Abstract
The discovery of the ellagitannin geraniin was made exactly 40 years ago. It is a secondary metabolite found in plants and is categorised as a hydrolysable tannin under the huge family of polyphenolic compounds. At present, the occurrence of geraniin has been verified in at least 71 plant species, many of which are used in traditional medicine. Hence, like other polyphenols, geraniin has also received widespread interest as a research focus to unearth its beneficial biological effects and therapeutic values apart from understanding its chemical properties, biosynthesis and interaction with the body system. Indeed, it has been demonstrated that geraniin possesses antioxidant, antimicrobial, anticancer, cytoprotective, immune-modulatory, analgesic properties besides exerting promising therapeutic effects on hypertension, cardiovascular disease and metabolic dysregulation. The objective of this review is to summarise the current knowledge about the basic chemistry, natural sources, isolation techniques, biosynthesis, pharmacokinetics and pharmacodynamics of geraniin. With reference to this information, the clinical significance, obstacles and future perspectives in geraniin research will also be scrutinised.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polyphenolic compounds which are secondary metabolites found ubiquitously in plants are probably among the most extensively studied phytochemicals. This is not only because of their pharmaceutical potential, but also due to their distinctive properties as food preservatives, natural colouring and flavouring agents as well as cosmetic products (Ignat et al. 2011). Polyphenolic compounds can be sub-divided into flavonoids, phenolic acids, tannins, stilbenes and lignans. In the tannin subgroup, there are generally three major classes, namely, hydrolysable tannins, non-hydrolyzable/condensed tannins and phlorotannins. Among the three classes, hydrolysable tannins are one of the earliest polyphenolic compounds that were subjected to quantitative and qualitative analysis about a century ago (Fisher 1914). In this context, it is also the class where the compound of interest in this review—the ellagitannin geraniin belongs.
The use of geraniin-containing herbs in folk medicine has been broadly documented. In Japan, Geranium thunbergii which has been long-known for its richness in geraniin, is certified as an official antidiarrheal drug (Luger et al. 1998). Geranium bellum which is known as “Pata de leόn” in Mexico, is used to treat fever, pain and gastrointestinal disorders (Velazquez-Gonzalez et al. 2014). Likewise, “amla” or fruits of Phyllanthus emblica are also used for similar ailments in addition to being a preventive measure for peptic ulcers and alopecia in India (Baliga and Dsouza 2011). Furthermore, in traditional Tibetan medicine, the roots of various Geranium spp. are collectively termed “li ga dur” and are used as an effective treatment for swelling in the limbs (Kletter and Kriechbaum 2001). Geranium wilfordii is also widely employed in traditional Chinese medicine for the therapy of rheumatism, osteoporosis and diarrhoea (Liu et al. 2010). Undeniably, the abundant occurrence of geraniin has been identified in a number of plant species, ranging from small flowering annual herbs to perennial woody shrubs or trees. At present, there is, yet very limited evidence to show its presence in fruits and vegetables. Nevertheless, considering the fact that ellagitannins have been discovered in different foods of plant origins like nuts, berries and grapes (Clifford and Scalbert 2000; Landete 2011), it is likely that geraniin may also exist in these functional foods.
Like many other polyphenolic compounds, geraniin is well-known for its potent antioxidant properties. Studies have shown that the antioxidant capacity of intact geraniin is at least fourfold to that of ascorbic acid while the metabolites of geraniin have displayed even more powerful antioxidant activities than geraniin itself (Ito 2011; Ishimoto et al. 2012). Aside from that, numerous biological activities have also been reported, including antihypertensive (Cheng et al. 1994; Lin et al. 2008), apoptotic (Lee et al. 2008), antiviral (Yang et al. 2007, 2012), liver protective (Londhe et al. 2012), anti-hyperglycaemic (Palanisamy et al. 2011a) and antidiabetic (Chung et al. 2014) activities. Hence, it is believed that geraniin may harbour therapeutic values which are worth investigating.
In this context, the aim of this review is to outline the properties of geraniin in terms of its chemistry, natural sources and available techniques for geraniin isolation and purification. Further discussion will also be carried out with reference to the current understanding of the pharmacological and biological effects of geraniin together with its putative mechanisms of action. This information may help to shed some light on the clinical significance of geraniin besides providing new perspectives in geraniin research.
Basic chemistry of the ellagitannin geraniin
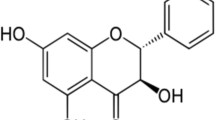
The chemical formula of geraniin is C41H28O27 with a molecular mass of 952.64 g/mol. Upon crystallization, geraniin exists as a hydrate with a chemical formula of C41H28O27·7H2O (Okuda et al. 1982; Luger et al. 1998). Generally, ellagitannins are characterised as polyphenolic compounds with various numbers of hexahydroxydiphenoyl (HHDP) units attached to a sugar moiety. In addition, other acyl units like galloyl and sanguisorboyl moieties may also be found in ellagitannins. This gives a huge structural variety to ellagitannins because there are different combinations of acyl units to the sugar moiety and more importantly, due to the high tendency monomeric ellagitannins to form their dimeric or oligomeric counterparts (Niemetz and Gross 2005). Geraniin seems to be a typical ellagitannin since it is made up of common acyl moieties, namely a galloyl, a HHDP and a dehydrohexahydrodiphenoyl (DHHDP) group, esterified to a glucose molecule (Fig. 1).
Like many hydrolysable tannins, geraniin is water-soluble and readily undergoes hydrolysis in the presence of hot water, weak acids and weak bases. Under these treatments, the ester linkages will be hydrolysed and the HHDP and DHHDP units will undergo spontaneous rearrangement to yield water-insoluble, bislactone ellagic acids (Fig. 1) (Clifford and Scalbert 2000). Furthermore, geraniin can also precipitate water-soluble proteins and alkaloids. The reactivity is comparable to other tannins of similar molecular size (Okuda et al. 1985). Yet, unlike many tannins, geraniin gives almost no astringent mouth feeling (Okuda et al. 2009).
At aqueous state, the DHHDP unit of geraniin readily isomerises into a mixture of hydrated five- and six-membered hemiacetalic rings (Fig. 1). In addition, it is also exceedingly prone to chemical modifications. The DHHDP moiety can be oxidatively cleaved as exemplified by phyllanthusiins A, B and C which are some of the oxidative metabolites of geraniin (Fig. 2) (Yoshida et al. 1992). Moreover, the DHHDP moiety also allows geraniin to undergo condensation reactions with numerous compounds like ascorbic acid, acetone and ortho-phenylenediamine to form ascorgeraniin (elaeocarpusin), phyllanthusiin D and a phenazine derivative respectively (Fig. 2) (Okuda et al. 2009; Okuda and Ito 2011). These condensation products can be prepared under mild laboratory conditions without any enzymatic intervention, suggesting that some of these compounds may form naturally in plants (Okuda et al. 1986). Indeed, it has been shown that ascorgeraniin co-exists with geraniin in several plant species like Geranium thunbergii (Nonaka et al. 1986; Okuda et al. 1986) and Euphorbia watanabei (Amakura and Yoshida 1996) while an analog of ascorgeraniin, putranjivain A (Fig. 2) has also been successfully isolated from some euphorbiaceous plants (Lin et al. 1990a). Many of the aforementioned chemical reactions do not only occur under controlled procedures in the laboratory, but have also been observed in plants in the field (Klumpers et al. 1994; Viriot et al. 1994), clearly pointing out the high lability of geraniin.
Chemically modified products of geraniin via oxidation (phyllanthusiins A, B, and C) and condensation with ascorbic acid (ascorgeraniin and putranjivain A), acetone (acetonylgeraniin/phyllanthusiin D) and ortho-phenylenediamine (a phenazine derivative of geraniin). Asterisk the compound has been isolated from natural sources
Natural sources and isolation of the ellagitannin geraniin
The earliest documented attempt to extract the ellagitannin geraniin from plant species (Geranium thunbergii) can be traced back to mid-1970s by Okuda et al. (1975). However, at that time, the compound was simply termed Tannin 1 because it was one of the two major tannins found in the ethyl acetate fraction of Geranium thunbergii extract (Okuda et al. 1975). The official nomenclature, geraniin, was assigned to Tannin 1 in 1976, according to genus Geranium after its crystallization from Geranium thunbergii plant extracts (Okuda et al. 1976). Since then, the occurrence of geraniin has been confirmed in at least 71 different plant species from 26 genera and across 9 families which are summarised in Fig. 3. The prevalence of geraniin in a vast diversity of plant species suggests that it may be a key player in plant survival. In fact, various theories have been proposed to justify why plants invest their metabolic energy in tannin biosynthesis (Kraus et al. 2003), some of which include functions as herbivore deterrent (Butler 1989; Schultz 1989), pathogen defence (Field and Lettinga 1992; Schultz et al. 1992), ecological succession (Schimel et al. 1998), regulation of plant homeostasis (Scalbert 1991; Feucht and Treutter 1999), wound healing (Walkinshaw 1999) and resistance against abiotic stressors (Chalker-Scott and Krahmer 1989). Nonetheless, there is no conclusive remark about the exact function of each tannin in plants because empirical findings yield mixed results. Thus, the biological role of gerannin in plants also remains largely uncertain.
Generally, most of the screening work of geraniin distribution was done by Okuda et al. (1980) using high performance liquid chromatography (HPLC) of acetonitrile–water plant extracts. They ascertained the presence of geraniin in 41 plant species. The content of geraniin in different plants varies greatly, ranging from as low as 0.01 % in Breynia nivosa to more than 10 % in some Geranium spp. (Okuda et al. 1980). Based on Fig. 3, among the nine families in which geraniin-producing plants are found, Euphorbiaceae, Geraniaceae and Phyllanthaceae give rise to the highest number of such plant species. Geraniin-producing species are found most abundantly in the genus Geranium, in which 18 species were identified to date, followed closely by 15 Euphorbia spp. and 8 Phyllanthus spp. There is no distinctive geographical region for geraniin-producing plants since their habitat ranges from tropical to temperate areas. However, the synthesis of geraniin may be subjected to slight temporal variation (Okuda et al. 1980), notably for the plants in temperate areas probably due to the seasonal changes throughout the year.
As mentioned earlier, geraniin has been identified in both herbaceous and woody plants. The plant part used for geraniin extraction also varies considerably, including whole plant, leaf, herbaceous stem, tree bark, root, fruit skin and seed but the leaf is the most frequently used plant part, probably because it is easier to harvest, process and tends to give higher yield. Geraniin can also be extracted from fruit flesh of Indian gooseberry (P. emblica) (Liu et al. 2008, 2012b). This points to the likelihood of geraniin occurrence in other edible fruits. Aside from that, it is also worth highlighting that geraniin and other phenolic compounds have been isolated from suspension cell cultures of geraniin-synthesizing plants like G. thunbergii (Ishimaru and Shimomura 1991; Yazaki et al. 1991) and S. sebiferum (Neera and Ishimaru 1992; Neera et al. 1992). The yield of geraniin isolated from the hairy root culture was about 70 % of that extracted from the roots of the mother plant (Ishimaru and Shimomura 1991). However, certain conditions have been reported to enhance geraniin production of leaf callus cultures of S. sebiferum, including the use of Murashige-Skoog medium without NH4NO3, addition of 2,4-dichlorophenoxyacetic acid and benzyladenine to culture medium and placing the cultures under light (Neera et al. 1992). Such a cell culture system is undoubtedly useful for the studies of factors affecting geraniin biosynthesis. This will not only provide insight into the biological functionality of geraniin in plants, but also offer an alternative to manipulate the pathway for large-scale geraniin production, assuming the practical uses of geraniin are more prevalent in the future.
The reported percentage yield of ellagitannin geraniin extraction differs significantly (Table 1). This is attributable to both the difference of original geraniin content in the plant species and the diverse geraniin extraction and purification protocols. However, it is noted that Geranium spp. are more consistent in giving higher percentage yield, suggesting that Geranium spp. are excellent natural sources of the compound. In terms of extraction techniques, conventional solvent extraction is commonly utilised. However, more recent techniques, particularly microwave-assisted extraction, has also been proven to be feasible to obtain geraniin from plant materials (Yang et al. 2010, 2013). To separate the plant crude extract, most research groups prefer gel filtration chromatography as the first step to obtain small molecule metabolites followed by further separation with reverse-phase column chromatography to obtain geraniin at a higher purity. Generally, extraction procedures involving intense heating should be avoided considering the high susceptibility of geraniin to be hydrolysed into corilagin, ellagic acid and gallic acid (Luger et al. 1998). The isolation techniques of geraniin from various plant materials and species are summarised in Table 1.
Biosynthesis of the ellagitannin geraniin
Thus far, there is no published literature specifically about the biosynthesis of ellagitannin geraniin. Nonetheless, most hydrolysable tannins seem to share a common precursor, 1,2,3,4,6-penta-O-galloyl-β-d-glucopyranose or pentagalloylglucose (PGG), suggesting that the synthesis of geraniin in plants may be somewhat similar. The biosynthesis of hydrolysable tannins has been summarised comprehensively in other reviews (Niemetz and Gross 2005; Gross 2008; Pouysegu et al. 2011).
