Abstract
In the last decade the prevalence of diabetes has escalated globally and it is estimated that the number of diabetic people will increase to 642 million by 2040. Although numerous classes of pharmaceutical drugs are available to treat Type ll diabetes, they manifest certain side effects. PTP1B has attracted significant interest as an important therapeutic agent and has been validated to target diabetes and obesity. Fungi, in general, produce secondary metabolites with some amazing chemical and structural diversity and are recognized to be a valuable source for therapeutic molecules. In this review, the focus is on describing the PTP1B effects and their potential as anti-diabetic agents for the various metabolites isolated from fungi.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hyperglycemia is the hallmark of diabetes mellitus. Despite serious efforts to address this issue, the incidence of diabetes mellitus (type-2 diabetes mellitus) and the deaths attributed due to diabetes are rapidly increasing on an international scale. Insulin is an important hormone that performs multiple functions, primarily to maintain normal glucose index within the human body (Hussain et al. 2019; Shrestha et al. 2019). Protein tyrosine phosphatase (PTP) 1B is involved in the down-regulation of insulin and leptin signaling and thus is an emerging therapeutic target for the management of diabetes and obesity (Krishnan et al. 2018).
Both protein tyrosine phosphatases (PTPs) as well as its kinases (PTKs) are signaling enzymes which play vital roles in crucial cellular pathways such as metabolic regulation, mitosis, apoptosis and cell proliferation by controlling phosphorylation of proteins. PTKs actually catalyze the phosphorylation process of tyrosine residues of proteins whereas PTPs hydrolyze the tyrosine phosphate to consequently reverse this action (Shi et al. 2019). PTP1B is a member of the non-receptor PTP family and is widely expressed in the cytoplasmic face of the endoplasmic reticulum. PTP1B mediates the dephosphorylation process that leads to inactivation of insulin and leptin signaling pathways. In connection with insulin signaling, PTP1B results in dephosphorylation of the active insulin receptor (IR) and its downstream targets of the substrate of the insulin receptor while in the case of leptin signaling, it antagonizes Janus kinase (Jak2) activity, which is directly downstream from the leptin receptor. PTP1B negatively regulates signaling pathways of both insulin and leptin and consequently is involved in glucose and lipid homeostasis. In a high fat animal diet model, ablation of the PTPN1 gene that encodes PTP1B resulted in increased insulin sensitivity and resistance to obesity (Shi et al. 2019).
PTP1B has attracted significant interest with an enormous therapeutic potential and is a validated target against diabetes and obesity. PTP1B is also associated with dendritic cell-based cancer immunotherapy since it is involved in the progression of various types of cancers (Xu et al. 2019). Since PTPs exhibit a high degree of structure similarity in their active sites, selectivity and high affinity for PTP1B are major challenges in the development of PTP1B inhibitors as drugs (Zhang et al. 2007a). During the past two decades, various efforts have been made to discover potential PTP1B inhibitors with broad structural diversity where it was found that most of the inhibitors incorporated phosphotyrosine (pTyr) mimetics in order to develop the essential binding ability at the PTP1B catalytic site. In addition, novel PTP1B inhibitors can overcome these limitations if they were able to target alternative binding sites other than the catalytic site of PTP1B (Tang et al. 2018).
Natural products are an important source in lead compounds for the fight against cancer, bacterial, malaria, fungal infections, autoimmune disorders, and cardiovascular diseases (Bills and Gloer 2016; Newman and Cragg 2016). Additional numerous natural product derived agricultural chemicals have been reported (Bills and Gloer 2016; Asolkar et al. 2013; Rimando and Duke 2006). Fungi are capable to generate interesting and novel secondary metabolites with fascinating chemical diversity from simple starting materials viz., sugars, organic acids, terpenes, amino acids, pyrimidines, and purines (González-Medina et al. 2017). Fungi are in particular, abundant sources of lead compounds and have spectacularly contributed for the beneficiation of human and animal health. The best known examples are penicillins, Cephalosporin C, cephalosporins, pleuromutilin, griseofulvin, echinocandin B, enfumafungin, lovastatin, cyclosporin A, myriocin, ergotamine, ergocryptine, mizoribine, and gibberellic acid (Bills and Gloer 2016).
A literature survey revealed that numerous natural products have been reported to possess PTP1B inhibitory effects. Moreover several reviews dealing with PTP1B inhibitor drug development, have been published. However, the majority of them centered on synthetic PTP1B inhibitors (Zhang et al. 2007b; Combs 2010; Nichols et al. 2006; Taylor and Hill 2004; Taylor 2003; Lee and Wang 2007; Mohler et al. 2009; Thareja et al. 2012). In addition Zhao et al. (2018) published a review on natural products which showed PTP1B inhibition (Zhao et al. 2018) and earlier, Wang et al. (2015) published a review about natural and semisynthetic PTP1B inhibitors covering the literature from 2009 to 2014. Additionally, Jiang et al. (2012) reviewed the natural PTP1B inhibitors from 2002 to 2011 while two reviews have been published on PTP1B inhibitory effects of marine natural products (Ezzat et al. 2018; Zhou et al. 2017). The current literature review aims to give an overview on PTP1B inhibitory effects of secondary metabolites derived from various fungi.
We have used various databases as an integral part of our literature search methodology available from the scientific literature (Science Finder, Springer, Science direct, PubMed, Scopus, and Google Scholar) in order to collect and collate all available literature reports. In addition, our search utilized keywords such as: “protein tyrosine phosphatase 1B” or “PTP1B” (alone or in combination), “fungus” or “ fungi” with an additional keyword “protein tyrosine phosphatase 1B” or “PTP1B” among others.
Alkaloids
Indole and indole diketopiperazine alkaloids
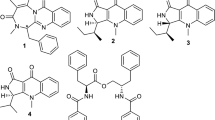
Indole-terpenoids, penerpenes A (1) and B (2) along with paxilline (3) (Fig. 1) and emindole SB (4), were reported from the fungus Penicillium sp. and their structures were established via spectroscopic and ECD analysis. Among these compounds, emindole SB (4: IC50: 0.7 µM) was the most potent PTP1B inhibitor followed by compounds 1 (IC50: 1.7 µM) and 2 (IC50: 2.4 µM). On the other hand, paxilline (3) was moderately active with IC50: 10.6 µM (Kong et al. 2019) (Table 1). Paxilline (3) was first isolated from P. paxilli (Cole et al. 1974) and showed various biological effects (Knaus et al. 1994; Zhou and Lingle 2014; McLeay et al. 1999). Emindole SB (4) was previously reported from the fungi Emericella striata (Nozawa et al. 1988), Albophoma yamanshiensis (Huang et al. 1995), Aspergillus oryzae (Qiao et al. 2010).
Fructigenine A (5) (Fig. 2) is a unique indole alkaloid which features a reverse-prenyl moiety was originally isolated from various strains of Penicillium spp. (Arai et al. 1989; Sohn et al. 2013), and exhibited PTP1B effects with IC50: 10.7 µM (Sohn et al. 2013). The diketopiperazine alkaloid, echinulin (6) was reported from the fungus Eurotium sp. (Sohn et al. 2013) along with several Aspergillus spp. (Bräse et al. 2009; Liang et al. 2018) and is reported to possess moderate PTP1B inhibition with IC50: 29.4 µM (Sohn et al. 2013).
Two indole diketopiperazine dimers, SF5280-451 (7) and SF5280-415 (8) (Fig. 2) were isolated from Aspergillus sp. and demonstrated PTP1B activity with IC50: 12.9 and 14.2 µM, respectively (Cho et al. 2018). The SAR study for these compounds showed that an exchange of the benzyl group (compound 7) or the iso butyl group (compound 8) does not effect the activity to any great extent. Indole-terpenoids, penerpenes E (9: IC50: 14 µM), F (10: IC50: 27 µM) and H (11: IC50: 23 µM) were reported from the fungus Penicillium sp. and possesses activity towards PTP1B. Moreover compound 9 possesses a core with an unusual 6/5/5/6/6/5/5 heptacyclic framework (Zhou et al. 2019). 7-Hydroxypaxilline-13-ene (12) was also isolated from Penicillium sp. (Zhou et al. 2019; Ariantari et al. 2019) and this paxilline analog illustrated PTP1B effects with IC50: 13 µM (Zhou et al. 2019).
Amauromine (13) was initially reported from the fungus Amauroascus sp. (Takase et al. 1984, 1985) and the subsequent synthesis of this compound, due to its unusual chemical structure, was later achieved to unambiguously confirm the assigned structure (Mueller and Stark 2016; Takase et al. 1986). Amauromine (13) was recently reported from the fungus Malbranchea circinate and demonstrated PTP1B effects with IC50: 15.3 µM (Rangel-Grimaldo et al. 2020). Malbrancheamide (14) was isolated from the fungi Penicillium sp., Aspergillus sp. and Malbranchea aurantiaca (Martinez-Luis et al. 2006; Figueroa et al. 2008; Miller et al. 2008; Rangel-Grimaldo et al. 2020) and possesses significant PTP1B effects with IC50: 14.5 µM (Rangel-Grimaldo et al. 2020).
