Abstract
Cyclodextrins are cyclic oligosaccharides consisting of d-glucopyranose units bound via α-(1,4)-glycosidic linkages. They are obtained from starch enzymatic degradation by the action of cyclodextrinases or glycosyltransferases. Among the modified-cyclodextrins, 2-hydroxypropylated and methylated β-cyclodextrins are produced on industrial scale. Particularly, methylated β-cyclodextrins are more suitable than native β-cyclodextrins to form stable inclusion complexes with organic molecules of low molecular weight, making these complexes more soluble in aqueous solutions. Cyclodextrins have often been considered useful carriers of antitumor- and immuno-regulatory drugs, and they have also been used as additives for food industry. It is also important to note that cyclodextrins are used for improving bioactive compound production in plant cell cultures because of cyclodextrins ability as “hosts” of bioactive compounds favoring their accumulation in aqueous media. In fact, the treatment of plant cell cultures with cyclodextrins and their derivatives increases the production of secondary metabolites such as resveratrol, ajmalicine, serpentine, lutein, arachidin, among other antioxidant compounds, enhancing the capability of plant in vitro cultures to produce high levels of different bioactive compounds which have beneficial properties for human health. In addition, metabolomic, transcriptomic, and proteomic studies have been carried out on both control and cyclodextrins-treated cell cultures offering important clues about how cyclodextrins are capable of substantially increasing the production of bioactive compounds in plant in vitro cultures. This review focuses on the effect of cyclodextrins on both bioactive compound production and their accumulation, which could be of great interest for chemical and pharmaceutical industries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
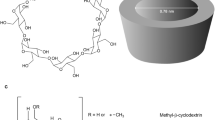
Cyclodextrins (CD) are cyclic oligosaccharides consisting of d-glucopyranose units bound via α-(1,4)-glycosidic linkages (Fig. 1). CD can be classified as α-, β, or γ-CD cyclohexa-, hepta or octaamylose, respectively (Fig. 1). They are obtained from starch enzymatic degradation by the action of a group of amylases such as cyclodextrinases or glycosyltransferases (Gentili 2020). Moreover, there are other types of CD which are generated synthetically by the modification of their functional groups. As regard to the CD structures, they have a hydrophilic outer surface and a hydrophobic inner cavity. The hydrophilic character comes from the OH groups while hydrophobicity of the internal cavity is due to the presence of ethereal oxygen from the glucopyranose units and the hydrocarbon skeleton of CD structure. These features give CD the ability of hosting several compounds via non-covalent interactions (Landy et al. 2012).
On the other hand, the water solubility at room temperature of the different α, β and γ-CD corresponds to 13, 2 and 26% (w/w), respectively (Atwood et al. 1996). The addition of alcohols or acetonitrile increase the solubility of CD in water; however, these compounds are insoluble in most organic solvents except for pyridine, dimethyl formamide and dimethyl sulfoxide. In addition, CD can be modified to improve their ability of formation of inclusion complexes or increase their solubility in both, organic and aqueous solutions. Among the modified-CD, 2-hydroxypropylated and methylated β-CD are produced at industrial scale. Particularly, methylated β-CD are more suitable than the native β-CD to form stable inclusion complexes with organic molecules of low molecular weight making them more soluble in aqueous solutions. The most common inclusion complexes (that is, bioactive compound-CD) are of type 1:1, 1:2 and 2:1. Several studies have shown that β-CD form inclusion complexes of 1:1 type with resveratrol and gallates, being the phenolic ring inserted into the β-CD cavity (Morales et al. 1998; Martínez-Alonso et al. 2015).
On the other hand, β-CD are considered useful carriers of antitumor- and immuno-regulatory drugs (Gidwani and Vyas 2014). Also, α-, β- and γ-CD can be used as additives for alimentary use since they were included in the European list as E-457, E-459 and E-458, respectively. Several reviews have described the use of CD in food industry and their impact on the compound sequestration, food packaging and maintenance of sensory food (Cravotto et al. 2006; Astray et al. 2009).
It is also important to highlight the use of β-CD for improving bioactive compound production in plant cell cultures (Ramírez-Estrada et al. 2016; Bru et al. 2006), because of CD ability as “hosts” of bioactive compounds favoring their accumulation in aqueous medium (Bru et al. 2006). Moreover, β-CD increase the ability of hydrophobic compounds to cross cell membranes, and they act as elicitors in plant cell cultures due to their structural similarity to pectic oligosaccharides which are released from cell walls as a result of fungal infection, and thus, promoting the biosynthesis of plant bioactive compounds (Bru et al. 2006; Almagro et al. 2016; Zamboni et al. 2006). In this way, several studies have reported the capability of methylated-β-CD to increase the production of some bioactive compounds (Torres and Corchete 2016; Miras-Moreno et al. 2016; Rojas et al. 2005; Almagro et al. 2014a; Belchí-Navarro et al. 2012; Tisserant et al. 2016, among others). This review has been focused on the action of β-CD producing and accumulating compounds which result of great interest for chemical and pharmaceutical industries.
Use of cyclodextrins to increase the production of phenolic compounds in plant in vitro cultures
Phenolic compounds are considered one of the most important secondary metabolites in plants (Randhir et al. 2004). These compounds, which are derived from the shikimate, pentose phosphate, and phenylpropanoid pathways, are involved in key physiological and morphological processes for the plants. In fact, they play an important role in plant growth and development, mainly participating in plant defense reactions (Cheynier 2012) since their biosynthesis increases against insect and pathogen attack, high levels of UV radiation or even wounding (Kennedy and Wightman 2011). As regards their structure, phenolic compounds are formed by an aromatic ring bearing one or more hydroxyl groups (Chirinos et al. 2009), formally named as simple phenols or polyphenols, if they contain more than one phenol unit. In general, phenolic compounds can be classified in simple phenols, lignans, lignins, tannins, coumarins, phenolic acids and flavonoids (Soto-Vaca et al. 2012).
Phenolic compounds have human health benefits due to their physiological properties, such as anti-atherogenic, anti-microbial, anti-inflammatory, anti-thrombotic, antioxidant, anti-allergenic, vasodilatory and cardioprotective effects (Manach et al. 2005; Puupponen-Pimiä et al. 2001). Owing to the multiple beneficial properties of phenolic compounds for the humans, it is important to describe strategies that allow a sustainable production of these compounds. In this way, methylated-β-CD have been widely used in order to increase the production of phenolic compounds in different plant in vitro systems (Belchí-Navarro et al. 2012; Zamboni et al. 2006; Vidal-Limón et al. 2018; Tisserant et al. 2016; Hidalgo et al. 2017). More specifically, numerous studies have shown that methylated-β-CD increased the production of trans-resveratrol, a simple molecule of stilbene nature (3,5,4′-trihydroxystilbene) which has beneficial effects on human health (Vang et al. 2011). Morales et al. (1998) described for the first time that β-CD increased trans-resveratrol production in Vitis vinifera cell cultures. These authors observed that 5 mM dimethylated-β-CD induced the biosynthesis of trans-resveratrol reaching the maximal levels of 10 mg/g DW at 48 h of incubation in V. vinifera cv Gamay (Table 1). Bru et al. (2006) also observed that 50 mM methylated-β-CD caused higher levels of trans-resveratrol in the same cultivar grown in darkness (337.40 mg/g DW) than in V. vinifera cv Monastrell grown under light conditions (8.89 mg/g DW or in V. vinifera cv Gamay (305.5 mg/g DW) (Table 1). Moreover, the capability of dimethylated-β-CD to induce the production of trans-resveratrol was also tested in other different Vitis cell cultures (Zamboni et al. 2006). The levels of trans-resveratrol were of 0.51, 4.31, 225.22 and 911.25 mg/L in V. vinifera cv. Pinot Noir, V. vinifera cv. Merzling, V. amurensis and V. riparia × V. berlandieri, respectively (Table 1). Considering the variability of the response to dimethylated-β-CD treatment in the four grape genotypes analyzed, it was confirmed that it is very important to select what genotypes can naturally produce high levels of trans-resveratrol. Likewise, Zamboni et al. (2009) analyzed the transcriptome changes of V. riparia × V. berlandieri cell cultures in response to dimethylated-β-CD at 2 and 6 h after treatment by using microarray analysis. At both time points, these authors identified a specific set of induced genes belonging to the general phenylpropanoid metabolism, including stilbenes and hydroxycinnamates, and defense proteins such as pathogenesis-related proteins. At 6 h, they