The overview of ellagitannin biosynthesis is illustrated in Fig. 4. Briefly, PGG biosynthesis comprises two essential building blocks: β-d-glucose and gallic acid. The first step involves the esterification of gallic acid to glucose, yielding 1-O-galloyl-β-d-glucose or β-glucogallin. This process was first described by Gross (1983a) using transferases isolated from oak leaves and uridine diphosphate glucose (UDP-glucose) as the activated donor molecule. Strikingly, by using the same oak leaf enzyme preparations, it was later demonstrated that β-glucogallin possesses dual functionality as both the acyl donor and acyl acceptor for the formation of digalloylglucose without the need of additional cofactors (Gross 1983b). β-glucogallin remains as the principal acyl donor for higher degrees of esterification catalysed by β-glucogallin-dependent galloyltransferases up to PGG following a remarkably specific metabolic pattern (β-glucogallin → 1,6-digalloylglucose → 1,2,6-trigalloylglucose → 1,2,3,6-tetragalloylglucose → 1,2,3,4,6-pentagalloylglucose) (Schmidt et al. 1987; Cammann et al. 1989; Gross and Denzel 1991; Hagenah and Gross 1993). The highly selective acylation sequence of glucopyranosides suggests a combination of differential reactivity of primary and secondary hydroxyl groups, neighbour-activation effects and steric hindrance at work (Williams and Richardson 1967).
Subsequently, PGG acts as the immediate precursor for the formation of HHDP residues through oxidative biaryl coupling of adjacent galloyl units. Using radioactively-labelled PGG (Rausch and Gross 1996), the enzyme catalysing the oxidative C–C coupling was identified as one of the members from the group of laccase-type phenol oxidases (EC 1.10.3.2) and named “pentagalloylglucose: O2 oxidoreductase” (Niemetz and Gross 2003). The HHDP moiety can be located at the 2,3-, 4,6-, 1,6-, 3,6- and/or 2,4-positions of the glucopyranose core (Immel and Khanbabaee 2000). In geraniin, it is postulated that the bridging HHDP units form at both the 2,4- and 3,6-positions of the glucopyranose ring in its 1C4 conformation, followed by further oxidation of the 2,4-HHDP glucoside to yield a DHHDP residue (Pouysegu et al. 2011). The enzymatic catalysis of the HHDP residue oxidation, however, is not well-understood and thus, requires further investigation.
Pharmacokinetics and bioavailability of the ellagitannin geraniin
Existing evidence on the metabolism of geraniin upon oral ingestion is fairly limited, but the pharmacokinetic studies of other ellagitannins may help to provide a better overview for geraniin. Generally, intact geraniin is rarely found in the circulation after oral dosing. This is anticipated because of its large molar mass which does not facilitate simple diffusion very effectively and so, degradation of geraniin into smaller metabolites is crucial for the absorption. However, it is noteworthy that some of the large hydrolysable tannins like corilagin (M.W. 636 g/mol) and punicalagin (M.W. 1084 g/mol) do get absorbed and excreted at a small extent upon oral consumption in rats (Cerda et al. 2003; Ito 2011). In this context, the absorption and metabolism of geraniin may occur at two distinct regions of the gastrointestinal tract, namely (1) the stomach and proximal small intestine as well as (2) the distal small intestine and colon, the latter of which seems to play a more predominant role in the absorption. The proposed metabolism of geraniin into smaller metabolites is illustrated in Fig. 5.
Proposed metabolism of geraniin into different metabolites in the presence of intestinal microflora. Metabolic pathways highlighted by solid line may also take place in the stomach and/or proximal small intestine while the metabolites highlighted by dotted line are found in the serum or urine after the consumption of geraniin and other ellagitannins
Partial hydrolysis of geraniin produces corilagin while complete hydrolysis yields gallic acid and ellagic acid. All these hydrolytic products have been detected in serum and/or urine upon consumption of ellagitannin-rich functional food (Hodgson et al. 2000; Ito 2011), According to Seeram et al. (2006), in human subjects, the plasma concentration of ellagic acid peaked at about 1 h post oral administration of pomegranate juice which is high in ellagitannin content, suggesting that at least part of the ellagitannin are rapidly hydrolysed and absorbed in the stomach and/or small intestine. Daniel et al. (1991) demonstrated that it is in the small intestine that free ellagic acid is released from crude ellagitannin extract and the reaction is dependent on the mild alkaline pH in the small intestine and free from the interference of pancreatic enzymes and bile salts. In the same study, it was also shown that ellagitannin is relative stable under physiological gastric conditions. Although these findings are not established specifically based on geraniin, considering how most ellagitannins share similar basic chemistry, it is reasonable to extrapolate the information to geraniin. Absorption of free ellagic acid in small intestine is rather poor (<1 % of the total ingested amount) (Stoner et al. 2005). The absorption capacity is probably diminished by the high, irreversible binding of ellagic acid to macromolecules like proteins and DNA in the intestinal epithelium (Whitley et al. 2003).
Hydrolysis of geraniin as well as other catabolic reactions like decarboxylation and removal of hydroxyl groups continue in the large intestine where intestinal bacteria seem to play an integral role in the process. Various hydrolytic products including corilagin, ellagic acid, gallic acid and brevifolincarboxylic acid as well as decarboxylated products such as pyrogallol and brevifolin were found when geraniin was subjected to anaerobic incubation with rat fecal suspension (Ito 2011). Further incubation for more than 6 h affords various hydroxyl-6H-benzopyran-6-one derivatives (urolithins) (Ito et al. 2008; Ito 2011). The timing of urolithin production corresponds precisely to the upsurge of serum and urine urolithin concentration which occur only after 6–12 h after single dosing of geraniin in rats (Ito et al. 2008; Ito 2011). This suggests that metabolites generated from intestinal microbial degradation of geraniin are subsequently absorbed into bloodstream at the large intestine. Nevertheless, not all the bacterial urolithin products are absorbed. Intestinal bacteria transform geraniin or ellagic acid into urolithins with different numbers of hydroxyl groups, ranging from monohydroxy-urolithin (urolithin B), dihydroxy-urolithin (urolithin A), trihydroxy-urolithin (urolithin C) up to tetrahydroxy-urolithin (urolithins D and E) (Ito et al. 2008; Garcia-Villalba et al. 2013), but only urolithins A, B and C are consistently found in the serum and urine (Cerda et al. 2004; Ito et al. 2008). Basically, intestinal bacteria-facilitated geraniin breakdown is a gradual process because complete clearance is not achieved even after 72 h (Ito et al. 2008). The hydrolysis reaction appears to be the rate-limiting reaction as conversion of ellagic acid to urolithins is much more efficient in comparison to conversion of ellagitannins to urolithins (Gonzalez-Barrio et al. 2011).
Another evidence that supports the involvement of colon microbes in geraniin metabolism is the marked interpersonal variation of the circulatory and excretory urolithins detected in human volunteers after drinking high-ellagitannin fruit juices (Seeram et al. 2006; Gonzalez-Barrio et al. 2010). Such an observation is attributable to the variability of colonic microbiota. In fact, not only does the urolithin profile show person-to-person differences, large inter-individual variations have also been detected in terms of the quantity of urolithins (Seeram et al. 2006), latency between ellagitannin ingestion and presence of urolithins in circulation (Gonzalez-Barrio et al. 2010) and the total urinary excretion of urolithin (Cerda et al. 2004), indicating how strong the dependence of ellagitannin geraniin metabolism is on the gut microflora.
As pointed out earlier, a number of metabolites, namely gallic acid, pyrogallol, ellagic acid and urolithins, are absorbed into the body. The metabolic pathways of these metabolites upon absorption is outlined in Fig. 6. Generally, compounds with ortho-dihydroxyl group will be swiftly methylated by the action of catechol-O-methyl transferase (COMT) to form a methyl ether group. In ellagic acid, there are two such groups and hence, two methyl moieties are added to form dimethylellagic acid. Gallic acid, pyrogallol and urolithin C can also be converted by COMT (Yasuda et al. 2000; Ito 2011). Aside from that, hydroxylation of urolithins A and B is also a likely in vivo metabolic process so that they become more susceptible to further conjugation and urinary excretion as evidenced by the escalated expression and activity of cytochrome P450 1A1 (CYP1A1) in ellagic acid-treated colon cancer cell lines (Gonzalez-Sarrias et al. 2009). Irrespective of methylation, virtually all the metabolites are subjected to sulphate or glucuronide conjugation. The conjugates are then delivered to either the liver or kidney for elimination.
No study has reported the detection of intact geraniin in the circulation or urine post oral administration. Absorption takes place after pH-induced hydrolysis and microbial transformation. Some metabolites, notably urolithins, display exceedingly good absorption efficacy whereas others like corilagin, gallic acid, ellagic acid and pyrogallol are absorbed to a much lesser extent (Ito 2011). The bioavailability of the metabolites, however, varies substantially. In rats, approximately 12.4 % of the total geraniin was excreted in urine as urolithins over 72 h (Ito et al. 2008) whereas in humans, the total urinary excretion of metabolites upon ellagitannin ingestion ranged from 0.7 to 52.7 % (Cerda et al. 2003). Once again, this reinforces the notion that the bioavailability is highly dependent on the bacterial composition of colonic microbiota. Despite the absorption, no evidence has shown the deposition of the metabolites in major organs like the adipose tissues, muscles, heart and brain. Accumulation of the metabolites in their conjugated form was detected in the liver and kidney (Espin et al. 2007). This shows the involvement of these organs in the elimination of geraniin metabolites. Indeed, a large amount and variety of urolithin conjugates were found in bile juice, confirming the active role of the hepatic drug clearance system in addition to urinary excretion (Espin et al. 2007). Such an enterohepatic circulation is held responsible for the long persistency of urolithins in the circulation.
In short, like other ellagitannins, there is limited evidence, showing that geraniin is bioavailable upon oral dosing. However, the compound is subjected to extensive degradation and metabolism mainly by the intestinal bacteria to afford various metabolites which are subsequently absorbed and persists in the body for a relatively long duration. These metabolites, particularly urolithins, may be the key players that account for the bioactivity of geraniin as well as other ellagitannins.
Biological effects of the ellagitannin geraniin
Over four decades of geraniin research, a large variety of bioactivities has been reported for the polyphenolic compound, ranging from antioxidant, antimicrobial, anticancer, anti-inflammatory, antihypertensive, antihyperglycaemic, antidiarrheal, anaesthetic to protective effects on different organs. Different approaches, including in vitro cell culture study, in vivo animal study as well as direct chemical and enzymatic assays have been employed to attain these findings. These bioactivities of geraniin are summarised in Table 2. Looking into these bioactive properties of geraniin may allow us to characterise the natural product and its mechanisms of action. The bioactivities of geraniin have also been summarised in a review by Perera et al. (2015).
Antioxidant properties
High antioxidant activity has been consistently reported in the crude extract of geraniin-rich plants. The crude ethanolic extract of N. lappaceum peels has a 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging capacity comparable to that of vitamin C (Palanisamy et al. 2008). Such a powerful radical scavenging capacity has also been confirmed in the plant extract of the same (Thitilertdecha and Rakariyatham 2011) or different geraniin-rich plant species (Yang et al. 2010; Arina and Rohman 2013). Palanisamy et al. (2008) further tested the pro-oxidant capacity of the plant extract as some strong antioxidant agents, particularly vitamin C, also possess pro-oxidant properties through their interaction with catalytic metal ions to generate harmful radicals (Carr and Frei 1999). It was demonstrated that the peel extract of N. lappeceum has the lowest pro-oxidant capacity in comparison to vitamin C and α-tocopherol (Palanisamy et al. 2008). The pro-oxidant profile is similar to Emblica TM, a commercial antioxidant with exceedingly low pro-oxidant activity derived from the fruits of another geraniin-rich plant, P. emblica (Liu et al. 2008, 2012b). The consistency in the detection of strong antioxidant and low pro-oxidant activities of geraniin-rich plant extracts strongly suggest that geraniin may play an integral role to these bioactivities.
