig. 7). Using spectroscopic methods and electronic circular dichroism (ECD), the structures and absolute configurations were confirmed to be: 5-carboxymethyl-3′,4′, 7-trihydroxyflavone (104), (2S)-5-carboxymethyl-3′,4′, chiral isomers, 7-trihydroxyflavonone (105a), (2R)-5-carboxymethyl-3′,4′, 7-trihydroxyflavonone (105b), (2S)-5-carboxymethyl-4′, 7-dihydroxyflavonone (106), 5- carbomethoxymethyl-4′, 7-dihydroxyflavone (107), and a new chromone named 5-carboxymethyl-7-hydroxychromone (108) (Zou et al. 2016a, b). The carboxymethyl subunit was positioned on C-5 in all the isolated compounds, which had previously not been reported for the genus Selaginella (Zou et al. 2016a, b). The antibacterial effect of all the isolates were also evaluated against a range of Gram-negative and Gram-positive bacterial strains. 106 and 107 exhibited significant broad-spectrum antimicrobial activities against E. coli, with an MIC value of 25 µg/mL, while compound 108 was most active against Gram-positive bacteria, with an MIC value of 12.5 µg/mL against S. aureus and S. pneumoniae (Zou et al. 2016a, b). As part of the continuous search for new bioactive molecules from the genus Selaginella, S. uncinata was phytochemically investigated. Two new flavonoids, uncinataflavones A (109) and B (110), were isolated from 75% ethanolic extract after chromatographic purification (Zou et al. 2016a, b). Compounds 109 and 110 were apigenin derivatives with aryl substituents at the C-6 position. Biological activities for the new compounds were not reported (Zou et al. 2016a, b). The phytochemical studies of S. involven yielded six novel apigenin derivatives, involvenflavones A–F, with 3′-aryl substitute (Long et al. 2015). Involvenflavones A–F (111–115) were also analysed for their protective effect against the injury of human umbilical vein endothelial cell (HUVECs) caused by high amounts of glucose. In the MTT viability test, all the compounds were shown to have potent protective effect in vitro at 3 µM (Long et al. 2015). The biosynthetic pathways for these compounds were proposed to be derived from the coupling reaction between apigenin and phenols, with a radical mechanism (Long et al. 2015).
Diterpenoids
The large-scale chromatographic separation and purification of the methanolic extract of S. moellendorffii and subsequent NMR and ECD analysis of pure compounds resulted in the identification of modified abietene diterpenoid (3S,4S,5R,10S)-18(4 → 3)-abeo-3,4,12,18-tetrahydroxy-8,11,13-abietatrien-7-one (116), and two novel dimers, selaginedorffones A (117) and B (118) (Ke et al. 2018) (Fig. 8). Compounds 117 and 118 possessed a new cyclohexene moiety that is believed to be biosynthesized from two modified abietene diterpenoid units. The new compounds were evaluated for their growth inhibitory properties against HL-60 human leukaemia, A-549 human lung-cancer, SW480 human rectal-cancer, SMMC-7721 human liver-cancer, and MCF-7 human breast-cancer cell lines. Compounds 116 and 117 did not exhibit any activities against the tested cell lines. Compound 118 was cytotoxic towards the MCF-7 cells with an IC50 value of 9.0 μM, while it did not exhibit any significant activity against the other tested cell lines (Ke et al. 2018).
Alkaloids
Following a positive reaction to Dragendorff’s reagent, eight new pyrrolidinoindoline alkaloids (119–126) were purified and characterized from the methanol extract of S. moellendorfii (Wang et al. 2009). These unique alkaloids (Fig. 8), possessed a 3-carboxybut-2-enyl side chain at the 3a position and two N8-methyl groups. Compounds 119, 122, and 124 were evaluated for antibacterial, cytotoxic, and acetylcholinesterase inhibitory activities, but did not exhibit any significant activities at 200 µg/mL (Wang et al. 2009). A new pyrrole alkaloid (127) was isolated from the butanolic extract of S. delicatula together with two new adenine analogues, delicatulines A and B. The compounds were evaluated for their inhibitory activities on HBV surface antigen and HBV DNA in HepAD38 cells and were found to possess only weak or no inhibitory activity (Yao et al. 2019a, b).
Lignans and neolignans
Selamoellenin A (128), isolated from S. moellendorffii Hieron, exhibited a protective effect even at low doses against high glucose concentration-induced injury of HUVECs at 0.01 nM concentrations (Zeng et al. 2017). Further phytochemical studies of the ethanolic extract of Chinese ethnic medicinal plant S. moellendorffii led to the isolation of three novel lignans, selamoellenins B–D (129–131) together with 11 known compounds (Zhu et al. 2018) (Fig. 9). The natural products were evaluated for their in vitro antitumor activities against four human cancer cell lines (hepatocellular, colon, gastric and urinary bladder carcinoma; MGC-803, A549, HepG2, T24) by the MTT viability assay using 5-fluorouracil (5-Fu) as positive control. The new compounds 130 and 131 decreased the T24 viability with IC50 values of 22.53 and 25.85 µM, respectively, while compound 129 was less cytotoxic, with an IC50 of 45.43 µM (Zhu et al. 2018). Three new compounds named pictalignan A–C (132–134) and three known derivatives, syringaresinol (135), 3,3′,5-trimethoxy-4′,7-epoxy-8,5′-neoligan-4′,9,9′-triol (136), 4,9-dihydroxy-4′,7-epoxy-8′,9′-dinor-8,5′-neolignan7′-oic acid (137) were isolated from a 75% aqueous ethanol extract of S. picta, (Cheng et al. 2018). Compounds 132–134 exhibited moderate activity against HT-22 cells injured by L-glutamate treatment (Cheng et al. 2018). Compound 132 exhibited a better protective effect in the range of 10–15 µM than the positive control, dimethyl fumarate (Cheng et al. 2018).
The pyrrolidinoindoline alkaloid selaginellic acid, which was previously isolated from the whole plant S. moellendorffii, exhibited antiplatelet activity. This interesting bioactivity led Long and co-workers to further investigate this plant (Zhuo et al. 2016). Chromatographic purification of the methanolic extract of S. moellendorffii resulted in the isolation of two new neolignans, selaginellol (138) and selaginellol 4′-O-β-D-glucopyranoside (139) (Zhuo et al. 2016). Compounds 138 and 139 were evaluated for platelet aggregation activity induced by ADP or collagen, but did not show any significant activity (Zhuo et al. 2016). Tamariscinols U–W (140–142) which belong to the dihydrobenzofuran-type norneolignans were isolated from the ethanolic extract of S. tamariscina. These compounds were tested for their inhibitory effects against the mushroom tyrosinase. Tamarisconol U (IC50 = 5.75 µM) was three times more active than the positive control, kojic acid (He et al. 2019). Seven new neolignans, sinensiols A–G (143–149) together with two known compounds (150–151), were isolated from the whole plant of S. sinensis (Wang et al. 2007; Chen et al. 2019a, b). The planar structures of these compounds were determined by the analysis of spectroscopic data, and the absolute configuration was established by a comparison of the experimental CD data. Sinensiols A–D are dimers that are sesquilignans. All the isolated compounds were evaluated for their cytotoxicity against A549 and HepG2 human cancer cell lines, however, no growth inhibition of the cancer cells was observed (Wang et al. 2007; Chen et al. 2019a, b).
