Abstract
Rhodiola rosea L. is a worldwide popular plant with adaptogenic activities that have been and currently are exploited in the traditional medicine of many countries, as well as, examined in a number of clinical trials. More than 140 chemical structures have been identified which belong to several natural product classes, including phenylpropanoid glycosides, phenylethanoids, flavonoids and essential oils, and are mainly stored in the rhizomes and the roots of the plant. A number of mechanisms contribute to the adaptogenic activities of R. rosea preparations and its phytochemical constituents. Among them, the intrinsic inducible mammalian stress responses and their effector proteins, such as heat shock protein 70 (Hsp70), are the most prominent. Due to its popular medicinal use, which has led to depletion of its natural habitats, R. rosea is now considered as endangered in most parts of the world. Conservation, cultivation and micropropagation are all implemented as potential preservation strategies. A number of in vitro systems of R. rosea are being developed as sources of pharmaceutically valuable secondary metabolites. These are greatly facilitated by advances in elucidation of the biosynthetic pathways and the enzymes, which catalyse the production of these secondary metabolites in the plant. In addition, biotechnological approaches show promise towards achieving sustainable production of R. rosea secondary metabolites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rhodiola rosea L. (roseroot or golden root) family Crassulaceae, is a multipurpose medicinal plant with well-established adaptogenic properties, able to increase the body’s nonspecific resistance and normalize its functions in response to different stressors of emotional, mental, and physical origin (Olsson et al. 2009). R. rosea extracts, as well as, its valuable pharmacologically active substances, such as salidroside, tyrosol, and rosavins, are extensively studied for their neuroprotective (Chen et al. 2008), hepatoprotective (Wu et al. 2009), antioxidant (Chen et al. 2009), antiviral (Wang et al. 2009), anticancer (Hu et al. 2010) and anti-inflammatory activities (Guan et al. 2011a). Scientific research and clinical studies have confirmed the safety of R. rosea and its effectiveness as a psychostimulant, general strengthener, and antistress agent (Chiang et al. 2015). Understanding the signalling and regulatory system functioning on cellular level is important in elucidation the defence mechanism and the specific features of the adaptogenic activity (Volkova et al. 2013).
The pronounced physiological and pharmacological activities of R. rosea lead to indiscriminate harvesting and depletion of its natural habitats (Bai et al. 2014). Therefore, it is a priority plant with vulnerable to critically endangered status, strictly forbidden for harvesting (Platikanov and Evstatieva 2008; Cuerrier et al. 2015) and included in the Red List of protected plant species in many countries throughout the world (Mossberg and Stenberg 2003; Sidjimova et al. 2014).
Hence, the application of plant in vitro systems as a sustainable platform for biotechnological production of pharmaceuticals is a promising alternative. In vitro systems possess numerous advantages, including biosynthesis of biosafe metabolites according to the good manufacturing practices (GMP) and independency of the environmental factors (Marchev et al. 2014; Grech-Baran et al. 2015).
The aim of this review is to summarize the current status of R. rosea in terms of its medicinal use with emphasis on the responsible molecular mediators and relevant application in clinical practice. Special attention to the in vitro systems as a tool for secondary metabolite biosynthesis is assigned. The proposed biosynthetic pathways of salidroside and cinnamyl alcohol glycosides, as well as, several considerations, including nutrient medium optimization, precursor feeding and genetic engineering with the aim of enhancing their yields are reviewed in details. The prospects of future research regarding the pharmacological profiles of R. rosea extracts and pure compounds, as well as, the demand of exploring the biosynthetic pathway of R. rosea secondary metabolites are highlighted, as well.
Botany of Rhodiola rosea
Rhodiola rosea L. (synonyms: Sedum rosea (L.) Scop., belongs to the Crassulaceae family, Sedoideae subfamily, Sedeae tribe, Umbilicinae subtribe (Engler and Melchior 1964). Some species of the genus were previously classified to the genus Sedum L., from which they were separated later (Hooker and Jackson 1895–1974). The genus Rhodiola consists of 136 accepted species (Grech-Baran et al. 2015), occurring mainly in Asia and Europe (Brown et al. 2002), and the most widespread species in Europe is R. rosea.
The name of the plant can be traced back to the ancient Greek physician Dioscorides who first wrote about medicinal applications of rodia riza in 77 C.E. in De Materia Medica (Mell 1938). Linnaeus renamed it as Rhodiola rosea referring to the rose-like fragrance of the fresh cut rootstock (Linnaeus 1749).
R. rosea L. is a dioecious, perennial herbaceous plant, native to East Asia, Russia, Japan, Korea and Southern China (Martin et al. 2010). In the mountains it grows at altitudes ca. 2000–2600 m (Platikanov and Evstatieva 2008) under very severe conditions (e.g. intensive UV radiation, low oxygen concentration, alpine climate with low temperatures and growth on poor soils—mountain limestone, granite and sandstone regions or rock crevices; Ling-ling et al. 2007). The natural area of distribution of R. rosea is wide and includes most of the boreal and temperate parts of the Northern hemisphere. In Europe, the species occurs in the cool temperature regions of the Northern, Central and South-eastern parts of the continent (Tutin 1964). Eastward it spreads to Central and Northern Asia (the subarctic and Siberia, Altai and the mountains of Mongolia; Fu and Ohba 2001), and in North America—in the mountains of Alaska, Canada, and the continental United States (Small and Catling 1999).
R. rosea has a very well-developed root system. The root is spindle-shaped and becomes thicker at the base, developing into short rhizomes (Hegi 1963). The above-ground part consists of several shoots appearing from the thick root (Mossberg and Stenberg 2003). Leaves are fleshy orbicular-ovate to linear-oblong, dentate, glabrous and almost waxy with a green-bluish colour (Mossberg and Stenberg 2003).
Flowers are unisexual, rarely hermaphrodite and usually 4-merous (Tutin 1964; Mossberg and Stenberg 2003) and form inflorescences arranged in terminal cymes (Tutin 1964). The flowers are yellow in colour with sometimes reddish nuance and with a pleasant scent (Mossberg and Stenberg 2003).
After the flowering period each female flower develops the fruits, represented by four red follicles. Fruits mature in July–September but this period is highly variable depending on the altitude and other ecological factors. Chromosome number is 2n = 22. Seeds are brown and lanceolate (Hegi 1963).
Phytochemical composition of Rhodiola rosea
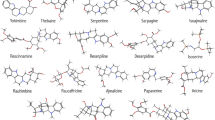
The initial study of roseroot phytochemical profile began in the 1960s. To date, HPLC-, GC-MS- and NMR-based phytochemical profiling have identified the structures of approximately 140 compounds, belonging to different natural product classes (Panossian et al. 2010; Joset et al. 2011). Most of its pharmaceutically active secondary metabolites (Table 1) are mainly stored in the rhizomes and roots. A large number of flavonoid derivatives have been isolated from the rhizomes and the aerial parts of the plant (Kurkin et al. 1985), as well as coumarins, lactones (Furmanowa et al. 1995), phenolic acids (Brown et al. 2002) and cyanogenic glycosides, i.e. lotaustralin (Akgul et al. 2004). Some of the main chemical compounds are structurally presented on Fig. 1.
Salidroside (phenylethanoid glycoside) was first isolated in 1967 by Troshchenko and Kutikova along with its aglycone, tyrosol and was named rhodioloside. Rhodioloside was first identified in Salix triandra L. from which originated the name salidroside later (György 2006). Further, Zapesochnaya and Kurkin (1982) reported the isolation of rosin, rosarin and rosavin (phenylpropanoid glycosides), identified as cinnamyl alcohol glycosides and collectively called ‘‘rosavins’’. A phytochemical profile comparison of 21 Rhodiola species revealed the specificity of cinnamyl alcohol glycosides for R. rosea, which can distinguish it from all other Rhodiola species (Kurkin et al. 1986). Nowadays, rosin, rosavin, rosarin and salidroside are considered as diagnostic marker compounds of R. rosea (Panossian and Wagner 2005).