Quinoline and quinazolinone alkaloids
Marinamide (15) (Fig. 3) was produced by the fungus Aspergillus sp. and this compound inhibited PTPlB with an IC50: 23.3 µg/mL. In 2006, Feng and Lin isolated marinamide (15) and proposed the structure 16 for marinamide which turned out to be incorrect (Feng & Lin 2006). In 2010, She, Lin and co-workers reported on the isolation of marinamide from Penicillium sp. (Shao et al. 2010). A year later, Koenig and coworkers determined the correct structure for marinamide via X-ray spectroscopy (Elsebai et al. 2011) to be 15 which was further confirmed via total synthesis (Naveen et al. 2017). Additionally, viridicatol, a quinoline alkaloid (17) also demonstrated PTP1B inhibition with IC50: 64 µM (Sohn et al. 2013) which compound was isolated from the fungi Penicillium sp (Birkinshaw et al. 1963) and Eurotium sp. (Sohn et al. 2013).
Quinazolinone alkaloids have been reported from various fungi and demonstrated to possess a wide range of biological effects (Kshirsagar 2015). Quite recently, the two quinazolinone alkaloids, 18 and 19 (Fig. 3) were isolated from the fungus Malbranchea circinate and demonstrated PTP1B activity with IC50: 17.3 and 106.2 µM, respectively. Interesting, compound 18 was six fold more potent than 19 which would suggest that the C-15 hydroxyl group plays a crucial role in the activity (Rangel-Grimaldo et al. 2020). Notably, compounds 18 and 19 featured a quinazolinone core linked to an aromatic part (ring C) via the imine carbon and this type of framework is also present in sclerotigenin (Witt and Bergman 2002), circumdatin F (Rahbæk and Breinholt 1999), asperlicins (Tseng et al. 2010), and benzo-malvins (Clevenger et al. 2018; Jang et al. 2012).
Miscellaneous alkaloids
Terreusinone A (20) was produced by the fungus Cordyceps gracilioides and PTP1B has an inhibition of 12.5 µg/mL (Wei et al. 2015). Cyclopenol (21) is a benzodiazepine alkaloid which was reported from various Aspergillus sp. (Zhuravleva et al. 2016) and Penicillium sp. (Sohn et al. 2013; Bräse et al. 2009), and illustrated activity towards PTP1B with an IC50: 30 µM (Sohn et al. 2013). The pyridone alkaloid, fumosorinone (22) (Fig. 4) is an amorphous solid produced by the fungus Isaria fumosorosea and this alkaloid demonstrated significant PTP1B effects with an IC50: 14.0 µM. Furthermore, a kinetic study revealed that compound 22 is a noncompetitive inhibitor (Liu et al. 2015a, b).
Fumosorinone (22) furthermore demonstrated a significant similarity with the 2-pyridones viz., tenellin (Eley et al. 2007), aspyridone A (Xu et al. 2010), and desmethylbassianin (Heneghan et al. 2011). These compounds could conceivably share similar biosynthetic pathways (Liu et al. 2015a, b). In another study, Liu et al. reported that compound 22 reduces PTP1B expression, increases the insulin-provoked glucose uptake, and also decreases blood glucose and lipid levels in mice (Liu et al. 2015a, b). Moreover, fumosorinone (22) is also active towards other PTPs (Chen et al. 2015a, b). The fungus Isaria fumosorosea produces fumosorinone A (23), which inhibits the activity of PTP1B with IC50: 3.24 µM (Zhang et al. 2017). This report revealed that compound 23 is more potent than compound 22 and 4-times more potent than sodium orthovanadate (IC50 = 11.3 µM). Quite recently, Schobert and his group reported the total synthesis of fumosorinone A (23) (Bruckner et al. 2018).
Depsides
KS-506a (24) and KS-506m (25) (Fig. 5) were isolated from Micromucor ramannianus and evaluated for their PTP1B inhibition. Interestingly, compound 24 possesses potent PTP1B inhibition with an IC50: 4.9 µM and Ki: 2.7 µM (Table 1) while metabolite 25 proved to be moderately active IC50: 69.9 µM (Oh et al. 2004). Notably, compounds 24 and 25 were previously isolated from Mortierella vinacea (Kuroda 1988). In another report the fungus Cosmospora sp. was described to produce the glycosylated depside, aquastatin A (26) and this compound was shown to possess potent PTP1B effects with an IC50: 0.19 µM (Seo et al. 2009). In addition, compound 26 was converted into its methyl ester 27 whose PTP1B activity proved to unfortunately be less than (IC50: 17 µM). This indicated that the carboxylic acid moiety plays a crucial role in the inhibition mechanism (Zhang et al. 2008a; Na et al. 2006). Compound 26 after being subjected to enzymatic hydrolysis, produced compound 28 and this latter metabolite inhibited PTP1B with IC50: 0.22 µM). Further hydrolysis of 26 with NaOH produced product 29 which inhibited PTP1B with an IC50: 0.59 µM (Seo et al. 2009). This study suggest that the additional aromatic ring present in compound 26 has a very minor influence on PTP1B effects.
Trivaric acid (30) (Fig. 6) was initially reported to be isolated from lichen (Culberson et al. 1999) but later also from the fungus F10Z1082 (Sun et al. 2017a, b). Trivaric acid (30) illustrated very potent effects towards PTP1B with an IC50: 0.17 µM. Moreover, compound 30 possesses an insulin sensitizing activity in diabetic and normal mice (Sun et al. 2017a, b). In another study trivaric acid (30) inhibited PTP1B very strongly with an IC50: 173 nM. An in vivo study of this depside demonstrated that it increased insulin stimulation and glucose consumption. In addition compound 30 decreases glucose levels and boosts insulin resistance (Sun et al. 2017a, b). Similarly, nordivaricatic acid (31) and the divarinyl divarate (32) were reported as lichen metabolites (Zheng et al. 2012) and later isolated from the fungus F10Z1082 (Sun et al. 2017a, b). Depsides 31 and 32 strongly inhibited PTP1B with IC50: 0.51 and 0.72 µM respectively (Sun et al. 2017a, b). An SAR study of compounds 31 and 32 demonstrated that the carboxylic acid plays only a minor role in PTP1B effects.
Xanthones and anthraquinones
Bis-tetrahydroxanthones, asperdichrome (33) and secalonic acid F (34) (Fig. 7) were obtained from the fungus Aspergillus sp. Metabolites 33 (IC50: 6.0 µM) and 34 (IC50: 9.6 µM) demonstrated significant activity towards PTP1B (Yamazaki et al. 2016). Secalonic acid F (34) was previously reported from Aspergillus sp. (Andersen et al. 1977) and the lichen Diploicia canescens (Millot et al. 2009). Secalonic acid F (34) was recently isolated from Aspergillus sp. and illustrated PTP1B effects with IC50: 5.9 µM (Rotinsulu et al. 2017). 4-Isoprenylravenelin (35) was produced by the fungus Malbranchea circinate and illustrated activity towards PTP1B with an IC50: 13.9 µM (Rangel-Grimaldo et al. 2020). Anthraquinone glycosides 36–39 were produced by the fungus Malbranchea circinate and these metabolites possess a D-ribofuranosyl group. Moreover, all these compounds demonstrated PTP1B effects with IC50 values ranging from 25.5 to 92.5 µM. Metabolites 37 (IC50: 27.9 µM) and 39 (IC50: 25.5 µM) illustrated better effects than compounds 36 and 38 (Rangel-Grimaldo et al. 2020). 6-O-Methylalaternin (40) was produced by the fungi Stemphylium globuliferum (Debbab et al. 2009) and Alternaria sp. (Qi 2019) and were shown to possess potent PTP1B effects with IC50: 0.62 µM (Qi 2019).
Azaphilones
Pinophilin C (41) (Fig. 8) was produced by the fungus Cordyceps sp. (Wei et al. 2015; Isaka et al. 2001) and illustrated PTP1B inhibition with 6.8 µg/mL (Wei et al. 2015) (Table 2). Deflectins C1-C3 (42–44), D1-D2 (45,46), E (47), 1a-1c (48–50), 2a (51), and 2b (52) were reported to be isolated from the fungus Aspergillus deflectus. Azaphilones 42–52 possess a wide spread of activity towards PTP1B with IC50: 2.1–40.4 µM. Among these metabolites, compounds 50 and 52 were the most potent with IC50: 2.1 and 2.6 µM. An SAR study showed that the presence of the γ-lactone ring with the double bonds at C-8/C-12, the length of the aliphatic side chain and the presence of a C-2’ methyl on the side chain all play a positive role in the PTP1B activity (Huo et al. 2020). Compounds 48–52 were previously isolated from Aspergillus deflectus (Musso et al. 2010; Anke et al. 1981; Gao et al. 2013).
Cerebrosides
Glycosphingolipids (cerebrosides) were first reported by the German physician J. L. W. Thudichum in the second half of the nineteenth century (Thudichum 1884). The structure of the actual cerebroside isolated by J. L. W. Thudichum was determined by Carter (Carter et al. 1965). Cerebrosides are crucial and important constituents in a wide range of organs and tissues in biological systems. Cerebrosides mainly comprise two parts viz., a sugar part (red part) and a ceramide nucleus while the latter in turn comprises of a long-chain „sphingoid base“ (blue part) and an amide-linked long-chain fatty acid (green part).