Saponins and sterols
The butanolic soluble fraction of the ethanolic extract of S. uncinata exhibited significant anti-anoxic activity. After solvent–solvent partitioning and chromatographic purification, two new steroidal saponin derivatives (Fig. 10), (3β,7β,12β,25R)spirost-5-ene-3,7,12-triol-3-O-α-L-rhamnopyranosyl-(1 → 2)-O-[α-L-rhamnopyranosyl-(1 → 4)]-O-β-D-glucopyranoside (152) and (2α,3β,12β, 25R)-spirost-5-ene-2,3,12-triol-3O-α-L-rhamnopyranosyl-(1 → 2)-O-[α-L-rhamnopyranosyl(1 → 4)]-O-β-D-glucopyranoside (153) were isolated. This was the first report of steroidal saponins isolated from S. uncinate (Zheng et al. 2013). The anoxic PC12 cell assay was utilized to determine the protective effect against anoxia of compounds 152 and 153. The tested compounds exhibited a potent protective effect (Zheng et al. 2013). The ethanolic extract of S. tamariscina yielded three new sterols, 3β,16α-dihydroxy-5α,17β-cholestan-21-carboxylic acid (154), 3 β-acetoxy-16α-hydroxy-5α,17β-cholestan-21carboxylic acid (155) and 3β-(3-hydroxybutyroxy)-16α-hydroxy5a,17β-cholestan-21-carboxylic acid (156) (Fig. 10). Compounds 154–157 were tested for their antiproliferative effects in human leukemia HL-60 cells. All three sterols exhibited weak cell growth inhibition compared to 5-fluorouracil (Gao et al. 2007).
Biological activities
The pharmacological profile of compounds isolated from various species of Selaginella has been summarised in Tables 1, 2, 3, 4 and 5 below. It is worthwhile to mention here that most publications only reported the biological activity of extracts, however, a number of research groups also reported the biological activities of the pure compounds. The disadvantage of such an approach is the uncertainty in the active compound responsible for the manifested activity, on the other hand it could better mimic potential synergic effect of the various compounds in the same plant. Each table provides details, comprised of the plant species, whether a pure compound or extract were tested, the test subject and the relevant activities.
Anticancer activity
The toxicity of extracts or pure compounds isolated from the Selaginella species was tested against up to 40 different cell lines (Table 1). To date, of approximately 700 species of the Selaginella genus (Bieski et al. 2015), only nine were characterized for cytotoxicity activity to the best of our knowledge. These nine species include S. convolute, S. delicatula, S. doederleinii, S. labordei, S. moellendorffii, S. picta, S. pulvinata, S. tamariscina, and S. uncinata. Usually water, ethanol, ethyl acetate, chloroform and hexane were used as extracting solvents; although the essential oil was prepared and tested as well. Half of the published papers deal with crude extracts and the rest reported the isolation and testing of pure compounds, which can further lead to determining the mechanism of anticancer activity at the molecular level. Unfortunately, in 97 percent of published papers, the mode of action could be simple toxicity rather than anticancer activity, as most of the publications only dealt cell lines derived from carcinomas and did not use nonmalignant cell lines as controls. Likewise, none of the current publications compared the Selaginella extracts compounds with some of the drugs commercially used for the treatment of selected cancers. Therefore, such results can hardly prove anticancer activity, because the simple cytotoxicity of the compounds is not excluded. When a control cell line was involved, it was usually represented by human dermal fibroblasts (HDF), mouse neuronal (HT-22) or monkey kidney (Vero) cells. However, both the cancer cell line and the nonmalignant cell line should be preferably of human origin and of the same tissue origin to ensure that the selective anticancer effect is not caused by species or tissue differences in sensitivity (Calderon-Montano et al. 2014). Ideally, at particular concentration within the therapeutic window, the control cells should continue to grow and proliferate, while the cancer cells are inhibited. However, the data for such a conclusion is frequently missing, except in a paper by Wang et al. (Wang et al. 2015a, b), which showed that the ethyl acetate extract of Selaginella doederleinii was 2–4 times more toxic for e.g. cervical, hepatocellular and prostatic adenocarcinoma than for African green monkey kidney cells.
The toxicity of Selaginella extracts was previously reported for hepatocellular, alveolar, skin, nasopharyngeal, prostatic, and oesophageal squamous cells, cervical, bone, and oral squamous cells, Lewis lung carcinoma and leukaemia cells with the IC50 ranging from 3 mg/L (ethyl acetate extract, cervical and colon adenocarcinoma) (Li et al. 2014a, b) to 800 mg/L (water extract, leukaemia) (Ahn et al. 2006). In addition, the Selaginella ethyl acetate extract was not toxic for the monkey kidney cells at up to 150 mg/L (Wang et al. 2015a, b). However, the crude extracts usually contain many biologically active compounds that could act additionally, synergistically or antagonistically.
Pure compounds isolated from Selaginella that were tested for cancer cell line toxicity included the following compounds: (3R)-5,6,7-trihydroxy-3-isopropyl-3-methylisochroman-1-one; 2,3-dihydroamentoflavone 7,4′-dimethyl ether; 2″,3″-dihydro-3′, 3‴-biapigenin; 2″,3″dihydrorobustaflavone 7,4′, -dimethyl ether; 3′,3‴-binaringenin; 3β-(3-hydroxybutyroxy)-16α-hydroxy-5α,17β-cholestan-21-carboxylic acid; 3β,16α-dihydroxy-5α,17β-cholestan-21-carboxylic acid; 3β-acetoxy-16α-hydroxy-5α,17β-cholestan-21-carboxylic acid; 5-carboxymethyl-4′,7-dihydroxyflavone ethyl ester; 7, 4′,7″,4‴-tetra-O-methyl-amentoflavone; 7″-O-methylamentoflavone; amentoflavone; diselaginellin B (37); ginkgetin; heveaflavone; hinokiflavone; isoginkgetin; neocryptomerin; pictalignan A–C (128–130); pulvinatabiflavone; robustaflavone; robustaflavone 4′,4‴-dimethyl ether; robustaflavone 4′-methyl ether; selagibenzophenone B (41); selaginedorffone B (115); selaginellins (A (2), C (4), M (14), N (15)); sumaflavone; and α-tocopheryl quinone. (3R)-5,6,7-trihydroxy-3-isopropyl-3-methylisochroman-1-one seems to be the most active compound, with an IC50 value for human colon adenocarcinoma of 10 nM. This isochroman is followed by ginkgetin, hinokiflavone and robustaflavone 4′,4‴-dimethyl ether with IC50 values for human colon, alveolar basal epithelial cells, gastric adenocarcinoma and mouse leukaemia of around 1 µM.
Several papers described the isolation of the same compounds from different Selaginella species and reported comparable anticancer activities. Amentoflavone isolated from S. tamariscina and S. doederleinii exhibited IC50 in the range from 5 to 125 µM for human breast, cervical, alveolar, lung, nasopharyngeal carcinoma and leukaemia cells; in addition, at several times lower concentration (1.8 µM), it protected human dermal fibroblasts against UV irradiation. Hinokiflavone isolated from S. tamariscina and S. moellendorffii was active against human gastric, hepatocellular, cervical, breast carcinoma and glioma, with IC50 ranging from 1 µM to 39 µM. Finally, robustaflavone 4′-methyl ether isolated from both S. moellendorffii and S. delicatula exhibited activity against human alveolar and lung carcinoma, with IC50 ranging from 5 to approx. 50 µM.
Mechanisms of the anticancer activity
Most scientific publications aimed at determining the mechanism of activity of different types of Selaginella extracts. Since an extract is a mixture of many secondary metabolites, the synergistic, antagonistic and additive effects of such complex mixtures will also influence the biological activities. Only few authors tested the effects and mechanisms of action of pure substances. As shown in the previous chapter, Selaginella species are a rich source of unique compounds and only a limited number of these compounds have had their mechanism of action in living cells determined. For the crude Selaginella extracts, two main mechanisms were predicted. The first one is based on p53-mediated apoptosis, the second one on metalloproteinase inhibition.