Indeed, using ferric reducing/antioxidant power (FRAP) assay, it is known that the antioxidant capacity of geraniin is about fivefold to sixfold more potent than L-ascorbic acid and trolox (Sudjaroen et al. 2012). Much of this antioxidant properties is probably attributable to its free radical scavenging ability towards numerous damaging radicals like DPPH radicals, superoxide radicals, hydroxyl radicals and nitric oxide radicals as shown in Table 2. Tabata et al. (2008) showed that geraniin is comparable to or even better than epigallocatechin gallate, a strong antioxidant found in green tea in terms of free radical scavenging (Tabata et al. 2008). Furthermore, to give an idea of how strong the radical scavenging activity of geraniin is, in most of the studies, the 50 % inhibition concentration (IC50) of geraniin is about 7–14 times smaller than that of positive controls including ascorbic acid (Lin et al. 2008), butylated hydroxyanisole (BHA) (Liu et al. 2008) and butylated hydroxytoluene (BHT) (Lin et al. 2008), implying that geraniin is more effective at preventing oxidation than most of the widely used antioxidant food additives. In one particular study, it was even demonstrated that geraniin is about 87 times stronger than BHT in scavenging DPPH radicals (Thitilertdecha et al. 2010). The beneficial radical scavenging properties also confers cytoprotective effects in a human foreskin fibroblast cell line against oxidative and nitrosative stress-induced cell death (Ling et al. 2012). The mechanism of radical scavenging activity is not clearly characterised. However, it is generally accepted that most of the polyphenolic compounds scavenge free radicals via hydrogen atom transfer mechanism (Leopoldini et al. 2011; Meo et al. 2013). Phenolic compounds have a high tendency to donate a hydrogen atom to a radical, transforming the original radical into a harmless molecule, but itself to a phenoxyl radical (Leopoldini et al. 2011). Despite the formation of another radical, phenoxyl radicals are non-damaging because they have aromatic structures that facilitate resonance stabilization of the radical (Leopoldini et al. 2011). These phenoxyl radicals may further couple with another free radical to quench the reactivity of both radicals, a characteristic known as chain-breaking antioxidant activity. Such an activity has been reported in some of the natural polyphenols like catechin, quercetin and gallic acid (Roginsky 2003), but it is unsure whether the free radical scavenging activity of geraniin acts in a similar manner.
Apart from free radical scavenging activity, geraniin can also inhibit xanthine oxidase which is a reactive oxygen species (ROS)-generating enzyme (Lin et al. 2008). However, considering the protein-precipitating effect of geraniin, it is likely that the inhibitory effect may be caused by the precipitation of the enzyme (Sudjaroen et al. 2012). Geraniin also possesses an inhibitory effect on lipid peroxidation (Liu et al. 2008; Thitilertdecha et al. 2010). This may explain the reduction of malondialdehye level which an end product of the decomposition of certain lipid peroxidation products (Janero 1990) in animals after geraniin treatment (Islam et al. 2008; Jin and Sun 2011). In addition, geraniin treatment has been shown to enhance glutathione (GSH) levels as well as reduce intracellular ROS levels and cell death rates in a concentration- and time-dependent manner in human hepatocarcinoma cells exposed to hydrogen peroxide-induced oxidative stress (Wang et al. 2015). These beneficial effects might be due to geraniin-induced nuclear translocation of nuclear factor-erythroid 2-related factor 2 (Nrf2), which is a transcription factor responsible for the regulation of various detoxifying antioxidant genes, presumably via PI3K/AKT and ERK1/2 signalling pathways (Wang et al. 2015). Thus, aside from scavenging free radicals directly, at least in in vitro cell culture studies, the antioxidant activity of geraniin may also be augmented by its ability to trigger certain signalling pathways that eventually lead to the upregulation of antioxidant enzymes.
The antioxidant effect of geraniin has also been detected in tetrachloromethane (CCl4)-induced acute liver failure rats (Islam et al. 2008) and sarcoma S180-tumor cell-inoculated mice (Jin and Sun 2011) as evidenced by significant escalation in GSH and catalase activity in liver tissues for the former as well as increase in superoxide dismutase (SOD) activity in the red blood cells for the latter. These improvements in oxidative status were also associated with better outcomes like improved liver function and suppressed tumor growth in comparison to the untreated counterparts. As such, it is likely that the antioxidant activity of geraniin may be translated into therapeutic effects in certain diseases. However, our current understanding in this aspect is still limited and further investigation should be carried out to unearth the clinical potential of geraniin.
Antimicrobial activity
Crude extracts of geraniin-rich plants also display promising antimicrobial activity against some common human pathogens like Staphylococcus aureus and Escherichia coli (Thitilertdecha et al. 2008; Yang et al. 2013) as well as dengue virus 2 (Lee et al. 2013). Further investigations using purified geraniin from plant materials confirmed the antibacterial activities with a minimum inhibitory concentration (MIC) ranging from µg/mL to mg/mL (Table 2). Furthermore, Funatogawa et al. (2004) also demonstrated that most hydrolysable tannins, including geraniin, possess antibacterial activity against Helicobacter pylori. The activity is likely to be bactericidal because the number of viable cells decreased exponentially after prolonged exposure to another hydrolysable tannin, tellimagradin I. In the same study, Funatogawa et al. (2004) showed that hydrolysable tannins could disrupt liposomal membranes and subsequently, release the liposomal content in a dose-dependent manner. This may be one of the mechanisms of action which accounts for the bactericidal activity against H. pylori. The fact that most hydrolysable tannins exhibited similar effects on the growth of H. pylori and liposomal membranes disruption suggests that the antibacterial activity lies in the intrinsic properties of hydrolysable tannins. It is also worth mentioning that most of the antibacterial screening tests were not carried out at physiological gastric conditions, particularly acidic pH. As low pH is very likely to jeopardise the structural integrity of most hydrolysable tannins, further work is required to examine whether hydrolysable tannins, including geraniin, can retain its antibacterial properties against H. pylori under normal gastric conditions.
Based on Table 2, geraniin also demonstrates potent antiviral properties against some of the most devastating pathogenic viruses. Firstly, hepatitis B virus (HBV) is the major causative agent of acute and chronic liver diseases, of which the latter will lead to cirrhosis and is associated with increased risk of hepatocellular carcinoma (Tsukuma et al. 1993). In this context, geraniin intervention greatly reduced the liberation of HBV e (HBeAg) and surface (HBsAg) antigens from HBV-transfected cell lines (Huang et al. 2003; Li et al. 2008). Using in vitro enzymatic inhibition assays, geraniin also exhibited remarkable inhibitory effects on several enzymes of retroviral origin such as protease, integrase and most importantly, reverse transcriptase (Kakiuchi et al. 1985; Xu et al. 2000; Notka et al. 2004). Co-incubation of geraniin and human immunodeficiency virus (HIV) successfully inhibited virus uptake and replication in MT4-T-lymphoid cells, an outcome which is attributable to the blockade of CD4-gp120 binding by geraniin (Notka et al. 2003, 2004). Moreover, in the presence of small concentrations of geraniin, infection caused by human enterovirus 71 (EV71), herpes simplex virus (HSV) and Coxsackie B2 was significantly reduced (Corthout et al. 1991; Yang et al. 2007, 2012). In one animal study (Yang et al. 2012), 1 mg/kg of geraniin was intraperitoneally administered to mice which were exposed to a lethal dose EV71. The outcome was astonishing. Geraniin treatment enhanced the survival rate of the mice by about 40 % besides extending the survival time and reducing the severity of the symptoms, strongly pointing out the in vivo therapeutic effect of geraniin against EV71.
In addition to antibacterial and antiviral activities, some studies also reported antifungal and antiprotozoal effects of geraniin. Geraniin treatment effectively inhibits the growth of Candida pseudotropicalis and Candida albicans at a MIC of 2 mg/mL (Adesina et al. 2000; Gohar et al. 2003). This is of paramount clinical importance because Candida spp. are the most common pathogens of fungal infection, principally among immunocompromised patients. When Plasmodium falciparum-infected erythrocytes were subjected to geraniin treatment, proliferation of P. falciparum was inhibited in a dose-dependent manner (Ndjonka et al. 2012). Moreover, geraniin therapy effectively impaired the viability of amastigotes of Leishmania donovani residing within murine macrophage cells at an IC50 of <0.4 µg/mL (Kolodziej et al. 2001). This is much more powerful than the clinically used antileishmanial drug, sodium stibogluconate (IC50, 7.9 µg/mL). As such, the antifungal and antimalarial effects of geraniin undoubtedly merit further investigation to understand the precise mode of action and explore potential clinical significance, particularly in the treatment of candidiasis, malaria and leishmaniasis.
Apart from human pathogens, geraniin treatment also exerts antibacterial effects on plant bacteria in in vitro settings. For instance, when tested on Streptomyces scabies, which is a microorganism that causes common scab in potatoes, geraniin could reliably inhibit the growth of the potato pathogens (Ushiki et al. 1998). A similar inhibitory effect was also observed against Ralstonia solanacearum, a plant pathogen that is capable of infecting a wide range of crops and eventually causing wilting and dying (Ooshiro et al. 2011) as well as fish pathogens like Aeromonas salmonica and Pseudomonas fluorescens (Kurihara et al. 1993). Thus, geraniin is not only valuable in fighting against some human infections, but also harbours incredible potential in agricultural aspects in term of disease prevention in large-scale crop plantations and fish farming.
Anticancer activity
Treatment with geraniin in a wide variety of cancer cell lines of both murine and human origins is able to impair the viability and impede the proliferation of the tumor cells. The anticancer activity of geraniin has also been recognised in one in vivo study using adenocarcinoma tumor cell xenografts onto nude mice (Li et al. 2013). Several mechanisms of action have been postulated to describe the anticancer properties of geraniin. Firstly, geraniin treatment induces apoptotic cell death of the cancer cells in a time- and dose-dependent pattern (Lee et al. 2008; Li et al. 2013). When being exposed to geraniin, the expression of Fas ligand is upregulated, promoting ligand-receptor interaction with Fas death receptor (Lee et al. 2008). This process initiates the pro-apoptotic signalling cascades, including the proteolysis of pro-caspase-8 to active caspase-8, liberation of cytochrome c from mitochondria into the cytoplasm, activation of caspase-9 and most importantly, caspase-3 (Lee et al. 2008; Li et al. 2013). Geraniin-facilitated induction of caspase 3 activity leads to apoptotic cell death by promoting DNA fragmentation, obstructing DNA repair besides inducing the cleavage of focal adhesion kinase which is an anti-apoptotic protein (Lee et al. 2008). It may be added that expression of the p53 tumor suppressor gene is also enhanced upon geraniin treatment (Jin and Wang 2010). Activated p53 will help to initiate programmed cell death. Furthermore, it will also promote cell cycle arrest which has been observed in certain geraniin-treated cancer cell lines (Li et al. 2013; Vassallo et al. 2013). Therefore, it is apparent that geraniin affects several pivotal signalling pathways in cell growth to interfere the proliferation and survival of malignant cells.
Secondly, the anti-cancer properties of geraniin are also linked to its interaction with heat shock protein 90 (Hsp90). Under physiological condition, Hsp90 is a chaperone protein that ensures proper protein folding to preserve precise biological activity, maintains protein structural integrity against environmental stresses and targets misfolded or non-functional proteins for proteolytic degradation (Neckers and Ivy 2003). In cancer cells, Hsp90 is imperative in prolonging cancer cell survival via the stabilization of oncoproteins (Solit and Rosen 2006). In this context, geraniin is able to bind to Hsp90 and triggers a dose-dependent inhibitory effect on the ATPase and chaperone activities of Hsp90 (Vassallo et al. 2013). This contributes to the reduction of the level of several client proteins of Hsp90 in tumour cells, including Raf-1, pAkt and EGFR which are active in cell fate determination like proliferation, differentiation, apoptosis and survival (Vassallo et al. 2013). The inhibitory effect of geraniin on Hsp90 is reported to be similar to that of 17-(allylamino)-17-demethoxygeldanamycin, an experimental drug which targets Hsp90 in cancer treatment (Vassallo et al. 2013). As such, it is believed that geraniin-dependent Hsp90 inhibition may be further exploited for the disruption of tumour progression.
One recent study reported that geraniin treatment may also be beneficial in the prevention of migration and metastasis of cancer cells (Ko 2015). In the study, transforming-growth factor β-1 (TGF-β1) was used to potentiate the metastatic properties of A549 lung cancer cells by inducing some of the key changes like morphological transformation from epithelial to mesenchymal phenotype, improved motility, enhanced invasiveness and resistance to anoikis which is a type of programmed cell death caused by the detachment of cells from the surrounding extracellular matrix (Ko 2015). All TGF-β1-induced metastatic processes are annihilated by geraniin treatment (Ko 2015). According to the evidence, geraniin may possess both tumoristatic and tumoricidal characteristics besides preventing malignant cell migration and these substantiate further exploration on the clinical use of geraniin as a cancer treatment.
Immuno-modulatory effect
The generation of free radicals is one of the most essential and fundamental process in our immune system. In small and regulated quantity, ROS and reactive nitrogen species (RNS) help to launch cascades of immune responses to fend off pathogens and destroy malignant cells. On the contrary, uncontrolled overproduction of free radicals is responsible for the pathogenesis of a number of inflammatory and autoimmune diseases like cardiovascular disease, diabetes mellitus, Alzheimer’s disease, Parkinson’s disease and rheumatoid arthritis (Valko et al. 2007). As mentioned earlier, geraniin treatment confers antioxidant effect via its free radical scavenging capacity. This also allows geraniin to exert certain influences onto immune responses.