also observed a down-regulation of the genes involved in both cell wall loosening and cell division. Moreover, a high increase in trans-resveratrol production was observed when dimethylated-β-CD and methyl jasmonate (MJ) were jointly added to V. vinifera cv. Monastrell cell cultures (Lijavetzky et al. 2008). Dimethylated-β-CD and MJ also increased the expression of genes involved in trans-resveratrol biosynthetic pathway (this means, phenylalanine ammonia lyase, cinnamate 4-hydroxylase, 4-coumarate CoA ligase and stilbene synthase) but only dimethylated-β-CD enhanced the production of trans-resveratrol (over 57 mg/g DW) (Table 1). The joint action of dimethylated-β-CD and MJ provoked the highest values of extracellular resveratrol which was almost one order of magnitude higher than in the presence of dimethylated-β-CD alone (about 364.8 mg/g DW, Table 1), and this production was highly correlated with maximum expression values for genes of trans-resveratrol biosynthetic pathway. Belchí-Navarro et al. (2012) analyzed the effect of different factors on extracellular trans-resveratrol production in V. vinifera cv Monastrell cell cultures, concluding that the optimal conditions for its maximal production (304.7 mg/g DW) were the elicitation with 50 mM randomly methylated-β-CD, 100 µM MJ, and 20 g/L sucrose in the culture medium during 168 h of treatment using a cell density of 10 g DW/L (Table 1). In order to dissect the basis of the interactions among the elicitation responses triggered by randomly methylated-β-CD and MJ, a transcriptional analysis of V. vinifera cell cultures treated with both elicitors individually, or in combination was performed (Almagro et al. 2014a). The results showed that the induction of genes encoding enzymes from stilbene biosynthetic pathway induced by randomly methylated-β-CD alone was strongly increased in the presence of MJ, which correlated with their effects on trans-resveratrol production. Likewise, cell cycle regulation and protein translation were more highly down-regulated in randomly methylated-β-CD-treated cells than in MJ-treated cells, and this response was increased in the presence of both elicitors. Salicylic acid and jasmonate signaling transcription factors were activated only in the presence of randomly methylated-β-CD and MJ, respectively (Almagro et al. 2014a). Moreover, the combined action of randomly methylated-β-CD and MJ provoked the up-regulation of MYB15, NAC and WRKY transcription factors, protein kinases and calcium signal transducers. The results obtained by Almagro et al. (2014a) suggested that both elicitors provoked an activation of the secondary metabolism (mainly in the production of trans-resveratrol) in detriment of primary metabolism. Apart from the studies on the transcriptomic profile, Belchí-Navarro et al. (2019a) analyzed the changes in the secretome of V. vinifera cv Monastrell cell cultures elicited with MJ and/or randomly methylated-β-CD by using label-free quantitative approaches. Treatments with randomly methylated-β-CD reinforced the defensive arsenal and enhanced the accumulation of peroxidase V, β-1,3-glucanase and xyloglucan endotransglycosylase, whereas the presence of randomly methylated-β-CD and MJ increased the accumulation of proteins such as peroxidase IV, reticulin oxidase, heparanase and xyloglucan endotransglycosylase. Therefore, these proteins could be used as potential defense biomarkers in grapevine. Similar studies were carried out by Martínez-Esteso et al. (2009) in V. vinifera cv Gamay cell cultures treated with randomly methylated-β-CD and/or MJ. The results showed 25 spots of proteins which were differentially expressed in 2-D gels, and they were identified as secretory peroxidases, β-1,3-glucanases, thaumatin-like proteins, chitinase-III, SGNH plant lipase-like, xyloglucan endotransglycosylase NtPR27-like, and subtilisin-like protease. In addition, a chitinase III and a class III secretory basic peroxidase were strongly induced in the presence of randomly methylated-β-CD alone or in combination with MJ. Some of the proteins induced under randomly methylated-β-CD treatment were also induced in other species by activators of systemic acquired resistance, which is considered a form of plant immunity (Cusido et al. 2014 and references therein). These results suggested that treatment with randomly methylated-β-CD resembles the effect of systemic acquired resistance induction agents in grapevine cell cultures.
On the other hand, trans-resveratrol (72 mg/L at day 13) and γ-viniferin (48.4 mg/L at day 20) were obtained from V. labrusca L cell cultures treated with 800 µM MJ and 13 mM methylated-β-CD (Nivelle et al. 2017). Moreover, Lambert et al. (2019) observed that a scale-up to 20 l-stirring-bioreactor gave similar growth rates to those observed in shake flask cultures, with a high production of trans-resveratrol (4230 mg/L) and γ-viniferin (760 mg/L) in V. labrusca cell cultures treated with 500 µM MJ and 50 mM methylated-β-CD.
Moreover, the same elicitation conditions (50 mM methylated-β-CD and 100 µM MJ) were used in V. vinifera Pinot Noir hairy root cultures for 96 h (Tisserant et al. 2016). In this study elicited V. vinifera cv Pinot Noir hairy roots produced and secreted to the culture medium, a wide variety of stilbenes being ε-viniferin and trans-resveratrol the two major compounds although other stilbenes were also detected in a low concentration (piceatannol, pallidol, scirpusin A, maackin, and vitisin B). The total sum of stilbenes presented in the culture medium of V. vinifera cv Pinot Noir hairy roots was 9.2 mg/g fresh weight (FW) whereas the intracellular production, in these hairy roots was 3.1 mg/g FW (Table 1). Similarly, the elicitation of Arachis hypogaea hairy root cultures with 100 μM MJ and 6.87 mM methylated-β-CD led to an increase of trans-resveratrol (5.02 µg/g DW), arachidin-1 (7.81 µg/g DW), and arachidin-3 (17.19 µg/g DW) in the culture medium (Table 1). In addition, methylated-β-CD and MJ induced a synergistic effect on stilbene synthase gene expression, which could explain the higher levels of trans-resveratrol found when these elicitors were jointly added in comparison with A. hypogaea hairy root cultures were treated with methylated-β-CD or MJ alone (Yang et al. 2015). Furthermore, Pietrowska-Borek et al. (2014) observed that diadenosine triphosphate (5 µM Ap3A) in combination with randomly methylated-β-CD enhanced both, the transcript level of genes encoding enzymes involved in phenylpropanoid pathway, and the extracellular production of trans-resveratrol (over 80 mg/g DW) in V. vinifera cv Monastrell (Table 1). In fact, the expression analysis of transcripts showed that both elicitors increased the expression levels of phenylalanine ammonia lyase, cinnamate 4-hydroxylase, 4-coumarate CoA ligase and stilbene synthase. The maximum accumulation of transcripts was found for the stilbene synthase gene at 12 h of treatment, being 15-fold and 20-fold higher in Ap3A and randomly methylated-β-treated cells, respectively, than in control cells. More recently, Vidal-Limón et al. (2018) observed that perfluorodecalins (a fluorocarbon in which all the hydrogen atoms are replaced by fluorine atoms) in combination with 100 µM MJ and 50 mM randomly methylated-β-CD increased trans-resveratrol levels (98.3 mg/g DW) compared to the joint action of 100 µM MJ and 50 mM randomly methylated-β-CD (90.3 mg/g DW), which means a trans-resveratrol production enhanced of 8% (Table 1). Besides, to analyze the relationship between gene expression and trans-resveratrol production, the expression levels of genes encoding enzymes involved in the trans-resveratrol biosynthesis were also studied. The results indicated that the phenylalanine ammonia lyase, cinnamate 4-hydroxylase, 4-coumarate CoA ligase and stilbene synthase genes were strongly up-regulated in the elicitation conditions, with maximum expression observed in the MJ and randomly methylated-β-CD treatment, with or without perfluorodecalins. The elicitors led a reprogramming of the gene expression in V. vinifera cell cultures, which likely accounts for the differentially increased production of trans-resveratrol.
Metabolic engineering is another strategy that has been used to increase the production of stilbenes under β-CD elicitation (Pilaisangsuree et al. 2018). Thus, a highly stable and productive hairy root cultures from peanut was transformed with Agrobacterium rhizogenes strain K599, and elicited with 6.87 mM methylated-β-CD being the productivity of trans-resveratrol (64.46 µg/g DW), arachidin-1 (223.81 µg/g DW), and arachidin-3 (20.18 µg/g DW) increased in the culture medium (Pilaisangsuree et al. 2018) (Table 1). In addition, the combination of 6.87 mM methylated-β-CD and 100 µM MJ slightly increased the production of trans-resveratrol (71.95 µg/g DW) in this transgenic hairy root line (Pilaisangsuree et al. 2018). Moreover, heterologous resveratrol production in Silybum marianum cell cultures was carried out using CD strategy (Hidalgo et al. 2017). For that, S. marianum cell cultures were transformed with stilbene synthase gene from V. vinifera, which allowed an accumulation of trans-resveratrol (12 mg/L) in the extracellular medium at 72 h of treatment in the presence of 30 mM randomly methylated-β-CD (Table 1).