The anticancer activity of Selaginella crude extracts is usually attributed to decreasing the bcl-2/bax mRNA level ratio (Ahn et al. 2006; Jing et al. 2009; Wang et al. 2015a, b). Bax (bcl-2 associated X protein) promotes cell death through permeabilization of the mitochondrial outer membrane in response to different cellular stresses (Khodapasand et al. 2015). This means that it functions as an apoptotic activator, which results in the release of cytochrome C and other pro-apoptotic factors from the mitochondria and activation of caspases (esp. caspase-3 Ahn et al. 2006; Li et al. 2014a, b; Wang et al. 2015a, b) and caspase-9 (Sui et al. 2016)). Bax is involved in p53-mediated apoptosis, as its expression is upregulated by the tumour suppressor protein p53. The p53 protein is a transcription factor that regulates not only bax, but also many other downstream target genes (Wang et al. 2015a, b). In contrast to Bax, Bcl-2 (derived from B-cell lymphoma where the protein was first discovered) prevents apoptosis by inhibiting the activity of Bax (Khodapasand et al. 2015). The ratio of bcl-2/bax mRNA level is therefore crucial for triggering the programmed death of cancer cells or inhibiting tumour growth by arresting the cell cycle at the S phase (Jing et al. 2009; Sui et al. 2016). Nowadays, many drugs activating bax are in clinical use for the treatment of cancer (Liu et al. 2016). A decrease in the gene expression of cyclooxygenase (COX-2), lipoxygenase (5-LOX, 12-LOX), 5-lipoxigenase-activating protein (FLAP) and survivin was demonstrated to accompany the decrease in bcl-2/bax mRNA level ratio (Wang et al. 2015a, b). The inhibition of COX, LOX and FLAP blocks the formation of prostaglandins and leukotrienes, and thus limits the inflammatory process (Martel-Pelletier et al. 2003) and prevents necrosis (Wallach and Kovalenko 2014). The role of survivin lies in the inhibition of caspase. Its inhibition leads to no negative regulation of apoptosis. Metalloproteinases (MMPs) are involved in the breakdown of extracellular matrix in normal physiological processes; however, their increased expression was demonstrated in several types of tumours (Zucker et al. 1995; Morini et al. 2000; Groblewska et al. 2012). Tissue endogenous natural inhibitors of metalloproteinases (TIMPs; among them TIMP-1 and TIMP-2 were best characterised) form complexes with MMPs and inhibit cancer dissemination (Groblewska et al. 2012). Several authors demonstrated that Selaginella extracts inhibit MMP-1, (Lee et al. 2008a, b) MMP-2 and MMP-9 (Hsin et al. 2013) enzyme activity as well as their expression (Hsin et al. 2013; Yang et al. 2013a, b) and increase both TIMP-1 (Yang et al. 2007) and TIMP-2 expression (Hsin et al. 2013) through Erk1/2 (extracellular signal-regulated kinase) and a p38-dependent pathway (Hsin et al. 2013). Yang et al. demonstrated that the regulation of MMP-2 and MMP-9 expression is based on the regulation of their promoters (Yang et al. 2013a, b). The same authors further showed that Selaginella extract inhibits the phosphorylation of p38 and Akt. (Hsin et al. 2013). Similarly to MMPs, urokinase plasminogen activator (u-PA) expression was decreased in cancer cells as well; meanwhile, plasminogen activator inhibitor-1 (PAI-1), was increased (Yang et al. 2007).
Among the biflavonoids occurring in Selaginella species, sumaflavone and amentoflavone exhibited significant MMP-1 inhibitory activity. The IC50 values were approximately 10 × lower than the IC50 of retinoic acid (10 µM), which served as a positive control (Lee et al. 2011). Moreover, the cells treated with amentoflavone exhibited a series of cellular alterations related to apoptosis (Lee et al. 2011; Pei et al. 2012) and signs of mitochondrial dysfunctions—the reduction of mitochondrial inner-membrane potential, the release of cytochrome c from mitochondria, and activation of caspase 3 (Pei et al. 2012) and caspase 9 (Lee et al. 2011). Amentaflavone decreased the expression of Bcl-2, but elevated the apoptotic factor Bax (Lee et al. 2011). Similarly to amentoflavone, the apoptosis was induced by diselaginellin B (Cao et al. 2017). The findings that selaginellin (1) and selaginellin M (14) inhibited cytochromes P450 (CYP) with IC50 values of around 1 µM for CYP2C8 followed by other less inhibited cytochromes (CYP2C9 and CYP2J2) (Zanger and Schwab 2013) confirm the concept that extracts are mixtures of substances with complex effects. As the cytochromes P450 are the major source of variability in drug pharmacokinetics, their inhibition significantly slows down the biotransformation of most substances, including various bioactive compounds from Selaginella extract (Zanger and Schwab 2013).
Some recent publications demonstrated specific anticancer activity of Selaginella extracts. It was shown that hinokiflavone obtained from Selaginella tamariscina (Beauv.) triggers apoptosis and may inhibit the migration of breast cancer cells in Balb/c nude mice (Huang et al. 2020). Extract from S. tamariscina was reported to downregulate the growth of human lung cancer cells both in vitro and in vivo by specifically inhibiting aldo–keto reductase (Jung et al. 2017). Amentoflavone was identified as the compound responsible for this activity. Delicaflavone isolated from Selaginella doederleinii Hieron triggered apoptosis via a mitochondrial pathway and the inhibition of MAPK signalling in HeLa cells (Yao et al. 2019a, b), and inhibited several kinases involved in signalling pathways of colorectal cancer cells (Yao et al. 2020). An oral proliposomes formulation was proposed to overcome the solubility-related limitation for the oral delivery of a biflavonoids extract from S. doederleinii. (Chen et al. 2019a, b) An alternative might be amorphous solid dispersion, which exhibited acceptable pharmacokinetics in rats (Chen et al. 2020).
Tackling drug resistance
Multidrug resistance (MDR) is a major challenge for the 21th century in cancer chemotherapy (Chambers et al. 2019). Drug efflux pumps and detoxification enzymes, such as aldo–keto reductases (AKRs), are typical proteins involved in the resistance of tumours (Jung et al. 2017). Overcoming such resistance with natural compounds, especially flavonoids, has been previously reported (Viktorova et al. 2019). Many flavonoids commonly consumed in the daily diet or dietary supplements are present in many evolutionary distinct plant species, including Selaginella. Therefore, Jung et al. (Jung et al. 2017) focused their attention on the modulation of MDR by S. tamariscina ethanol extract and amentoflavone, which is the dominant flavonoid in this species. Both the extract and flavone were able to inhibit human aldo–keto reductase AKR1B10 in a dose-dependent manner, with IC50 equal to 8 mg/L and 2 µM, respectively. In addition, the authors confirmed the anti-proliferative activity of lung cancer cells overexpressing AKR1B10 when cultivated with the extract or amentoflavone combined with doxorubicin. This observation leads to the conclusion that the extract and amentoflavone synergistically increase the doxorubicin anti-proliferative effect in human lung cancer cells overproducing AKR1B10. S. tamariscina ethanol extract also significantly inhibited lung tumour growth in mice (Zhu et al. 2017).
Animal testing
As the in vitro demonstration of cellular apoptosis is inaccurate for predicting the real antitumor activity of the compounds or extracts in vivo, Selaginella extracts were administered to mice (Yang et al. 2007; Le et al. 2012; Wang et al. 2015a, b) and rats (Chen et al. 2018). The results showed considerable antitumor activity of Selaginella extracts without toxicity for non-cancer cells (up to 10 g/kg) (Le et al. 2012). The oral administration of extract did not prevent Lewis lung carcinoma formation; however, it produced a significant inhibition of tumour growth (Yang et al. 2007; Le et al. 2012). The doses of 50 and 150 mg/kg/day of total biflavonoids extract inhibited the mouse tumour growth by 40 and 54%, respectively (Yao et al. 2017a, b). Moreover, the immune response of the mouse (TNF-α and IFN-γ production) was significantly enhanced (Yao et al. 2017a, b). Similarly, Sui et al. observed alveolar tumour growth inhibition accompanied by reduced expression of antigen Ki67 and reduced microvascular density (Sui et al. 2016). A decrease in Ki67 activity leads to an inhibition of ribosomal RNA synthesis (Rahmanzadeh et al. 2007) and microvascular density correlating with the aggressiveness of several cancers (Iakovlev et al. 2012). Lewis lung carcinoma metastases of rats treated with Selaginella extract (3 g/kg/day) decreased by 79% in 30 days without any apparent signs of toxicity (Yang et al. 2007).