A large number of cerebrosides have been reported from plants and fungi and have been shown to possess a diverse range of biological activities (Tan and Chen 2003). Cordycerebroside B (53) (Fig. 9) was isolated from the fungus Cordyceps militaris and inhibited PTP1B with an IC50: 4.68 µM. This activity was higher than the standard ursolic acid (IC50: 5.15 µM) (Sun et al. 2019). Furthermore, cerebroside 54 was isolated from the fungi, Cordyceps militaris (Sun et al. 2019), Schizophyllum commune (Kawai and Ikeda 1983) and Cortinarius tenuipes (Tan et al. 2003), and inhibited PTP1B with an IC50: 16.9 µM (Sun et al. 2019). Cordycerebroside A (55) was obtained from the fungus Cordyceps militaris (Sun et al. 2019; Chiu et al. 2016) and was shown to possess activity towards PTP1B with an IC50: 10.4 µM (Sun et al. 2019) (Table 2). On the other hand, soya-cerebroside I (56) was initially reported as a plant metabolite (Kim et al. 2001) but quite recently this compound was isolated from the fungus Cordyceps militaris (Sun et al. 2019) and reported to show PTP1B inhibition with an IC50: 18.9 µM.
Triterpenes and steroids
Russula lepida is included in the subdivision of higher fungi viz., Basidiomycotina. (Clericuzio et al. 2012) after a phytochemical investigation of fruiting bodies of R. lepida led to the isolation of seco-cucurbitane triterpenes 57 and 58 (Maarisit et al. 2017) (Fig. 10). Notably, compound 57 demonstrated potent PTP1B effects with an IC50: 0.4 µM which is higher than the standard oleanolic acid (IC50: 1.1 µM) (Table 2). Moreover, metabolite 58 was moderately active with an IC50: 20.4 µM (Maarisit et al. 2017). An SAR study showed that the CH2OH group (present in compound 57) causes a larger effect than the carboxylic acid group (present in compound 58). A C25 sterol bearing the C-5/C-8 peroxide group, phomasterol A (59) was produced by the fungus Phoma sp. and illustrated activity towards PTP1Bc with an IC50 : 25 µM (Chen et al. 2015a, b).
Sesquiterpenes
The sesquiterpenes, clitocybulols C (60: IC50: 36.0 µM), G (61: IC50: 49.5 µM), and L (62: IC50: 38.1 µM) (Fig. 11) were reported from the fungus Pleurotus cystidiosus and illustrated activity towards PTP1B (Tao et al. 2016a, b). The SAR study of compounds 60–62 revealed that the CH2OAc and CH2OH groups enhance the PTP1B effects and activity was decreased when these groups was replaced by a methyl group (compound 61). Ylangene-type sesquiterpenes, postinins A (63) and B (64) were isolated from the fungus Postia sp. Metabolite 63 illustrated significant effects towards PTP1B with IC50: 1.6 µg/mL while compound 64 was less effective with IC50: 6.2 µg/mL (Fan et al. 2014). Bisabolene sesquiterpenes, pleurotins A (65) and E (66) were isolated from the fungus Pleurotus citrinopileatus and both compounds exhibited PTP1B inhibition with IC50: 32.1 and 30.5 µM respectively (Tao et al. 2016a, b) (Table 2).
Isocoumarin-based metabolites
Two isocoumarin analogs, phelligridins H (67: IC50: 3.1 µM) and I (68: IC50: 3.0 µM) (Fig. 12) were isolated from the fungus Phellinus igniarius and both possess good PTP1B activity (Wang et al. 2007). Cladosporamide A (69) was isolated from the fungus Cladosporium sp. and illustrated moderate activity towards PTP1B with IC50: 48 µM (Rotinsulu et al. 2018). Asperentin B (70) was produced by the fungus Aspergillus sydowii (Wiese et al. 2017) and its structure was noted to be quite close to that of asperentin (Scott et al. 1971). Asperentin B (70) illustrated significant PTP1B effects with an IC50: 2.0 µM (Table 2) and these effects were six fold higher than the standard suramin (IC50: 11.8 µM). In addition, compound 70 was only weakly active towards Propionibacterim acnes and demonstrated no effects towards Xanthomonas campestris, Septoria tritici, Candida albicans, Staphylococcus lentus, HT29 and HepG2 cancer cells (Wiese et al. 2017).
Meroterpenoids
Meroterpenoids are a class of secondary metabolites originating from a biosynthetic pathway between a hybrid polyketide or non-polyketide and terpenoid (Peng and Qiu 2018; Geris and Simpson 2009; Matsuda and Abe 2016). Merosesquiterpenes, verruculide A (71), chrodrimanins A (72) and H (73) (Fig. 13) were reported from the fungus Penicillium verruculosum. Compounds 71 (IC50: 8.4 µM) and 72 (IC50: 8.5 µM) inhibited PTP1B almost to the same level. Notably, metabolite 73 was less effective with IC50: 14.9 µM (Yamazaki et al. 2015). In another report the meroterpenoid, preaustinoid A6 (74) was reported from the fungus Penicillium sp. and inhibited PTP1B with an IC50: 17.6 µM. In addition, a kinetic study revealed that compound 74 is a noncompetitive inhibitor having a Ki: 17.0 µM (Park et al. 2019). It is also noted that preaustinoid A3 (75) is produced by various fungal species of Penicillium sp. (Park et al. 2019; Fill et al. 2007) and possesses PTP1B activity with IC50: 58.4 µM (Park et al. 2019).
Chrodrimanins O (76), R (77), S (78) and compound 79 are all produced by the fungus Penicillium sp. Metabolite 76 contains the unusual trichlorinated pattern bearing an uncommon dichloromethine moiety. Furthermore, metabolites 76–79 illustrated PTP1B activity with IC50 ranging from 39.6 to 71.6 µM. However, these compounds were not active towards HepG2, A549, and Hela cancer cells (Kong et al. 2017). Meroterpenoid, furanoaustinol (80) is produced by the fungus Penicillium sp. and this metabolite weakly inhibited PTP1B IC50: 77.2 µ M (Park et al. 2018).
Biphenyl ethers
The fungus Phoma sp. produces the biphenyl ether, 1-methoxy-3,5′-dimethyl-2,3′-oxybiphenyl-5,1′,2′-triol (81) and in an anti-PTP1B assay, 81 (IC50: 13.0 µM) (Fig. 14; Table 3) displayed significant activity towards PTP1B (Sumilat et al. 2017a, b). Cyperine (82) initially reported as a herbicide isolated from Alternaria sp. (Singh and Pandey 2019) as well from the plant (Cyperus rotundus) (Sharma and Rani 2016). In 2017, cyperine (82) was reported to be isolated from the fungus Phoma sp. and illustrated PTP1B activity with an IC50: 17.0 µM (Sumilat et al. 2017a, b). In comparison with the activity of oleanolic acid (positive control; IC50 = 1.3 µM), compounds 81 and 82 appear to be good candidates for further studies. A mode of action evaluation based on Huh-7 cells, demonstrated that only compound 82 exhibited 26% growth inhibition (Sumilat et al. 2017a, b).
Palmarumycins and naphthopyranones
Palmarumycins have been isolated from various fungi and reported to possess interesting biological activities (Macías-Rubalcava et al. 2008; Schlingmann et al. 1996; Dong et al. 2008; Martinez-Luis et al. 2008; Seephonkai et al. 2002; Chu et al. 1994, 1995; Singh et al. 1994; Sakemi et al. 1995; Wipf et al. 2001; Powis et al. 2006). Guignardin C (83) was obtained from the fungus Guignardia sp. and inhibited PTP1B with an IC50: 25.7 µM. In addition, compounds 83 possesses antimicrobial activity towards Staphylococcus aureus (Ai et al. 2014). The naphtho-γ-pyrone, rubrofusarin B (84) (Fig. 15) is produced by various fungi viz., Aspergillus sp. (Huang et al. 2010, 2011; Sakurai et al. 2002; Zhang et al. 2008a; Song et al. 2004; Xiao et al. 2014; Zhan et al. 2007; Kim et al. 2020), Alternaria sp. (Shaaban et al. 2012), and Cladosporium herbarum (Ye et al. 2005). This compound inhibited PTP1B with IC50: 6.5 µM (Kim et al. 2020) (Table 3).
Additionally TMC-256A1 (85) was obtained from various Aspergillus sp. (Zhang et al. 2008b; Zahn et al. 2007; Huang et al. 2007; Sakurai et al. 2002; Kim et al. 2020) and illustrated PTP1B effects with IC50: 5.1 µM (Kim et al. 2020). The SAR study of compounds 84 and 85 revealed that the methyl group in compound 84 does not alter the PTP1B effects. Interestingly, aurasperone F (86) (Shaaban et al. 2012; Bouras et al. 2005, 2007; Kim et al. 2020) and fonsecin (87) (Shaaban et al. 2012; Zhang et al. 2008a; Ehrlich et al. 1984; Bouras et al. 2005; Zhan et al. 2007; Sakurai et al. 2002; Kim et al. 2020) are both quite active and strongly inhibited PTP1B with IC50: 7.9 and 3.3 µM respectively (Kim et al. 2020). Notably, the effects of fonsecin (87) was higher than the standard ursolic acid (IC50: 4.3 µM (Kim et al. 2020).