Roseroot also accumulates a relatively small amount of essential oil, mostly in its roots and rhizomes. According to different studies, the composition of the essential oil varies but the most frequently identified compounds are geraniol, n-decanol, myrtenol, trans-pinocarveol (Rohloff 2002; Héthelyi et al. 2005; Shatar et al. 2007; Evstatieva et al. 2010).
A remarkable deviation in the active metabolite contents and constituents was observed in a study aiming to analyze the marker compounds in individuals of an Austrian R. rosea wild population (Mirmazloum et al. 2015a). The recently published results, along with the growing number of reports on the authenticity and quality control of R. rosea products (Xin et al. 2015; Booker et al. 2015) are increasingly highlighting the increasing importance of in vitro systems optimization for reliable and homogenous plant material production.
Medicinal use and ethnopharmacology of Rhodiola rosea: a focus on the molecular mediators
R. rosea is widely recognized as an adaptogen, i.e. a substance, which elicits a state of increased overall resistance, thereby allowing the organism to counteract and adapt to conditions of extreme stress. In accordance with the European Medicines Agency Assessment Scale, adaptogens refer as herbal preparations that increase attention and endurance in fatigue; reduce stress-induced impairments and disorders related to the neuro-endocrine and immune systems (Panossian and Wikman 2010), while resent investigations redefine them as “metabolic regulators which increase the ability of an organism to adapt to environmental stressors and prevent damage to the organism by such stressors” (Panossian 2013). Concerning plants, criteria for adaptogenic herbs include a high level of safety and normalization of body functions regardless of the nature of stressors (Panossian 2013). Documents regarding the medicinal use and ethnopharmacology of R. rosea date back to the 1700s. The use of the root of this plant for treatment of various conditions, including headaches, hernia, and diseases of the skin and kidney, were reported in Linné’s Materia Medica, the first Swedish Pharmacopeia, and a book of useful plants from Iceland, which has been previously reviewed (Panossian et al. 2010). In the nineteenth century, R. rosea was used in France, Germany and many other European countries as a folk medicine to fight fatigue.
More recently, R. rosea has emerged as one of the most popular plant adaptogens utilized in Europe, especially in Russia, where it has been recommended by the Pharmacological Committee of the Ministry of Health as a stimulant to improve stamina, memory, and mood (Saratikov and Krasnov 2004). In Siberia, R. rosea is used to increase physical endurance, work productivity, longevity, treat fatigue and depression, and enhance resistance to high-altitude sickness (Panossian et al. 2010). Similarly, in the traditional medicine of China and Tibet, R. rosea is commonly used for the treatment of high-altitude sickness and hypoxia. Furthermore, various R. rosea preparations are used worldwide as dietary supplements and claimed to “contribute to optimal mental and cognitive activity” (Khanum et al. 2005).
These numerous health beneficial effects are supported by a gene expression profiling in a human neuroglia cell line (T98G) after exposure to a SHR-5 R. rosea extract at 40 µg/mL (Panossian et al. 2014). The analysis of the data has revealed multiple transcriptional alterations, affecting the expression of 1062 genes. The most significant changes in gene expression are associated with cardiovascular, metabolic, gastrointestinal, neurological, endocrine, behavioral, and psychological parameters. Pathway analysis showed changes affecting communication between innate and adaptive immune cells, eNOS signalling, altered T and B cell signalling in rheumatoid arthritis, axonal guidance signalling, G-protein coupled receptor signalling, glutamate receptor signalling, ephrin receptor signalling, cAMP-mediated signalling, and atherosclerosis signalling.
Findings from the gene expression profiling are in close alignment with previous investigations demonstrating the antioxidant, anti-hypoxic, immunomodulatory, cardioprotective and neuroprotective activities of R. rosea preparations. Many of the experimental studies on the medicinal properties of R. rosea have been extensively reviewed recently (Grech-Baran et al. 2014; Chiang et al. 2015). It is becoming increasingly clear that several mechanisms contribute to the adaptogenic activities of R. rosea preparations and its phytochemical components (Panossian and Wikman, 2009; Panossian et al. 2010). In this section, it is focused on reports that link to the health beneficial effects of extracts of R. rosea and salidroside with specific molecular mediators and cellular processes that are common in the pathogenesis of chronic diseases.
Oxidative stress and the ensuing tissue damage are involved in the pathogenesis of essentially all chronic diseases, such as neurodegenerative, cardiovascular and immune system diseases. Preparations of R. rosea (or pure salidroside) protect against oxidative damage caused by hydrogen peroxide in numerous experimental systems involving different cell types. At concentrations ranging from 1 to 10 µg/mL, R. rosea extract exhibited a protective effect against H2O2-induced oxidative stress in human neuroblastoma cells (IMR-32) and human osteosarcoma cells (Schriner et al. 2009). At the same concentration range, a standardized R. rosea extract (containing 3 % salidroside and 40 % phenolic compounds) protected human cortical neurons (HCN 1-A). Exposure to H2O2 resulted in 60 % cell death, while after a 24-h pre-treatment, the extract prevented the reduction in neuronal viability by 55–60 %. The levels of lactate dehydrogenase (LDH) decreased by 25–50 %, compared to the H2O2-treated cells (Palumbo et al. 2012). Salidroside (10–100 µM) restored the survival of human neuroblastoma cells (SH-SY5Y) up to 76 % in comparison with the cell death (57.5 %) induced by H2O2 (Zhang et al. 2007). Salidroside (at concentrations between 40 and 80 µM) was also able to attenuate the H2O2-induced cell death by 33.6 and 47.0 % in human bone marrow derived endothelial progenitor cells and to restore the reactive oxygen species production to nearly physiological levels (Tang et al. 2014). It also had a protective effect in human endothelial cells (EVC-304; Zhao et al. 2013), human hepatocytes (HL-7702; Guan et al. 2011b) and human fetal lung fibroblasts (Mao et al. 2010). At a concentration of 100 µg/mL salidroside attenuated by 51.5 % the H2O2-induced apoptosis in human umbilical vein endothelial cells (HUVECs; Xu et al. 2013), through inhibition of endothelial nitric oxide synthase (eNOS), adenosinemonophosphate-activated protein kinase (AMPK), and Akt, as well as, the redox sensitive transcription factor, NF-kappa B (Xing et al. 2014). In hippocampal neurons salidroside (60–240 µM) decreased LDH from 52.62 to 30.11 % and lowered the cell death by 35 % (Chen et al. 2009), while in rat cardiomyoblasts H9c2 cells (concentration of salidroside 266.6 µM) this effect was more pronounced—69.7 % attenuation in cell death and decrease of LDH from 107.7 to 37 % (Zhu et al. 2011). Salidroside (100 µM) restored the pro- and anti-apoptotic balance in the rat pheochromocytoma (PC12) cell line (Cai et al. 2008) and in primary cortical neuronal culture via an increase of Bcl-2 (57.6 % compared to H2O2 treatment) and a decrease of Bax (213.3 % compared to H2O2 treatment) (Shi et al. 2012).
In addition to its protective effects against oxidative stress, salidroside (10–100 µM) also protects against hypoxia-mediated damage in cultured cardiomyocytes and PC12 cells (Zhang et al. 2009; Zhong et al. 2014), and in vivo (Huang et al. 2015). The latter study examined the effect of salidroside in a mouse model of pulmonary arterial hypertension induced by chronic hypoxia. Salidroside (at a dose of 32 mg/kg) attenuated the right ventricular hypertrophy and pulmonary arterial remodelling. By examining the apoptosis factors, Bax, Bcl-2, cytochrome c, and caspase 9, it was found that salidroside reversed the hypoxia-mediated apoptosis resistance. In addition, salidroside treatment upregulated the expression of adenosine A2A receptor, a member of the G protein-coupled receptor (GPCR) family, which has a protective function against pulmonary arterial remodelling (Xu et al. 2011).