As pharmacokinetics is one of the major parameters affecting the success of drugs, Chen et al. administered five biflavonoids (amentoflavone, robustaflavone, 2″,3″-dihydro-3′,3″-biapigenin, 3′,3″-binaringenin and delicaflavone) orally or intravenously in rats. After intravenous administration, the plasma concentration of all biflavonoids rapidly decreased and cleared out within 12 h. The distribution of 3′,3‴-binaringenin, delicaflavone and 2″,3″-dihydro-3′,3‴-biapigenin between plasma and the rest of the body after oral administration (Vd) were greater than those of amentoflavone and robustaflavone, indicative of a relatively greater tissue distribution. For the oral administration, the total biflavonoids were detected at a much lower plasma level (Chen et al. 2018). The mean oral bioavailability (F) was highest for delicaflavone (2.5%), followed by 2″,3″-dihydro-3′,3‴-biapigenin (1%). The lowest bioavailability was observed for amentoflavone (0.3%) (Chen et al. 2018). Unfortunately biflavonoids, being members of the flavonoid group, share the group’s low bioavailability which limits their effects on health (Thilakarathna and Rupasinghe 2013). Many compounds are known for their biological activities in vitro; however, because of their poor solubility, rapid metabolism, or a combination of both, they are usually poorly bioavailable upon oral administration. In such cases, several mechanisms of drug-delivery systems could be used to increase drug penetration into the target place of action. One of them is nano-engineering which was successfully used for the delivery of flavonoids isolated from S. bryopteris (Bhargava et al. 2017). Both in vitro and in vivo studies with nano-encapsulated flavonoids exhibited increased mito-protective activity in a dose-dependent manner when exposed to a carcinogen, and an inhibitory effect on tumour growth in mice (Bhargava et al. 2017).
Antimicrobial activity
To date, only 18 reports exist on the antimicrobial activity of Selaginella sp. Moreover, in comparison to anticancer properties, the antimicrobial activity of Selaginella species is not significant, and a small number of papers on this topic originate from only a few research groups. Moreover, some of the currently published papers lack the use of appropriate controls (e.g. evaluation of the antimicrobial activity of the pure solvents which were used for plant extraction). Considering the number of known Selaginella species, only some have been determined as antimicrobial. As with its other activities, the antimicrobial activity of Selaginella is mostly tested using crude extracts of plants. Moreover, essential oils prepared from several Selaginella species were also tested for their antimicrobial activity (Wang et al. 2018). Selaginella bryopteris extract was also used as a reducing agent for silver nanoparticle synthesis. This green synthesis produced antimicrobial particles; however, their activity was caused by the silver rather than by the plant extract, as published by the authors (Baskaran et al. 2018).
Selaginella extracts were found to be active against both bacteria and yeasts with no selectivity for Gram-positive or negative bacteria. The sensitivity of the particular strain used rather than the extraction solvent used is a major factor affecting antimicrobial activity results (Table 2). The minimal concentrations inhibiting visible growth (MIC) of bacteria are in the range of 0.4–6.3 mg/mL, and the same concentrations for the yeast lie in the range of 0.04–2.5 mg/mL. Non-polar extracts exhibited slightly better activity than the polar extracts. Joo et al. also reported on antimicrobial activity against the causative agent of acnes—Propionibacterium acnes (Joo et al. 2008). The MIC inhibiting the growth of these anaerobic bacteria was relatively low compared to the previously mentioned aerobic microorganisms (MIC = 0.25 mg/mL).
Amentoflavone, which is commonly isolated from Selaginella, exhibited antimicrobial activity besides anticancer activity. Despite the concentrations of amentoflavone inhibiting half of cancer cells (IC50) being relatively low (tens of µg/mL), (Lee et al. 2011; Pei et al. 2012; Li et al. 2014a, b) the effective concentrations inhibiting the visible growth of bacteria (MIC) are about ten times lower (Hwang et al. 2012). The same shift in effective doses inhibiting bacterial and cancer cells is evident for selaginellin (1) (Cao et al. 2010a, b; Zhang et al. 2012). Both amentoflavone and isocryptomerin inhibited human pathogenic bacteria (Lee et al. 2009a, b; Hwang et al. 2013) and drug-resistant strains (e.g. 10 µg/mL of isocryptomerin inhibited methicillin-resistant S. aureus (Lee et al. 2009a, b)). Moreover, Hwang et al. reported a synergistic effect of both amentoflavone (Hwang et al. 2013) and isocryptomerin (Lee et al. 2009a, b) when applied in binary drug therapy together with some clinically used antibiotics (ampicillin, cefotaxime and chloramphenicol). This synergistic effect could eliminate the multidrug resistance phenotype of bacteria.
The mechanism of the activity of amentoflavone on Candida albicans and cancer cells may be the same, as both are eukaryotic cells. Amentoflavone was shown to cause disruption of the mitochondrial functions in a number of studies (Lee et al. 2011; Hwang et al. 2012; Pei et al. 2012). Extract of S. tamariscina was reported to eliminate four strains of Microcystis aeruginosa that form cyanobacterial bloom (Lee et al. 2020). Amentoflavone was identified as the major active compound in the extract responsible for this activity. Fractions of S. convolute activated the nociceptive peripheral pathway, resulting in an antinociceptive effect (de Sa et al. 2012; Oliveira-Macedo et al. 2019).
Neuroprotective activity
Oxidative stress is an imbalance between the concentration of reactive oxygen (synthesised as by-product of metabolism) and the way in which the organism can destroy this reactive species. A high concentration of these reactive species causes many different diseases (for example Parkinson’s disease, atherosclerosis, and heart attack). The rather poor antioxidant potential of Selaginella species is mentioned above; therefore, some neuroprotective activity could be suggested. However, to date, there have only been a few articles about the neuroprotective activity of Selaginella.
Experiments with mice treated with ethanol extract from S. convulata support the theory of the traditional use of this plant as an analgesic agent. In addition, the extract did not affect motor coordination (de Sa et al. 2012). Extract from the same plant was used for the preparation of ZnO nanoparticles. Both the extract and the nanoparticles possessed muscle relaxant activity (Xu et al. 2020). Moreover, the aqueous extract of S. delicatula was tested on a Drosophila model mimicking Parkinson’s disease symptoms. The extract offered concentration-dependent protection against rotenone-induced lethality, ROS production and protein carbonyl and hydroperoxide levels. Simultaneously, the extract restored the activity of antioxidant enzymes (superoxide dismutase, glutathione reductase), glutathione-S-transferase and membrane-bound enzymes (NADH-cytochrome c reductase and succinate dehydrogenase), suggesting its propensity to protect mitochondrial integrity. Finally, the extract normalized the activity levels of acetylcholinesterase and dopamine depletion. These findings suggest that the extract has concentration-dependent neuroprotective potencies (Girish 2012) and reduces mitochondrial dysfunction (Chandran and Muralidhara 2013).
Immunomodulation activity
Inflammation is a complex biological response of an organism to the presence of foreign agents. This protective reaction includes the immune system and blood circulation. The function of this reaction is to eliminate damage, remove foreign agents and initiate the tissue repair process. Many substances can affect the immune system and thereby regulate inflammatory processes via many mechanisms. Several Selaginella extracts previously demonstrated the ability to modulate the immune system. The oral administration of S. bryopteris methanol extract at doses of up to 2 g/kg did not exhibit marked toxicity in in vivo models, and local treatment resulted in a reduction in the redness and swelling of inflammation, which was also achieved by the aqueous extract of S. moellendorffii (Zhao et al. 2017). This study also revealed the mechanism of its anti-inflammatory effect, which is the reduction of nitrate oxide (NO) accumulation (an important mediator of the inflammatory process, production inducible by nitric oxide synthesis) and inhibition of anti-inflammatory cytokine production in the tissue. Indomethacin (a reference anti-inflammatory drug) has a similar mechanism (Paswan et al. 2017). Selagin, originating from S. participens, exhibited thymus growth-stimulatory activity in adult mice; moreover, it protected immunocompromised mice against Aspergillus fumigatus infection, although without any direct antifungal activity in vitro (Gayathri et al. 2011). The ethanol extract of S. tamariscina was able to inhibit the production of inflammatory mediators and pro-inflammatory cytokines in a dose-dependent manner. Amentaflavone, one of the main active substances of Selaginella, inhibited the expression of nitrate oxide synthase by NF-κB deactivation (Woo et al. 2005). At the same time, the extract showed potential for free radical neutralization and prevented their formation (Won et al. 2018).