Polyketides
The polyketides, penostatins A (88), B (89), C (90) and J (91) (Fig. 16) produced by the fungus Isaria tenuipes were tested for their biological activity. It was found that compounds 88–91 possess significant effects towards PTP1B with IC50 values ranging from 0.37 to 43.6 µM. Among these compounds, penostatin B (89) was the most potent inhibitor with an IC50: 0.37 µM followed by penostatin A (88: IC50: 12.5 µM) (Chen et al. 2014). The SAR study of compounds 88 and 89 revealed that the stereochemistry of the hydroxyl group plays an important role in the PTP1B effects. Polyketides, trichoketides A (92) and B (93) are produced by the fungus Trichoderma sp. together with a few C-8 epimers. Octaketides 92 and 93 possess PTP1B effects with IC50: 53.1 and 65.1 µM, respectively (Yamazaki et al. 2015). Similarly, trichodermaketones C (94) and D (95) are produced by various Trichoderma sp. (Song et al. 2010; Mukhopadhyay et al. 1996; Yamazaki et al. 2015a) and these compounds also inhibited PTP1B with IC50: 68.0 and 55.9 µM (Table 3), respectively (Yamazaki et al. 2015a).
The fungus Aspergillus sp. produces the polyketides, (±)-tylopilusin D (96) and funalenone (97) (Fig. 17). Both compounds possess PTP1B activity with IC50 values of 8.1 and 6.1 µM, respectively (Kim et al. 2020). Botcinin A (98) and B (99) are produced by the fungus Botryotinia sp. In a PTP1B evaluation study, metabolite 99 illustrated significant inhibition with an IC50: 53.6 µM, while metabolite 98 was only a weak inhibitor (IC50 = 340.7 µM) (Table 3). An SAR study of compounds 98 and 99 demonstrated that the length of the alkyl chain plays a crucial role of PTP1B effects. The authors observed that botcinins A (98) and B (99) were unstable in MeOH and slowly transformed into botcinin D (100) and botcinin G (101) respectively. The metabolite 101 was two-fold less active (IC50 = 96.2 µM) than compound 99. Similarly, 100 was also less active (IC50 = 461.2 µM) than its natural precursor 98 (Kim et al. 2012).
Ascochitine (102) (Fig. 18) was reported to be isolated from various fungal species of Ascochyta sp. (Seibert et al. 2006; Kim et al. 2016; Bertini et al. 1956; Oku and Nakanishi 1963; Venkatasubbaiah and Chilton 1992) and found to possess PTP1B activity with an IC50: 38.5 µM (Seibert et al. 2006). The polyketides, tanzawaic acids A (103) and B (104) were isolated from Penicillium sp. (Quang et al. 2014; Shin et al. 2016) and demonstrated activity towards PTP1B with IC50 values of 8.2 µM and 30 µM respectively (Quang et al. 2014). The fungus Phoma sp. produces (10’S)-verruculide B (105) which was shown to possess PTP1B effects with an IC50: 13.7 µM. Moreover, this compound was also active towards SHP1 and TCPTP (Gubiani et al. 2017).
Penicillium sp. produced the pyrone-derived metabolite, penstyrylpyrone (106) which demonstrated good PTP1B inhibitory effects with an IC50: 5.28 µM (Lee et al. 2013). The chaetocyclinone derivative, anhydrofulvic acid (107) was produced initially by Penicillium sp. (Lösgen et al. 2007) and possesses antimicrobial effects towards Candida sp. (Fujita et al. 1999). The latter compound 107 was also reported from various Penicillium sp. Yamauchi et al. (1984) synthesized this compound to confirm its assigned structure. Lee et al. (2013) reported that anhydrofulvic acid (107) possesses inhibitory effects towards PTP1B with an IC50: 1.90 µM and this compound was more potent than the standard ursolic acid (IC50: 3.1 µM).
Cryptosporioptide A (108) (Fig. 19) was produced by the fungi Cordyceps gracilioides (Wei et al. 2015) and Cryptosporiopsis sp. (Tousif et al. 2014). This compound illustrated reasonable PTP1B inhibition with an IC50: 7.3 µg/mL (Wei et al. 2015). The polyketide, neglectine A (109), was reported from the fungus Pestalotiopsis neglecta and illustrated PTP1B inhibitory effects with an IC50: 6.7 µg/mL (Gao et al. 2019) (Table 4).
Micellaneous
Butanolide A (110) (Fig. 20) was reported from the fungus Penicillium sp. and illustrated moderate inhibitory effects towards PTP1B with an IC50: 27.4 µM (Zhou et al. 2018). Betulactone B (111) was reported from the fungus Lenzites betulinus and demonstrates PTP1B inhibitory effects with an IC50: 21.5 µg/mL (Wen et al. 2017). Talarodride (112) was produced by the fungus Talaromyces purpurogenus and inhibited PTP1B by 76% (Zhao et al. 2019).
Monodictyphenone (113) (Fig. 21) was produced by the fungus Penicillium albobiverticillium and was shown to possess moderate PTP1B inhibitory effects with an IC50: 36 µM (Sumilat et al. 2017a, b). Flavoglaucin (114) was isolated from Eurotium sp. (Gao et al. 2011; Sohn et al. 2013) and showed PTP1B inhibition with an IC50: 13.4 µM (Sohn et al. 2013). Prenylflavanone, (2S)-7,4’-dihydroxy-5-methoxy-8-(γ,γ-dimethylallyl)-flavanone (115) was initially isolated as a plant metabolite (Kang et al. 2000) and later from the fungus Cladosporium sp. and illustrated moderate inhibitory effects towards PTP1B with IC50: 11 µM (Table 4) (Rotinsulu et al. 2018).
The fungus Isaria fumosorosea produces the most intriguing peptide, beauvericin (116) (Fig. 22), which exhibited very low inhibitory activity with an IC50 value (0.59 µM) against PTP1B. Interestingly, this compound’s inhibitory effects was 22-times more potent than sodium orthovanadate (IC50 = 11.3 µM) which is used as reference compound (Zhang et al. 2017). In another investigation, the fungus Aspergillus sp. afforded the very interesting cyclic peptide, malformin A1 (117) which inhibited PTP1B with an IC50: 5.2 µM (Kim et al. 2020).
A mixture of arugosin N (118) and its 1,6,10-trihydroxy-8-methyl-2-(3-methyl-2-butenyl)dibenz[b,e]oxepin-11(6H)-one (119) were produced by the fungi Malbranchea circinate (Rangel-Grimaldo et al. 2020) and Talaromyces flavus (Sun et al. 2016). The epimeric mixture of compounds 118 and 119 has been reported to possess PTP1B inhibitory effects with an IC50: 30.3 µM (Rangel-Grimaldo et al. 2020) (Table 3). Divaric acid (120) and 2,4-dihydroxy-6-[(3E,5E)-nona-3,5-dien-1-yl] benzoic acid (121) were produced by the fungus F10Z1082 and these compounds possess PTP1B inhibitory effects with IC50: 1.58 and 4.27 µM respectively. The PTP1B activity of metabolite 120 was 2.7-fold more potent than that of metabolite 121, indicating that that the length and double bonds of the carbon side chain on C-6 of the aromatic ring might be crucial for activity (Sun et al. 2017a, b).
Conclusion and future perspective
Fungal metabolites play a vitally important and sustainable role in initiating drug development. In this context, the last two decades have been observed as a most fruitful period in isolating low molecular weight antidiabetic compounds from fungi. The fascinating research on PTP1B inhibitors that has been accomplished based on fungal metabolites cannot be underestimated. Moreover some of these fungal metabolites possess PTP1B effects with IC50: < 1 µM and notably the depside analog trivaric acid (30) inhibited PTP1B very strongly with an IC50: 173 nM. These active fungal metabolites furnish new chemical entities for developing novel candidates for the treatment of diabetes and obesity. Notably, further synthetic and biological studies on these chemical templates are necessary in order to obtain intriguing SAR information. Furthermore, the mode of actions of these compounds have not yet been fully investigated and future research should focus on establishing the mechanism of action of these active compounds.
It has been reported that an insufficient number of lead compounds have reached clinical trials. A benzothiophene derivative (Liu 2003) and ertiprotafib (Xue et al. 2019) have failed in the Phase II clinical trial because of their unsatisfactory side effects. Some reports showed that two more PTP1B inhibitors viz., SI-1436 (Lantz et al. 2010) and JTT-551 (Ito et al. 2014) have reached clinical trials. The major issues which are associated with PTP1B drug development is their cell permeability, bioavailability coupled with their poor selectivity. Although some of these fungal metabolites illustrated significant PTP1B effects, very few semi-synthetic secondary metabolites have been synthesized for PTP1B activity employing natural product scaffolds. Furthermore, computational studies illustrated that natural products cover larger areas of chemical space than synthetic compounds. In addition, natural products comprise higher numbers of stereogenic centers along with greater diversity in heteroatomic rings. Moreover, natural products have inspired synthetic protocols which can therefor access lead compounds with better potency, selectivity, lipophilicity and bioavailability.