The effects of salidroside on the family of transcription factors hypoxia-inducible factors 1 (HIF1) have been examined, as well. The HIF1 family members are central regulators of oxygen homeostasis and mediators of cellular responses to hypoxia (Semenza 2012; Kenneth and Rocha 2008). One study found that salidroside (200 µM) treatment reduced the hypoxia-induced levels of HIF1 in SH-SY5Y cells (Li et al. 2010). In contrast, another study reported that in human embryonic kidney fibroblasts (HEK293T) and human hepatocellular carcinoma cells (HepG2) salidroside (at concentrations up to 100 µM) induced the accumulation HIF1 (by inhibiting its degradation) and the subsequent gene expression of erythropoietin from its transcription regulatory element, hypoxia response element (HRE; Zheng et al. 2012). Accumulation of HIF1 was also observed in cultured human umbilical vein endothelial cells after pre-treatment with salidroside (100 μg/mL), followed by exposure to H2O2 (Xu et al. 2013).
Several molecular mechanisms contribute to the adaptogenic activity of R. rosea (Panossian and Wikman 2009; Panossian et al. 2010). One of the key mediators is the inducible molecular chaperone, heat shock protein 70 (Hsp70) (Panossian et al. 2009; Hernández-Santana et al. 2014). Moreover, it has been proposed that Hsp70 can be used as a molecular biomarker for adaptogenic activity (Asea et al. 2013). Hsp70 inhibits the expression of inducible nitric oxide synthase (iNOS; Hauser et al. 1996) and interferes with the function of the glucocorticoid receptor directly by participating in its unfolding (Kirschke et al. 2014). Hsp70 inhibits the stress-activated c-Jun N-terminal protein kinase 1 (JNK1), thereby regulating JNK-mediated cell signalling (Mosser et al. 1997; Yaglom et al. 1999; Simar et al. 2012; Kim et al. 2015) in a manner independent of its chaperone activity (Yaglom et al. 1999). Consequent to inhibition of the glucocorticoid receptor and iNOS expression, Hsp70 affects the levels of circulating cortisol and nitric oxide (NO). This in turn, prevents stress-induced ATP depletion leading to increased mental and physical performance and endurance (Panossian and Wikman 2009).
Hsp70 is transcriptionally inducible as part of two major cytoprotective mechanisms, termed the heat shock response and the phase 2 response, which can be triggered by various stimuli, including thermal, osmotic, oxidative and electrophilic stress, and allow cells and organisms to adapt and survive under unfavourable conditions (Talalay 2000; Akerfelt et al. 2010; Morimoto 2011; Dayalan et al. 2015). Induction of the heat shock response and the phase 2 response is controlled by two central regulators, heat shock factor 1 (HSF1) and nuclear factor-erythroid 2-related factor (NRF2), respectively. Emerging evidence suggests that, at least under certain conditions, R. rosea may cause activation of HSF1 and/or NRF2 in mammalian cells. In human neuroglia cells, silencing of HSF1 leads to a significant suppression of the enhanced Hsp70 expression promoted by a standardized preparations containing R. rosea or salidroside (Panossian et al. 2012). One study has reported that the antioxidant activity of R. rosea is most likely independent of NRF2 activation: although there was a modest induction in reporter gene expression, there were no obvious changes in the levels of NRF2 target proteins (Schriner et al. 2009). Nevertheless, it is noteworthy that salidroside (administered at daily doses of 50, 100, or 200 mg/kg for 28 days) was recently found to inhibit pulmonary fibrosis in rats treated with bleomycin, which was accompanied by stabilization of NRF2 and increased levels of its downstream target proteins NAD(P)H:quinone oxidoreductase 1 (NQO1), heme oxygenase 1 (HMOX1), and suppression of pro-inflammatory responses (Tang et al. 2015). In addition, salidroside (at concentrations ranging from 10 to 50 µM) protects H9c2 cardiomyocytes against ischemic damage caused by oxygen and glucose deprivation/re-oxygenation (Zheng et al. 2014). This protective effect is accompanied by an increase in NRF2 target gene expression and is diminished by knockdown of NRF2. It is thus possible that the cytoprotective activity of R. rosea preparations under certain stress conditions is mediated, at least in part, through activation of HSF1 and/or NRF2.
The ability to inhibit the activity of enzymes, which participate in the degradation of monoamines, and consequently to affect the levels of serotonin, dopamine and norepinephrine in the cerebral cortex, hypothalamus and the brain stem (Stancheva and Mosharrof 1987), to prevent catecholamine release and the subsequent increase in the levels of cAMP (Maslova et al. 1994), and to activate opioid receptors have also been reported to provide functional means by which R. rosea extracts may exert their adaptogenic activity (Lishmanov et al. 1997).
In summary, the available experimental evidence has shown that exposure to R. rosea extracts or salidroside causes multiple transcriptional and functional alterations in the mammalian cell with clear benefits to the immune, cardiovascular, and nervous systems, provide scientific support for its continued medicinal use.
Clinical trials
R. rosea appears in the official Pharmacopoeia of many European countries, e.g. Sweden, France, Estonia and Russia (Cuerrier et al. 2015) and the products registered under the EU’s traditional herbal medicinal products (THMP) scheme must adhere to the GMP, good agricultural and collection practices (GACP). THMP must be produced in licensed GMP facilities and correspond to the requirements of the European Pharmacopeia. The analytical testing must be according to good laboratory practice (GLP), demonstrating both safety and quality (Booker et al. 2015). According to the European Medicines Agency (EMA), the herbal preparations of R. rosea are used in the form of dry extracts (drug extract ratio 1.5–5:1), extraction solvent ethanol 67–70 %. The pharmaceutical form should be described according to the European Pharmacopeia full standard term, which single dose varies between 144 and 200 mg, whereas the daily dose varies between 144 and 400 mg (Committee on herbal medicinal products 2012a). The preparations Rosenrot and Arctic Root (based on SHR-5 extract) are currently registered as THMP (Panossian et al. 2010). In 1985 SHR-5 was registered in Sweden as a natural remedy and as THMP in 2008 (Panossian and Wikman 2015). Vitango (based on R. rosea extract WS 1375), was registered in the Netherlands as a traditional herbal medicinal product in 2009 (Hung et al. 2011). R. rosea extracts used in clinical trials are mostly standardized to min. 3 % rosavins and 0.8–1 % salidroside, similarly to their naturally occurring ratio (Brown et al. 2002). More than 46 companies worldwide sell R. rosea products and over 30 companies supply them as food ingredients. R. rosea is traded in preparations such as alcoholic extracts, tablets, capsules or in combination with other medicinal plants and/or honey (Platikanov and Evstatieva 2008).
After numerous placebo-controlled randomized clinical trials, R. rosea proved to be safe in acute and subacute toxicity studies (Panossian and Wikman 2010; Hung et al. 2011). An acute oral toxicity study was performed with alcoholic extract from R. rosea (cultivated in Alberta, Canada), containing ca. 2.7 % of rosavins. Treatment with concentration of 50 mg/kg during 7- and 28-days repeated oral dose studies in rats showed no toxic effects. Nevertheless, the limit of the safety dose was not achieved (Semple 2010). Repeated administration of R. rosea extract for 10 days decreased LD50 of 40 % ethanol in mice from 24.1 to 55.2 mL/kg. Salidroside was able to shorten (from 100 to 19 %) the duration of benzene induced sleep in rats (Saratikov and Krasnov 2004). An LD50 value of 3.36 g/kg in animals was reported by Brown et al. (2002) for extract from R. rosea grown in Russia. According to unpublished results from the Swedish Herbal Institute, after 90 days administration of SHR-5 R. rosea extract (0.14–1.43 g/kg) in piglets no inflammation response was observed. The same extract had no toxicity on the central nervous system in mice (0.1–0.5 g/kg) and no change in the body weight or behavior was observed (1.0–3.4 g/kg), as well (Committee on herbal medicinal products 2012b). In vivo study revealed that salidroside had no genotoxic effect at concentration of 1.5 g/kg when administrated daily for 3 ays on mice (Zhu et al. 2010). Adverse events (AEs) due to R. rosea intake are rare and mild (sleepiness and cold extremities; Aslanyan et al. 2010; Hung et al. 2011). R. rosea has the potential to enhance the action of stimulating medications, therefore, it should not be used concomitantly with such medications. The use of R. rosea is also not recommended during pregnancy or lactation (Ross 2014). Most of the clinical studies with R. rosea have been conducted in Russia and Sweden where it has already been established as a psychostimulant, general strengthener, and an antistress agent (Ross 2014).