In this context, several studies have shown that treatment with geraniin on murine macrophages modulates the secretion of proinflammatory cytokines such as tumour necrosis factor (TNF), interleukin-8 and interferon (Kolodziej et al. 2001; Okabe et al. 2001; Fujiki et al. 2003; Park et al. 2007). The activities of some inflammatory mediator-producing enzymes like 5-lipoxygenase (5-LOX) and inducible nitric oxide synthase (iNOS) are also affected by the presence of geraniin (Pan et al. 2000; Park et al. 2007). Furthermore, in activated macrophages, geraniin compromises the activity of nuclear factor kB (NF-kB), which is a key signalling factor in cellular inflammatory response (Pan et al. 2000; Park et al. 2007). On the other hand, Ushio et al. (1991) demonstrated that geraniin-treated macrophages participated more actively in phagocytosis of yeasts in comparison to non-treated counterpart. Based on this evidence, geraniin appears to possess both the immuno-stimulating and immuno-suppressing effects. The dual functionality requires further clarification in order to utilise geraniin in the modulation of immune response.
Cytoprotective activity
Studies have also shown that treatment with geraniin may build a strong defensive barrier against chemical and radioactive assaults besides conferring protective effects for different tissues. One of the most significant cellular protective effect of geraniin is hepatoprotective activity. The liver plays an irreplaceable role in the detoxification and elimination of harmful drugs. Hence, despite the fact that the liver has an enormous regenerative capacity, long-term hepatocyte injury will inevitably lead to liver failure which is potentially fatal. Pretreatment with extracts from Geranium schiedeanum, which is a geraniin-rich plant decreased thioacetamide-induced hepatotoxicity by 66 % (Gayosso-de-Lucio et al. 2014). The beneficial effects of geraniin treatment in liver protection have been replicated in various in vitro and in vivo models of chemically-induced liver injury. Generally, geraniin helps to inhibit lipid peroxidation besides restoring liver function as evidenced by improved bilirubin level and aminotransferase activities which are deranged by liver toxins like peroxidised corn oil, tetrachloromethane and alcohol (Okuda et al. 1983; Kimura et al. 1984; Nakanishi et al. 1999; Londhe et al. 2012). The extent of programmed cell death in injured liver is also diminished by the presence of geraniin (Londhe et al. 2012).
The hepatoprotective activities are possibly related to the antioxidant effect because Londhe et al. (2012) reported significant changes in the activity of some key antioxidative enzymes in the liver, namely catalase, SOD, GSH peroxidase and GSH reductase. This is reasonable as the drug detoxification processes tend to release free radicals that pose tremendous oxidative and nitrosative stresses to the hepatocytes (Jaeschke et al. 2002). This causes liver damage which is further exacerbated by the activation of macrophages (Kupffer cells) and infiltration of neutrophils, leading to the onset of both necrotic and apoptotic cell death (Jaeschke et al. 2002; Jaeschke 2011). By removing free radicals and enhancing the activities of antioxidative enzymes, it is very likely that geraniin is able to protect the liver from the insults from hepatotoxins.
Recently, the osteoprotective effect of geraniin has been acknowledged. Co-incubation of geraniin and osteoclasts, which is a type of bone cells that cause bone resorption (breakdown of bone materials), results in a dose-dependent inhibitory effect on the differentiation and maturation of osteoclasts (He et al. 2013; Xiao et al. 2015). To understand the mechanism by which geraniin inhibits osteoclast differentiation, it is important to know that osteoclasts are developed from macrophages upon induction by TNF-related cytokine receptor activator of nuclear factor kB ligand (RANKL) and polypeptide growth factor colony-stimulating factor-1 (CSF-1) (Yasuda et al. 1998; Lacey et al. 1998). Treatment with geraniin markedly downregulates the expression of RANKL-induced osteoclast-specific genes, inhibits NF-kB and extracellular signal-regulated kinase (ERK) signalling pathways and suppresses the activity of key osteoclast transcriptional factor, NFATc1 and c-Fos (Xiao et al. 2015). As a result, osteoclastogenesis is severely suppressed. This is directly translated into the reduction in bone resorption and particle-induced osteolysis in both in vitro and in vivo settings (He et al. 2013; Xiao et al. 2015). According to another in vivo study in which female rats were subjected to bilateral ovariectomy to simulate postmenopausal osteoporosis, geraniin treatment not only modulated a number of circulatory biomarkers which are involved in bone formation, resorption and remodelling, but also rescued low-estrogen-induced bone loss by maintaining bone density, mineral content and calcium content (Lu et al. 2015). In short, the osteoprotective effect of geraniin may have some practical uses in terms of ageing-related bone resorption, wear particle-induced peri-prosthetic osteolysis, postmenopausal osteoporosis as well as other degenerative bone diseases.
Furthermore, geraniin protects from cellular injury caused by ionising radiation, evidenced by the geraniin-mediated reduction in DNA fragmentation and cell death post exposure to γ-radiation (Londhe et al. 2009; Kang et al. 2011; Bing et al. 2013). Such a radioprotective effect is also observed in an in vivo study in which radiosensitive tissues like splenocytes and jejunal crypt cells are spared from the deleterious effects of whole body irradiation after geraniin pretreatment (Bing et al. 2013). This is attributable to the radical scavenging properties of geraniin because the upsurge of ROS level upon γ-radiation exposure is reversed by geraniin which minimises damage to vital intracelluler targets like proteins, membrane lipids and DNA (Londhe et al. 2009; Kang et al. 2011). As a result, the activation of proapoptotic signalling pathway is diminished by geraniin even after γ-irradiation (Bing et al. 2013).
Based on two preliminary studies, it is likely that geraniin may also exhibit neuprotective effects, particularly against Alzheimer’s disease via the inhibition of prolyl endopeptidase (Lee et al. 2007) and β-secretase (Youn and Jun 2013). Prolyl endopeptidase is a serine protease that inactivates various neuropeptides via proteolysis. Exaggerated upregulation of prolyl endopeptidase in the hippocampus takes place in the pre-plaque phase (prior to β-amyloid plaque formation) in both senescence-accelerated (Fukunari et al. 1994) and amyloid precursor protein-transfected mice (Roßner et al. 2005) to induce a seemingly “accelerated aging” effect. This suggests that prolyl endopeptidase may be a major factor that contributes to initial cognitive impairment in aging progress and Alzheimer’s pathogenesis. Secretase has long been known to play a predominant role in the formation of amyloid-β which accounts for cognitive impairments in Alzheimer’s patients. Being able to inhibit these enzymes, geraniin seems to be an attractive compound to preserve cognitive capability. However, it should be noted that the studies by Lee et al. (2007) and Youn and Jun (2013) are enzymatic studies. Several issues, in particular the ability of geraniin to penetrate blood–brain barrier and the absorption by brain cells, require further investigations. In short, current evidence in this aspect is still limited.
Therapeutic effects in metabolic disorders
In vivo studies have shown that geraniin can effectively act as an antihypertensive agent even after a single dose of the polyphenolic compound (Cheng et al. 1994; Lin et al. 2008). The underlying mechanism is probably the inhibition of the angiotensin-converting enzyme (ACE) whose activity is to convert angiotensin I to II to promote vasoconstriction and aldosterone secretion (Ueno et al. 1988; Lin et al. 2008). By inhibiting ACE, geraniin treatment encourages vasorelaxation and lowers blood pressure. Geraniin may also interfere with the sympathetic pathway by reducing circulating noradrenaline in a dose-dependent manner (Cheng et al. 1994). The noradrenaline-lowering effect is not abolished by adrenalectomy, indicating that geraniin may directly interact with sympathetic nerve terminals to either reduce noradrenaline release or enhance reuptake efficiency to prevent spillover (Cheng et al. 1994). In contrast, when acetonylgeraniin, a by-product of geraniin extraction in the presence of acetone, is used to treat spontaneous hypertensive rats (SHRs) with induced orthostatic hypotension, the orthostatic hypotension is prevented and the serum noradrenaline is elevated (Hsu et al. 1994). Like geraniin, the effects of acetonylgeranin remain unaffected by adrenalectomy (Hsu et al. 1994). Based on these findings, it can be deduced that the DHHDP unit in geraniin may be the functional group that interacts with the sympathetic nerve terminals because a slight structural modification by acetone is sufficient to reverse its impacts on blood pressure regulation and circulating noradrenaline level.
Crude extract from rambutan (N. lappaceum) peels which is rich in geraniin is effective in inhibiting carbohydrate hydrolysing enzymes: α-glucosidase and α-amylase (Palanisamy et al. 2011b). It was later confirmed that the inhibitory effect is due to geraniin (Palanisamy et al. 2011a). Therefore, geraniin has the potential to interrupt carbohydrate digestion and glucose absorption which subsequently suppresses postprandial hyperglycaemia. Furthermore, in vitro assays demonstrated that geraniin significantly inhibits aldol reductase activity and the formation of advanced glycation end products (AGEs), both of which play a crucial role in the onset of diabetic complications (Palanisamy et al. 2011a). For diabetic treatment, the crude plant extracts from geraniin-rich P. nururi and peels of N. lappaceum could normalise lipid profile and glycaemic status in streptozotocin-induced diabetic rodents (Rani and Kumar 2015; Muhtadi et al. 2015). In another study, treatment with rambutan peel extract could control weight gain and inhibit adipocyte hypertrophy by interfering with the expression of peroxisome proliferator-activated receptor-γ (PPARγ), a key nuclear receptor for lipid metabolism and adipogenesis (Lestari et al. 2015). The beneficial health effects are indeed contributed by geraniin because in high-fat diet-induced obese rats, supplementation with geraniin also successfully ameliorates diet-induced metabolic dysregulations in glucose and lipid metabolisms besides significantly reducing visceral fat depots without notable side effect (Chung et al. 2014). Hence, considering the widespread prevalence of metabolic syndrome and type 2 diabetes mellitus, geraniin treatment may be employed to safely mitigate the metabolic dysfunction.
In addition, recent research shows that geraniin may help to prevent the formation of thrombus by inhibiting platelet aggregation (Chen et al. 2012). Chen et al. (2012) reported that geraniin is able to inhibit platelet aggregation induced by arachidonic acid, adenosine diphosphate and platelet activating factor in a dose-dependent way. Similar findings were found using an ex vivo study design in which platelets isolated from plasma of rabbits given intragastric 5 mg/kg of geraniin were used. Platelet aggregation induced by the three compounds was significantly inhibited at 60–120 min post geraniin administration (Chen et al. 2012). This points out that metabolites of geraniin, notably ellagic acid, may also possess antiplatelet aggregation activity. As the three compounds activate platelet aggregation via different mechanisms, it is assumed that the inhibitory effect of geraniin on platelet aggregation is non-selective. During thrombus formation, activated neutrophils will promote platelet activation and aggregation and in response, activated platelets will also encourage neutrophils rolling and adhesion to the thrombus-forming site (Kim et al. 2013). Such a platelet-neutrophil crosstalk is also abolished by geraniin (Chen et al. 2012). Furthermore, treatment with geraniin also inhibits the activity of plasminogen activator inhibitor-1 (PAI-1) (Yuan et al. 2012). As indicated by the name, PAI-1 is the principal inhibitor of tissue plasminogen activator (tPA) which is a major enzyme in blood clot breakdown and is clinically used to treat ischaemic stroke. By inhibiting PAI-1, geraniin is able to promote fibrinolysis to degrade blood clots. This is particularly useful for the treatment of thromboembolic events as well as atherosclerosis.
Analgesic and antinociceptive properties
Some geraniin-rich plants have been used in folk medicine as a pain reliever, indicating its potential analgesic and antinociceptive (inhibition of the pain perception) activity. Indeed, several studies reported that the plant extracts from the genus Phyllanthus has a potent, dose-dependent and long-lasting inhibitory effect on pain sensation (Santos et al. 1994, 1995). Furthermore, geraniin pretreatment given via both the oral route and intraperitoneal injection is able to reduce the writhing movements or abdominal contractions, which is interpreted as reduction in nociception, after acetic acid-induced abdominal pain in mice (Miguel et al. 1996; Velazquez-Gonzalez et al. 2014). However, geraniin is less effective in comparison to common analgesic agents, aspirin and acetaminophen (Miguel et al. 1996). Martini et al. (2000) demonstrated that geraniin could inhibit the binding of a GTP analogue to rat cerebral cortex membrane proteins. This implies that geraniin treatment has an inhibitory effect on the GTP-dependent G protein activation which is a critical step in the activity of G-protein coupled signal transduction. As a result, the activity of metabotropic glutamate receptor which is one of the many G-protein-coupled receptors and is responsible in pain perception, is also inhibited (Montana and Gereau 2011). Thus, it is possible that geraniin may act as an analgesic drug by exerting a modulatory effect on the activation of metabotropic glutamate receptor in the glutaminergic excitatory pathways.