On the other hand, lignans are a class of phenolic compounds produced by oxidative dimerization of two phenylpropanoid units. Lignans are found in nature mainly in their free form, while their glycosylated forms are minority (Saleem et al. 2005). Lignans are found in different plant families and tissues such as seeds, roots, stems, rhizomes, leaves, and fruits. However, these plant sources do not provide sufficient commercial quantities of lignans which are often used for their biological properties. More specifically, podophyllotoxin, a lignan biosynthesized in roots and rhizomes of Podophyllum sp. is an effective drug for the treatment of venereal wart condyloma acuminatum. In addition, podophyllotoxin and their analogues have anti-HIV, immunomodulatory, anti-leishmaniasis, antipsoriasis, antiasmatic, and antimalarial activities (Qian Liu et al. 2007 and references therein). In this sense, Woerdenbag et al. (1990) observed an increase of 0.05% on day 13 of incubation when P. hexandrum cell cultures were treated with β-CD and feeding with 3 mM coniferyl alcohol which formed inclusion complexes with them. Likewise, silymarin, a group of flavolignans of the milk thistle S. marianum increased until 45 mg/L in the culture medium when S. marianum cell cultures were elicited with methylated-β-CD, whereas the combination of this elicitor with 100 µM MJ increased even more its production (75 mg/L), which means 5.2 times greater than those of control treatments (Belchí-Navarro et al. 2011) (Table 1). The gene expression of chalcone isomerase, flavanone 3-hydroxylase, flavonol 3´-hydroxylase and cinnamyl alcohol dehydrogenase involved in flavonolignan biosynthesis was notably induced under CD elicitation, indicating that the presence of methylated-β-CD acted as true elicitors on the flavonolignan production in S. marianum cell cultures (Torres and Corchete 2016). Moreover, the DIGE technique was used to detect statistically significant changes in the proteome of S. marianum cell cultures elicited with methylated-β-CD (Corchete and Bru 2013). The results showed that MJ and methylated-β-CD, separately or in combination, activated the expression of defense-related proteins and proteins related to transport mechanisms which accompanies extracellular accumulation of secondary metabolites. Remarkably, a Ras related protein Rab11C like protein of the Rab family was up-regulated by the three elicitor treatments, pointing to a possible involvement of a vesicular transport mechanism in trafficking of secondary metabolites in plant cell cultures.
In another study, Capsicum frutescens cell cultures accumulated vanillin when these cell cultures were fed with its precursor isoeugenol (Rao and Ravishankar 1999). Maximum levels of vanillin were 14.15 mg/L at day 6 in 2.5 mM isoeugenol-treated immobilized cells. The addition of 2.5 mM β-CD and 2.5 mM isoeugenol resulted in an increase in the production of vanillin (22.97 mg/L) at day 4 of elicitation, which means 1.62 times higher than in cells treated only with isoeugenol (Rao and Ravishankar 1999) (Table 1). In the same way, the maximum levels of eugenol, isoeugenol and vanillin in D. carota cell cultures treated with randomly methylated-β-CD were 21.25, 75.99 and 380.22 µg/L, respectively (Miras-Moreno et al. 2016) (Table 1). These authors described for the first time the production of eugenol and isoeugenol in plant cell cultures in a CD-treatment. Furthermore, the application of 50 mM 2-methyl-β-CD to Pimpinella anisum cell cultures also led to a strong extracellular accumulation of two phenolic compounds, eugenin and bergapten (150 and 8 mg/L, respectively), being their accumulation 50 and 8 times more than those found in control cells (Soto-Argel et al. 2018) (Table 1).
Finally, β-CD are also useful to increase flavonoids and anthraquinones in plant in vitro cultures from Crassulaceae family (Perassolo et al. 2016; García-Pérez et al. 2019). García-Pérez et al. (2019) analyzed the effects of methylated-β-CD and hydroxypropylated-β-CD on the extra- and intracellular accumulation of flavonoids and phenols in Bryophyllum sp. cell suspensions. Their results showed CD increased the extracellular production of polyphenols favoring their accumulation outside the cells. The maximal levels of total phenolic compounds were detected, both inside and outside the cells, in the presence of methylated-β-CD at day 7 of treatment (233 and 130 gallic acid equivalents/L), whereas both types of CD (β-methyl- and hydroxypropyl-CD) induced the same levels of intra- and extracellular flavonoids (57 and 35 mg catechin equivalent/L, respectively). Perassolo et al. (2016) showed that 100 µM MJ and 20 mM hydroxypropylated-β-CD enhanced intracellular anthraquinone levels in both Rubia tinctorum (10 mg/g DW) and Morinda citrifolia cell cultures (13.25 mg/g DW) (Table 1). However, the combination of both elicitors did not increase the release of anthraquinones to the culture media. Jaisi and Panichayupakaranant (2020) also observed the combination of chitosan (150 mg/L) with methylated-β-CD (2 mM) significantly increased plumbagin production (14.33 mg/g DW) in Plumbago indica L. root cultures as compared to untreated root cultures (1.76 mg/g DW) (Table 1).
Taking into account all of the above, it can be concluded that modified β-CD are agents capable of increasing the biosynthesis of phenolic compounds in plant in vitro cultures, that sometimes even increase the gene expression related to their biosynthetic pathways, and therefore, act as true elicitors. Moreover, as indicated previously, the chemical structure of modified β-CD allowed them to form inclusion complexes with hydrophobic phenolic compounds, favoring their excretion from cells and facilitating their separation from the culture medium (Belchí-Navarro et al. 2012).
Use of cyclodextrins to increase the production of alkaloids in plant in vitro cultures
Alkaloids are secondary metabolites which contain nitrogen in their structure, frequently in a heterocyclic ring (Roberts 2013). Alkaloids can be isolated from higher plants that belong to different plant families, but also from fungi, algae, bacteria, and even from mammals (Liu et al. 2019). However, the main sources of alkaloids are plants with flowers, mainly dicotyledonous species. The biosynthetic pathways of alkaloids in plants involve many steps, which are catalyzed by different enzymes that belong to an extensive range of protein families. The functional characterization of the enzymes involved in alkaloid biosynthetic pathways has been intensively studied in recent years (Tatsis et al. 2017; Parage et al. 2016; Carqueijeiro et al. 2018).
Alkaloids are involved in plant defense reactions against pathogens and herbivores (Ramawat and Mérillon 2013). This group of secondary metabolites has very complex molecular structures, and numerous physiological activities in both animals and humans. Due to their wide range of biological activities, these compounds have been used as pharmaceutical compounds. In fact, numerous studies have revealed that alkaloids can be used against different types of cancer, including breast and human melanoma and colon, liver, oral, and pancreatic cancer (Yoo et al. 2019; Zhang et al. 2019; Wang et al. 2018). Owing to their wide range of biological activities, several strategies to increase alkaloid production have been carried out including the use of plant in vitro cultures, and the metabolic engineering of alkaloid biosynthetic pathways (Almagro et al. 2013). More specifically, 50 mM methylated-β-CD were able to enhance the production of ajmalicine reaching values of 216 ± 18 mg/L in Catharanthus roseus cell cultures (Almagro et al. 2011, Table 2). In addition, the joint use of methylated-β-CD and MJ induced the maximal levels of ajmalicine which increased linearly until 192 h of treatment (930 ± 16 mg/L, Table 2). Moreover, Almagro et al. (2014b) also observed that randomly methylated-β-CD significantly enhanced the gene expression (geraniol-10-hydroxylase, tryptophan decarboxylase, secologanin synthase and strictosidine synthase), as well as ajmalicine and catharanthine production in C. roseus cell cultures treated with 100 µM MJ and/or 50 mM methylated-β-CD for 8 days. The presence of both elicitors induced a significant synergistic reprogramming of gene expression in C. roseus cell cultures, which is probably the reason why high production levels of catharanthine and ajmalicine were detected under these elicitation conditions. In the same way, Zhou et al. (2015) also confirmed that 10 mM β-CD and 150 µM MJ induced the highest yields of ajmalicine (58.98 mg/L), catharanthine (1.76 mg/L) and vindoline (7.45 mg/L) in C. roseus cambial meristematic cell cultures growth in 5-L stirred airlift bioreactors (Table 2). They also observed that both elicitors increased the expression of geraniol synthase, geraniol 10-hydroxylase, tryptophan decarboxylase, strictosidine synthase, strictosidine β-d-glucosidase, and desacetoxyvindoline-4-hydroxylase, ORCA3, loganic acid O-methyltransferase, and iridoid synthase genes which are involved in monoterpenoid indole alkaloid biosynthetic pathways (Zhou et al. 2015). Moreover, the highest levels of gene expression were detected in the presence of β-CD alone or in combination with MJ (Zhou et al. 2015).