Mental health conditions
Olsson et al. (2009) assessed the efficacy of the standardized extract SHR-5 (2.43 % salidroside) of roots of R. rosea in the treatment of individuals suffering from stress-related fatigue. The participants (males and females), aged between 20 and 55 years, classified in two groups, experienced difficulties equivalent to the criteria of “fatigue syndrome”. After 28 days of treatment with SHR-5 extract (576 mg extract/day), a significant positive change was recorded in terms of Pines’ burnout scale (physical, emotional and mental exhaustion), mental health, attention and quality of life. No side effects or toxicology were reported (Olsson et al. 2009). Reduction of fatigue and improvement in quality of life in a set of tests collectively calculated as a “Total Fatigue Index”, during 42 days of treatment of physicians on night shift (18–55 years of age) was reported. The daily dose of R. rosea extract (2.8 % total rosavins) was 364 mg, but the authors noticed some AEs, which were used to recommend higher doses of the extract to confirm its effectiveness in further studies (Punja et al. 2014). Intake of SHR-5 standardized extract at 340 mg/day had lower antidepressant effect than sertraline. This extract showed significantly less AEs and was better tolerated in patients with mild to moderate depression (Mao et al. 2015).
Mental performance
Acute R. rosea ingestion (3 mg/kg) decreased the heart rate response to sub-maximal exercise and improved endurance exercise performance, mood and cognitive function in 15 recreationally active college women (21 ± 0.09 years; Buckley and Lewis 2009). Aslanyan et al. (2010) evaluated the single dose effect of ADAPT-232 (fixed combination of dried extracts form roots of R. rosea, berries of Schizandra chinensis (Turcz.) Baill and roots Eleutherococcus senticosus Maxim) on mental performance. Twenty out of forty healthy females (between 20 and 68 years of age) received a single tablet of ADAPT-232 (270 mg). After 2 h the ADAPT-232 group experienced improved attention and increased speed and accuracy during a cognitive test (evaluated by d2 Test of Attention). Experiment participants also had lower percentage of error, improved accuracy, quality of work and degree of care in stressful conditions (Aslanyan et al. 2010). Daily doses of 300 mg extract (3 % rosavins and 1 % salidroside) for 28 days increased the general intelligence in healthy volunteers aged between 26 and 56 years. It was concluded that the mechanisms of action included cholinesterase inhibition, anti-oxidant and anti-inflammatory activities, increased blood flow and energy metabolism (Stough et al. 2011).
Physical performance
Dosing strategy of R. rosea preparations for 1 month may attenuate muscle damage and inflammation. After consumption of 340 mg standardized R. rosea extract RHODAX (30 mg of “actives”, i.e., rosavins plus salidroside) twice per day for 30 days by young untrained participants, decreased exercise-induced inflammation and muscle damage during a 6-day period of intense exercise was observed. The blood concentrations of C-reactive protein (CRP) and creatinine kinase (CK), serving as inflammation markers, were significantly lower in the treated group in comparison with the control (Abidov et al. 2004). Contrary to the expectations, in experienced male and female runners, R. rosea extract (total rosavins >5 % and salidroside >1.8 %; PoliNat SL, Las Palmas, Spain) did not attenuate the post-race muscle damage, soreness, and inflammation experienced by the runners, or the decrement in muscle function (Shanely et al. 2014). Treatment with salidroside (600 mg/day) exhibited a protective effect on epirubicin-induced early left ventricular regional systolic dysfunction in patients with histologically confirmed breast cancer. At a higher cumulative dose of epirubicin (300 mg/m2), the strain rate was normalized only with the intake of salidroside (Zhang et al. 2012).
Preservation strategies of R. rosea
The natural habitats of R. rosea are almost completely exhausted because of indiscriminate harvesting of R. rosea plants for their valuable pharmacological substances (Ling-ling et al. 2007; Bai et al. 2014). That is why this species is with high conservation value throughout its area of distribution. It is one of the priority species for conservation in many European countries including Finland, Lithuania, Sweden, Norway and Iceland (Cuerrier et al. 2015). R. rosea is protected species with endangered status in Great Britain, Czech Republic, Bosnia and Herzegovina (Platikanov and Evstatieva 2008); critically endangered in Bulgaria (Sidjimova et al. 2014) and vulnerable in Slovakia (Galambosi 2006), where its collection is strictly forbidden (Platikanov and Evstatieva 2008). It is also a subject of protection in many former Soviet Union republics, as well as, the Komi Republic (Taskaev 1999), the Central Urals, Arkhangelsk, Nenetz and Khanty Mansiysk Autonomous Area of the Russian Federation, including the Republic of Karelia (Kotiranta et al. 1998). R. rosea is also included in the Red Data Book of several countries, including Bulgaria (Sidjimova et al. 2014), Ukraine (Didukh 2009), Västra Götaland County (Scandinavia) and Göteborg and Bohuslän counties in Sweden (Mossberg and Stenberg 2003).
There are several strategies developed for the preservation of R. rosea, including field cultivation and micropropagation with the aim of restoring R. rosea to its natural habitats (Tasheva and Kosturkova 2010).
Field cultivation
The first publications on the cultivation of R. rosea date back to 1970s when a collection was established in the experimental station of Upper Altai Pedagogical Institute (Dneprovskii et al. 1975). Cultivation started with transferring whole plants which were used subsequently for seed production. Extensive collections have been established during the last few decades in different parts of the former Soviet Union in Tomsk, Siberia, Petersburg and Syktyvkar. Successful cultivation of the species was reported in Russia, Scandinavia (Sweden, Finland and Norway), Alpine Countries (Austria, Italy, Germany and Switzerland), Carpathian Countries (Poland, Romania, Bulgaria, Moldova, Czech Republic and Slovak Republic), as well as, other countries, e.g. Estonia, Great Britain and Mongolia (Galambosi 2015).
Kudryavtseva and Viracheva (2006) reported long-term results (1938–2006) of cultivation of 15 Rhodiola species in the Kola Peninsula, the Northern Russia. The results showed that R. rosea is a long living plant, with a life span from 40 to 70 years and tolerant to the unfavourable ecological conditions (Kudryavtseva and Viracheva 2006). Technology for cultivation of R. rosea in Bulgaria was developed by Platikanov and Evstatieva (2008). They considered the ecological conditions in Bulgaria as unfavourable for seed propagation, mostly due to higher temperatures and lower air and soil humidity in comparison to other parts of its areal. Therefore, they recommended vegetative propagation by direct rooting of cuttings of 2–5 cm length (Platikanov and Evstatieva 2008). Mineral fertilization with N, P and K increased the raw material yield about 30–40 % and that of phenylpropanoids after 2 years of cultivation (Buchwald et al. 2015).
Notably, most of the products on the market are based on raw material collected from wild populations from the Altai region. There are several organizations of different sizes in various parts of the world, including Alaska (http://www.alaskarhodiolaproducts.com; date of access 09.12.2015), Alberta (Alberta Rhodiola Rosea Growers Organization), and Finland (http://personal.inet.fi/koti/sini.marjanen/rreng.htm; date of access 09.12.2015), whose members are cultivating roseroot.
The main disadvantage of the field-cultivated R. rosea plants is that the accumulation of the secondary metabolites occurs after 5–7 years of cultivation (Grech-Baran et al. 2015) and the adaptation of the plants is difficult due to the specific climate conditions (Platikanov and Evstatieva 2008).