Asthma is a chronic inflammatory disease characterised by inflammation, bronchial hyperactivity and airway obstruction. Flavonoids from S. uncinata weakened airway hyper-reactivity in ovalbumin-induced asthma in rats. T-cell-associated cytokine levels as well as IgE and IgA were decreased, while IFN-γ was elevated in treated rats (Yu et al. 2017). The effect of Selaginella extracts on asthma was also tested in vitro. Phosphodiesterase-4 (PDE4) catalyses the hydrolysis of secondary messengers (cyclic adenosine monophosphate and guanosine monophosphate) and is associated with respiratory diseases. There are many inhibitors of this enzyme, but only roflumilast is approved for the treatment of chronic obstructive pulmonary disease. Selaginpulvilins B (43) and C (44) proved to be potent inhibitors of this enzyme. Their IC50 values were 0.11 ± 0.02 and 0.18 0.02 μM respectively, and thus were more effective than roflumilast (IC50 0.54 ± 0.04 µM) (Liu et al. 2014). Nanotechnology is emerging in the field of medicinal plant research. This is mainly because this technology offers solutions in the field of transport or stability of the active ingredients. The oral administration of silver nanoparticles synthetized in the presence of S. myosurus extract significantly reduced paw edema in a mouse model (Kedi et al. 2018).
A recent paper indicated that robustaflavone-4′-dimethyl ether isolated from S. uncinata downregulates the activation of neutrophils by inhibiting the cytokine receptor FLT3-mediatied AKT and MAPK pathways (Wu et al. 2020). The nanoparticles prepared by using Selaginella tamariscina Carbonisata extracts were shown to activate the fibrinogen system, resulting in haemostatic effects in rat and mouse tail amputation and scratch models (Zhao et al. 2020). Similarly, silver nanoparticles prepared by green technology using S. bryopteris extract exhibited anticoagulant and antiplatelet properties together with antimicrobial effects (Dakshayani et al. 2019).
Other activities
The number of papers describing antiviral (Table 3) and antiparasitic (Table 4) activity of the Selaginella species is even lower. Whole extracts were mainly used, although a paper described the activity of unique chemical entities (Kunert et al. 2008; Cao et al. 2010a, b). Promising extracts sometimes exhibited significant toxicity, which probably limits their future use. As the methodology is broad, it is difficult to compare the results or distinguish between a selective effect against given pathogen from a general protective effect of the extracts on the cells in the test. Antioxidant activity (Table 5) of the Selaginella species has only minor potential for two reasons. One is that the total antioxidant activity is relatively low compared to the controls, second, the main compound (if identified) is usually a common (bi)flavonoid, which could be extracted from many other plant species or may be present in the daily diet. Biflavonoids isolated from S. uncinata were suggested to be natural antidiabetic agents, as they acted as potent inhibitors of protein tyrosine phosphatase 1B, causing insulin resistance by interfering with the intracellular insulin signalling pathway (Dwi 2011).
Conclusions
We sought to provide a comprehensive review summarising current knowledge about the chemical composition and biological activities of Selaginella species. The Selaginellaceae family has been shown to provide a remarkably rich panel of compounds potentially useful in medical applications including flavonoids, lignans, terpenes and a large group of unique compounds named selaginellins. Notably, numerous novel compounds were also identified from various Selaginella extracts. In the course of this review, the “coincidental simultaneous assignment” of the same trivial name to different structures was also uncovered. As suggested by Ramabharathi and Schuehly (2014), it is also our belief that appropriate rules should be adhered to when giving a trivial name to natural products.
The diverse structural classes of compounds isolated from the genus Selaginella exhibited a wide range of interesting biological activities as indicated in various sections above. Analysis of the biological activities (Fig. 11a) revealed that almost half the publications explored the cytotoxic effects of the extracts or pure compounds obtained from Selaginella. This was closely followed by their antimicrobial activities. Further analysis of Selaginella as producers of new compounds together with their anticancer and antimicrobial activity identified four main Selaginella species (S. tamariscina, S. moellendorffii, S. pulvinata, and S. doederleinii) as sources of new compounds with a high number of anticancer or antimicrobial activities (Fig. 11b.). As most of the potentially active compounds are present at very low concentrations in the extracts, further fractionation and purification of the crude extracts may provide positive results and avoid concentration limits and, in some cases, the antagonistic effect of the compound in complex mixtures. Due to the interesting pharmacological effects of the extracts and secondary metabolites, further research in this field is highly desirable.
References
Ahn SH, Mun YJ, Lee SW et al (2006) Selaginella tamariscina induces apoptosis via a caspase-3-mediated mechanism in human promyelocytic leukemia cells. J Med Food 9(2):138–144
Baskaran X, Vigila AVG, Zhang SZ, Feng SX, Liao WB (2018) A review of the use of pteridophytes for treating human ailments. J Zhejiang Univ Sci B 19:1–35
Bhargava A, Pathak N, Seshadri S et al (2017) Pre-clinical validation of mito-targeted nano-engineered flavonoids isolated from Selaginella bryopteris (Sanjeevani) as a novel cancer prevention strategy. Anti-Cancer Agents Med Chem 18:1860–1874
Bieski IG, Leonti M, Arnason JT et al (2015) Ethnobotanical study of medicinal plants by population of Valley of Juruena Region Legal Amazon, Mato Grosso, Brazil. J Ethnopharmacol 173:383–423
Calderon-Montano JM, Burgos-Moron E, Lopez-Lazaro M (2014) The in vivo antitumor activity of cardiac glycosides in mice xenografted with human cancer cells is probably an experimental artifact. Oncogene 33(22):2947–2948
Cao Y, Chen J-J, Tan N-H et al (2010a) Structure determination of selaginellins G and H from Selaginella pulvinata by NMR spectroscopy. Magn Reson Chem 48(8):656–659
Cao Y, Chen JJ, Tan NH et al (2010b) Antimicrobial selaginellin derivatives from Selaginella pulvinata. Bioorg Med Chem Lett 20(8):2456–2460
Cao Y, Yao Y, Huang X-J et al (2015) Four new selaginellin derivatives from Selaginella pulvinata: mechanism of racemization process in selaginellins with quinone methide. Tetrahedron 71(10):1581–1587
Cao Y, Zhao M, Zhu Y et al (2017) Diselaginellin B, an unusual dimeric molecule from Selaginella pulvinata, inhibited metastasis and induced apoptosis of SMMC-7721 human hepatocellular carcinoma cells. J Nat Prod 80(12):3151–3158
Chambers CS, Viktorova J, Rehorova K et al (2019) Defying multidrug resistance! Modulation of related transporters by flavonoids and flavonolignans. J Agric Food Chem 68:1763–1779
Chandran G, Muralidhara M (2013) Neuroprotective effect of aqueous extract of Selaginella delicatula as evidenced by abrogation of rotenone-induced motor deficits, oxidative dysfunctions, and neurotoxicity in mice. Cell Mol Neurobiol 33(7):929–942