All these results demonstrated that β-CD were able to increase the production of alkaloids, at least monoterpenoid indole alkaloids, in C. roseus cell cultures.
Use of cyclodextrins to increase the production of terpene in plant in vitro cultures
Terpenes are a wide group of plant natural products including at least 30.000 different compounds with a wide variety of chemical structures (Connolly and Hill 1991). In fact, hundreds of different terpenes are classified according to the number of carbons they have in the molecule, as monoterpene (C10), sesquiterpene (C15), diterpene (C20), triterpene (C30), tetraterpenes (C40), and polyterpenes (more than C40) (Degenhardt et al. 2009 and references therein). The wide variety of terpenoid compounds is mainly attributed to the action of terpene synthases. In fact, this high terpene diversity is due to the great number of different terpene synthases existing in nature, and the fact that these enzymes can biosynthesize multiple compounds. These terpene synthases transform the squalene skeleton and acyclic prenyl diphosphates into a high amount of cyclic and acyclic forms. Terpenes are found in different parts of plants such as roots, leaves, fruits, flowers, and barks, and these compounds can be produced in differentiated tissues and cells (Fojtová et al. 2008). Terpenes are considered secondary metabolites since they are produced by plants as a part of defense mechanisms against pathogens, and for their brilliant colour acting as pollinator attractants (Gershenzon and Dudareva 2007). In addition, terpenes also have beneficial effects on human health because they have antitumoral, neuroprotective or antiinflammatory activities (Cho et al. 2017).
The most commonly strategies used to obtain terpenes are their direct extraction from plant raw material, and the use of in vitro cultures (Tange et al. 2017). In this context, different elicitors including CD have been used to enhance the production of terpenes (De Alwis et al. 2009; Zare-Hassani et al. 2019) in plant in vitro cultures (Komaraiah et al. 2003; Sabater-Jara et al. 2010, 2014a, b; Durante et al. 2011; Rizzello et al. 2014). In fact, different CD have been used to enhance the production of monoterpenes and sesquiterpenes in plant in vitro cultures. Chakraborty and Chattopadhyay (2008) described that Mentha piperita cell cultures were able to yield up to 100 and 148 mg/L of menthol (an important monoterpene found in essential oils) in the presence of 60 mM γ-CD alone or in combination with 35 µM menthone, respectively, in comparison with 77 mg/L found in control treatment (Table 2). The treatment of hairy root cultures of Solanum tuberosum with an extract of Rhizoctonia bataticola, MJ and β-CD individually or in combination induced the formation of sesquiterpenes (Komaraiah et al. 2003). These elicitors also increased the production of sesquiterpenes in a dose-dependent manner. Thus, in the presence of 100 mg/L β-CD separately or in combination with 2 ml/L R. bataticola, the final concentration of rishitin, lubimin, phytuberin and phytuberol was 213, 171, 50 and 100 µg/g DW, respectively at 48 h of treatment (Komaraiah et al. 2003) (Table 2). Moreover, the elicitation of Capsicum annum cell cultures with randomly methylated-β-CD resulted in an increasing of the production of aromadendrene and solavetivone, reaching 46.1 and 63.7 µg/g DW, respectively, after 96 h of treatment (Sabater-Jara et al. 2010). In this case, the joint treatment with randomly methylated-β-CD and MJ provoked a rise of aromadendrene and solavetivone of 2353 and 1094 µg/g DW, respectively (Table 2). In fact, the combined treatment enhanced the production of sesquiterpenes more than 10 times respect to the action of randomly methylated β-CD alone.
On the other hand, Durante et al. (2011) also observed that 50 mM dimethylated-β-CD provoked an important enhancement of total artemisinin released to the culture medium (7.11 mg/g DW) in Artemisia annua cell cultures. Moreover, in this A. annua cell cultures, the joint action of 100 µM MJ and dimethylated-β-CD also stimulated artemisinin accumulation (7.76 mg/g DW) but the action of both elicitors was not significantly different (Table 2). In addition, Durante et al. (2011) also showed that different genes involved in artemisinin biosynthetic pathway were up-regulated in the presence of MJ and dimethylated-β-CD. In fact, CYP71AV1 expression was enhanced about two-fold when A. annua cell cultures were treated with MJ and dimethylated-β-CD. The expression levels of artemisinic aldehyde Δ11(13) reductase and AaWRKY1 were also up-regulated between 30 min and 48 h after treatment. These results suggested that the CYP71AV1, artemisinic aldehyde Δ11(13) reductase and AaWRKY1 gene expression could be involved in the production of artemisinin in A. annua cell cultures.
On the other hand, CD have also been used to increase the production of diterpenes (C20) and triterpenes (C30) in Taxus sp. and S. lycopersicum cell cultures. In fact, Sabater-Jara et al. (2014a) observed an enhanced production of taxanes in T. media cell cultures treated with 50 mM randomly methylated-β-CD, hydroxypropylated-β-CD or γ-CD. These authors showed that the maximal levels of taxanes were found when Taxus cell cultures were elicited with 50 mM randomly methylated-β-CD (15.90 mg/L) individually or in combination with MJ (140 mg/L) at day 16 of incubation (Table 2). Gene expression was not increased by the elicitation with randomly methylated-β-CD alone, but when these Taxus cell cultures were treated with both elicitors, a synergistic effect on the accumulation of transcripts was observed. In a similar way, taxane biosynthesis was significantly increased by the addition of randomly methylated-β-CD and 1 µM coronatine to the culture medium of T. media and T. globosa (Ramirez-Estrada et al. 2015). The total production of taxanes in T. globosa was lower than that of T. media cell cultures being the main taxanes found in T. globosa 10-deacetyltaxol and cephalomannine while in T. media were baccatin III and taxol. The high transcript levels involved in taxane biosynthesis in T. media cells was strongly correlated with the high production of taxanes (Table 2). Also, Kashani et al. (2018) used 50 mM methylated-β-CD and 1 µM coronatine to promote the production of paclitaxel (303.75 µg/g DW) in T. baccata cell cultures (Table 2). In addition, the expression of deacetylbaccatin III-10-O-acetyltransferase- DBAT, baccatin III-3-amino 13-phenylpropanoyl-CoA transferase- BAPT, and debenzoyltaxol N-benzoyl transferase, key genes involved in paclitaxel biosynthesis, increased by the joint action of coronatine and methylated-β-CD in T. baccata cell cultures.
The fact that taxanes form inclusion complexes with different CD provoked a decrease of the cellular toxicity, as well as a decrease of the extracellular enzymatic degradation of these bioactive compounds allowing a high accumulation of taxanes in the culture medium.
Recently, Vidal-Limón et al. (2018) described a new strategy for enhancing taxane production in T. media cell cultures, combining 50 mM randomly methylated-β-CD, 1 µM coronatine and 575 ml/L perfluorodecalins. The total taxane levels accumulated in elicited T. media cell cultures was 3.3-fold higher than in the control treatments. As regards to gene expression in elicited T. media cell cultures, a strong positive relationship was detected between the taxol production and the transcript levels of all genes analyzed.
On the other hand, the addition of 50 mM randomly methylated-β-CD to S. lycopersicum cv Micro-Tom cell cultures led to a release of triterpenes, such as phytosterols (mainly isofucosterol and β-sitosterol) and taraxasterol, a tomato fruit cuticular triterpene, to the culture medium. The production of isofucosterol, β-sitosterol, and taraxasterol was around 35, 20 and 219 µg/g DW (Briceño et al. 2012) (Table 2). These authors also analyzed the extracellular proteome of S. lycopersicum cv Micro-Tom cell cultures elicited with randomly methylated-β-CD and MJ. The presence of amino acid sequences homologous to a cationic peroxidase, pathogenesis-related 1 and 5 proteins, and a biotic cell death-associated protein were detected, which suggested that the joint action of randomly methylated-β-CD and MJ triggered the expression of proteins that mediate defense responses in S. lycopersicum cv Micro-Tom. In the same way, D. carota cell cultures produced high levels of phytosterols (95.69 ± 10.48 mg/L, that is 6.53 ± 0.58 mg/g DW) which were secreted to the culture medium in the presence of 50 mM of randomly methylated-β-CD at 144 h of treatment (Sabater-Jara and Pedreño 2013) (Table 2). These D. carota cell cultures were used to analyze the effect of MJ and randomly methylated-β-CD, separately or in combination, on the induction of defense responses, particularly in the accumulation of pathogenesis-related proteins (Sabater-Jara et al. 2014b). Under elicitation conditions, the presence of amino acid sequences homologous to glycoproteins and Leucine-Rich Repeat domain-containing proteins, which play an essential role in defense against pathogens, were detected. Also, some tryptic peptides similar to a thaumatin-like protein and a reticuline oxidase-like protein, which are related with defense responses against abiotic stress and fungi, respectively, were found. These data indicated that MJ and randomly methylated-β-CD played a role in mediating defense-related enzymes expression in D. carota cell cultures.