Another strategy for the establishment of PTP1B inhibitors is that these can be used in combination therapy by utilizing PTP1B inhibitors for treatment regarding diabetes/obesity protocols. These combined treatment strategies could have a synergistic effect and may be more effective than either treatment alone. Literature revealed that a PTP1B inhibitor is used with anti-ErbB2 antibodies to treat breast cancer (Julien et al. 2007). In another study, Delibegovic et al. (2009) proposed that the combined treatment of PPARγ agonist and PTP1B inhibition could be more effective for diabetes and obesity. In view of the outstanding value of PTP1B drug targeting, only a few screening methods are available for testing for PTP1B inhibition viz., fluorescence method, colorimetric method employing p-nitrophenyl phosphate (PNPP) as substrate and scintillation proximity assay (SPA). However, those technologies could lead to false positive results in some cases which could create more difficulties in PTP1B drug discovery. Therefore, further high-throughput methods are essential in order to screen lead PTP1B inhibitors more efficiently and accurately. The information provided in this literature review may help to achieve the goal of establishing therapeutic drugs for diabetes and obesity with more efficacy.
References
Ai W, Wei X, Lin X et al (2014) Guignardins A–F, spirodioxynaphthalenes from the endophytic fungus Guignardia sp. KcF8 as a new class of PTP1B and SIRT1 inhibitors. Tetrahedron 70:5806–5814
Andersen R, Buechi G, Kobbe B, Demain AL (1977) Secalonic acids D and F are toxic metabolites of Aspergillus aculeatus. J Org Chem 42:352–353
Anke H, Kemmer T, Höfle G (1981) Deflectins, new antimicrobial azaphilones from Aspergillus deflectus. J Antibiot 34:923–928
Arai K, Kimura K, Mushiroda T et al (1989) Structures of fructigenines A and B, new alkaloids isolated from Penicillium fructigenum Takeuchi. Chem Pharm Bull 37:2937–2939
Ariantari NP, Ancheeva E, Wang C et al (2019) Indole Diterpenoids from an Endophytic Penicillium sp. J Nat Prod 82:1412–1423
Asolkar RN, Cordova-Kreylos AL, Himmel P et al (2013) Discovery and development of natural products for pest management. ACS Symp Ser 1141:17–30
Bertini S (1956) Su di un composto ad antibiotica prodotto da Ascochyta pisi Lib. Annali Sperimentaz Agraria (Roma) 11:545–556
Bills GF, Gloer JB (2016) Biologically active secondary metabolites from the fungi. Microbiol Spectrum 4(6):1–32 (FUNK-0009-2016)
Birkinshaw J, Luckner M, Mohammed Y (1963) Studies in the biochemistry of micro-organisms. 114. Viridicatol and cyclopenol, metabolites of Penicillium viridicatum Westling and Penicillium cyclopium Westling. Biochem J 89:196
Bouras N, Mathieu F, Coppel Y, Lebrihi A (2005) Aurasperone F–a new member of the naphtho-gamma-pyrone class isolated from a cultured microfungus, Aspergillus niger C-433. Nat Prod Res 19:653–659
Bouras N, Mathieu F, Coppel Y et al (2007) Occurrence of naphtho-gamma-pyrones-and ochratoxin A-producing fungi in French grapes and characterization of new naphtho-gamma-pyrone polyketide (aurasperone G) isolated from Aspergillus niger C-433. J Agric Food Chem 55:8920–8927
Bräse S, Encinas A, Keck J, Nising CF (2009) Chemistry and biology of mycotoxins and related fungal metabolites. Chem Rev 109:3903–3990
Bruckner S, Weise M, Schobert R (2018) Synthesis of the Entomopathogenic Fungus Metabolites Militarinone C and Fumosorinone A. J Org Chem 83:10805–10812
Capon RJ, Stewart M, Ratnayake R et al (2007) Citromycetins and bilains A–C: new aromatic polyketides and diketopiperazines from Australian marine-derived and terrestrial Penicillium spp. J Nat Prod 70:1746–1752
Carter H, Johnson P, Weber E (1965) Glycolipids. Annu Rev Biochem 34:109–142
Chen YP, Yang CG, Wei PY et al (2014) Penostatin derivatives, a novel kind of protein phosphatase 1B inhibitors isolated from solid cultures of the entomogenous fungus Isaria tenuipes. Molecules 19:1663–1671
Chen C, Cao M, Zhu S et al (2015a) Discovery of a novel inhibitor of the protein tyrosine phosphatase Shp2. Sci Rep 5:17626
Chen ZM, Fan QY, Chen HP et al (2015b) A novel C25 sterol peroxide from the endophytic fungus Phoma sp. EA-122. Z Naturforsch 70c:93–96
Chiu CP, Liu SC, Tang CH et al (2016) Anti-inflammatory cerebrosides from cultivated Cordyceps militaris. J Agric Food Chem 64:1540–1548
Cho KH, Sohn JH, Oh H (2018) Isolation and structure determination of a new diketopiperazine dimer from marine-derived fungus Aspergillus sp. SF-5280. Nat Prod Res 32:214–221
Chu M, Truumees I, Patel MG et al (1994) A novel class of antitumor metabolites from the fungus Nattrassia mangiferae. Tetrahedron Lett 35:1343–1346
Chu M, Truumees I, Patel M et al (1995) Sch 50673 and Sch 50676, two novel antitumor fungal metabolites. J Antibiot 48:329–331
Clericuzio M, Cassino C, Corana F et al (2012) Terpenoids from Russula lepida and R. amarissima (Basidiomycota, Russulaceae). Phytochemistry 84:154–159
Clevenger KD, Ye R, Bok JW et al (2018) Interrogation of benzomalvin biosynthesis using fungal artificial chromosomes with metabolomic scoring (FAC-MS): discovery of a benzodiazepine synthase activity. Biochemistry 57:3237–3243
Cole RJ, Kirksey JW, Wells JM (1974) A new tremorgenic metabolite from Penicillium paxilli. Can J Microbiol 20:1159–1162
Combs AP (2010) Recent advances in the discovery of competitive protein tyrosine phosphatase 1B inhibitors for the treatment of diabetes, obesity, and cancer. J Med Chem 53:2333–2344
Culberson CF, LaGreca S, Johnson A et al (1999) Trivaric acid, a new tridepside in the Ramalina americana chemotype complex (lichenized Ascomycota: Ramalinaceae). Bryologist 102:595–601
Debbab A, Aly AH, Edrada-Ebel R et al (2009) Bioactive metabolites from the endophytic fungus Stemphylium globuliferum isolated from Mentha pulegium. J Nat Prod 72:626–631
Delibegovic M, Zimmer D, Kauffman C et al (2009) Liver-specific deletion of protein-tyrosine phosphatase 1B (PTP1B) improves metabolic syndrome and attenuates diet-induced endoplasmic reticulum stress. Diabetes 58:590–599
Depew KM, Marsden SP, Zatorska D et al (1999) Total synthesis of 5-N-acetylardeemin and amauromine: Practical routes to potential MDR reversal agents. J Am Chem Soc 121:11953–11963
Ding Y, Wet JRD, Cavalcoli J et al (2010) Genome-based characterization of two prenylation steps in the assembly of the stephacidin and notoamide anticancer agents in a marine-derived Aspergillus sp. J Am Chem Soc 132:12733–12740
Dong JY, Song HC, Li JH et al (2008) Ymf 1029A – E, Preussomerin Analogues from the Fresh-Water-Derived Fungus YMF 1.01029. J Nat Prod 71:952–956
Ehrlich KC, DeLucca AJ, Ciegler A (1984) Naphtho-gamma-pyrone production by Aspergillus niger isolated from stored cottonseed. Appl Environ Microbiol 48:1–4
Eley KL, Halo LM, Song Z et al (2007) Biosynthesis of the 2-pyridone tenellin in the insect pathogenic fungus Beauveria bassiana. ChemBioChem 8:289–297
Elsebai MF, Rempel V, Schnakenburg G et al (2011) Identification of a potent and selective cannabinoid CB1 receptor antagonist from Auxarthron reticulatum. ACS Med Chem Lett 2:866–869
Ezzat SM, Bishbishy MHE, Habtemariam S et al (2018) Looking at Marine-Derived Bioactive Molecules as Upcoming Anti-Diabetic Agents: A Special Emphasis on PTP1B Inhibitors. Molecules 23:3334
Fan QY, Dong ZJ, Li ZH et al (2014) Two new ylangene-type sesquiterpenoids from cultures of the fungus Postia sp. J Asian Nat Prod Res 16:254–258
Figueroa M, González MDC, Mata R (2008) Malbrancheamide B, a novel compound from the fungus Malbranchea aurantiaca. Nat Prod Res 22:709–714
Fill TP, Pereira GK, dos Santos RMG, Rodrigues-Fo E (2007) Four additional meroterpenes produced by Penicillium sp found in association with Melia azedarach. Possible biosynthetic intermediates to austin. Z Naturforsch 62b:1035–1044