Chen JJ, Duh CY, Chen JF (2005) New cytotoxic biflavonoids from Selaginella delicatula. Planta Med 71(7):659–665
Chen B, Wang X, Zou Y et al (2018) Simultaneous quantification of five biflavonoids in rat plasma by LC-ESI-MS/MS and its application to a comparatively pharmacokinetic study of Selaginella doederleinii Hieron extract in rats. J Pharm Biomed Anal 149:80–88
Chen B, Wang X, Lin D et al (2019a) Proliposomes for oral delivery of total biflavonoids extract from Selaginella doederleinii: formulation development, optimization, and in vitro-in vivo characterization. Int J Nanomed 14:6691–6706
Chen X, Xu P-S, Zou Z-X et al (2019b) Sinensiols B–G, six novel neolignans from Selaginella sinensis. Fitoterapia 134:256–263
Chen B, Wang X, Zhang Y et al (2020) Improved solubility, dissolution rate, and oral bioavailability of main biflavonoids from Selaginella doederleinii extract by amorphous solid dispersion. Drug Deliv 27(1):309–322
Cheng F, Xu KP, Liu LF et al (2018) New neolignans from Selaginella pieta and their protective effect on HT-22 cells. Fitoterapia 127:69–73
Cheng X-L, Ma S-C, Yu J-D et al (2008) Selaginellin A and B, two novel natural pigments isolated from Selaginella tamariscina. Chem Pharm Bull 56(7):982–984
Dakshayani SS, Marulasiddeshwara BM, Kumar MNS et al (2019) Antimicrobial, anticoagulant and antiplatelet activities of green synthesized silver nanoparticles using Selaginella (Sanjeevini) plant extract Int. J Biol Macromol 131:787–797
de Sa PG, Nunes XP, de Lima JT et al (2012) Antinociceptive effect of ethanolic extract of Selaginella convoluta in mice. BMC Complement Altern Med 12:187
Duraiswamy H, Nallaiyan S, Nelson J et al (2010) The effect of extracts of Selaginella involvens and Selaginella inaequalifolia leaves on poultry pathogens. Asian Pac J Trop Med 3(9):678–681
Dwi A (2011) Review: natural products from Genus Selaginella (Selaginellaceae). Nusantara Bioscie 3:44–58
Gang W, Hua LS, Lian ZH et al (2017) Phytochemical screening, antioxidant, antibacterial and cytotoxic activities of different extracts of Selaginella doederleinii. Bangl J Bot 46:1193–1201
Gao LL, Yin SL, Li ZL et al (2007) Three novel sterols isolated from Selaginella tamariscina with antiproliferative activity in leukemia cells. Planta Med 73(10):1112–1115
Gayathri V, Asha VV, John JA et al (2011) Protection of immunocompromised mice from fungal infection with a thymus growth-stimulatory component from Selaginella involvens, a fern. Immunopharm Immunot 33(2):351–359
Girish C (2012) Propensity of Selaginella delicatula aqueous extract to offset rotenone-induced oxidative dysfunctions and neurotoxicity in Drosophila melanogaster: implications for Parkinson’s disease. Neurotoxicology 33(3):444–456
Groblewska M, Siewko M, Mroczko B et al (2012) The role of matrix metalloproteinases (MMPs) and their inhibitors (TIMPs) in the development of esophageal cancer. Folia Histochem Cytobiol 50(1):12–19
He X-R, Xu L-Y, Jin C et al (2019) Tamariscinols U–W, new dihydrobenzofuran-type norneolignans with tyrosinase inhibitory activity from Selaginella tamariscina. Phytochem Lett 34:79–83
Hsin CH, Wu BC, Chuang CY et al (2013) Selaginella tamariscina extract suppresses TPA-induced invasion and metastasis through inhibition of MMP-9 in human nasopharyngeal carcinoma HONE-1 cells. BMC Complement Altern Med 13:234
Huang Y, Liu X, Wu D et al (2017) The discovery, complex crystal structure, and recognition mechanism of a novel natural PDE4 inhibitor from Selaginella pulvinata. Biochem Pharmacol 130:51–59
Huang W, Liu C, Liu F et al (2020) Hinokiflavone induces apoptosis and inhibits migration of breast cancer cells via EMT signalling pathway. Cell Biochem Funct. https://doi.org/10.1002/cbf.3443
Hwang IS, Lee J, Jin HG et al (2012) Amentoflavone stimulates mitochondrial dysfunction and induces apoptotic cell death in Candida albicans. Mycopathologia 173(4):207–218
Hwang JH, Choi H, Woo ER et al (2013) Antibacterial effect of amentoflavone and its synergistic effect with antibiotics. J Microbiol Biotechnol 23(7):953–958
Iakovlev VV, Gabril M, Dubinski W et al (2012) Microvascular density as an independent predictor of clinical outcome in renal cell carcinoma: an automated image analysis study. Lab Invest 92(1):46–56
Jing Y, Tang AZ, Liu J et al (2009) Effects of Selaginella doederleinii on human nasopharyngeal carcinoma TW03 cells in vitro and its mechanism. Zhong Yao Cai 32(12):1864–1867
Joo SS, Jang SK, Kim SG et al (2008) Anti-acne activity of Selaginella involvens extract and its non-antibiotic antimicrobial potential on Propionibacterium acnes. Phytother Res 22(3):335–339
Jung YJ, Lee EH, Lee CG et al (2017) AKR1B10-inhibitory Selaginella tamariscina extract and amentoflavone decrease the growth of A549 human lung cancer cells in vitro and in vivo. J Ethnopharmacol 202:78–84
Ke LY, Zhang Y, Xia MY et al (2018) Modified abietane diterpenoids from whole plants of Selaginella moellendorffii. J Nat Prod 81(2):418–422
Kedi PBE, Meva FE, Kotsedi L et al (2018) Eco-friendly synthesis, characterization, in vitro and in vivo anti-inflammatory activity of silver nanoparticle-mediated Selaginella myosurus aqueous extract. Int J Nanomed 13:8537–8548
Khodapasand E, Jafarzadeh N, Farrokhi F et al (2015) Is Bax/Bcl-2 ratio considered as a prognostic marker with age and tumor location in colorectal cancer? Iran Biomed J 19(2):69–75
Kim JH, Cho CW, Tai BH et al (2015) Soluble epoxide hydrolase inhibitory activity of Selaginellin derivatives from Selaginella tamariscina. Molecules 20(12):21405–21414
Kunert O, Swamy RC, Kaiser M et al (2008) Antiplasmodial and leishmanicidal activity of biflavonoids from Indian Selaginella bryopteris. Phytochem Lett 1(4):171–174
Le MH, Do TT, Hoang TH et al (2012) Toxicity and anticancer effects of an extract from Selaginella tamariscina on a mice model. Nat Prod Res 26(12):1130–1134
Le DD, Nguyen DH, Zhao BT et al (2017) PTP1B inhibitors from Selaginella tamariscina (Beauv.) Spring and their kinetic properties and molecular docking simulation. Bioorg Chem 72:273–281
Lee CW, Choi HJ, Kim HS et al (2008a) Biflavonoids isolated from Selaginella tamariscina regulate the expression of matrix metalloproteinase in human skin fibroblasts. Bioorg Med Chem 16(2):732–738
Lee NY, Min HY, Lee J et al (2008b) Identification of a new cytotoxic biflavanone from Selaginella doederleinii. Chem Pharm Bull 56(9):1360–1361
Lee J, Choi Y, Woo ER et al (2009a) Antibacterial and synergistic activity of isocryptomerin isolated from Selaginella tamariscina. J Microbiol Biotechnol 19(2):204–207
Lee J, Choi Y, Woo ER et al (2009b) Isocryptomerin, a novel membrane-active antifungal compound from Selaginella tamariscina. Biochem Bioph Res Commun 379(3):676–680
Lee S, Kim H, Kang JW et al (2011) The biflavonoid amentoflavone induces apoptosis via suppressing E7 expression, cell cycle arrest at sub-G(1) phase, and mitochondria-emanated intrinsic pathways in human cervical cancer cells. J Med Food 14(7–8):808–816
Lee J, Kim M, Jeong SE et al (2020) Amentoflavone, a novel cyanobacterial killing agent from Selaginella tamariscina. J Hazard Mater 384:121312
Li J, Lei X, Chen KL (2014a) Comparison of cytotoxic activities of extracts from Selaginella species. Pharmacogn Mag 10(40):529–535