Micropropagation
Protocols for in vitro plant organogenesis, regeneration and propagation were established. The key points in this approach are the establishment of suitable media for induction, multiplication and rooting of the shoots, their adaptation into greenhouses and acclimatization in the natural habitats (Tasheva and Kosturkova 2010, 2012a; Ghiorghită et al. 2011). The suitable concentration and combination of the plant growth regulators depend on the genotype, the ecotype, the explant type and its stage of development. Even a soft computing model for prediction of the optimal nutrient media which demonstrate the best results for growing and rooting of R. rosea in vitro cultures has been developed by Simeonova et al. (2013).
Regarding the micropropagation of R. rosea, Ghiorghită et al. (2011) concluded that the most suitable explants were shoot apices and nodes cultivated on hormone-free MS medium. Another efficient hormonal variant for micropropagation is also MS medium supplemented with NAA and Kin or IAA and Zea characterized with very intense rhizogenesis (Ghiorghită et al. 2011). The most appropriate media for shoot induction and multiplication were MS medium containing Zea and IAA, whereas rhizogenesis and root induction was achieved on MS medium supplemented with IAA, IBA and GA3, suggesting that IAA and IBA in combination have synergistic effect (Tasheva and Kosturkova 2010). Further acclimatization of the plantlets into a greenhouse (22–24 °C, PFD of 40 µM photons/m2 s1 (16/8 d/n) and 90 % relative humidity and a 1:1:2 mixture of perlite:peat:soil; Tasheva and Kosturkova 2010) or into septic environment in a hydroponic system was performed (Ghiorghită et al. 2011). Both approaches ensured high survival of the plantlets: 85 and 90 %, respectively (Tasheva and Kosturkova 2010; Ghiorghită et al. 2011). When R. rosea plants were transferred in Rhodope Mountains (area Beglika, Bulgaria 1525 meters altitude) 70 % of the plants survived (Tasheva and Kosturkova 2010), which was a great success in comparison with other reports, where during the first year 73.5 % of the plants survived and their number dropped to 57 % in the second year when transferred in Ceahlău Mountains, Romania (1750 meters altitude) (Ghiorghită et al. 2011). The micropropagated plants in Rhodope Mountains contained higher levels of salidroside (0.61–0.64 %) in comparison with the wild plant (0.36–0.44 %), while those in Ceahlău Mountains had different leaf colour (light green) in comparison to green-gray colour of the native individuals (Tasheva and Kosturkova 2010; Ghiorghită et al. 2011).
Rhodiola rosea in vitro systems as a source of pharmaceutically valuable secondary metabolites. Biosynthetic pathway of salidroside and cinnamyl alcohol glycosides
Plant in vitro systems have been endorsed as sustainable perspective for production of high-value phytochemicals. These compounds are being produced under ecofriendly controlled process parameters according to the GMP, thus ultimately providing continuous production of biosafe, bioactive and stable natural products. This approach eliminates the influence of environmental and seasonal factors and does not threaten the natural populations of rare and protected plant species. Moreover, several strategies for enhancement of the desired metabolites could be applied, facilitating down-stream processes and resulting in increased yields of the target metabolites (Marchev et al. 2014; Grech-Baran et al. 2015). At present there are a limited number of reports concerning secondary metabolite production from calli and suspension cultures of R. rosea. Scientific publications for induction and cultivation of hairy roots of this species are still lacking. The secondary metabolites biosynthesized by callus and suspension cultures of R. rosea (Table 2) include phenylpropanoids, e.g. rosarin, rosavin, rosin, triandrin, (Furmanowa et al. 1998; György et al. 2004, 2005; Krajewska-Patan et al. 2007a); phenylethanoids, e.g. salidroside, tyrosol (Krajewska-Patan et al. 2007a); phenolic acids, e.g. gallic, chlorogenic, caffeic and p-coumaric acid (Furmanowa et al. 1998; Krajewska-Patan et al. 2007a; Kurkin et al. 1991); proanthocyanidins, e.g. catechin, epicatechin, epigallocatechin, gallate epicatechin, gallate epigallocatechin (Gryszczyńska et al. 2012).
R. rosea is growing very slowly. The cultivation of this species aiming high levels of biologically active substances is quite long and takes from 5 to 7 years (Furmanowa et al. 1999; Galambosi 2006) and very often the biosynthesis of salidroside is in the low range, between 0.13 and 1.6 % DW (Furmanowa et al. 1999; Linh et al. 2000; Platikanov and Evstatieva 2008), and that of rosavins is between 0.1 and 3.0 % DW (Furmanowa et al. 1999; Saunders et al. 2013), frequently with high deviation in the content between different species or even intraspecially due to heterozygosis, morphological and chemical variability (Weglarz et al. 2008; Mirmazloum and György 2012). Along with that, these substances are found in lower amounts in the field-cultivated R. rosea in comparison to the naturally growing plants (Ma et al. 2008). To meet the demand for roseroot metabolites, there have been many attempts for chemosynthesis of salidroside and rosavins but all were ineffective due to high production costs (Ma et al. 2007).
These challenges could be overcome by developing biotechnological methods to improve the production of R. rosea secondary metabolites through in vitro culture systems (Lan et al. 2013; Mirmazloum et al. 2014). However, the in vitro produced metabolites are in lower amounts (György et al. 2004; György and Hohtola 2009), and in some cases even failed to be produced by in vitro systems (Martin et al. 2010) or needed specific requirements for their biosynthesis, such as addition of precursors (Grech-Baran et al. 2013). There are also few reports for the successful biosynthesis of salidroside in microorganisms (Bai et al. 2014). One of the highlighted reasons for the low salidroside biosynthesis for example, is the low efficiency of glycosylation and the non-synchronization of UDP-glycosyltransferase activity with tyrosol accumulation (Xu et al. 1998a; Grech-Baran et al. 2013). As a result, there is a considerable interest in the regulation of R. rosea secondary metabolites by modulating the expression of the endogenous enzymes at the rate limiting steps of the biosynthetic pathway (by overexpressing the pathway genes or/and by eliminating competing pathways and feedback inhibition) or by introduction of new enzymes (Ma et al. 2007, 2008; Bai et al. 2014). The metabolic engineering approach is possible but dependent on the discovery of the genes expressing the relevant enzymes (Lan et al. 2013).
Proposed biosynthetic pathway of salidroside
Salidroside is a tyrosol 8-O-β-d-glucoside (Ma et al. 2008; Zhang et al. 2011) and mostly accumulates in rhizomes and roots of R. rosea (György et al. 2009). It is formed as a product of dehydration between the hemiacetal hydroxyl of glucose and the ethanol hydroxyl of tyrosol (4-hydroxyphenylethanol) (Ling-ling et al. 2007). The biosynthetic pathway of salidroside (Fig. 2, arrow types B-G) is still elusive and its regulation is not very well understood (Ma et al. 2008; Bai et al. 2014).
Proposed biosynthetic pathway of salidroside and cinnamyl alcohol glycosides in Rhodiola spp. (modified after Ling-ling et al. 2007; Zhang et al. 2011; Mirmazloum and György 2012). Enzymes abbreviation: PAL phenylalanine ammonia-lyase (EC: 4.3.1.5), TAL tyrosine ammonia-lyase (EC: 4.3.1.23), PTAL phenylalanine/tyrosine ammonia-lyase (EC: 4.3.1.25), TyrDC tyrosine decarboxylase (EC: 4.1.1.25), HPA histidinol-phosphate transaminase (EC: 2.6.1.9), AAT aromatic-amino-acid transaminase (EC: 2.6.1.57), HPPD: 4-hydroxyphenylpyruvate decarboxylase (EC: 4.1.1.80), CAO primary-amine oxidase (EC: 1.4.3.21), AAD aryl-alcohol dehydrogenase (EC: 1.1.1.90), 4CL 4-coumarate coenzyme A:ligase (EC: 6.2.1.12), C 4 H trans-cinnamate 4-monooxygenase (EC: 1.14.13.11), CCR cinnamoyl-CoA reductase (EC: 1.2.1.44), CAD cinnamyl alcohol dehydrogenase (EC: 1.1.1.195), PCD P-Coumaric acid decarboxylase (EC: 4.1.1.-.)