Miras-Moreno et al. (2016) also analyzed the effect of randomly methylated-β-CD on α-tocoferol production in D. carota cell cultures (Miras-Moreno et al. 2016). The results showed that the extracellular α-tocoferol production at the end of treatment was 599.61 µg/L that means 27.27 µg/g DW. These levels of α-tocopherol represented only around 8% of α-tocopherol total content because the highest levels of this compound were accumulated inside the cells (92%). In a similar way, Almagro et al. (2016) demonstrated that the presence of randomly methylated-β-CD did not enhance the production of tocopherols in Linum usitatissimum cell cultures, and only a rise in the levels of tocopherols was detected when these cell cultures were treated with 50 mM hydroxypropylated-β-CD in combination with 40 µM (Z)-3-hexenol (257 µg/g DW) or 1 mg/L β-glucan (174 µg/g DW).
Terpenoids including other compounds which are involved in essential plant processes such as photosynthesis (carotenoids) and respiration (ubiquinone Q9 and Q10), and they also have important benefits for human health due to their strong antioxidant activities (Gershenzon and Dudareva 2007). The main carotenoids intake from the diet includes xanthophylls such as lutein and zeaxanthin, and carotenes as β-carotene. These compounds prevent the appearance of some diseases as well as certain type of cancers (Krinsky and Johnson 2005).
The ability of dimethylated-β-CD to enhance the biosynthesis of carotenoids was also showed in A. annua L. cell cultures at 7 days of elicitation (Rizzello et al. 2014). In this study, the extracellular levels of β-carotene (10 µg/L) and lutein (140 µg/L) were lower than those found by Miras-Moreno et al. (2016) at day 7 of treatment in D. carota cell cultures treated with randomly methylated-β-CD (21.93 µg/L and 424.15 µg/L for β-carotene and lutein, respectively) (Table 2). However, at the same time, the intracellular levels of lutein and β-carotene (around 270 µg/g and 21 µg/g DW, respectively) were similar in A. annua and D. carota cell cultures treated with these different types of β-CD methylated (Rizzello et al. 2014; Miras-Moreno et al. 2016). Real-Time PCR analysis showed that the expression of 1-deoxy-d-xylulose-5-phosphate reductoisomerase gene in A. annua cell cultures treated with dimethylated-β-CD was 2.5-fold higher than in control cells, indicating that dimethylated-β-CD led to an enhancement of carotenoid levels due to the activation of the plastidial isoprenoid biosynthetic pathway. Furthermore, 50 mM randomly methylated-β-CD were also able to increase the release of lutein in cultures of the fungus Mucor circinelloides reaching levels of 4 µg/L in absence of 300 µM MJ and 18 µg/L in its presence (Sánchez-Pujante et al. 2017).
Mechanism of action of cyclodextrins
CD and derivatives have been often used for their ability to complex non polar compounds in their hydrophobic cavity to overcome their poor solubility in aqueous solutions, and in the case of compounds of phenolic nature, to avoid their oxidation, favoring their accumulation (Bru et al. 2006). This is particularly relevant when MJ is currently used as elicitor, especially in grapevine cell cultures where it induces the biosynthesis of stilbenes since the latter are oxidized in the culture medium by the action of peroxidases if CD are not present (Morales et al. 1998). Despite the fact that many researchers have tried to elucidate the mechanism by which these two elicitors act synergistically on living cell systems, the available information is fragmented because some of the processes involved in living cellular systems remains uncertain and are complex (Almagro et al. 2014b; Sabater-Jara et al. 2014a). Therefore, a large part of these studies has been carried out in vitro, in the absence of living cells. In fact, López-Nicolás et al. (2013) described the interaction of β-CD/MJ complexes comparing with those formed from β-CD/stilbene (trans-resveratrol) in aqueous solutions, in tests carried out in the absence of plant cell systems, without considering the possibility of binary and ternary complexes formed at the same time. Oliva et al. (2018) evaluated both β-CD/trans-resveratrol and β-CD/MJ interactions independently, and then considered the case in which the three compounds (β-CD, MJ and trans-resveratrol) interacted with each other. For it, they performed a complete structural characterization of both randomly methylated-β-CD and β-CD/trans-resveratrol complexes as well as the same types of CD forming complexes with MJ (that is, randomly methylated-β-CD and β-CD/MJ). The results obtained from their phase solubility diagrams, their structural analysis by nuclear magnetic resonance (NMR), and the determination of their thermodynamic parameters using isothermal titration calorimetry (ITC) demonstrated that trans-resveratrol much more increased its solubility and chemical stability in the presence of randomly methylated-β-CD than those randomly methylated-β-CD complexes formed with MJ. However, when the three compounds (CD, trans-resveratrol and MJ) were together in aqueous solution, no ternary complex was observed by either NMR or ITC. Oliva et al. (2018) concluded that the main benefits of using randomly methylated-β-CD resided in this increase in solubility and chemical stability of trans-resveratrol due to its very strong affinity by this type of CD, and the possibility to use high CD concentrations in the culture medium. These results strongly support the joint action of MJ and CD as elicitors on biosynthesis of trans-resveratrol through the signaling pathways in grapevine cells, since the presence of randomly methylated-β-CD/MJ complexes could even enhance the solubility of MJ in the culture medium, and so, provoke a highest elicitation response.
Apart from the production of metabolites, randomly methylated-β-CD were also able to activate different defense responses (Almagro et al. 2014a). In fact, Zamboni et al. (2009) and Almagro et al. (2014a) observed early defense responses induced by the presence of methylated-β-CD, which mediated the activation of different protein phosphatases and/or protein kinases in Vitis cell cultures, using pharmacological approaches, and obtaining similar results to other authors (Belchí-Navarro et al. 2013). In addition, randomly methylated-β-CD also induced different cascades of signal transduction which in turn, activated several families of transcription factors able to regulate the expression of genes encoding enzymes involved in biosynthetic pathways of both trans-resveratrol and defense-related proteins (Almagro et al. 2014a; Belchí-Navarro et al. 2019a). Moreover, randomly methylated-β-CD not only enhanced the production of secondary metabolites by inducing the gene expression of their biosynthetic pathways but also allowed the accumulation of these secondary metabolites in the culture medium, thereby reducing feedback inhibition. This fact prevented the cell death provoked by the high concentrations of secondary metabolites accumulated in the culture medium (Sabater-Jara and Pedreño 2013; Almagro et al. 2011; Sabater-Jara et al. 2014a; Briceño et al. 2012; Zamboni et al. 2009).
On the other hand, some studies have been carried out to improve understanding of intracellular signaling pathways involved in the production of secondary metabolites in plant cell cultures treated with randomly methylated-β-CD (Almagro et al. 2012; Belchí-Navarro et al. 2019a, b). Thus, Belchí-Navarro et al. (2019b) confirmed the involvement of hydrogen peroxide and nitric oxide in the trans-resveratrol production induced by randomly methylated-β-CD and MJ in V. vinifera cell cultures. In addition, hydrogen peroxide was mainly detected in cytoplasmic areas, and in the nucleoplasm while nitric oxide was detected in all the cellular compartments, mainly in the cytoplasmic organelles and nucleus. In the same way, Almagro et al. (2012) analyzed the early signal transduction pathways triggered by MJ and randomly methylated-β-CD in tobacco cell cultures. Their results indicated that MJ and randomly methylated-β-CD provoked a Ca2+ cytosolic rise promoted by Ca2+ influx through Ca2+-permeable channels. Moreover, oxidative burst induced by randomly methylated-β-CD was less pronounced than when tobacco cells are incubated with MJ alone. Moreover, the combined treatment increased nitric oxide levels, although to a lesser extent that in MJ-treated cells since the treatment with randomly methylated-β-CD alone did not trigger this accumulation. All these results showed the existence of different intracellular signaling pathways for MJ and randomly methylated-β-CD. In addition, randomly methylated-β-CD might act by regulating the signaling pathways led by MJ alone since when tobacco cell cultures were treated with both elicitors, the strong oxidative and nitrosative bursts decreased.
The combination of MJ and methylated-β-CD was also used by Somboon et al. (2019) to investigate the defense responses in peanut hairy root cultures. They described a dramatic increase in non-enzymatic antioxidant activities and phenolic compounds after MJ plus methylated-β-CD treatment. This finding revealed the presence of a defense mechanism against excessive reactive oxygen species (ROS) generation after the combined treatment in hairy root cultures. In addition, stilbene compounds such as trans-resveratrol, trans-archidin-1 and trans-arachidin-3 were also detected after MJ and methylated-β-CD elicitation. In fact, the jointly action of both elicitors resulted in a high metabolite content of stilbene compounds in the culture medium in a similar way as previously described by Yang et al. (2015).