Li S, Zhao M, Li Y et al (2014b) Preparative isolation of six anti-tumour biflavonoids from Selaginella doederleinii Hieron by high-speed counter-current chromatography. Phytochem Anal 25(2):127–133
Li D, Qian Y, Tian YJ et al (2017) Optimization of ionic liquid-assisted extraction of biflavonoids from Selaginella doederleinii and evaluation of its antioxidant and antitumor activity. Molecules 22(4):586
Lian R, Li J, Ma HM et al (2013) Effect of ethanol extract of Selaginella doederleinii Hieron on the proliferation of nasopharyngeal carcinoma CNE-1 and C666–1 cells. Afr J Tradit Complement Altern Med 10(6):490–493
Lin LC, Kuo YC, Chou CJ (2000) Cytotoxic biflavonoids from Selaginella delicatula. J Nat Prod 63(5):627–630
Liu X, Luo HB, Huang YY et al (2014) Selaginpulvilins A–D, new phosphodiesterase-4 inhibitors with an unprecedented skeleton from Selaginella pulvinata. Org Lett 16(1):282–285
Liu Z, Ding Y, Ye N et al (2016) Direct activation of bax protein for cancer therapy. Med Res Rev 36(2):313–341
Liu R, Zou H, Zou ZX et al (2018a) Two new anthraquinone derivatives and one new triarylbenzophenone analog from Selaginella tamariscina. Nat Prod Res 32:1–6
Liu X, Tang G-H, Weng H-Z et al (2018b) A new selaginellin derivative and a new triarylbenzophenone analog from the whole plant of Selaginella pulvinata. J Asian Nat Prod Res 20(12):1123–1128
Long HP, Zou H, Li FS et al (2015) Involvenflavones A-F, six new flavonoids with 3′-aryl substituent from Selaginella involven. Fitoterapia 105:254–259
Ma LY, Ma SC, Wei F et al (2003) Uncinoside A and B, two new antiviral chromone glycosides from Selaginella uncinata. Chem Pharm Bull 51(11):1264–1267
Macedo L, de Oliveira RG, Souza GR et al (2018) Chemical composition, antioxidant and antibacterial activities and evaluation of cytotoxicity of the fractions obtained from Selaginella convoluta (Arn.) Spring (Selaginellaceae). Biotechnol Biotechnol Equip 32(2):506–512
Martel-Pelletier J, Lajeunesse D, Reboul P et al (2003) Therapeutic role of dual inhibitors of 5-LOX and COX, selective and non-selective non-steroidal anti-inflammatory drugs. Ann Rheum Dis 62(6):501
Morini M, Mottolese M, Ferrari N et al (2000) The α3β1 integrin is associated with mammary carcinoma cell metastasis, invasion, and gelatinase B (MMP-9) activity. Int J Cancer 87:336–342
Nguyen PH, Ji DJ, Han YR et al (2015a) Selaginellin and biflavonoids as protein tyrosine phosphatase 1B inhibitors from Selaginella tamariscina and their glucose uptake stimulatory effects. Bioorg Med Chem 23(13):3730–3737
Nguyen PH, Zhao BT, Ali MY et al (2015b) Insulin-mimetic selaginellins from Selaginella tamariscina with protein tyrosine phosphatase 1B (PTP1B) inhibitory activity. J Nat Prod 78(1):34–42
Oliveira-Macedo LAR, Pacheco AGM, Lima-Saraiva SRG et al (2019) Fractions of Selaginella convoluta (Arn.) Spring (Selaginellaceae) attenuate the nociceptive behavior events in mice. Braz J Biol 80:57–65
Paswan SK, Gautam A, Verma P et al (2017) The Indian magical herb “Sanjeevni” (Selaginella bryopteris L.)—A promising anti-inflammatory phytomedicine for the treatment of patients with inflammatory skin diseases. J Pharmacopunct 20(2):93–99
Pei JS, Liu CC, Hsu YN et al (2012) Amentoflavone induces cell-cycle arrest and apoptosis in MCF-7 human breast cancer cells via mitochondria-dependent pathway. In Vivo 26(6):963–970
Queiroz DP, Carollo CA, Kadri MC et al (2016) vivo antileishmanial activity and chemical profile of polar extract from Selaginella sellowii. Mem Inst Oswaldo Cruz 111(3):147–154
Ramabharathi V, Schuehly W (2014) How to deal with nomenclatoral ambiguities of trivial names for natural products?—A clarifying case study exemplified for “corymbosin.” Nat Prod Commun 9(1):57–60
Rahmanzadeh R, Huttmann G, Gerdes J et al (2007) Chromophore-assisted light inactivation of pKi-67 leads to inhibition of ribosomal RNA synthesis. Cell Prolif 40(3):422–430
Rizk YS, Fischer A, Cunha Mde C et al (2014) In vitro activity of the hydroethanolic extract and biflavonoids isolated from Selaginella sellowii on Leishmania (Leishmania) amazonensis. Mem Inst Oswaldo Cruz 109(8):1050–1056
Robles-Zepeda RE, Velazquez-Contreras CA, Garibay-Escobar A et al (2011) Antimicrobial activity of northwestern Mexican plants against Helicobacter pylori. J Med Food 14(10):1280–1283
Rojas A, Bah M, Rojas JI et al (1999) Spasmolytic activity of some plants used by the Otomi Indians of Querétaro (México) for the treatment of gastrointestinal disorders. Phytomedicine 6(5):367–371
Sit NW, Chan YS, Chuah BL et al (2017) Antiviral, antifungal and antibacterial activities of the Chinese medicinal plants Houttuynia cordata, Lobelia chinensis and Selaginella uncinata SE. Asian J Trop Med 48(3):616–627
Sui Y, Li S, Shi P et al (2016) Ethyl acetate extract from Selaginella doederleinii Hieron inhibits the growth of human lung cancer cells A549 via caspase-dependent apoptosis pathway. J Ethnopharmacol 190:261–271
Tan G-S, Xu K-P, Li F-S et al (2009) Selaginellin C, a new natural pigment from Selaginella pulvinata Maxim (Hook et Grev.). J Asian Nat Prod Res 11(12):1001–1004
Teinkela JEM, Noundou XS, Nguemfo EL et al (2018) Biological activities of plant extracts from Ficus elastica and Selaginella vogelli: an antimalarial, antitrypanosomal and cytotoxity evaluation. Saudi J Biol Sci 25(1):117–122
Thilakarathna SH, Rupasinghe HPV (2013) Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 5(9):3367–3387
Verma M, Gangwar M, Sahai M et al (2015) Antimicrobial activity of phytochemicals isolated from Selaginella bryopteris. Chem Nat Compd 51(2):341–345
Viktorova J, Dobiasova S, Rehorova K et al (2019) Antioxidant, anti-inflammatory, and multidrug resistance modulation activity of silychristin derivatives. Antioxidants 8:303–322
Wallach D, Kovalenko A (2014) Keeping inflammation at bay. eLife 3:e02583
Wang YZ, Chen H, Zheng XK et al (2007) A new sesquilignan from Selaginella sinensis (Desv.) Spring. Chin Chem Lett 18(10):1224–1226
Wang Y-H, Long C-L, Yang F-M et al (2009) Pyrrolidinoindoline alkaloids from Selaginella moellendorfii. J Nat Prod 72(6):1151–1154
Wang G, Yao S, Cheng L et al (2015a) Antioxidant and anticancer effection of the volatile oil from various habitats of Selaginella doederleinii Hieron. Technol Health Care 23(Suppl 1):S21-27
Wang JZ, Li J, Zhao P et al (2015b) Antitumor activities of ethyl acetate extracts from Selaginella doederleinii Hieron in vitro and in vivo and Its possible mechanism. Evid Based Complement Alternat Med 2015:865714
Wang G, Ma XK, Li D et al (2018) Chemical composition of essential volatile oils of Selaginella spp and their antibacterial activity. Bangladesh J Bot 47(3):719–726
Won AN, Kim SA, Ahn JY et al (2018) HO-1 Induction by Selaginella tamariscina extract inhibits inflammatory response in lipopolysaccharide-stimulated RAW 264.7 macrophages. Evid Based Complement Alternat Med 2018:7816923
Woo ER, Lee JY, Cho IJ et al (2005) Amentoflavone inhibits the induction of nitric oxide synthase by inhibiting NF-κB activation in macrophages. Pharmacol Res 51(6):539–546
Woo S, Kang KB, Kim J et al (2019) Molecular networking reveals the chemical diversity of selaginellin derivatives, natural phosphodiesterase-4 inhibitors from Selaginella tamariscina. J Nat Prod 82:1820–1830