Other biological activities of geraniin
Although G. thunbergii which is a geraniin-rich plant has long been accepted as an official antidiarrheal drug in Japan, empirical findings supporting the antidiarrheal activity of geraniin is fairly limited. It is generally assumed that the antidiarrheal activity of geraniin is accountable to its astringency, a characteristic to shrink or constrict mucous membranes and body tissues (Ofuji et al. 1998), but the exact mechanism is not known. Based on Kan and Taniyama (1992), geraniin can decrease the contraction frequency of the small and large intestines when being subjected to contractile agents like histamine and acetylcholine. Such a modulatory effect on intestinal contractility may allow geraniin to exhibit antidiarrheal effect. Geraniin has also been shown to accelerate the progress of wound healing by stimulating the proliferation of fibroblasts and differentiation of keratinocytes (Agyare et al. 2011). It can also prevent gastric ulceration by inhibiting back diffusion of acid and inducing gastric mucus generation (Hung et al. 1995). Based on these findings, the traditional medicinal use of geraniin-rich plants in wound healing and the treatment of diarrhoea and peptic ulcer seems to be fairly justifiable. However, further investigations are highly encouraged to elucidate these bioactivities of geraniin.
Potential therapeutic significance, challenges and future research focus
Amazingly, a huge amount of insightful work has been conducted in attempts to understand the pharmacokinetics and pharmacodynamics of geraniin as well as the respective underlying mechanisms. Generally, it is assumed that geraniin is safe to be used on living tissues and organisms because to date, there is no acute toxicity or significant adverse effect which are remotely associated with the use of geraniin in both in vitro and in vivo studies. In most normal cell lines, geraniin can be tolerated at a considerably high concentration (up to 100 µM) without significantly jeopardising the cell viability (Agyare et al. 2011; Kang et al. 2011; Bing et al. 2013). In mice, 30 mg/kg of geraniin had been given intraperitoneally to facilitate full bioavailability and yet, no side effect was reported (Miguel et al. 1996). However, it is highly recommended to conduct a comprehensive risk assessment, at least in animal models, in order to examine the possible acute and chronic side effects of the natural compound. This information will be valuable when geraniin or its metabolites are tested in clinical settings.
Additionally, based on Table 2, geraniin possesses a wide variety of biological activities. A large portion of these findings are established according to the results from in vitro studies like biochemical or enzymatic assays and cell culture studies. Therefore, the conclusions from in vitro enzymatic assays should be analysed conscientiously. This is because, like other polyphenolic compounds, geraniin is a strong protein precipitant. In enzymatic assays, it will precipitate and denature proteins, rendering the tested enzymes non-functional. Although specific enzyme inhibitory effect cannot be ruled out, the fact that geraniin can suppress the activity of a number of structurally and functionally diverse enzymes like human digestive enzymes, enzymes from the central nervous system and of viral origin, strongly points out that non-selective inhibition due to protein precipitation is a more likely mechanism for the observed inhibitory effect. Clinically, the use of non-selective enzyme inhibitors is less appealing because it tends to interact with off-target enzymes, leading to undesirable side effects. Furthermore, as the enzyme inhibitory actions of geraniin are almost established exclusively based on in vitro studies, it is also uncertain whether the same effect can be replicated in vivo. Hence, for future phenolic compound research, it is advisable to take the protein precipitation effect into consideration, especially when studying enzyme inhibition.
In spite of the issue about in vitro enzymatic assays, most of the bioactivities of geraniin found in in vitro studies are reproducible using ex vivo or in vivo study designs, signifying that its biological effects are largely preserved even in complex physiological matrices. However, the evidence from in vivo studies is still limited. Also, for in vivo geraniin research, the most popular test objects are rodents and no human subject has been employed thus far. Hence, most of the bioactivities are inferred based on animal studies and whether or not it will work as anticipated in human body is still unknown.
For in vivo animal study, the most widely employed mode of administrations are intraperitoneal and oral administration routes, of which the former ensures full bioavailability. Conversely, as outlined previously, oral ingestion of geraniin does not facilitate its absorption in the intact form. Therefore, it is increasingly recognised that the resulting bioactivities following oral intake of geraniin are attributable to the derivatives of geraniin such as ellagic acid and urolithins. In fact, a large number of studies have documented the anti-inflammatory, anticarcinogenic, antioxidant and antimicrobial effects of ellagic acid and urolithins (Landete 2011; Espin et al. 2013). Such a striking similarity in the biological functionality of the parental compound and its metabolites also suggests that these activities are due to a common intrinsic trait shared between these compounds which is unaffected by the degradation of geraniin. One likely speculation is the free radical scavenging activity which is found not only in geraniin and its metabolites, but also in many other polyphenolic compounds. This is because several biological effects of geraniin, namely antioxidant, cytoprotective and immunomodulatory effects, which are closely linked to the free radical scavenging properties are also consistently demonstrated in geraniin metabolites as well as other polyphenolic compounds.
Nevertheless, there are also some in vivo bioactivities which seem to implicate unique mechanisms that are independent from the antioxidant activity of geraniin, for instance, the therapeutic effect against metabolic dysregulation as well as anticoagulant, antiplatelet, analgesic and anticancer properties. Amongst these, the underlying mechanisms for anticancer effects are more clearly elaborated which involve the induction of apoptosis, destabilization of oncoprotein via Hsp90 interaction and inhibition of metastatic transformation. On the other hand, the discovery of the other bioactivities are relatively recent and the mechanisms are not explicitly defined, thus requiring further exploration. It may be added that the promising beneficial effects on metabolic dysfunction and anticoagulation are some of the most exciting features of geraniin as they can be further exploited for the therapy of some highly prevalent diseases, notably metabolic syndrome, type 2 diabetes mellitus and cardiovascular diseases. In short, due to the pleitropic effects, ellagitannin geraniin seems to be an attractive target drug in the treatment of multiple diseases but there are still a lot of uncertainties in the biological functionality of geraniin which are worth investigating.
One of the biggest challenges for the clinical application of geraniin is the low bioavailability upon oral ingestion, which is largely because of its large molecular size and is complicated by low solubility in gastric fluid (Elendran et al. 2015). To be considered as an oral drug candidate, effective formulation and transport system should be created to enhance the bioavailability of geraniin. Apart from that, isolation of geraniin from natural sources is a tedious and time-consuming procedure. Therefore, another challenge and prospective focus in geraniin research is to develop synthetic version of the hydrolysable tannin and its functional derivatives like ellagic acid and urolithins. Some of the subunits of geraniin like corilagin (Yamada et al. 2008), HHDP and DHHDP motifs (Pouysegu et al. 2011) have been chemically synthesized but there is no report about total synthesis of geraniin. The ability to chemically synthesise geraniin and its derivatives could offer several advantages such as bypassing the cumbersome isolation step, large-scale production of the compounds besides allowing the modifications of certain functional groups to enhance their bioactivities. Nonetheless, artificial synthesis of these compounds is only economically feasible if the practical applications and clinical values of geraniin are proven to be tremendous.
Conclusion
After almost 40 years of geraniin research, the science community has established a fundamental understanding of the hydrolysable tannin in terms of its natural sources, purification techniques, biosynthesis and pharmacokinetics. Additionally, like many other polyphenolic compounds, several promising and clinically significant bioactivities have also been discovered, making the ellagitannin geraniin an attractive research candidate so as to fully explore its therapeutic effects. Nevertheless, current evidence is still inadequate to support the clinical use of geraniin due to a few issues. Firstly, there is no thorough assessment about the potential short- and long-term harmful impacts of geraniin ingestion, making the safety of geraniin consumption debatable. Next, the underlying mechanisms by which it exerts the observed beneficial effects remain largely mysterious. This aspect definitely requires more investigations because the knowledge of a drug’s mechanism may not only facilitate better monitoring on the drug effects and prediction of the potential adverse effects, but also allow us to catch a glimpse into the complex pathogenesis of diseases like malignancy and metabolic dysfunction on which we could base to develop more effective drugs to tackle the target pathway. There is also a large room for improvement to optimise drug delivery and bioavailability if the hydrolysable tannin is to be considered as an oral medication. Last but not least, large-scale synthesis of geraniin is a must to ensure the feasibility of commercialization. Considering these challenges and predicaments, it can be said without fear of contradiction that we are still far from putting geraniin into actual clinical use and yet, the potent bioactivities found based on preliminary studies are a solid indicator that future input in geraniin research may be worthwhile after all.
Abbreviations
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl
- IC50 :
-
50 % inhibition concentration
- 5-LOX:
-
5-Lipoxygenase
- AGEs:
-
Advanced glycation end products
- ACE:
-
Angiotensin-converting enzyme
- COMT:
-
Catechol-O-methyl transferase
- CSF-1:
-
Colony-stimulating factor-1
- CYP1A1:
-
Cytochrome P450 1A1
- DHHDP:
-
Dehydrohexahydrodiphenoyl
- EV71:
-
Enterovirus-71
- ERK:
-
Extracellular signal-regulated kinase
- FRAP:
-
Ferric reducing antioxidant power
- GSH:
-
Glutathione
- Hsp90:
-
Heat shock protein 90
- HBV:
-
Hepatitis B virus
- HBeAg:
-
Hepatitis B virus e antigen
- HBsAg:
-
Hepatitis B virus surface antigen
- HSV:
-
Herpes simplex virus
- HHDP:
-
Hexahydroxydiphenoyl
- HPLC:
-
High performance liquid chromatography
- HIV:
-
Human immunodeficiency virus
- iNOS:
-
Inducible nitric oxide synthase
- LPS:
-
Lipopolysaccharide
- MIC:
-
Minimum inhibitory concentration
- NF-kB:
-
Nuclear factor kB
- Nrf2:
-
Nuclear factor-erythroid 2-related factor 2
- ORAC:
-
Oxygen radical absorbance capacity
- PGG:
-
Pentagalloylglucose
- PPAR:
-
Peroxisome proliferator-activated receptor
- PAI-1:
-
Plasminogen activator inhibitor-1
- RNS:
-
Reactive nitrogen species
- ROS:
-
Reactive oxygen species
- RANKL:
-
Receptor activator of nuclear factor kB ligand
- SOD:
-
Superoxide dismutase