On the other hand, plant cells display a wide variety of defense mechanisms to alleviate the deleterious effects of ROS. These mechanisms involve the activation of both endogenous antioxidant enzymes and non-enzymatic low-molecular-weight antioxidants such as glutathione and α-tocopherol, phenolic compounds, and pathogenesis-related proteins. The antioxidant enzymes as well as the non-enzymatic antioxidant systems that are launched against oxidative stress caused by the presence of methylated-β-CD and MJ were also differentially induced in peanut hairy root cultures (Somboon et al. 2019). In fact, superoxide dismutase activity was significantly decreased at long post-treatment with MJ and methylated-β-CD compared to control, but not significantly at short times. This finding was consistent with results previously described by Pilaisangsuree et al. (2018) which showed a down-regulation of CuZn-superoxide dismutase antioxidant enzyme gene expression, which indicated that MJ could regulate the suppression of hydroxypropylated-β-CD-responsive antioxidant enzyme gene expression. Therefore, the MJ and β-CD elicitation might be mediated through different signaling pathways, and trigger differential protein immunity, which could be controlled by plant signaling molecules to ensure complete cell protection against external stress.
Taking into consideration the importance of the mechanism by which CD are able to increase transport of secondary metabolites outside of cells, Martínez-Márquez et al. (2017) cloned a tau class glutathione-S-transferase (GSTU-2) from the cDNA of grapevine cells treated with MJ and methylated-β-CD, and it was used for Agrobacterium-mediated grapevine cell transformation. The non-elicited lines that overexpressed GSTU-2 produced extracellular trans-resveratrol while this metabolite was not detected in wild-type cell cultures. The transient expression of the GSTU-2-GFP fusion proteins showed localization in the plasma membrane in grapevine cells, and the immunoprecipitation of HA-tagged GSTU-2 revealed its interaction with HIR, a plasma membrane-bound protein. All these results indicated that GSTU-2 was involved in the transport of trans-resveratrol from the cells to extracellular medium. Moreover, Sabater-Jara et al. (2014a) showed a remarkable enhancement in the expression of a gene encoding a putative ABC protein when T. media cell cultures were elicited with MJ and randomly methylated-β-CD. This ABC gene likely encodes an integral and highly stable membrane protein that is involved in the transfer of paclitaxel from the cells to the culture medium (Sabater-Jara et al. 2014a; Cusido et al. 2014).
Conclusions
The treatment with native CD and their derivatives increases the production of secondary metabolites, enhancing the capability of plant in vitro cultures to produce high levels of different bioactive compounds. Moreover, CD has been also shown to interact positively with other elicitors such as MJ or coronatine provoking a synergistic effect on the production of different plant secondary metabolites such as stilbenoids and indole alkaloids. In this way, CD act as true elicitors by activating the biosynthetic pathway of certain metabolites such as resveratrol, or as metabolite extractors from cells, increasing the solubility of these metabolites in aqueous media since CD are able to form inclusion complexes with them.
Besides, the identification of the genes induced and repressed in response to CD treatments in plant in vitro cultures have been crucial to elucidate the mode of action of CD. Comparative metabolomic, transcriptomic, and proteomic studies have been carried out on both control and CD-treated cell cultures offering important clues about how CD are capable of substantially increasing the production of bioactive compounds in plant in vitro cultures. However, more studies are needed so as to truly understand how CD alone or in combination with other elicitors are capable of substantially altering defense responses in plant cells.
Abbreviations
- CD:
-
Cyclodextrins
- DW:
-
Dry weight
- FW:
-
Fresh weight
- GSTU:
-
Tau class glutathione-S-transferase
- ITC:
-
Isothermal titration calorimetry
- MJ:
-
Methyl jasmonate
- NMR:
-
Nuclear magnetic resonance
- ROS:
-
Reactive oxygen species
References
Almagro L, Perez AL, Pedreño MA (2011) New method to enhance ajmalicine production in Catharanthus roseus cell cultures based on the use of cyclodextrins. Biotechnol Lett 33:381–385
Almagro L, Bru R, Pugin A, Pedreño MA (2012) Early signaling network in tobacco cells elicited with methyl jasmonate and cyclodextrins. Plant Physiol Biochem 51:1–9
Almagro L, Sottomayor M, Pedreño MA (2013) Bioproduction of terpenoid indole alkaloids from Catharanthus roseus cell cultures. In: Ramawat KG, Merillon JM (eds) Natural products: phytochemistry, botany and metabolism of alkaloids, phenolics and terpenes, handbook of natural products. Springer, Berlin, pp 85–117
Almagro L, Carbonell-Bejerano P, Belchí-Navarro S, Bru R, Martínez-Zapater JM, Lijavetzky D, Pedreño MA (2014a) Dissecting the transcriptional response to elicitors in Vitis vinifera cells. PLoS ONE 9(10):e109777
Almagro L, Gutierrez J, Pedreño MA, Sottomayor M (2014b) Synergistic and additive influence of cyclodextrins and methyl jasmonate on the expression of the terpenoid indole alkaloid pathway genes and metabolites in Catharanthus roseus cell cultures. Plant Cell Tiss Org 119:543–551
Almagro L, García-Pérez P, Belchí-Navarro S, Sánchez-Pujante PJ, Pedreño MA (2016) New strategies for the use of Linum usitatissimum cell factories for the production of bioactive compounds. Plant Physiol Biochem 99:73–78
Astray G, Gonzalez-Barreiro C, Mejuto JC, Rial-Otero R, Simal-Gandara J (2009) A review on the use of cyclodextrins in foods. Food Hydrocoll 23:1631–1640
Atwood JL, Davies JED, Macnicol DD, Vogtle F (1996) Cyclodextrins. Compr Supramol Chem Cyclodext 3:6–32
Belchí-Navarro S, Pedreño MA, Corchete P (2011) Methyl jasmonate increases silymarin production in Silybum marianum (L.) Gaernt cell cultures treated with β-cyclodextrins. Biotechnol Lett 33:179–184
Belchí-Navarro S, Almagro L, Lijavetzky D, Bru R, Pedreño MA (2012) Enhanced extracellular production of trans-resveratrol in Vitis vinifera suspension cultured cells by using cyclodextrins and methyl jasmonate. Plant Cell Rep 31:81–89
Belchí-Navarro S, Almagro L, Sabater-Jara AB, Fernández-Pérez F, Bru R, Pedreño MA (2013) Early signaling events in grapevine cells elicited with cyclodextrins and methyl jasmonate. Plant Physiol Biochem 62:107–110
Belchí-Navarro S, Almagro L, Bru-Martinez R, Pedreño MA (2019a) Changes in the secretome of Vitis vinifera cv. Monastrell cell cultures treated with cyclodextrins and methyl jasmonate. Plant Physiol Biochem 135:520–527
Belchí-Navarro S, Rubio MA, Pedreño MA, Almagro L (2019b) Production and localization of hydrogen peroxide and nitric oxide in grapevine cells elicited with cyclodextrins and methyl jasmonate. J Plant Physiol 237:80–86
Briceño Z, Almagro L, Sabater-Jara AB, Calderón AA, Pedreño MA, Ferrer MA (2012) Enhancement of phytosterols, taraxasterol and induction of extracellular pathogenesis-related proteins in cell cultures of Solanum lycopersicum cv Micro-Tom elicited with cyclodextrins and methyl jasmonate. J Plant Physiol 169:1050–1058
Bru R, Sellés S, Casado-Vela J, Belchí-Navarro S, Pedreño MA (2006) Modified cyclodextrins are chemically defined glucan inducers of defense responses in grapevine cell cultures. J Agr Food Chem 54:65–71
Carqueijeiro I, Brown S, Chung K, Dang TT, Walia M, Besseau S, Munsch T (2018) Two tabersonine 6, 7-epoxidases initiate lochnericine-derived alkaloid biosynthesis in Catharanthus roseus. Plant Physiol 177:1473–1486