Fujita KI, Nagamine Y, Ping X et al (1999) Mode of action of anhydrofulvic acid against Candida utilis ATCC 42402 under acidic condition. J Antibiot 52:628–634
Gao J, León F, Radwan MM et al (2011) Benzyl derivatives with in vitro binding affinity for human opioid and cannabinoid receptors from the fungus Eurotium repens. J Nat Prod 74:1636–1639
Gao JM, Yang SX, Qin JC (2013) Azaphilones: chemistry and biology. Chem Rev 113:4755–4811
Gao WB, Han LP, Xie FX et al (2019) A New Polyketide-Derived Metabolite with PTP1B Inhibitory Activity from the Endophytic Fungus Pestalotiopsis neglecta. Chem Nat Compd 55:1056–1058
Geris R, Simpson TJ (2009) Meroterpenoids produced by fungi. Nat Prod Rep 26:1063–1094
González-Medina M, Owen JR, El-Elimat T et al (2017) Scaffold Diversity of Fungal Metabolites. Front Pharmacol 8:180–191
Gubiani JR, Wijeratne EK, Shi T et al (2017) An epigenetic modifier induces production of (10′ S)-verruculide B, an inhibitor of protein tyrosine phosphatases by Phoma sp. nov. LG0217, a fungal endophyte of Parkinsonia microphylla. Bioorg Med Chem 25:1860–1866
Heneghan MN, Yakasai AA, Williams K et al (2011) The programming role of trans-acting enoyl reductases during the biosynthesis of highly reduced fungal polyketides. Chem Sci 2:972–979
Huang XH, Nishida H, Tomoda H et al (1995) Terpendoles, Novel AC AT Inhibitors Produced by Albophoma yamanashiensis. J Antibiot 48:5–11
Huang HB, Feng XJ, Liu L et al (2010) Three dimeric naphtho-γ-pyrones from the mangrove endophytic fungus Aspergillus tubingensis isolated from Pongamia pinnata. Planta Med 76:1888–1891
Huang HB, Xiao ZE, Feng XJ et al (2011) Cytotoxic Naphtho-γ-pyrones from the Mangrove Endophytic Fungus Aspergillus tubingensis (GX1-5E). Helv Chim Acta 94:1732–1740
Huo C, Lu X, Zheng Z et al (2020) Azaphilones with protein tyrosine phosphatase inhibitory activity isolated from the fungus Aspergillus deflectus. Phytochemistry 170:112224
Hussain H, Green IR, Abbas G et al (2019) Protein tyrosine phosphatase 1B (PTP1B) inhibitors as potential anti-diabetes agents: patent review (2015–2018). Expert Opin Ther Pat 29:689–702
Isaka M, Tanticharoen M, Kongsaeree P, Thebtaranonth Y (2001) Structures of Cordypyridones A – D, Antimalarial N-Hydroxy-and N-Methoxy-2-pyridones from the Insect Pathogenic Fungus Cordyceps n ipponica. J Org Chem 66:4803–4808
Ito M, Fukuda S, Sakata S et al (2014) Pharmacological effects of JTT-551, a novel protein tyrosine phosphatase 1B inhibitor, in diet-induced obesity mice. J Diabetes Res 680348
Jang JP, Jang JH, Soung NK et al (2012) Benzomalvin E, an indoleamine 2, 3-dioxygenase inhibitor isolated from Penicillium sp. FN070315. J Antibiot 65:215–217
Jiang CS, Liang LF, Guo YW (2012) Natural products possessing protein tyrosine phosphatase 1B (PTP1B) inhibitory activity found in the last decades. Acta Pharmacol Sin 33:1217–1245
Julien SG, Dube N, Read M et al (2007) Protein tyrosine phosphatase 1B deficiency or inhibition delays ErbB2-induced mammary tumorigenesis and protects from lung metastasis. Nature Genet 39:338–346
Kawai G, Ikeda Y (1983) Chemistry and functional moiety of a fruiting-inducing cerebroside in Schizophyllum commune. Biochim Biophys Acta Lipids Lipid Metab 754:243–248
Kim JS, Byun JH, Kang SS (2001) Isolation of soya-cerebroside I from the roots of Trichosanthes kihlowii. Nat Prod Sci 7:27–32
Kim MY, Sohn JH, Jang JH et al (2012) Two new botcinin derivatives encountered in the studies of secondary metabolites from the marine-derived fungus Botryotinia sp. SF-5275. J Antibiot 65:161–164
Kim W, Peever TL, Park JJ et al (2016) Use of metabolomics for the chemotaxonomy of legume-associated Ascochyta and allied genera. Sci Rep 6:20192
Kim DC, Minh Ha T, Sohn JH et al (2020) Protein tyrosine phosphatase 1B inhibitors from a marine-derived fungal strain Aspergillus sp. SF-5929. Nat Prod Res 34:675–682
Knaus HG, McManus OB, Lee SH et al (1994) Tremorgenic indole alkaloids potently inhibit smooth muscle high-conductance calcium-activated potassium channels. Biochemistry 33:5819–5828
Kong FD, Zhang RS, Ma QY et al (2017) Chrodrimanins O–S from the fungus Penicillium sp. SCS-KFD09 isolated from a marine worm. Sipunculusnudus Fitoterapia 122:1–6
Kong FD, Fan P, Zhou LM et al (2019) Penerpenes A–D, Four Indole Terpenoids with Potent Protein Tyrosine Phosphatase Inhibitory Activity from the Marine-Derived Fungus Penicillium sp. KFD28. Org Lett 21:4864–4867
Krishnan N, Konidaris KF, Gasser G, Tonks NK (2018) A potent, selective, and orally bioavailable inhibitor of the protein-tyrosine phosphatase PTP1B improves insulin and leptin signaling in animal models. J Biol Chem 293:1517–1525
Kshirsagar U (2015) Recent developments in the chemistry of quinazolinone alkaloids. Org Biomol Chem 13:9336–9352
Kuroda K (1988) KS-506 compounds and process for the production thereof. Eur Pat Appl EP 335:386
Lantz KA, Hart SGE, Planey SL et al (2010) Inhibition of PTP1B by trodusquemine (MSI-1436) causes fat-specific weight loss in diet-induced obese mice. Obesity 18:1516–1523
Lee S, Wang Q (2007) Recent development of small molecular specific inhibitor of protein tyrosine phosphatase 1B. Med Res Rev 27:553–573
Lee DS, Jang JH, Ko W et al (2013) PTP1B inhibitory and anti-inflammatory effects of secondary metabolites isolated from the marine-derived fungus Penicillium sp. JF-55. Mar drugs 11:1409–1426
Liang TM, Fang YW, Zheng JY, Shao CL (2018) Secondary metabolites isolated from the gorgonian-derived fungus Aspergillus ruber and their antiviral activity. Chem Nat Compd 54:559–561
Liu G (2003) Protein Tyrosine Phosphatase 1B Inhibition: Opportunities and Challenges. Curr Med Chem 10:1407–1421
Liu L, Zhang J, Chen C et al (2015a) Structure and biosynthesis of fumosorinone, a new protein tyrosine phosphatase 1B inhibitor firstly isolated from the entomogenous fungus Isaria fumosorosea. Fungal Genet Biol 81:191–200
Liu ZQ, Liu T, Chen C et al (2015b) Fumosorinone, a novel PTP1B inhibitor, activates insulin signaling in insulin-resistance HepG2 cells and shows anti-diabetic effect in diabetic KKAy mice. Toxicol Appl Pharmacol 285:61–70
Lösgen S, Schlörke O, Meindl K et al (2007) Structure and biosynthesis of chaetocyclinones, new polyketides produced by an endosymbiotic fungus. Eur J Org Chem 2007:2191–2196
Maarisit W, Yamazaki H, Kanno SI et al (2017) Protein tyrosine phosphatase 1B inhibitory properties of seco-cucurbitane triterpenes obtained from fruiting bodies of Russula lepida. J Nat Med 71:334–337
Macías-Rubalcava ML, Hernández-Bautista BE, Jiménez-Estrada M et al (2008) Naphthoquinone spiroketal with allelochemical activity from the newly discovered endophytic fungus Edenia gomezpompae. Phytochemistry 69:1185–1196
Martínez-Luis S, Rodríguez R, Acevedo L et al (2006) Malbrancheamide, a new calmodulin inhibitor from the fungus Malbranchea aurantiaca. Tetrahedron 62:1817–1822
Martínez-Luis S, Della-Togna G, Coley PD et al (2008) Antileishmanial constituents of the Panamanian endophytic fungus Edenia sp. J Nat Prod 71:2011–2014
Matsuda Y, Abe I (2016) Biosynthesis of fungal meroterpenoids. Nat Prod Rep 33:26–53
McLeay L, Smith B, Munday-Finch S (1999) Tremorgenic mycotoxins paxilline, penitrem and lolitrem B, the non-tremorgenic 31-epilolitrem B and electromyographic activity of the reticulum and rumen of sheep. Res Vet Sci 66:119–127
Miller KA, Welch TR, Greshock TJ et al (2008) Biomimetic total synthesis of malbrancheamide and malbrancheamide B. J Org Chem 73:3116–3119
Millot M, Tomasi S, Studzinska E et al (2009) Cytotoxic constituents of the lichen Diploicia canescens. J Nat Prod 72:2177–2180