- tPA:
-
Tissue plasminogen activator
- TGF:
-
Transforming growth factor
- TNF:
-
Tumour necrosis factor
- UDP:
-
Uridine diphosphate
References
Adesina SK, Idowu O, Ogundaini AO, Oladimeji H, Olugbade TA, Onawunmi GO, Pais M (2000) Antimicrobial constituents of the leaves of Acalypha wilkesiana and Acalypha hispida. Phytother Res 14:371–374
Agyare C, Lechtenberg M, Deters A, Petereit F, Hensel A (2011) Ellagitannins from Phyllanthus muellerianus (Kuntze) Exell.: geraniin and furosin stimulate cellular activity, differentiation and collagen synthesis of human skin keratinocytes and dermal fibroblasts. Phytomedicine 18:617–624
Amakura Y, Yoshida T (1996) Tannins and related polyphenols of Euphorbiaceous plants. XIV. Euphorbin I, a new dimeric hydrolysable tannin from Euphorbia watanabei. Chem Pharm Bull 44:1293–1297
Arina NB, Rohman A (2013) The phenolic contents and antiradical activity of Indonesian Phyllantus urinaria L. Int Food Res J 20:1119–1124
Baliga MS, Dsouza JJ (2011) Amla (Emblica officinalis Gaert), a wonder berry in the treatment and prevention of cancer. Eur J Cancer Prev 20:225–239
Bing SJ, Ha D, Kim MJ, Park E, Ahn G, Kim DS, Ko RK, Park JW, Lee NH, Jee Y (2013) Geraniin down regulates gamma radiation-induced apoptosis by suppressing DNA damage. Food Chem Toxicol 57:147–153
Butler LG (1989) Effects of condensed tannin on animal nutrition. In: Hemingway RW, Karchesy JJ (eds) Chemistry and significance of condensed tannins. Plenum Press, New York
Cammann J, Denzel K, Schilling G, Gross GG (1989) Biosynthesis of gallotannins: β-glucogallin-dependent formation of 1,2,3,4,6-pentagalloylgucose by enzymatic galloylation of 1,2,3,6-tetragalloylglucose. Arch Biochem Biophys 278:58–63
Carr A, Frei B (1999) Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J 13:1007–1024
Cerda B, Llorach R, Ceron JJ, Espin JC, Tomas-Barberan FA (2003) Evaluation of the bioavailability and metabolism in the rats of punicalagin, an antioxidant polyphenol from pomegranate juice. Eur J Nutr 42:18–28
Cerda B, Espin JC, Parra S, Martinez P, Tomas-Barberan FA (2004) The potent in vitro antioxidant ellagitannins from pomegranate juice are metabolised into bioavailable but poor antioxidant hydroxyl-6H-dibenzopyran-6-one derivatives by the colonic microflora of healthy humans. Eur J Nutr 43:205–220
Chalker-Scott L, Krahmer RL (1989) Microscopic studies of tannin formation and distribution in plant tissues. In: Hemingway RW, Karchesy JJ (eds) Chemistry and significance of condensed tannins. Plenum Press, New York
Chen P, Li F, He B, Wu H, Yang J, Zhang X, Shen Z (2012) Effects of geraniin on platelet aggregation and interactions between platelets and neutrophils. J Kunming Med Univ 33:4–10
Cheng JT, Chang SS, Hsu FL (1994) Antihypertensive action of geraniin in rats. J Pharm Pharmacol 46:46–49
Chung APYS, Ton SH, Gurtu S, Palanisamy UD (2014) Ellagitannin geraniin supplementation ameliorates metabolic risks in high-fat-diet-induced obese Sprague Dawley rats. J Funct Foods 9:173–182
Clifford MN, Scalbert A (2000) Ellagitannins—nature, occurrence and dietary burden. J Sci Food Agric 80:1118–1125
Corthout J, Pieters LA, Claeys M, Vanden Berghe DA, Aj Vlietnck (1991) Antiviral ellagitannins from Spondias mombin. Phytochemistry 30:1129–1130
Daniel EM, Ratnayake S, Kinstle T, Stoner GD (1991) The effects of pH and rat intestinal contents on the liberation of ellagic acid from purified and crude ellagitannins. J Nat Prod 54:946–952
Elendran S, Lee WW, Prankerd R, Palanisamy UD (2015) The physicochemical properties of geraniin, a potential antihyperglycemic agent. Pharm Biol 8:1–8
Espin JC, Gonzalez-Barrio R, Cerda B, Lopez-Bote C, Rey AI, Tomas-Barberan FA (2007) Iberian pig as a model to clarify obscure points in the bioavailability and metabolism of ellagitannins in humans. J Agric Food Chem 55:10476–10485
Espin JC, Larrosa M, Garcia-Conesa T, Tomas-Barberan FA (2013) Biological significance of urolithins, the gut microbial ellagic acid-derived metabolites: the evidence so far. Evid Based Complement Altern Med 2013:270418
Fecka I, Cisowski W (2005) Tannins and flavonoids from the Erodium cicutarium Herb. Z Naturforsch B 60:555–560
Feucht W, Treutter D (1999) The role of Flavan-3-ols and proanthocyanidins in plant defense. In: Dakshini KMM, Foy CL (eds) Principles and practices in plant ecology—allochemical interactions. CRC Press, Boca Raton
Field JA, Lettinga G (1992) Toxicity of tannic compounds to microorganisms. In: Hemingway RW, Laks PE (eds) Plant polyphenols. Synthesis, properties, significance. Plenum Press, New York
Filho VC, Santos AS, De Campos RO, Miguel OG, Yunes RA, Ferrari F, Messana I, Calixto JB (1996) Chemical and pharmacological studies of Phyllanthus caroliniensis in mice. J Pharm Pharmacol 48:1231–1236
Fisher E (1914) Synthesis of depsides, lichen-substances and tannins. J Am Chem Soc 36:1170–1201
Foo LY (1993) Amariin, a di-dehydrohexahydroxydiphenoyl hydrolysale tannin from Phyllanthus amarus. Phytochemistry 33:487–491
Foo LY (1995) Amariinic acid and related ellagitannins from Phyllanthus amarus. Phytochemistry 39:217–224
Foo LY, Wong H (1992) Phyllanthusiin D, an unusual hydrolysable tannin from Phyllanthus amarus. Phytochemistry 31:711–713
Fujiki H, Suganuma M, Kurusu M, Okabe S, Imayoshi Y, Taniguchi S, Yoshida T (2003) New TNF-α releasing inhibitors as cancer preventive agents from traditional herbal medicine and combination cancer prevention study with EGCG and sulindac or tamoxifen. Mutat Res 523–524:119–125
Fukunari A, Kato A, Sakai Y, Yoshimoto T, Ishiura S, Suzuki K, Nakajima T (1994) Colocalization of prolyl endopeptidase and amyloid β-peptide in brains of senescence-accelerated mouse. Neurosci Lett 176:201–204
Funatogawa K, Hayashi S, Shimomura H, Yoshida T, Hatano T, Ito H, Hirai Y (2004) Antibacterial activity of hydrolysable tannins derived from medicinal plants against Helicobacter pylori. Microbiol Immunol 48:251–261
Garcia-Villalba R, Beltran D, Espin JC, Selma MV, Tomas-Barberan FA (2013) Time course production of urolithins from ellagic acid by human gut microbiota. J Agric Food Chem 61:8797–8806
Gayosso-de-Lucio JA, Torres-Valencia JM, Cerda-Garcia-Rojas CM, Joseph-Nathan P (2010) Ellagitannins from Geranium potentillaefolium and G. bellum. Nat Prod Commun 5:531–534
Gayosso-De-Lucio J, Bautista M, Velazquez-Gonzalez C, De la O Arciniega M, Morales-Gonzalez JA, Benedi J (2014) Chemical composition and hepatotoxic effect of Geranium schiedeanum in a thioacetamide-induced liver injury model. Pharmacogn Mag 10(suppl S3):S574–S580
Gohar AA, Lahloub MF, Niwa M (2003) Antibacterial polyphenol from Erodium glaucophyllum. Z Naturforsch C 58:670–674
Gonzalez-Barrio R, Borges G, Mullen W, Crozier A (2010) Bioavailability of anthocyanins and ellagitannins following consumption of raspberries by healthy humans and subjects with an ileostomy. J Agric Food Chem 58:3933–3939
Gonzalez-Barrio R, Edwards CA, Crozier A (2011) Colonic catabolism of ellagitannins, ellagic acid, and raspberry anthocyanins: in vivo and in vitro studies. Drug Metab Dispos 39:1680–1688
Gonzalez-Sarrias A, Azorin-Ortuno M, Yanez-Gascon MJ, Tomas-Barberan FA, Garcia-Conesa MT, Espin JC (2009) Dissimilar in vitro and in vivo effects of ellagic acid and its microbiota-derived metabolites, urolithins, on the cytochrome P450 1A1. J Agric Food Chem 57:5623–5632
Gross GG (1983a) Partial purification and properties of UDP-glucose: vanillate 1-O-glucosyl transferase from oak leaves. Phytochemistry 22:2179–2182
Gross GG (1983b) Synthesis of mon-, di- and trigalloyl-β-d-glucose by β-glucogallin-dependent gallolytransferases from oak leaves. Z Naturforsch C 38:519–523
Gross GG (2008) from lignins to tannins: forty years of enzyme studies on the biosynthesis of phenolic compounds. Phytochemistry 69:3018–3031
Gross GG, Denzel K (1991) Biosynthesis of gallotannins. β-glucogallin-dependent galloylaytion of 1,6-digalloylglucose to 1,2,6-trigalloylglucose. Z Naturforsch C 46:389–394
Hagenah S, Gross GG (1993) Biosynthesis of 1,2,3,6-tetra-O-galloyl-β-d-glucose. Phytochemistry 32:637–641
He B, Hu M, Li SD, Yang XT, Lu YQ, Liu JX, Chen P, Shen ZQ (2013) Effects of geraniin on osteoclastic bone resorption and matrix metalloproteinase-9 expression. Bioorg Med Chem Lett 23:630–634
Hodgson JM, Morton LW, Puddey IB, Beilin LJ, Croft KD (2000) Gallic acid metabolites are markers of black tea intake in humans. J Agric Food Chem 48:2276–2280
Hsu FL, Lu FH, Cheng JT (1994) Influence of acetonylgeraniin, a hydrolysable tannin from Euphoria longana, on orthostatic hypotension in a rat model. Planta Med 60:297–300
Huang RL, Huang Yl OuJC, Chen CC, Hsu FL, Chang C (2003) Screening of 25 compounds isolated from Phyllanthus species for anti-human hepatitis B virus in vitro. Phytother Res 17:449–453
Hung CR, Chen JT, Neu SL (1995) Prophylactic effects of sucralfate and geraniin on ethanol-induced gastric mucosal damage in rats. Chin J Physiol 38:211–217
Ignat I, Volf I, Popa VI (2011) A critical review of methods for characterization of polyphenolic compounds in fruits and vegetables. Food Chem 126:1821–1835
Immel S, Khanbabaee K (2000) Atropdiastereoisomers of ellagitannin model compounds: configuration, conformation, and relative stability of d-glucose diphenoyl derivatives. Tetrahedron Asymmetry 11:2495–2507
Ishimaru K, Shimomura K (1991) Tannin production in hairy root culture of Geranium thunbergii. Phytochemistry 30:825–828
Ishimaru K, Yoshimatsu K, Yamakawa T, Kamada H, Shimomura K (1992) Phenolic constituents in tissue cultures of Phyllanthus Niruri. Phytochemistry 31:2015–2018
Ishimoto H, Tai A, Yoshimura M, Amakura Y, Yoshida T, Hatano T, Ito H (2012) Antioxdative properties of functional polyphenols and their metabolites assessed by an ORAC assay. Biosci Biotechnol Biochem 76:395–399
Islam A, Mazumder UK, Gupta M, Ghosal S (2008) Synergistic effects of geraniin and rutin in the antioxidant properties of major lignins in Phyllanthus amarus. Pharmacol Online 3:1024–1036
Ito H (2011) Metabolites of the ellagitannin geraniin and their antioxidant activities. Planta Med 77:1110–1115
Ito H, Iguchi A, Hatano T (2008) Identification of urinary and intestinal bacterial metabolites of ellagitannin geraniin in rats. J Agric Food Chem 56:393–400
Jaeschke H (2011) Reactive oxygen and mechanisms of inflammatory liver injury: present concepts. J Gastroenterol Hepatol 26:173–179
Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, Lemasters JJ (2002) Mechanisms of hepatotoxicity. Toxicol Sci 65:166–176
Janero DR (1990) Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med 9:515–540
Jin ZX, Sun RS (2011) Effects of geraniin in Geranium sibiricum L. on antioxidant function of S180 mice. Paper presented at the 2011 international conference on human health and biomedical engineering. Jilin Empire Garden Hotel Guohui Hall, China, 19–22 August 2011
Jin ZX, Wang BQ (2010) Induction of P53 genes in human hepatoma cells by geraniin. Paper presented at the 2010 3rd international conference on biomedical engineering and informatics. Yantai University, China, 16–18 October 2010
Kakiuchi N, Hattori M, Namba T, Nishizawa M, Yamagishi T, Okuda T (1985) Inhibitory effect of tannins on reverse transcriptase from RNA tumor virus. J Nat Prod 48:614–621
Kan S, Taniyama K (1992) Mechanism of inhibitory actions of Geranium thunbergii, tannic acid and geraniin on the motility of rat intestine. Shoyakugaku Zasshi 46:246–253
Kang SJ, Hong SS, Hwang BY, Ro JS, Lee KS, Towers GHN (2006) Phenolic compounds from the stems of Sapium japonicum. Nat Prod Sci 12:125–128
Kang KA, Lee IK, Zhang R, Piao MJ, Kim KC, Kim SY, Shin T, Kim BJ, Lee NH, Hyun JW (2011) Radioprotective effect of geraniin via the inhibition of apoptosis triggered by γ-radiation-induced oxidative stress. Cell Biol Toxicol 27:83–94