Salidroside and CAGs (rosin, rosavin and rosarin) have common precursors at the beginning of their biosynthetic pathway, including the l-amino acids phenylalanine (Phe) and tyrosine (Tyr) derived via the shikimate pathway which is the main biosynthetic pathway of phenolics in higher plants (Ma et al. 2008; Mirmazloum and György 2012).
The biosynthesis of salidroside can be divided into two stages: the biosynthesis of tyrosol, and the subsequent transfer of glucose to tyrosol in order to form salidroside (Ling-ling et al. 2007). In the available literature there are two different opinions concerning the biosynthesis of tyrosol: one is that tyrosol is presumably produced by a decarboxylase from a p-coumaric acid precursor, mainly deriving from Phe; and the second one is that the precursor of tyrosol is tyramine, which is synthesized from Tyr by tyrosine decarboxylase (Ma et al. 2008; Zhang et al. 2011).
According to the first view, the biosynthesis of tyrosol starts with deamination of Phe to trans-cinnamic acid (Ling-ling et al. 2007; Ma et al. 2008) by phenylalanine ammonia-lyase (PAL; EC: 4.3.1.5) (Ling-ling et al. 2007; Mirmazloum and György 2012). Phe and Tyr can inhibit their own synthesis through feedback inhibition of chorismate mutase (Ma et al. 2008). This reaction is an offshoot in primary and secondary metabolisms in plants, and PAL has key regulatory functions. Trans-cinnamic acid is hydroxylated at position 4 of the aromatic ring to form p-coumaric acid by trans-cinnamate 4-monoxydase (C4H; EC: 1.14.13.11). P-coumaric acid can be synthesized from Tyr as well by tyrosine ammonia-lyase (TAL; EC: 4.3.1.23), phenylalanine/tyrosine ammonia-lyase (PTAL; EC: 4.3.1.25) and also by PAL enzyme with TAL activity (Mirmazloum and György 2012) (Fig. 2, D type arrows). Two possibilities have been described for the transformation of p-coumaric acid to tyrosol. The first one (Fig. 2, C type arrows) is that p-coumaric acid is directly converted to tyrosol via decarboxylation by p-coumaric acid decarboxylase (pCD; EC: 4.1.1.-) (Ling-ling et al. 2007; Mirmazloum and György 2012). Nevertheless, there is still no confirmation available for the presence of pCD in plant species (Ma et al. 2008; Mirmazloum and György 2012). The second possibility (Fig. 2, F and G type arrows) for tyrosol biosynthesis includes the conversion of p-coumaric acid into p-coumarol-CoA by 4-coumarate-CoA ligase (4CL; EC: 6.2.1.12), p-coumaraldehyde by cinnamoyl-CoA reductase (CCR; EC: 1.2.1.44), and finally p-coumaryl alcohol by cinnamyl alcohol dehydrogenase (CAD; EC: 1.1.1.195). It has been proposed that p-coumaryl alcohol is further converted into tyrosol by still uncharacterized enzymes at least in two steps (indicated with question mark) (Ling-ling et al. 2007).
An important advance in clarifying the tyrosol biosynthesis was the overexpression of the endogenous PALrs1 gene in R. sachalinensis (Ma et al. 2008). As a result, there was a sharp decrease in Tyr, tyrosol and salidroside content in comparison with the non-transgenic plants. The amount of p-coumaric acid increased by 3.3-fold but it did not facilitate tyrosol biosynthesis. This is a clear evidence that tyrosol is not derived from Phe and that the observed reduction of salidroside biosynthesis correlates with the availability of Tyr (Ma et al. 2008). This conclusion is further supported by the lack of correlation between PALrs1 transcriptional expression and the accumulation of salidroside. PALrs1 transcription which was higher in calli than in stems and leaves and very low in roots, whereas the salidroside content was more pronounced in roots and calli and less in stems and leaves (Ma et al. 2008).
The second view for tyrosol biosynthesis suggests that it derives from Tyr (Fig. 2, B and E type arrows) (Ma et al. 2008; György et al. 2009; Zhang et al. 2011; Lan et al. 2013). Tyr is converted to tyramine by tyrosine decarboxylase (TyrDC; EC: 4.1.1.25) (Fig. 2, B type arrows). TyrDC has a decisive role and crucial function not only in the initial conversion of tyrosol, but also a key regulatory function in the salidroside biosynthesis pathway in general (Ma et al. 2008; György et al. 2009; Zhang et al. 2011; Lan et al. 2013). TyrDC has a genotype and tissue dependent expression. Its expression is significantly higher in the roots, than in leaves, stems or flowers in R. rosea and R. crenulata, which is in accordance with the high salidroside content in the roots (György et al. 2009; Lan et al. 2013). The salidroside content is significantly higher in transgenic hairy roots of the same species (Lan et al. 2013), as well as in R. sachalinensis (Zhang et al. 2011), containing TyrDC gene. This information supports the opinion that salidroside biosynthesis begins with the decarboxylation of Tyr by TyrDC which produces tyramine. Supporting evidences suggests that the addition of 1 mM Tyr or 1 mM tyrosol increased ninefold the salidroside content in cell cultures of R. sachalinensis, resulting in the highest ever reported salidroside content by Rhodiola cell suspension culture (154.95 mg/g), whereas the Phe did not exhibit the same effect (Xu et al. 1998b).
The next step in the tyrosol synthesis is the conversion of tyramine to 4-hydroxyphenylacetaldehyde (4-HPAA) by primary-amine oxidase (CAO; EC: 1.4.3.21) (Mirmazloum and György 2012). Another route for the biosynthesis of tyrosol is catalyzed by histidinol-phosphate transaminase (HPA; EC: 2.6.1.9) and aromatic-amino acid transaminase (AAT; EC: 2.6.1.57) which convert Tyr to 4-hydroxyphenylpyruvate (4-HPP) (Fig. 2, E type arrows). So far these enzymes have been reported only in Nicotiana tabacum L. and Vigna radiata (L.) R. Wilczek respectively. Afterwards, 4-HPP is converted to 4-HPAA by 4-hydroxyphenylpyruvate decarboxylase (HPPD: EC: 4.1.1.80) (Mirmazloum and György 2012). Aryl-alcohol dehydrogenase (AAD; EC: 1.1.190) is responsible for the formation of tyrosol from 4-HPAA, which has been recognized as the direct precursor of tyrosol, and the enzyme has been found in plants (Zhang et al. 2011). Recently, salidroside has been synthesized in recombinant Escherichia coli from glucose through 4-HPP (key intermediate in the yeast Ehrlich pathway, derived from Tyr by transamination). The key enzymes were the Saccharomyces cerevisiae pyruvate decarboxylase ARA10, which converted 4-HPP to 4-HPAA and the introduced plant-derived glycosyltransferase UGT73B6. A significant increase of salidroside biosynthesis was observed not only when the genes responsible for its biosynthesis were expressed, but also when the competitive pathways and negative regulation were eliminated, as well (Bai et al. 2014).
Natural products, such as phenylpropanoids, exist as glycosides in plants. The final step in salidroside (the storage form of tyrosol) biosynthesis is the transfer of a glucose molecule to tyrosol which is catalyzed by UDP-glycosyltransferase by means of deploying UDP-glucose as the glucose donor (Ling-ling et al. 2007). Glycosylation can alter the solubility and transport of the compounds within the cell, which stabilize the product, and modulate its bioactivity and storage (Mirmazloum and György 2012).