Wu XN, Yang Y, Zhang HH et al (2020) Robustaflavone-4′-dimethyl ether from Selaginella uncinata attenuated lipopolysaccharide-induced acute lung injury via inhibiting FLT3-mediated neutrophil activation. Int Immunopharmacol 82:106338
Xu K-P, Zou H, Li F-S et al (2011a) Two new selaginellin derivatives from Selaginella tamariscina (Beauv.) Spring. J Asian Nat Prod Res 13(4):356–360
Xu K-P, Zou H, Tan Q et al (2011b) Selaginellins I and J, two new alkynyl phenols, from Selaginella tamariscina (Beauv.) Spring. J Asian Nat Prod Res 13(2):93–96
Xu K-P, Li J, Zhu G-Z et al (2015) New selaginellin derivatives from Selaginella tamariscina. J Asian Nat Prod Res 17(8):819–822
Xu J, Yang L, Wang R et al (2019) The biflavonoids as protein tyrosine phosphatase 1B inhibitors from Selaginella uncinata and their antihyperglycemic action. Fitoterapia 137:104255
Xu K, Yan H, Cao M et al (2020) Selaginella convolute extract mediated synthesis of ZnO NPs for pain management in emerging nursing care. J Photochem Photobiol B 202:111700
Yang SF, Chu SC, Liu SJ et al (2007) Antimetastatic activities of Selaginella tamariscina (Beauv.) on lung cancer cells in vitro and in vivo. J Ethnopharmacol 110(3):483–489
Yang C, Shao Y, Li K et al (2012) Bioactive selaginellins from Selaginella tamariscina (Beauv.) Spring. Beilstein J Org Chem 8:1884–1889
Yang J-S, Lin C-W, Hsin C-H et al (2013a) Selaginellatamariscina attenuates metastasis via Akt pathways in oral cancer cells. PLoS ONE 8(6):e68035–e68035
Yang JS, Lin CW, Hsieh YS et al (2013b) Selaginella tamariscina (Beauv.) possesses antimetastatic effects on human osteosarcoma cells by decreasing MMP-2 and MMP-9 secretions via p38 and Akt signaling pathways. Food Chem Toxicol 59:801–807
Yao H, Chen B, Zhang YY et al (2017a) Analysis of the total biflavonoids extract from Selaginella doederleinii by HPLC-QTOF-MS and its in vitro and in vivo anticancer effects. Molecules 22(2):325–342
Yao W-N, Huang R-Z, Hua J et al (2017b) Selagintamarlin A: A Selaginellin analogue possessing a 1H-2-benzopyran core from Selaginella tamariscina. ACS Omega 2(5):2178–2183
Yao C-P, Zou Z-X, Zhang Y et al (2019a) New adenine analogues and a pyrrole alkaloid from Selaginella delicatula. Nat Prod Res 33(14):1985–1991
Yao W, Lin Z, Wang G et al (2019b) Delicaflavone induces apoptosis via mitochondrial pathway accompanying G2/M cycle arrest and inhibition of MAPK signaling cascades in cervical cancer HeLa cells. Phytomedicine 62:152973
Yao W, Lin Z, Shi P et al (2020) Delicaflavone induces ROS-mediated apoptosis and inhibits PI3K/AKT/mTOR and Ras/MEK/Erk signaling pathways in colorectal cancer cells. Biochem Pharmacol 171:113680
Yin D, Li J, Lei X et al (2014) Antiviral activity of total flavonoid extracts from Selaginella moellendorffii Hieron against coxsackie virus B3 in vitro and in vivo. Evid Based Complement Alternat Med 2014:950817
Yu B, Cai W, Zhang HH et al (2017) Selaginella uncinata flavonoids ameliorated ovalbumin-induced airway inflammation in a rat model of asthma. J Ethnopharmacol 195:71–80
Zanger UM, Schwab M (2013) Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther 138(1):103–141
Zeng W, Yao CP, Xu PS et al (2017) A new neolignan from Selaginella moellendorffii Hieron. Nat Prod Res 31(19):2223–2227
Zhang L-P, Liang Y-M, Wei X-C et al (2007) A new unusual natural pigment from Selaginella sinensis and Its noticeable physicochemical properties. J Org Chem 72(10):3921–3924
Zhang GG, Jing Y, Zhang HM et al (2012) Isolation and cytotoxic activity of selaginellin derivatives and biflavonoids from Selaginella tamariscina. Planta Med 78(4):390–392
Zhang J-S, Liu X, Weng J et al (2017) Natural diarylfluorene derivatives: isolation, total synthesis, and phosphodiesterase-4 inhibition. Org Chem Front 4(2):170–177
Zhang L, Zhang Y, Hou Y et al (2020) Total flavonoids of Selaginella pulvinata alleviates cognitive impairment in mice. Biomed Rep 13(2):8
Zhao P, Chen KL, Zhang GL et al (2017) Pharmacological basis for use of Selaginella moellendorffii in gouty arthritis: antihyperuricemic, anti-inflammatory, and xanthine oxidase inhibition. Evid Based Complement Alternat Med 2017:2103254
Zhao Y, Zhang Y, Kong H et al (2020) Haemostatic nanoparticles-derived bioactivity of from Selaginella tamariscina Carbonisata. Molecules 25(3):446
Zheng JX, Wang NL, Liu HW et al (2008) Four new biflavonoids from Selaginella uncinata and their anti-anoxic effect. J Asian Nat Prod Res 10(9–10):945–952
Zheng JX, Zheng Y, Zhi H et al (2011) New 3′,8″-linked biflavonoids from Selaginella uncinata displaying protective effect against anoxia. Molecules 16(8):6206–6214
Zheng J, Zheng Y, Zhi H et al (2013) Two new steroidal saponins from Selaginella uncinata (Desv.) Spring and their protective effect against anoxia. Fitoterapia 88:25–30
Zhoua XF, Tong GT, Wang XW et al (2016) Anti-proliferative constituents from Selaginella moellendorffii. Nat Prod Commun 11(5):623–626
Zhu B, Wang T-B, Hou L-J et al (2016) A new selaginellin from Selaginella moellendorffii inhibits hepatitis B virus gene expression and replication. Chem Nat Compd 52(4):624–627
Zhu QF, Bao Y, Zhang ZJ et al (2017) A biomimetic semisynthesis enables structural elucidation of selaginellin U: a tautomeric cyclic alkynylphenol from Selaginella tamariscina. R Soc Open Sci 4(7):170352
Zhu Y, Huang RZ, Wang CG et al (2018) New inhibitors of matrix metalloproteinases 9 (MMP-9): Lignans from Selaginella moellendorffii. Fitoterapia 130:281–289
Zhu Q-F, Shao L-D, Wu X-D et al (2019) Isolation, structural assignment of isoselagintamarlin A from Selaginella tamariscina and its biomimetic synthesis. Nat Prod Bioprospect 9(1):69–74
Zhuo JX, Wang YH, Su XL et al (2016) Neolignans from Selaginella moellendorffii. Nat Prod Bioprospect 6(3):161–166
Zou H, Yi ML, Xu KP et al (2016a) Two new flavonoids from Selaginella uncinata. J Asian Nat Prod Res 18(3):248–252
Zou Z, Xu P, Wu C et al (2016b) Carboxymethyl flavonoids and a chromone with antimicrobial activity from Selaginella moellendorffii Hieron. Fitoterapia 111:124–129
Zou Z, Xu K, Xu P et al (2017a) Seladoeflavones A-F, six novel flavonoids from Selaginella doederleinii. Fitoterapia 116:66–71
Zou Z, Xu P, Zhang G et al (2017b) Selagintriflavonoids with BACE1 inhibitory activity from the fern Selaginella doederleinii. Phytochemistry 134:114–121
Zucker S, Lysik RM, DiMassimo BI et al (1995) Plasma assay of gelatinase B: tissue inhibitor of metalloproteinase complexes in cancer. Cancer 76(4):700–708
Acknowledgements
This work was supported by the Czech National Program of Sustainability NPU I (LO1601), R. K. gratefully acknowledges the European Fund: European Structural and Investment Funds Operational Program Research, Development and Education (ChemJets UCT Prague, CZ.02.2.69/0.0/0.0/16_027/0008351).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the supplementary information.
Rights and permissions
About this article
Cite this article
Kumar, R., Viktorova, J., Krizkovska, B. et al. Structural diversity and biological activities of secondary metabolites isolated from the genus Selaginella. Phytochem Rev 20, 1209–1243 (2021). https://doi.org/10.1007/s11101-021-09743-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11101-021-09743-7