Kim KH, Barazia A, Cho J (2013) Real-time imaging of heterotypic platelet-neutrophil interactions on the activated endothelium during vascular inflammation and thrombus formation in live mice. J Vis Exp. doi:10.3791/50329
Kimura Y, Okuda H, Mori K, Okuda T, Arichi S (1984) Studies on the activities of tannins and related compounds from medicinal plants and drugs. IV. Effects of various extracts of Geranii Herba and geraniin on liver injury and lipid metabolism in rats fed peroxidised oil. Chem Pharm Bull 32:1866–1871
Kletter C, Kriechbaum M (2001) Li Ga Dur. In: Kletter C, Kriechbaum M (eds) Tibetan medicinal plants. Medpharm GmbH Scientific Publishers, Stuuttgart
Klocke JA, Wagenen BV, Balandrin MF (1986) The ellagitannin geraniin and its hydrolysis products isolated as insect growth inhibitors from semi-arid land plants. Phytochemistry 25:85–91
Klumpers J, Scalbert A, Janin G (1994) Ellagitannins in European oak wood: polymerization during wood ageing. Phytochemistry 36:1249–1252
Ko H (2015) Geraniin inhibits TGF-β1-induced epithelial-mesenchymal transition and suppresses A549 lung cancer migration, invasion and anoikis resistance. Bioorg Med Chem Lett 25:3529–3534
Kolodziej H, Kayser O, Kiderlen AF, Ito H, Hatano T, Yoshida T, Foo LY (2001) Antileishmanial activity of hydrolysable tannins and their modulatory effects on nitric oxide and tumour necrosis factor-α release in macrophages in vitro. Planta Med 67:825–832
Kraus TE, Dahlgren RA, Zasoski RJ (2003) Tannins in nutrient dynamics of forest ecosystems—a review. Plant Soil 256:41–66
Kumaran A, Karunakaran RJ (2006) Nitric oxide radical scavenging active components from Phyllanthus emblica L. Plant Foods Hum Nutr 61:1–5
Kurihara H, Kawabata J, Hatano M (1993) Geraniin, a hydrolysable tannin from Nymphaea tetragona Georgi (Nymphaeaceae). Biosci Biotechnol Biochem 57:1570–1571
Lacey DL, Timms E, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93:165–176
Landete JM (2011) Ellagitannins, ellagic acid and their derived metabolites: a review about source, metabolism, functions and health. Food Res Int 44:1150–1160
Lee SH, Jun M, Choi JY, Yang EJ, Hur JM, Bae KH, Seong YH, Huh TL, Song KS (2007) Plant phenolics as prolyl endopeptidase inhibitors. Arch Pharm Res 30:827–833
Lee JC, Tsai CY, Kao JY, Kao MC, Tsai SC, Chang CS, Huang LJ, Kuo SC, Lin JK, Way TD (2008) Geraniin-mediated apoptosis by cleavage of focal adhesion kinase through up-regulation of Fas ligand expression in human melanoma cells. Mol Nutr Food Res 52:655–663
Lee SH, Tang YQ, Rathkrishnan A, Wang SM, Ong KC, Manikam R, Payne BJ, Jaganath IB, Sekaran SD (2013) Effects of cocktail of four local Malaysian medicinal plants (Phyllanthus spp.) against dengue virus 2. BMC Complement Altern Med 13:192
Leopoldini M, Russo N, Toscano M (2011) The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem 125:288–306
Lestari SR, Djati MS, Rudijanto A, Fatchiyah F (2015) PPARγ expression by rambutan peel extract in obesity rat model-induced high-calorie diet. Asian Pac J Trop Biomed 5:812–817
Li J, Huang H, Zhou W, Feng M, Zhou P (2008) Anti-hepatitis B virus activities of Geranium carolinianum L. extracts and identification of the active components. Biol Pharm Bull 31:743–747
Li J, Wang S, Yin J, Pan L (2013) Geraniin induces apoptotic cell death in human lung adenocarcinoma A549 cells in vitro and in vivo. Can J Physiol Pharmcol 91:1016–1024
Lin JH, Ishimatsu M, Tanaka T, Nonaka G, Nishioka I (1990a) Tannins and related compounds. XCVI. Structures of macaranins and macarinins, new hydrolysable tannins possessing macaranoyl and tergalloyl ester groups, from the leaves of Macaranga sinensis (Baill.) Muell.-Arg. Chem Pharm Bull 38:1844–1851
Lin JH, Nonaka G, Nishioka I (1990b) Tannins and related compounds XCIV. Isolation and characterization of seven new hydrolysable tannins from the leaves of Macaranga tanarius (L.) Muell. Et Arg. Chem Pharm Bull 38:1218–1223
Lin SY, Wang CC, Lu YL, Wu WC, Hou WC (2008) Antioxidant, antisemicarbazide-sensitive amine oxidase, and anti-hypertensive activities of geraniin isolated from Phyllanthus urinaria. Food Chem Toxicol 46:2485–2492
Ling LT, Saito Y, Palanisamy UD, Cheng HM, Noguchi N (2012) Cytoprotective effects of geraniin against peroxynitrite- and peroxyl radical-induced cell death via free radical scavenging activity. Food Chem 132:1899–1907
Liu X, Cui C, Zhao M, Wang J, Luo W, Yang B, Jiang Y (2008) Identification of phenolics in the fruit of emblica (Phyllanthus emblica L.) and their antioxidant activities. Food Chem 109:909–915
Liu D, Su Z, Wang C, Gu M, Xing S (2010) Separation and purification of hydrolysable tannin from Geranium wilfordii Maxim by reversed-phase and normal-phase high-speed counter-current chromatography. J Sep Sci 33:2266–2271
Liu D, Ma Y, Wang Y, Su Z, Gu M, Janson JC (2011) One-step separation and purification of hydrolysable tannins from Geranium wilfordii Maxim by adsorption chromatography on cross-linked 12% agarose gel. J Sep Sci 34:995–998
Liu D, Ma Y, Gu M, Janson JC, Wang C, Xiao H (2012a) Liquid–liquid/solid three phase high-speed counter-current chromatography, a new technique for separation of polyphenols from Geranium wilfordii Maxim. J Sep Sci 35:2146–2151
Liu X, Zhao M, Wu K, Chai X, Yu H, Tao Z, Wang J (2012b) Immunomodulatory and anticancer activities of phenolics from emblica fruit (Phyllanthus emblica L.). Food Chem 131:685–690
Londhe JS, Devasagayam TPA, Foo LY, Ghaskadbi SS (2009) Radioprotective properties of polyphenols from Phyllanthus amarus Linn. J Radiat Res 50:303–309
Londhe JS, Devasagayam TPA, Foo LY, Shastry P, Ghaskadbi SS (2012) Geraniin and amariin, ellagitannins from Phyllanthus amarus, protect liver cells against ethanol induced cytotoxicity. Fitoterapia 83:1562–1568
Lu Y, He B, Zhang X, Yang R, Li S, Zhang Y, Yun Y, Yan H, Chen P, Shen Z (2015) Osteoprotective effect of geraniin against ovariectomy-induced bone loss in rats. Bioorg Med Chem Lett 25:673–679
Luger P, Weber M, Kashino S, Amakura Y, Yoshida T, Okuda T, Beurskens G, Dauter Z (1998) Structure of the tannin geraniin based on conventional X-ray data at 295 K and on synchrotron data at 293 and 123 K. Acta Crystallogr B 54:687–694
Ma YT, Chuang JI, Lin JH, Hsu FL (1997) Phenolics from Acalypha indica. J Chin Chem Soc 44:499–502
Mahdi ES, Noor AM, Sakeena MH, Abdullah GZ, Abdulkarim M, Abdul Sattar M (2011) Identification of phenolic compounds and assessment of in vitro antioxidants activity of 30% ethanolic extracts derived from two Phyllanthus species indigenous to Malaysia. Afr J Pharm Pharmacol 5:1967–1978
Martini LH, Souza CR, Marques PB, Calixto JB, Yunes RA, Souza DO (2000) Compounds extracted from Phyllantus and Jatropha elliptica inhibit the binding of [3H] glutamate and [3H]GMP-PNP in rat cerebral cortex membrane. Neurochem Res 25:211–215
Meo FD, Lemaur V, Cornil J, Lazzaron R, Duroux JL, Olivier Y, Trouillas P (2013) Free radical scavenging by natural polyphenols: atom versus electron transfer. J Phys Chem A 117:2082–2092
Miguel OG, Calixto JB, Santos ARS, Messana I, Ferrari F, Filho VC, Pizzolatti MG, Yunes RA (1996) Chemical and preliminary analgesic evaluation of geraniin and furosin isolated from Phyllanthus sellowianus. Planta Med 62:146–149
Montana MC, Gereau RW (2011) Metabotropic glutamate receptors as targets for analgesic: antagonism, activation, and allosteric modulation. Curr Pharm Biotechnol 12:1681–1688
Muhtadi, Primarianti AU, Sujono TA (2015) Antidiabetic activity of durian (Durio zibethinus Murr.) and rambutan (Nephelium lappaceum L.) fruits peels in alloxan diabetic rats. Procedia Food Sci 3:255–261
Nakanishi Y, Okuda T, Abe H (1999) Effects of geraniin on the liver in rats III-correlation between lipid accumulations and liver damage in CCl4-treated rats. Nat Med 53:22–26
Ndjonka D, Bergmann B, Agyare C, Zimbres FM, Luersen K, Hensel A, Wrenger C, Liebau E (2012) In vitro activity of extracts and isolated polyphenols from West African medicinal plants against Plasmodium falciparum. Parasitol Res 111:827–834
Neckers L, Ivy SP (2003) Heat shock protein 90. Curr Opin Oncol 15:419–424
Neera S, Ishimaru K (1992) Tannin production in cell cultures of Sapium sebiferum. Phytochemistry 31:833–836
Neera S, Arakawa H, Ishimaru K (1992) Tannin production in Sapium sebiferum callus cultures. Phytochemistry 31:4143–4149
Niemetz R, Gross GG (2003) Oxidation of pentagalloylglucose to the ellagitannin, tellimagrandin II, by a phenol oxidase from Tellima grandiflora leaves. Phytochemistry 62:301–306
Niemetz R, Gross GG (2005) Enzymology of gallotannin and ellagitannin biosynthesis. Phytochemistry 66:2001–2011
Nonaka G, Morimoto S, Nishioka I (1986) Elaeocarpusin, a proto-type of geraniin from Geranium thunbergii. Chem Pharm Bull 34:941–943
Notka F, Meier GR, Wagner R (2003) Inhibition of wild-type human immunodeficiency virus and reverse transcriptase inhibitor-resistant variants by Phyllanthus amarus. Antivir Res 58:175–186
Notka F, Meier G, Wagner R (2004) Concerted inhibitory activities of Phyllanthus amarus on HIV replication in vitro and ex vivo. Antivir Res 64:93–102
Ofuji K, Hara H, Sukamoto T, Yamashita S (1998) Effects of an antidiarrhoeica containing an extract from geranium herb on astringent action and short-circuit current across jejunal mucosa. Nihon Yakurigaku Zasshi 111:265–275
Okabe S, Suganuma M, Imayoshi Y, Taniguchi S, Yoshida T, Fujiki H (2001) New TNF-α releasing inhibitors, geraniin and corilagin, in leaves of Acer nikoense, Megusurino-ki. Biol Pharm Bull 24:1145–1148
Okuda T, Ito H (2011) Tannins of constant structure in medicinal and food plants—hydrolysable tannins and polyphenols related to tannins. Molecules 16:2191–2217
Okuda T, Yoshida T, Mori K (1975) Constituents of Geranium thunbergii SIEB. et ZUCC. II. Ellagitannins. Yakugaku Zasshi 95:1462–1466
Okuda T, Mori K, Hayashi N (1976) Constituents of Geranium thunbergii SIEB. et ZUCC. III. Application of determination methods of tannin activity with haemoglobin and of ellagitannin with nitrous acid. Yakugaku Zasshi 96:1143–1149
Okuda T, Yoshida T, Nayeshiro H (1977) Constituents of Geranium thunbergii SIEB. et ZUCC. IV. Ellagitannins. Structure of geraniin. Chem Pharm Bull 25:1862–1868
Okuda T, Mori K, Hatano T (1980) The distribution of geraniin and mallotusinic acid in the order geraniales. Phytochemistry 19:547–551
Okuda T, Yoshida T, Hatano T (1982) Constituents of Geranium thunbergii SIEB. et ZUCC. XII. Hydrated stereostructure and equilibration of geraniin. J Chem Soc Perkin Trans 1:9–14
Okuda T, Kimura Y, Yoshida T, Hatano T, Okuda H, Arichi S (1983) Studies on the activities of tannins and related compounds from medicinal plants and drugs. I. Inhibitory effects on lipid peroxidation in mitochondria and microsomes of liver. Chem Pharm Bull 31:1625–1631
Okuda T, Mori K, Hatano T (1985) Relationship of structures of tannins to the binding activities with haemoglobin and methylene blue. Chem Pharm Bull 33:1424–1433
Okuda T, Yoshida T, Hatano T, Ikeda Y, Shingu T, Inoue T (1986) Isolation of water-soluble tannins by centrifugal partition chromatography, and biomimetric synthesis of elaeocarpusin. Chem Pharm Bull 34:4075–4082
Okuda T, Yoshida T, Hatano T, Ito H (2009) Ellagitannins renewed the concept of tannins. In: Quideau S (ed) Chemistry and biology of ellagitannins: an underestimated class of bioactive plant polyphenols. World Scientific, Singapore
Ooshiro A, Kaji M, Katoh Y, Kawaide H, Natsume M (2011) Antibacterial activity of alkyl gallates and related compounds against Ralstonia solanacearum. J Pestic Sci 36:240–242
Palanisamy U, Ming CH, Masilamani T, Subramaniam T, Ling LT, Radhakrishnan AK (2008) Rind of the rambutan, Nephelium lappaceum, a potential source of natural antioxidants. Food Chem 109:54–63
Palanisamy UD, Ling LT, Manaharan T, Appleton D (2011a) Rapid isolation of geraniin from Nephelium lappaceum rind waste and its anti-hyperglycaemic activity. Food Chem 127:21–27