UDP-glycosyltransferase is also very important key regulatory enzyme in salidroside biosynthesis along with TyrDC enzyme (Ling-ling et al. 2007; Yu et al. 2011; Lan et al. 2013). UDP-glycosyltransferase activity is in correlation with the tyrosol availability and can be significantly upregulated by tyrosol addition as a precursor in the media (Grech-Baran et al. 2013) or treatment with MeJa (Lan et al. 2013). The expression of RcUDPGT was coordinated with the RcTYDC gene expression in R. crenulata (Lan et al. 2013) and the synchronized activity between TyrDC and UDP-glycosyltransferase in the hairy roots of R. kirilowii resulted in more pronounced synthesis of tyrosol and salidroside (Grech-Baran et al. 2013). Nevertheless, tyrosol accumulation was not synchronized with the tyrosol glucosyltransferase (TGase) activity in natural roots of R. kirilowii (Grech-Baran et al. 2013), which indicated that there might be other UGTs that are active toward tyrosol (Ma et al. 2007). UDP-glycosyltransferase is also a tissue specific enzyme and is more abundant in the roots of Rhodiola species and their in vitro cultures (Ma et al. 2007). The overexpression of UGT73B6 and UGT72B14 gene in R. sachalinensis was responsible for higher levels of salidroside (Ma et al. 2007; Yu et al. 2011), but compared to the UGT73B6 transgenic plants and calli (Ma et al. 2007), the hairy roots exhibited higher level of salidroside (Yu et al. 2011).
The accumulation of salidroside depends on the balance between its synthesis and degradation. The possible enzyme catalyzing its degradation is β-d-glucosidase (β-d-glucoside glucohydrolase; EC: 3.2.1.21) which is able to hydrolyze a range of glycosides. The enzyme has dual function: it can act as a hydrolase (hydrolyzes glycosides, releasing the glycosyl) or as a glucosyltransferase (transfers the glycosyl to other molecules, called aglycones). So far, it has remained unclear, if the dominant role of this enzyme is in the anabolism or catabolism of salidroside. It still needs to be clarified by observing its activity during different developmental stages of the plant and in different plant tissues (Ling-ling et al. 2007).
Proposed biosynthetic pathway of cinnamyl alcohol glycosides (CAGs)
The production of rosin and its derivatives in the genus Rhodiola is restricted to only a few species. Biosynthesis of phenolic glycosides occurs spontaneously in R. rosea roots and rhizomes (Grech-Baran et al. 2015). In the proposed biosynthetic pathway of CAGs (Fig. 2, A type arrows), trans-cinnamic acid is converted to cinnamoyl-CoA by 4-coumarate-CoA ligase (4CL; EC: 6.2.1.12) via a two-step reaction mechanism that involves the hydrolysis of ATP (György 2006). Further, the reduction of cinnamoyl-CoA to cinnamaldehyde formation is catalyzed by the enzyme cinnamoyl-CoA oxidoreductase (CCR; EC: 1.2.1.44). Afterwards cinnamyl alcohol dehydrogenase (CAD; EC: 1.1.1.195) reduces the cinnamaldehyde to cinnamyl alcohol. This enzyme has different isoforms, some of which have a preference towards one of the available substrates. The combination of isoforms varies depending on the developmental stage and the tissue. The enzyme(s) participating in the biosynthesis of cinnamyl alcohol glycosides have not been described. Rosin is the simplest glycoside in roseroot formed by attaching one molecule of glucose to cinnamyl alcohol. By connecting an arabinose or arabinofuranose molecule to rosin than rosavin and rosarin are formed, respectively. Conditional on the sugar type and the site of attachment, other glycosides may derive (Mirmazloum and György 2012). A very important precursor and inducer of the enzyme system responsible for the biosynthesis of CAGs is cinnamyl alcohol (CA). CAGs were not synthesized by non-transformed wild type (NTWT) and hairy roots from R. kirilowii unless CA was added to the media as a precursor at concentration of 2.5 mM in the presence of 1 % sucrose (Grech-Baran et al. 2014). It was concluded that CCR has greater impact on CAGs biosynthesis, than other enzymes, such as PAL (Mirmazloum et al. 2015b).
To sum up, the identification and regulation of the key enzymes is an important step in facilitating the metabolic flux flowing toward the downstream pathway leading to increased production of the end-products salidroside and CAGs, through overexpression of these enzymes at the rate-limiting points or by blocking enzymes at the branching points that can divert the metabolic flux to other secondary metabolites.
Biotechnological tools for secondary metabolites enhancement
The number of publications concerning in vitro systems induction from R. rosea is still limited. In vitro cultures from R. rosea are extensively used for biotransformation procedures in order to enhance the secondary metabolite production or for micropropagation of plants for the restoration of exhausted habitats (Krajewska-Patan et al. 2008; Ghiorghită et al. 2011). There are many factors affecting the in vitro culture induction from R. rosea, including plant ecotype, type of explants, nutrient medium compositions (plant growth regulators, carbon source and inorganic salts), light, temperature, and presence of precursors and elicitors (Grech-Baran et al. 2015). The available literature reveals that obtaining in vitro cultures from this plant is complicated task and faces many obstacles beginning with the sensitivity of the explants towards disinfection agents and reaches to obtaining viable calli lines and producing the desired compounds (Ghiorghită et al. 2011). The first problem could be solved by short and soft sterilization procedure (3–6 min treatment with chloramine-T, 5 %) or by using in vitro explants obtained from sterile seedlings (Tasheva and Kosturkova 2010; Ghiorghită et al. 2011). The explant type can influence not only the callus morphology, but also its ability to produce secondary metabolites. Axially bud originated callus line produced approximately twofold more rosavin than the hypocotyle originated callus line, the main feature of which was the high level of rosin: 1.2-fold higher than the axially bud callus (Krajewska-Patan et al. 2007a). BAP seems to favor the fast growth of the callus tissue. Apical buds and internode fragments, leaves and leaf disks inoculated on media containing BAP (0.2–1.5 mg/L) in combination with IAA (0.1 mg/L) (Tasheva and Kosturkova 2010), NAA (0.5 mg/L) (György and Hohtola 2009), or 2.4-D (0.5–1.0 mg/L) (Ghiorghită et al. 2011) were the most appropriate explants type and media compositions for induction of intensively growing green compact calli. But when 2.4-D was applied at higher concentrations (2 mg/L) spontaneous intense rhizogenesis and formation of sporadic shoots was observed (Ghiorghită et al. 2011).
Optimization of the nutrient medium by reduction of the concentration of sucrose from 30 to 20 g/L increased the callus growth two–threefold, but no salidroside was synthesized (Tasheva and Kosturkova 2014). Rosavin was also not produced when sucrose was the single carbon source, but when 10 g of sucrose were replaced with glucose, the CAGs content increased twofold (György et al. 2005). In contrast, glucose addition simultaneously with tyrosol had no positive effect on salidroside production, compared to the single tyrosol feeding (György et al. 2005). Positive effect of antibiotics (cefotaxime and carbenicillin) has been reported to increase the in vitro biomass growth of R. rosea calli (Mirmazloum et al. 2015c).
Regardless of the specific phytohormone combination or optimization of the nutrient composition, many reports confirm that secondary metabolites in R. rosea callus cultures are very often either not synthesized at all or synthesized in lower amounts in comparison to the intact plant tissue (Furmanowa et al. 1998, 1999; György et al. 2004; Martin et al. 2010). Many authors overcome this problem by applying different biotechnological strategies, including precursor feeding and genetic manipulation of R. rosea in vitro cultures. Since secondary metabolites are considered as products linked with differentiation, in the case of R. rosea most authors prefer working with compact callus aggregates (CCA) instead of homogenous cell suspension. Compact callus aggregates are spherical, smooth surfaced clumps displaying some level of cellular differentiation and no dispersed cells are observed when transferred in liquid media (György et al. 2004). Along with the type of precursor, the optimum amount and time of addition are also important. CA is one of the most effective precursors that increases or induces the biosynthesis of CAGs. When added at concentrations of 2 or 2.5 mM at the beginning of the cultivation of CCAs, the rosin content was induced and reached 0.72 % DW (György et al. 2004) and the content of rosavin reached 1.01 % (Furmanowa et al. 1999). In the control samples, none of these metabolites were produced (Furmanowa et al. 1999; György et al. 2004). After feeding R. rosea callus culture with CA, several new glycosides as products of biotransformation were identified (György et al. 2004; Tolonen et al. 2004). With addition of 2.5 mM CA, a remarkable increase in the content of rosin in the hypocotyle originated callus was observed, whereas the axially bud originated callus line produced 1.2-fold less rosin and twofold more rosavin compared to the first line. The content of salidroside in hypocotyle originated callus increased to concentration similar to the intact plant, whereas the amount of tyrosol and chlorogenic acid were 20-fold higher (Krajewska-Patan et al. 2007a). The content of salidroside can be enhanced by exogenous addition of p-tyrosol as a precursor. Its addition (5 mM) in solid and liquid CAA culture increased the level of salidroside reaching up to 4.3 % DW (Krajewska-Patan et al. 2007b).