Mohler ML, He Y, Wu Z et al (2009) Recent and emerging anti-diabetes targets. Med Res Rev 29:125–195
Mueller JM, Stark CB (2016) Diastereodivergent Reverse Prenylation of Indole and Tryptophan Derivatives: Total Synthesis of Amauromine, Novoamauromine, and epi-Amauromine. Angew Chem Int Ed 55:4798–4802
Mukhopadhyay T, Roy K, Sawant S et al (1996) On an Unstable Antifungal Metabolite from Trichoderma koningii. J Antibiot 49:210–211
Musso L, Dallavalle S, Merlini L et al (2010) Natural and semisynthetic azaphilones as a new scaffold for Hsp90 inhibitors. Bioorg Med Chem 18:6031–6043
Na M, Oh WK, Kim YH et al (2006) Inhibition of protein tyrosine phosphatase 1B by diterpenoids isolated from Acanthopanax koreanum. Bioorg Med Chem Lett 16:3061–3064
Naveen B, Ommi NB, Mudiraj A et al (2017) Total Synthesis of Penicinoline E, Marinamide, Methyl Marinamide and their Antimalarial Activity. ChemistrySelect 2:3256–3261
Newman DJ, Cragg GM (2016) Natural products as sources of new drugs from 1981 to 2014. J Nat Prod 79:629–661
Nichols AJ, Mashal RD, Balkan B (2006) Toward the discovery of small molecule PTP1B inhibitors for the treatment of metabolic diseases. Drug Dev Res 67:559–566
Nozawa K, Nakajima S, Kawai KI, Udagawa SI (1988) Isolation and structures of indoloditerpenes, possible biosynthetic intermediates to the tremorgenic mycotoxin, paxilline, from Emericella striata. J Chem Soc Perkin Trans 1:2607–2610
Oh H, Kim BS, Bae EY et al (2004) Inhibition of PTP1B by metabolites from Micromucor ramannianus var. angulisporus CRM000232. J Antibiot 57:528–531
Oku H, Nakanishi T (1963) A toxic metabolite from Ascochyta fabae having antibiotic activity. Phytopathology 53:1321–1325
Park JS, Quang TH, Yoon CS et al (2018) Furanoaustinol and 7-acetoxydehydroaustinol: new meroterpenoids from a marine-derived fungal strain Penicillium sp. SF-5497. J Antibiot 71:557–563
Park JS, Quang TH, Ngan NTT et al (2019) New preaustinoids from a marine-derived fungal strain Penicillium sp. SF-5497 and their inhibitory effects against PTP1B activity. J Antibiot 72:629–633
Peng X, Qiu M (2018) Meroterpenoids from Ganoderma species: A review of last five years. Nat Prod Bioprospect 8:137–149
Powis G, Wipf P, Lynch SM et al (2006) Molecular pharmacology and antitumor activity of palmarumycin-based inhibitors of Thioredoxin reductase. Mol Cancer Ther 5:630–636
Qi S (2019) Preparation of anthraquinone compound as enzyme inhibitors. CN109748838A
Qiao MF, Ji NY, Liu XH et al (2010) Indoloditerpenes from an algicolous isolate of Aspergillus oryzae. Bioorg Med Chem Lett 20:5677–5680
Quang TH, Ngan NTT, Ko W et al (2014) Tanzawaic acid derivatives from a marine isolate of Penicillium sp.(SF-6013) with anti-inflammatory and PTP1B inhibitory activities. Bioorg Med Chem Lett 24:5787–5791
Rahbæk L, Breinholt J (1999) Circumdatins D, E, and F: further fungal benzodiazepine analogues from Aspergillus ochraceus. J Nat Prod 62:904–905
Rangel-Grimaldo M, Macías-Rubalcava ML, González-Andrade M et al (2020) α-Glucosidase and Protein Tyrosine Phosphatase 1B Inhibitors from Malbranchea circinata. J Nat Prod. https://doi.org/10.1021/acs.jnatprod.9b01108
Rimando AM, Duke SO (2006) Natural products for pest management. ACS Symp Ser 927:2–21
Rotinsulu H, Yamazaki H, Miura T et al (2017) A 2, 4′-linked tetrahydroxanthone dimer with protein tyrosine phosphatase 1B inhibitory activity from the Okinawan freshwater Aspergillus sp. J Antibiot 70:967–969
Rotinsulu H, Yamazaki H, Sugai S et al (2018) Cladosporamide A, a new protein tyrosine phosphatase 1B inhibitor, produced by an Indonesian marine sponge-derived Cladosporium sp. J Nat Med 72:779–783
Sakemi S, Inagaki T, Kaneda K et al (1995) CJ-12, 371 and CJ-12, 372, Two Novel DNA Gyrase Inhibitors. J Antibiot 48:134–142
Sakurai M, Kohno J, Yamamoto K et al (2002) TMC-256A1 and C1, new inhibitors of IL-4 signal transduction produced by Aspergillus niger var niger TC 1629. J Antibiot 55:685–692
Schlingmann G, Matile S, Berova N et al (1996) Absolute stereochemistry of the diepoxins. Tetrahedron 52:435–446
Scott PM, van Walbeek W, MacLean WM (1971) Cladosporin, a new antifungal metabolite from Cladosporium cladosporioides. J Antibiot 24:747–755
Seephonkai P, Isaka M, Kittakoop P et al (2002) Evaluation of antimycobacterial, antiplasmodial and cytotoxic activities of preussomerins isolated from the lichenicolous fungus Microsphaeropsis sp. BCC 3050. Planta Med 68:45–48
Seibert SF, Eguereva E, Krick A et al (2006) Polyketides from the marine-derived fungus Ascochyta salicorniae and their potential to inhibit protein phosphatases. Org Biomol Chem 4:2233–2240
Seo C, Sohn JH, Oh H et al (2009) Isolation of the protein tyrosine phosphatase 1B inhibitory metabolite from the marine-derived fungus Cosmospora sp. SF-5060. Bioorg Med Chem Lett 19:6095–6097
Shaaban M, Shaaban KA, Abdel-Aziz MS (2012) Seven naphtho-γ-pyrones from the marine-derived fungus Alternaria alternata: structure elucidation and biological properties. Org Med Chem Lett 2:6
Shao CL, Wang CY, Gu YC et al (2010) Penicinoline, a new pyrrolyl 4-quinolinone alkaloid with an unprecedented ring system from an endophytic fungus Penicillium sp. Bioorg Med Chem Lett 20:3284–3286
Sharma K, Rani P (2016) A Holistic Ayurvedic Approach in Management of Sthaulya (Obesity). IJHSR 6:358–365
Shi T, Wijeratne EK, Solano C et al (2019) An isoform-selective PTP1B inhibitor derived from nitrogen-atom augmentation of radicicol. Biochemistry 58:3225–3231
Shin HJ, Pil GB, Heo SJ et al (2016) Anti-inflammatory activity of tanzawaic acid derivatives from a marine-derived fungus Penicillium steckii 108YD142. Mar drugs 14:14
Shrestha S, Seong SH, Park SG et al (2019) Insight into the PTP1B Inhibitory Activity of Arylbenzofurans: An In Vitro and In Silico Study. Molecules 24:2893
Singh AK, Pandey AK (2019) Fungal metabolites as a natural source of herbicide: a novel approach of weed management. J Nat Appl Sci 11:158–163
Singh SB, Zink DL, Liesch JM et al (1994) Preussomerins and deoxypreussomerins: Novel inhibitors of ras farnesyl-protein transferase. J Org Chem 59:6296–6302
Sohn JH, Lee YR, Lee DS et al (2013) PTP1B inhibitory secondary metabolites from marine-derived fungal strains Penicillium spp. and Eurotium sp. J Microbiol Biotechnol 23:1206–1211
Song Y, Li H, Ye Y et al (2004) Endophytic naphthopyrone metabolites are co-inhibitors of xanthine oxidase, SW1116 cell and some microbial growths. FEMS Microbiol Lett 241:67–72
Song F, Dai H, Tong Y et al (2010) Trichodermaketones A – D and 7-O-methylkoninginin D from the marine fungus Trichoderma koningii. J Nat Prod 73:806–810
Sumilat DA, Yamazaki H, Endo K et al (2017a) A new biphenyl ether derivative produced by Indonesian ascidian-derived Penicillium albobiverticillium. J Nat Med 71:776–779
Sumilat DA, Yamazaki H, Kanno SI et al (2017b) Biphenyl ether derivatives with protein tyrosine phosphatase 1B inhibitory activity from the freshwater fungus Phoma sp. J Antibiot 70:331–333
Sun W, Zhang B, Zheng H et al (2017a) Trivaric acid, a new inhibitor of PTP1b with potent beneficial effect on diabetes. Life Sci 169:52–64
Sun W, Zhuang C, Li X et al (2017b) Varic acid analogues from fungus as PTP1B inhibitors: Biological evaluation and structure–activity relationships. Bioorg Med Chem Lett 27:3382–3385
Sun J, Xu J, Wang S et al (2019) A new cerebroside from Cordyceps militaris with anti-PTP1B activity. Fitoterapia 138:104342
Takase S, Iwami M, Ando T et al (1984) Amauromine, a new vasodilator taxonomy, isolation and characterization. J Antibiot 37:1320–1323
Takase S, Kawai Y, Uchida I et al (1985) Structure of amauromine, a new hypotensive vasodilator produced by amauroascus sp. Tetrahedron 41:3037–3048