Palanisamy U, Manaharan T, Teng LL, Radhakrishnan AKC, Subramaniam T, Masilamani T (2011b) Rambutan rind in the management of hyperglycemia. Food Res Int 44:2278–2282
Pan MH, Lin-Shiao SY, Ho CT, Lin JH, Lin JK (2000) Suppression of lipopolysaccharide-induced nuclear factor-kB activity by theaflavin-3,3′-digallate from black tea and other polyphenols through down-regulation of IkB kinase activity in macrophages. Biochem Pharmacol 59:357–367
Park S, Han SU, Lee KM, Park KH, Cho SW, Hahm KB (2007) 5-LOX inhibitor modulates the inflammatory responses provoked by Helicobacter pylori infection. Helicobacter 12:49–58
Perera A, Appleton D, Ying LH, Elendran S, Palanisamy UD (2012) Large scale purification of geraniin from Nephelium lappaceum rind waste using reverse-phase chromatography. Sep Purif Technol 98:145–149
Perera A, Ton SH, Palanisamy UD (2015) Perspectives on geraniin, a multifunctional natural bioactive compound. Trends Food Sci Technol 44:243–257
Pouysegu L, Deffieux D, Malik G, Natangelo A, Quideau S (2011) Synthesis of ellagitannin natural products. Nat Prod Rep 28:853–874
Rakhimov RN, Abdulladzhanova NG, Kamev FG (2011) Phenolic compounds from Euphorbia canescens and E. franchetii. Chem Nat Compd 47:286–287
Rani S, Kumar B (2015) Efficacy of Phyllanthus niruri Linn. Extract in the management of type-2 diabetes mellitus associated hypercholesterolemia in mice diabetic model. Int J Curr Microbiol Appl Sci 4:507–513
Rausch H, Gross GG (1996) Preparation of [14C]-labelled 1,2,3,4,6-penta-O-galloyl-β-d-glucose and related gallotannins. Z Naturforsch C 51:473–476
Roßner S, Schulz I, Zeitschel U, Schliebs R, Bigl V, Demuth H-U (2005) Brain prolyl endopeptidase expression in aging, APP transgenic mice and Alzheimer's disease. Neurochem Res 30:695–702
Roginsky V (2003) Chain-breaking antioxidant activity of natural polyphenols as determined during te chain oxidation of methyl linoleate in triton X-100 micelles. Arch Biochem Biophys 414:261–270
Santos ARS, Filho VC, Niero R, Viana AM, Moreno FN, Compos MM, Yunes RA, Calixto JB (1994) Analgesic effects of callus culture extracts from selected species of phyllanthus in mice. J Pharm Pharmacol 46:755–759
Santos ARS, Filho VC, Yunes RA, Calixto JB (1995) Analysis of the mechanisms underlying the antinociceptive effect of the extracts of plants from the genus Phyllanthus. Gen Pharm 26:1499–1506
Scalbert A (1991) Antimicrobial properties of tannins. Phytochemistry 30:3875–3883
Schimel JP, Cates RG, Ruess R (1998) The role of balsam poplar secondary chemicals in controlling soil nutrient dynamics through succession in the Alaskan taiga. Biogeochemistry 42:221–234
Schmidt SW, Denzel K, Schilliung G, Gross GG (1987) Enzymatic synthesis of 1,6-digalloylglucose from β-glucogallin by β-glucogallin: β-glucogallin 6-0-galloyltransferase from oak leaves. Z Naturforsch C 42:87–92
Schultz JC (1989) Tannin–insect interactions. In: Hemingway RW, Karchesy KK (eds) Chemistry and significance of condensed tannins. Plenum Press, New York
Schultz JC, Hunter MD, Appel HM (1992) Antimicrobial activity of polyphenols mediates plant–herbivore interactions. In: Hemingway RW, Laks PE (eds) Plant polyphenols. Synthesis, properties, significance. Plenum Press, New York
Seeram NP, Henning SM, Zhang Y, Suchard M, Li Z, Heber D (2006) Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 hours. J Nutr 136:2481–2485
Solit DB, Rosen N (2006) Hsp90: a novel target for cancer therapy. Curr Top Med Chem 6:1205–1214
Stoner GD, Sardo C, Apseloff G, Mullet D, Wargo W, Pound V, Singh A, Sanders J, Aziz R, Casto B, Sun XL (2005) Pharmacokinetics of anthocyanins and ellagic acid in healthy volunteers fed freeze-dried black raspberries daily for 7 days. J Clin Pharmacol 45:1153–1164
Sudjaroen Y, Hull WE, Erben G, Wurtele G, Changbumrung S, Ulrich CM, Owen RW (2012) Isolation and characterization of ellagitannins as the major polyphenolic components of Longan (Dimocarpus longan Lour) seeds. Phytochemistry 77:226–237
Tabata H, Katsube T, Tsuma T, Ohta Y, Imawaka N, Utsumi T (2008) Isolation and evaluation of the radical-scavenging activity of the antioxidants in the leaves of an edible plant, Mallotus japonicus. Food Chem 109:64–71
Tanaka T, Nonaka G, Nishioka I (1986) Tannins and related compounds. Part 37. Isolation and structure elucidation of elaeocarpusin, a novel ellagitannin from Elaeocarpus sylvestris var. Ellipticus. J Chem Soc Perkin Trans 1:369–376
Thitilertdecha N, Rakariyatham N (2011) Phenolic content and free radical scavenging activities in rambutan during fruit maturation. Sci Hortic 129:247–252
Thitilertdecha N, Teerawutgulrag A, Rakariyatham N (2008) Antioxidant and antibacterial activities of Nephelium lappaceum L. extracts. LWT Food Sci Technol 41:2029–2035
Thitilertdecha N, Teerawutgulrag A, Kilburn JD, Rakariyatham N (2010) Identification of major phenolic compounds from Nephelium lappaceum L. and their antioxidant activities. Molecules 15:1453–1465
Tsukuma H, Hiyama T, Tanaka S, Nakao M, Yabuuchi T, Kitamura T, Nakanishi K, Fujimoto I, Inoue A, Yamazaki H, Kawashima T (1993) Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N Engl J Med 328:1797–1801
Ueno H, Horie S, Nishi Y, Shogawa H, Kawasaki M, Suzuki S, Hayashi T, Arisawa M, Shimizu M, Yoshizaki M, Morita N (1988) Chemical and pharmaceutical studies of medicinal plants in Paraguay. Geraniin, an angiotensin-converting enzyme inhibitor from “Paraparai Mi”, Phyllanthus Niruri. J Nat Prod 51:357–359
Ushiki J, Tahara S, Hayakawa Y, Tadano T (1998) Medicinal plants for suppression soil-borne plant disease: II. Suppressive effect of Geranium pratense L. on common scab of potato and identification of the active compound. Soil Sci Plant Nutr 44:157–165
Ushio Y, Fang T, Okuda T, Abe H (1991) Modification changes in function and morphology of cultured macrophages by geraniin. Jpn J Pharmacol 57:187–196
Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39:44–84
Vassallo A, Vaccaro MC, De Tommasi N, Paiz FD, Leone A (2013) Identification of the plant compound geraniin as a novel Hsp90 inhibitor. PLoS One 8:e74266
Velazquez-Gonzalez C, Carino-Cotes R, Gayosso de Lucio JA, Ortiz MI, Arciniega MDLO, Altamirano-Baez DA, Jumenes-Angeles L, Bautista-Avila M (2014) Antinociceptive and anti-inflammatory activities of Geranium bellum and its isolated compounds. BMC Complement Altern Med 14:506
Viriot C, Scalbert A, Herve du Penhoat CLM, Moutounet M (1994) Ellagitannins in woods of sessile oak and sweet chestnut dimerization and hydrolysis during wood ageing. Phytochemistry 36:1253–1260
Walkinshaw CH (1999) Constituent and induced tannin accumulations in roots of loblolly pines. In: Gross GG, Hemingway RW, Yoshida R, Branham S (eds) Plant polyphenols 2. Chemistry, biology, pharmacology, ecology. Kluwer/Plenum Publishers, New York
Wang P, Peng X, Wei ZF, Wei FY, Wang W, Ma WD, Yao LP, Fu YJ, Zu YG (2015) Geraniin exerts cytoprotective effect against cellular oxidative stress by upregulation of Nrf2-mediated antioxidant enzyme expression via PI3K/AKT and ERK1/2 pathway. Biochim Biophys Acta 1850:1751–1761
Whitley AC, Stoner GD, Darby MV, Walle T (2003) Intestinal epithelial cell accumulation of the cancer preventive polyphenol ellagic acid-extensive binding to protein and DNA. Biochem Pharmacol 66:907–915
Williams JM, Richardson AC (1967) Selective acylation of pyranosides-I. Benzoylation of methyl α-d-glycopyranosides of mannose, glucose and galactose. Tetrahedron 23:1369–1378
Wu QY, Zhou Y, Jin X, Guan Y, Xu M, Liu LF (2011) Chromatographic fingerprint and the simultaneous determination of five bioactive components of Geranium carolinianum L. water extract by high performance liquid chromatography. Int J Mol Sci 12:8740–8749
Xiao F, Zhai Z, Jiang C, Liu X, Li H, Qu X, Ouyang Z, Fan Q, Tang T, Qin A, Gu D (2015) Geraniin suppresses RANKL-induced osteoclastogenesis in vitro and ameliorates wear particle-induced osteolysis in mouse model. Exp Cell Res 330:91–101
Xu HX, Wan M, Dong H, But PPH, Foo LY (2000) Inhibitory activity of flavonoids and tannins against HIV-1 protease. Biol Pharm Bull 23:1072–1076
Xu M, Zha ZJ, Xl Qin, Zhang XL, Yang CR, Zhang YJ (2007) Phenolic antioxidants from the whole plant of Phyllanthus urinaria. Chem Biodivers 4:2246–2252
Yamada H, Nagao K, Dokei K, Kasai Y, Michihata N (2008) Total synthesis of (−)-corilagin. J Am Chem Soc 130:7566–7567
Yang CM, Cheng HY, Lin TC, Chiang LC, Lin CC (2007) The in vitro activity of geraniin and 1,3,4,6-tetra-O-galloyl-β-d-glucose isolated from Phyllanthus urinaria against herpes simplex virus type 1 and type 2 infection. J Ethnopharmacol 110:555–558
Yang YC, Li J, Zu YG, Fu YJ, Luo M, Wo N, Liu XL (2010) Optimization of microwave-assisted enzymatic extraction of corilagin and geraniin from Geranium sibiricum Linne and evaluation of antioxidant activity. Suppressive effect of Geranium pratense L. on common scab of potato and identification of the active compound. Food Chem 122:373–380
Yang Y, Zhang L, Fan X, Qin C, Liu J (2012) Antiviral effect of geraniin on human enterovirus 71 in vitro and in vivo. Bioorg Med Chem Lett 22:2209–2211
Yang YC, Yang ZW, Zhang ZH, Zu YG, Fu YJ (2013) Effect of acid hydrolysis in the microwave-assisted extraction of phenolic compounds from Geranium sibiricum Linne with the guidance of antibacterial activity. J Med Plant Res 7:819–830
Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T (1998) Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA 95:3597–3602
Yasuda T, Inaba A, Ohmori M, Endo T, Kubo S, Ohsawa K (2000) Urinary metabolites of gallic acid in rats and their radical-scavenging effects on 1,1-diphenyl-2-picrylhydrazyl radical. J Nat Prod 63:1444–1446
Yazaki K, Yoshida T, Okuda T (1991) Tannin production in cell suspension cultures of Geranium thunbergii. Phytochemistry 30:501–503
Yokozawa T, Chen CP, Dong E, Tanaka T, Nonaka GI, Nishioka I (1998) Study on the inhibitory effect of tannins and flavonoids against the 1,1-diphenyl-2-picrylhydrazyl radical. Biochem Pharmcol 56:213–222
Yoshida T, Chen L, Shingu T, Okuda T (1988) Tannins and related polyphenols of Euphorbiaceous plants. IV. Euphorbins A and B, novel dimeric dehydroellagitannins from Euphorbia hirta L. Chem Pharm Bull 36:2940–2949
Yoshida T, Itoh H, Matsunaga S, Tanaka R, Okuda T (1992) Tannins and related polyphenols of Euphorbiaceous plants. IX. Hydrolysable tannins with 1C4 glucose core from Phyllanthus flexuosus Muell. Arg Chem Pharm Bull 40:53–60
Yoshida T, Amakura Y, Liu YZ, Okuda T (1994) Tannins and related polyphenols of Euphorbiaceous plants. XI. Three new hydrolysable tannins and a polyphenol glucoside from Euphorbia humifusa. Chem Pharm Bull 42:1803–1807
Youn K, Jun M (2013) In vitro BACE1 inhibitory activity of geraniin and corilagin from Geranium thunbergii. Planta Med 79:1038–1042
Yuan X, Hu D, Zhu W, Xu X, Lu C (2012) Application of frontal affinity chromatography combined on-line with mass spectrometry to screening PAI-1 inhibitors from traditional Chinese medicine. Anal Methods 4:3744–3747
Zhang YJ, Abe T, Tanaka T, Yang CR, Kouno I (2001) Phyllanemblinins A-F, new ellagitannins from Phyllanthus emblica. J Nat Prod 64:1527–1532
Zhang YJ, Nagao T, Tanaka T, Yang CR, Okabe H, Kouno I (2004) Antiproliferative activity of the main constituents from Phyllanthus emblica. Biol Pharm Bull 27:251–255
Acknowledgments
The work was supported by ScienceFund of Malaysia Ministry of Science, Technology and Innovation (MOSTI Grant No.: 02-02-10-SF0249) as well as internal grant from the School of Science, Monash University Malaysia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Cheng, H.S., Ton, S.H. & Abdul Kadir, K. Ellagitannin geraniin: a review of the natural sources, biosynthesis, pharmacokinetics and biological effects. Phytochem Rev 16, 159–193 (2017). https://doi.org/10.1007/s11101-016-9464-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11101-016-9464-2