Chakraborty A, Chattopadhyay S (2008) Stimulation of menthol production in Mentha piperita cell culture. Vitro Cell Dev-Pl 44:518–525
Cheynier V (2012) Phenolic compounds: from plants to foods. Phytochem Rev 11:153–177
Chirinos R, Betalleluz-Pallardel I, Huamán A, Arbizu C, Pedreschi R, Campos D (2009) HPLC-DAD characterization of phenolic compounds from Andean oca (Oxalis tuberosa Mol.) tubers and their contribution to the antioxidant capacity. Food Chem 113:1243–1251
Cho KS, Lim YR, Lee K, Lee J, Lee JH, Lee IS (2017) Terpenes from forests and human health. Toxicol Res 33:97–106
Connolly JD, Hill RA (1991) Hemiterpenoids. In: Dictionary of Terpenoids 1-6. Springer US
Corchete P, Bru R (2013) Proteome alterations monitored by DIGE analysis in Silybum marianum cell cultures elicited with methyl jasmonate and methyl β-cyclodextrin. J Proteomics 85:99–108
Cravotto G, Binello A, Baranelli E, Carraro P, Trotta F (2006) Cyclodextrins as food additives and in food processing. Curr Nutr Food Sci 2:343–350
Cusido RM, Onrubia M, Sabater-Jara AB, Moyano E, Bonfill M, Goossens A, Pedreño MA, Palazon J (2014) A rational approach to improving the biotechnological production of taxanes in plant cell cultures of Taxus spp. Biotechnol Adv 32:1157–1167
De Alwis R, Fujita K, Ashitani T, Kuroda KI (2009) Volatile and non-volatile monoterpenes produced by elicitor-stimulated Cupressus lusitanica cultured cells. J Plant Physiol 166:720–728
Degenhardt J, Köllner TG, Gershenzon J (2009) Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry 70:1621–1637
Durante M, Caretto S, Quarta A, De Paolis A, Nisi R, Mita G (2011) β-Cyclodextrins enhance artemisinin production in Artemisia annua suspension cell cultures. Appl Microbiol Biotechnol 90:1905–1913
Fojtová J, Lojková L, Kubáň V (2008) GC/MS of terpenes in walnut tree leaves after accelerated solvent extraction. J Sep Sci 31:162–168
García-Pérez P, Losada-Barreiro S, Gallego PP, Bravo-Díaz C (2019) Cyclodextrin-elicited Bryophyllum suspension cultured cells: enhancement of the production of bioactive compounds. Int J Mol Sci 20:5180–5198
Gentili A (2020) Cyclodextrin-based sorbents for solid phase extraction. J Chrom A 1609:460654–460672
Gershenzon J, Dudareva N (2007) The function of terpene natural products in the natural world. Nat Chem Biol 3:408–416
Gidwani B, Vyas A (2014) Synthesis, characterization, and application of epichlorohydrin-β-cyclodextrin polymer. Colloid Surface B 114:130–137
Hidalgo D, Martínez-Márquez A, Cusidó R, Bru-Martínez R, Palazón J, Corchete P (2017) Silybum marianum cell cultures stably transformed with Vitis vinifera stilbene synthase accumulate t-resveratrol in the extracellular medium after elicitation with methyl jasmonate or methylated β-cyclodextrins. Eng Life Sci 17:686–694
Jaisi A, Panichayupakaranant P (2020) Enhanced plumbagin production in Plumbago indica root culture by simultaneous and sequential dual elicitations using chitosan with l-alanine and methyl-β-cyclodextrin. Bioresour Bioprocess 7(1):1–7
Kashani K, Javaran MJ, Sabet MS, Moieni A (2018) Identification of rate-limiting enzymes involved in paclitaxel biosynthesis pathway affected by coronatine and methyl-β-cyclodextrin in Taxus baccata L. cell suspension cultures. DARU 26:129–142
Kennedy DO, Wightman EL (2011) Herbal extracts and phytochemicals: plant secondary metabolites and the enhancement of human brain function. Adv Nutr 2:32–50
Komaraiah P, Reddy GV, Reddy PS, Raghavendra AS, Ramakrishna SV, Reddanna P (2003) Enhanced production of antimicrobial sesquiterpenes and lipoxygenase metabolites in elicitor-treated hairy root cultures of Solanum tuberosum. Biotechnol Lett 25:593–597
Krinsky NI, Johnson EJ (2005) Carotenoid actions and their relation to health and disease. Mol Aspects Med 26:459–516
Lambert C, Lemaire J, Auger H, Guilleret A, Reynaud R, Clément C, Courot E, Taidi B (2019) Optimize, modulate, and scale-up resveratrol and resveratrol dimers bioproduction in Vitis labrusca L. cell suspension from flasks to 20 l bioreactor. Plants 8:567–586
Landy D, Mallard I, Ponchel A, Monflier E, Fourmentin S (2012) Remediation technologies using cyclodextrins: an overview. Environ Chem Lett 10:225–237
Lijavetzky D, Almagro L, Belchi-Navarro S, Martínez-Zapater JM, Bru R, Pedreño MA (2008) Synergistic effect of methyl jasmonate and cyclodextrin on stilbene biosynthesis pathway gene expression and resveratrol production in Monastrell grapevine cell cultures. BMC Res Not 1:132–139
Liu YP, Liu QL, Zhang XL, Niu HY, Guan CY, Sun FK, Fu YH (2019) Bioactive monoterpene indole alkaloids from Nauclea officinalis. Bioorg Chem 83:1–5
López-Nicolás JM, Escorial Camps M, Pérez-Sánchez H, García-Carmona F (2013) Physicochemical and thermodynamic characterization of the encapsulation of methyl jasmonate by natural and modified cyclodextrins using reversed-phase high-pressure liquid chromatography. J Agric Food Chem 61:11347–11354
Manach C, Mazur A, Scalbert A (2005) Polyphenols and prevention of cardiovascular diseases. Curr Opin Lipidol 16:77–84
Martínez-Alonso A, Losada-Barreiro S, Bravo-Diaz C (2015) Encapsulation and solubilization of the antioxidants gallic acid and ethyl, propyl, and butyl gallate with β-cyclodextrin. J Mol Liq 210:143–150
Martínez-Esteso MJ, Sellés-Marchart S, Vera-Urbina JC, Pedreño MA, Bru-Martinez R (2009) Changes of defense proteins in the extracellular proteome of grapevine (Vitis vinifera cv. Gamay) cell cultures in response to elicitors. J Proteomics 73:331–341
Martínez-Márquez A, Martínez-Esteso MJ, Vilella-Antón MT, Sellés-Marchart S, Morante-Carriel JA, Hurtado E, Palazón J, Bru-Martínez R (2017) A tau class glutathione-S-transferase is involved in trans-resveratrol transport out of grapevine cells. Front Plant Sci 8:1457–1472
Miras-Moreno B, Almagro L, Pedreño MA, Sabater-Jara AB (2016) Enhanced accumulation of phytosterols and phenolic compounds in cyclodextrin-elicited cell suspension culture of Daucus carota. Plant Sci 250:154–164
Morales M, Bru R, Garcia-Carmona F, Barceló AR, Pedreño MA (1998) Effect of dimethyl-β-cyclodextrins on resveratrol metabolism in Gamay grapevine cell cultures before and after inoculation with shape Xylophilus ampelinus. Plant Cell Tiss Org 53:179–187
Nivelle L, Hubert J, Courot E, Jeandet P, Aziz A, Nuzillard JM, Renault JH, Clément C, Martiny L, Delmas D, Tarpin M (2017) Anti-cancer activity of resveratrol and derivatives produced by grapevine cell suspensions in a 14 L stirred bioreactor. Molecules 22:474–488
Oliva E, Mathiron D, Bertaut E, Landy D, Cailleu D, Pilard S, Djedaïni-Pilard F (2018) Physicochemical studies of resveratrol, methyl-jasmonate and cyclodextrin interactions: an approach to resveratrol bioproduction optimization. RSC Adv 8:1528–1538
Parage C, Foureau E, Kellner F, Burlat V, Mahroug S, Lanoue A, Besseau S (2016) Class II cytochrome P450 reductase governs the biosynthesis of alkaloids. Plant Physiol 172:1563–1577
Perassolo M, Smith ME, Giulietti AM, Talou JR (2016) Synergistic effect of methyl jasmonate and cyclodextrins on anthraquinone accumulation in cell suspension cultures of Morinda citrifolia and Rubia tinctorum. Plant Cell Tiss Org 124:319–330
Pietrowska-Borek M, Czekała Ł, Belchí-Navarro S, Pedreño MA, Guranowski A (2014) Diadenosine triphosphate is a novel factor which in combination with cyclodextrins synergistically enhances the biosynthesis of trans-resveratrol in Vitis vinifera cv. Monastrell suspension cultured cells. Plant Physiol Biochem 84:271–276