Takase S, Itoh Y, Uchida I et al (1986) Total synthesis of amauromine. Tetrahedron 42:5887–5894
Tan R, Chen J (2003) The cerebrosides. Nat Prod Rep 20:509–534
Tan JW, Dong ZJ, Liu JK (2003) New cerebrosides from the basidiomycete Cortinarius tenuipes. Lipids 38:81–84
Tang Y, Zhang X, Chen Z et al (2018) Novel benzamido derivatives as PTP1B inhibitors with anti-hyperglycemic and lipid-lowering efficacy. Acta Pharm Sin B 8:919–932
Tao QQ, Ma K, Bao L et al (2016a) Sesquiterpenoids with PTP1B inhibitory activity and cytotoxicity from the edible mushroom Pleurotus citrinopileatus. Planta Med 82:639–644
Tao QQ, Ma K, Bao L et al (2016b) New sesquiterpenoids from the edible mushroom Pleurotus cystidiosus and their inhibitory activity against α-glucosidase and PTP1B. Fitoterapia 111:29–35
Taylor SD (2003) Inhibitors of protein tyrosine phosphatase 1B (PTP1B). Curr Top Med Chem 3:759–782
Taylor SD, Hill B (2004) Recent advances in protein tyrosine phosphatase 1B inhibitors. Expert Opin Invest Drugs 13:199–214
Thareja S, Aggarwal S, Bhardwaj TR et al (2012) Kumar M. Protein tyrosine phosphatase 1B inhibitors: A molecular level legitimate approach for the management of diabetes mellitus. Med Res Rev 32:459–517
Thudichum JLW (1884) A treatise on the chemical constitution of the brain. London, Bailliere, Tindall and Cox
Tousif MI, Shazmeen N, Riaz N et al (2014) α-Glucosidase and lipoxygenase inhibitory derivatives of cryptosporioptide from the endophytic fungus Cryptosporiopsis sp. J Asian Nat Prod Res 16:1068–1073
Tseng MC, Yang HY, Chu YH (2010) Total synthesis of asperlicin C, circumdatin F, demethylbenzomalvin A, demethoxycircumdatin H, sclerotigenin, and other fused quinazolinones. Org Biomol Chem 8:419–427
Venkatasubbaiah P, Chilton W (1992) Phytotoxins of Ascochyta hyalospora, causal agent of lambsquarters leaf spot. J Nat Prod 55:461–467
Wang Y, Shang XY, Wang SJ et al (2007) Structures, biogenesis, and biological activities of pyrano [4, 3-c] isochromen-4-one derivatives from the fungus Phellinus igniarius. J Nat Prod 70:296–299
Wang LJ, Jiang B, Wu N et al (2015) Natural and semisynthetic protein tyrosine phosphatase 1B (PTP1B) inhibitors as anti-diabetic agents. RSC Adv 5:48822–48834
Wei PY, Liu LX, Liu T et al (2015) Three new pigment protein tyrosine phosphatases inhibitors from the insect parasite fungus Cordyceps gracilioides: terreusinone A, pinophilin C and cryptosporioptide A. Molecules 20:5825–5834
Wen CN, Chen HP, Zhao ZZ et al (2017) Two new γ-lactones from the cultures of basidiomycete Lenzites betulinus. Phytochem Lett 20:9–12
Wiese J, Aldemir H, Schmaljohann R et al (2017) Asperentin B, a new inhibitor of the protein tyrosine phosphatase 1B. Marine drugs 15:191
Wipf P, Hopkins TD, Jung JK et al (2001) New inhibitors of the thioredoxin–thioredoxin reductase system based on a naphthoquinone spiroketal natural product lead. Bioorg Med Chem Lett 11:2637–2641
Witt FA, Bergman J (2002) Oxygen analogues of the benzodiazepine alkaloids sclerotigenin and circumdatin F. J Heterocycl Chem 39:351–355
Xiao J, Zhang Q, Gao YQ et al (2014) Antifungal and antibacterial metabolites from an endophytic Aspergillus sp. associated with Melia azedarach. Nat Prod Res 28:1388–1392
Xu W, Cai X, Jung ME, Tang Y (2010) Analysis of intact and dissected fungal polyketide synthase-nonribosomal peptide synthetase in vitro and in Saccharomyces cerevisiae. J Am Chem Soc 132:13604–13607
Xu XY, Zhang XY, He F et al (2013) Two new compounds from gorgonian-associated fungus Aspergillus sp. Nat Prod Commun 8:1069–1070
Xu Q, Wu N, Li X et al (2019) Inhibition of PTP1B blocks pancreatic cancer progression by targeting the PKM2/AMPK/mTOC1 pathway. Cell death disease 10:1–15
Xue W, Tian J, Wang XS et al (2019) Discovery of potent PTP1B inhibitors via structure-based drug design, synthesis and in vitro bioassay of Norathyriol derivatives. Bioorg Chem 86:224–234
Yamauchi M, Katayama S, Todoroki T, Watanable T (1984) Total synthesis of fulvic acid. J Chem Soc Chem Commun 23:1565–1566
Yamazaki H, Nakayama W, Takahashi O et al (2015a) Verruculides A and B, two new protein tyrosine phosphatase 1B inhibitors from an Indonesian ascidian-derived Penicillium verruculosum. Bioorg Med Chem Lett 25:3087–3090
Yamazaki H, Saito R, Takahashi O et al (2015b) Trichoketides A and B, two new protein tyrosine phosphatase 1B inhibitors from the marine-derived fungus Trichoderma sp. J Antibiot 68:628–632
Yamazaki H, Ukai K, Namikoshi M (2016) Asperdichrome, an unusual dimer of tetrahydroxanthone through an ether bond, with protein tyrosine phosphatase 1B inhibitory activity, from the Okinawan freshwater Aspergillus sp. TPU1343. Tetrahedron Lett 57:732–735
Ye YH, Zhu HL, Song YC et al (2005) Structural Revision of Aspernigrin A, Reisolated from Cladosporium h erbarum IFB-E002. J Nat Prod 68:1106–1108
Zhan J, Gunaherath GKB, Wijeratne EK, Gunatilaka AL (2007) Asperpyrone D and other metabolites of the plant-associated fungal strain Aspergillus tubingensis. Phytochemistry 68:368–372
Zhang S, Zhang ZY (2007a) PTP1B as a drug target: recent developments in PTP1B inhibitor discovery. Drug discovery today 12:373–381
Zhang S, Zhang ZY (2007b) PTP1B as a drug target: recent developments in PTP1B inhibitor discovery. Drug Discov Today 12:373–381
Zhang Y, Ling S, Fang Y et al (2008a) Isolation, Structure Elucidation, and Antimycobacterial Properties of Dimeric Naphtho-γ-pyrones from the Marine Derived Fungus Aspergillus carbonarius. Chem Biodivers 5:93–100
Zhang YN, Zhang W, Hong D et al (2008b) Oleanolic acid and its derivatives: new inhibitor of protein tyrosine phosphatase 1B with cellular activities. Bioorg Med Chem 16:8697–8705
Zhang J, Meng LL, Wei JJ et al (2017) PTP1B inhibitors from the entomogenous fungi Isaria fumosorosea. Molecules 22:2058
Zhao BT, Nguyen DH, Le DD et al (2018) Protein tyrosine phosphatase 1B inhibitors from natural sources. Arch Pharm Res 41:130–161
Zhao JY, Wang XJ, Liu Z et al (2019) Nonadride and Spirocyclic Anhydride Derivatives from the Plant Endophytic Fungus Talaromyces purpurogenus. J Nat Prod 82:2953–2962
Zheng Z, Zhang S, Lu X et al (2012) Trivaric acid, a potent depside human leukocyte elastase inhibitor. Biol Pharm Bull 35:2247–2251
Zhou Y, Lingle CJ (2014) Paxilline inhibits BK channels by an almost exclusively closed-channel block mechanism. J Gen Physiol 144:415–440
Zhou Y, Zhang W, Liu X et al (2017) Inhibitors of Protein Tyrosine Phosphatase 1B from Marine Natural Products. Chem Biodivers 14:e1600462
Zhou Y, Li YH, Yu HB et al (2018) Furanone derivative and sesquiterpene from Antarctic marine-derived fungus Penicillium sp. S-1-18. J Asian Nat Prod Res 20:1108–1115
Zhou LM, Kong FD, Fan P et al (2019) Indole-Diterpenoids with Protein Tyrosine Phosphatase Inhibitory Activities from the Marine-Derived Fungus Penicillium sp. KFD28. J Nat Prod 82:2638–2644
Zhuravleva O, Kirichuk N, Denisenko V et al (2016) New kipukasin from marine isolate of the fungus Aspergillus flavus. Chem Nat Compd 52:266–268
Acknowledgements
The author H. Hussain is thankful to the Alexander von Humboldt Foundation for its generous support in providing the research opportunity in Germany which facilitated the writing of this review.
Author information
Authors and Affiliations
Corresponding authors
Additional information
This research work is dedicated to Prof. Dr. M. Iqbal Choudhary on his 60th birthday.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nazir, M., Saleem, M., Ali, I. et al. Fungal metabolites as anti-diabetic agents: emphasis on PTP1B inhibitors. Phytochem Rev 20, 119–143 (2021). https://doi.org/10.1007/s11101-020-09701-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11101-020-09701-9