Pilaisangsuree V, Somboon T, Tonglairoum P, Keawracha P, Wongsa T, Kongbangkerd A, Limmongkon A (2018) Enhancement of stilbene compounds and anti-inflammatory activity of methyl jasmonate and cyclodextrin elicited peanut hairy root culture. Plant Cell Tiss Org 132:165–179
Puupponen-Pimiä R, Nohynek L, Meier C, Kähkönen M, Heinonen M, Hopia A, Oksman-Caldentey KM (2001) Antimicrobial properties of phenolic compounds from berries. J Appl Microbiol 90:494–507
Qian Liu Y, Yang L, Tian X (2007) Podophyllotoxin: current perspectives. Curr Bioact Compd 3:37–66
Ramawat KG, Mérillon JM (2013) Natural products: phytochemistry, botany and metabolism of alkaloids, phenolics and terpenes (1541–2662). Springer, Heidelberg
Ramirez-Estrada K, Osuna L, Moyano E, Bonfill M, Tapia N, Cusido RM, Palazón J (2015) Changes in gene transcription and taxane production in elicited cell cultures of Taxus × media and Taxus globosa. Phytochemistry 117:174–184
Ramírez-Estrada K, Vidal-Limon H, Hidalgo D, Moyano E, Golenioswki M, Cusidó RM, Palazón J (2016) Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules 21:182–206
Randhir R, Lin YT, Shetty K, Lin YT (2004) Phenolics, their antioxidant and antimicrobial activity in dark germinated fenugreek sprouts in response to peptide and phytochemical elicitors. Asia Pac J Clin Nutr 13:295–307
Rao SR, Ravishankar GA (1999) Biotransformation of isoeugenol to vanilla flavour metabolites and capsaicin in suspended and immobilized cell cultures of Capsicum frutescens: study of the influence of β-cyclodextrin and fungal elicitor. Process Biochem 35:341–348
Rizzello F, De Paolis A, Durante M, Blando F, Mita G, Caretto S (2014) Enhanced production of bioactive isoprenoid compounds from cell suspension cultures of Artemisia annua L. using β-cyclodextrins. Int J Mol Sci 15:19092–19105
Roberts MF (2013) Alkaloids: biochemistry, ecology, and medicinal applications. Springer, New York
Rojas TR, Sampayo CAF, Vázquez BI, Franco CM, Cepeda A (2005) Study of interferences by several metabolites from Aspergillus spp. in the detection of aflatoxigenic strains in media added with cyclodextrin. Food Control 16:445–450
Sabater-Jara AB, Pedreño MA (2013) Use of β-cyclodextrins to enhance phytosterol production in cell suspension cultures of carrot (Daucus carota L.). Plant Cell Tiss Org 114:249–258
Sabater-Jara AB, Almagro L, Belchí-Navarro S, Ferrer MÁ, Barceló AR, Pedreño MA (2010) Induction of sesquiterpenes, phytoesterols and extracellular pathogenesis-related proteins in elicited cell cultures of Capsicum annuum. J Plant Physiol 167:1273–1281
Sabater-Jara AB, Almagro L, Pedreño MA (2014a) Induction of extracellular defense-related proteins in suspension cultured-cells of Daucus carota elicited with cyclodextrins and methyl jasmonate. Plant Physiol Biochem 77:133–139
Sabater-Jara AB, Onrubia M, Moyano E, Bonfill M, Palazón J, Pedreño MA, Cusidó RM (2014b) Synergistic effect of cyclodextrins and methyl jasmonate on taxane production in Taxus × media cell cultures. Plant Biotech J 12:1075–1084
Saleem M, Kim HJ, Ali MS, Lee YS (2005) An update on bioactive plant lignans. Nat Prod Rep 22:696–716
Sánchez-Pujante PJ, Miras-Moreno B, Soluyanova P, Garre V, Pedreño MA, Almagro L (2017) Production of fatty acid methyl esters and other bioactive compounds in elicited cultures of the fungus Mucor circinelloides. Mycol Prog 16:507–512
Somboon T, Chayjarung P, Pilaisangsuree V, Keawracha P, Tonglairoum P, Kongbangkerd A, Wongkrajang K, Limmongkon A (2019) Methyl jasmonate and cyclodextrin-mediated defense mechanism and protective effect in response to paraquat-induced stress in peanut hairy root. Phytochemistry 163:11–22
Soto-Vaca A, Gutierrez A, Losso JN, Xu Z, Finley JW (2012) Evolution of phenolic compounds from color and flavor problems to health benefits. J Agr Food Chem 60:6658–6677
Soto-Argel C, Hidalgo D, Palazon J, Corchete P (2018) Extracellular chromone derivatives in cell cultures of Pimpinella anisum. Influence of elicitation with methyl jasmonate and 2β-methyl cyclodextrins. Biotechnol Lett 40(2):413–418
Tange TO, Naesby M, Folly C, Delegrange F, Houghton-Larsen J, Carlsen S (2017) US Patent No. 9,834,800. Washington, DC: US Patent and Trademark Office
Tatsis EC, Carqueijeiro I, de Bernonville TD, Franke J, Dang TTT, Oudin A, Courdavault V (2017) A three enzyme system to generate the Strychnos alkaloid scaffold from a central biosynthetic intermediate. Nat Commun 8:1–10
Tisserant LP, Aziz A, Jullian N, Jeandet P, Clément C, Courot E, Boitel-Conti M (2016) Enhanced stilbene production and excretion in Vitis vinifera cv Pinot Noir hairy root cultures. Molecules 21:1703–1720
Torres M, Corchete P (2016) Gene expression and flavonolignan production in fruits and cell cultures of Silybum marianum. J Plant Physiol 192:111–117
Vang O, Ahmad N, Baile CA, Baur JA, Brown K, Csiszar A, Ma QY (2011) What is new for an old molecule? Systematic review and recommendations on the use of resveratrol. PLoS ONE 6(6):e19881
Vidal-Limón HR, Almagro L, Moyano E, Palazón J, Pedreño MA, Cusido RM (2018) Perfluorodecalins and hexenol as inducers of secondary metabolism in Taxus media and Vitis vinifera cell cultures. Front Plant Sci 9:335–350
Wang B, Dai Z, Yang XW, Liu YP, Khan A, Yang ZF, Luo XD (2018) Novel nor-monoterpenoid indole alkaloids inhibiting glioma stem cells from fruits of Alstonia scholaris. Phytomedicine 48:170–178
Woerdenbag HJ, van Uden W, Frijlink HW, Lerk CF, Pras N, Malingré TM (1990) Increased podophyllotoxin production in Podophyllum hexandrum cell suspension cultures after feeding coniferyl alcohol as a β-cyclodextrin complex. Plant Cell Rep 9:97–100
Yang T, Fang L, Nopo-Olazabal C, Condori J, Nopo-Olazabal L, Balmaceda C, Medina-Bolivar F (2015) Enhanced production of resveratrol, piceatannol, arachidin-1, and arachidin-3 in hairy root cultures of peanut co-treated with methyl jasmonate and cyclodextrin. J Agr Food Chem 63:3942–3950
Yoo ES, Choo GS, Kim SH, Woo JS, Kim HJ, Park YS, Nam JS (2019) Antitumor and apoptosis-inducing effects of piperine on human melanoma cells. Anticancer Res 39:1883–1892
Zamboni A, Vrhovsek U, Kassemeyer HH, Mattivi F, Velasco R (2006) Elicitor-induced resveratrol production in cell cultures of different grape genotypes (Vitis spp.). Vitis 45:63–68
Zamboni A, Gatto P, Cestaro A, Pilati S, Viola R, Mattivi F, Moser C, Velasco R (2009) Grapevine cell early activation of specific responses to DIMEB, a resveratrol elicitor. BMC Genom 10:363–376
Zare-Hassani E, Motafakkerazad R, Razeghi J, Kosari-Nasab M (2019) The effects of methyl jasmonate and salicylic acid on the production of secondary metabolites in organ culture of Ziziphora persica. Plant Cell Tiss Org 138:437–444
Zhang Y, Goto M, Oda A, Hsu PL, Guo LL, Fu YH, Hao XJ (2019) Antiproliferative aspidosperma-type monoterpenoid indole alkaloids from Bousigonia mekongensis inhibit tubulin polymerization. Molecules 24:1256–1266
Zhou P, Yang J, Zhu J, He S, Zhang W, Yu R, Huang X (2015) Effects of β-cyclodextrin and methyl jasmonate on the production of vindoline, catharanthine, and ajmalicine in Catharanthus roseus cambial meristematic cell cultures. Appl Microbiol Biotechnol 99:7035–7045
Acknowledgements
This work was supported by the Ministerio de Economía y Competitividad (BIO2017-82374-R) and Fundación Seneca-Agencia de Ciencia y Tecnología de la Región de Murcia (19876/GERM/15).
Authors’ contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Lorena Almagro and Maria Angeles Pedreño. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Almagro, L., Pedreño, M.Á. Use of cyclodextrins to improve the production of plant bioactive compounds. Phytochem Rev 19, 1061–1080 (2020). https://doi.org/10.1007/s11101-020-09704-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11101-020-09704-6