A promising and effective approach to enhance the secondary metabolite production by R. rosea in vitro cultures is the application of genetic engineering for regulation of their biosynthetic pathways through the enzyme activities involved. The most commonly employed method includes a transformation step, mediated by Agrobacterium tumefaciens with the aim to introduce DNA encoding definite enzyme activity into the plant genome. This approach concerning in vitro cultures of R. rosea is still in progress. Mirmazloum et al. (2014) developed a consistent method for genetic transformation of R. rosea cell cultures which can be used as a tool to enhance the pharmaceutically important metabolites through expression or suppression of the involved key genes in the plant genome. Several experiments performed with in vitro cultures of R. sachalinensis and R. crenulata confirmed that overexpression of genes encoding key biosynthetic enzymes, such as PAL, TyrDC and UDP-glycosyltransferase can successfully direct the biosynthetic pathway towards the target metabolites: salidroside and CAGs, and increase their concentrations (Ma et al. 2007; Yu et al. 2011; Zhang et al. 2011; Lan et al. 2013). Nevertheless, similar investigations based on R. rosea in vitro cultures are still missing in the available literature. Based on the analysis of the plant R. rosea, György et al. (2009) proved that TyrDC has an organ-specific expression. The expression of the gene encoding this enzyme was significantly higher in the roots, which corresponds with the fact that salidroside, accumulates preferentially in the underground parts of the plant. The expression in the leaves was lower than in the roots (György et al. 2009).
The choice of in vitro culture as a biotechnological tool for secondary metabolite production depends also on the localization of the target secondary metabolites in the intact plant (Martin et al. 2010). Since salidroside and CAGs are dominantly accumulated in the roots of the plant, therefore hairy roots should be a reliable alternative for their biosynthesis. Hairy roots are considered to be genetically and biochemically stable and hence are able to produce secondary metabolites similar to the intact plants: therefore, they can be used as a sustainable platform for in vitro biosynthesis of secondary metabolites (Georgiev et al. 2012; Marchev et al. 2014). The single report in the available literature for hairy root induction from R. rosea belongs to Tasheva and Kosturkova (2012b). In spite of the detailed investigations by using three different approaches for genetic transformation of R. rosea with A. rhizogenes ATCC 15834 and optimization of bacterial suspension density, co-cultivation time and antibiotic concentration, the authors obtained frustrating results. Currently, there are several reports for hairy roots obtained from Rhodiola species, including R. kirilowii, R. crenulata and R. sachalinensis. Once again, the secondary metabolite production faces the same problems as with callus and suspension cultures. Salidroside and CAGs were not produced form hairy roots of R. kirilowii without addition of the respective precursor tyrosol or CA (Grech-Baran et al. 2013, 2014). Nevertheless, the hairy roots either do not produce the whole spectrum of secondary metabolites, or produce them in lower amounts in comparison to the intact plant tissue (Grech-Baran et al. 2014). Precursor feeding (tyrosol, Tyr and Phe) and elicitor treatment (abiotic and biotic) increased significantly the expression of key enzymes involved in the salidroside biosynthetic pathway, thus enhancing approximately 2- to 3.5-times the salidroside concentration in comparison with wild type non-transformed roots of R. sachalinensis and R. crenulata (Zhou et al. 2007; Lan et al. 2013).
Conclusions and future perspectives
R. rosea is a plant species extensively applied as an adaptogenic, antifatigue, antidepressant, antioxidant, anti-inflammatory, antinociceptive, and anticancer agent, and modulator of immune functions. Application of a safe and effective alternative therapy utilizing natural products could be of public health relevance for many individuals unable, or unwilling, to use conventional therapies. Numerous clinical trials have demonstrated the applicability, safety and clinical relevance of R. rosea. Research on molecular mechanisms is essential for understanding the signalling and molecular network affected by adaptogens at cellular level in order to rationalize their beneficial effect on emotional behaviour, psychological, neurological and metabolite disorders, as well as, mental and physical performance under stress. It is essential to determine the pharmacological profile of each extract and pure compound, as well as, their contribution to the specific cellular functions associated with various diseases.
Regarding the protected status of R. rosea, in vitro cultures of this species are relevant and sustainable alternative for production of the pharmaceutically important salidroside and CAGs. Current optimization procedures of the growth medium components and the process of cell cultivation show promise to increase the biosynthesis of these secondary metabolites. Nevertheless, exploring the mechanisms that regulate the biosynthesis of these metabolites is a key factor for understanding and controlling their biosynthetic pathways in the plant, as well as, in cultures. These approaches, along with the emerging “omics” platforms (metabolomics in particular), could be successfully implemented in the manufacturing and chemical profiling of R. rosea preparations based on in vitro cultures.
Abbreviations
- 2.4-D:
-
2.4-Dichlorophenoxyacetic acid
- AEs:
-
Adverse events
- BAP:
-
6-Benzylaminopurine
- BAX:
-
Bcl-2-associated X protein
- Bcl-2:
-
B-cell lymphoma-2
- CA:
-
Cinnamyl alcohol
- cAMP:
-
Cyclic adenosine monophosphate
- DW:
-
Dry weight
- eNOS:
-
Endothelial nitric oxide synthase
- GA3 :
-
Gibberellic acid
- GC–MS:
-
Gas chromatography–mass spectroscopy
- GMP:
-
Good manufacturing practices
- HIF1:
-
Hypoxia-inducible factors 1
- HPLC:
-
High performance liquid chromatography
- Hsp70:
-
Heat shock protein 70
- IAA:
-
Indole-3-acetic acid
- IBA:
-
Indole-3-butyric acid
- Kin:
-
Kinetin
- MeJa:
-
Methyl jasmonate
- MS:
-
Murashige and Skoog
- NAA:
-
Naphtaleneacetic acid
- NMR:
-
Nuclear magnetic resonance
- NQO1:
-
NAD(P)H:quinone oxidoreductase 1
- Phe:
-
l-Phenylalanine
- THMP:
-
Traditional herbal medicinal products
- Tyr:
-
l-Tyrosine
- TyrDC:
-
Tyrosine decarboxylase
- UDP:
-
UDP-glucose:tyrosol glucosyltransferase
- Zea:
-
Zeatin
References
Abidov M, Grachev S, Seifulla R et al (2004) Extract of Rhodiola rosea radix reduces the level of C-reactive protein and creatinine kinase in the blood. Bull Exp Biol Med 138:63–64
Akerfelt M, Morimoto R, Sistonen L (2010) Heat shock factors: integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol 11:545–555
Akgul Y, Ferreira D, Abourashed E et al (2004) Lotaustralin from Rhodiola rosea roots. Fitoterapia 75:612–614
Asea A, Kaur P, Panossian A et al (2013) Evaluation of molecular chaperons Hsp72 and neuropeptide Y as characteristic markers of adaptogenic activity of plant extracts. Phytomedicine 20(14):1323–1329
Aslanyan G, Amroyan E, Gabrielyan E et al (2010) Double-blind, placebo-controlled, randomised study of single dose effects of ADAPT-232 on cognitive functions. Phytomedicine 17:494–499
Avula B, Wang Y, Ali Z et al (2009) RP-HPLC determination of phenylalkanoids and monoterpenoids in Rhodiola rosea and identification by LC-ESI-TOF. Biomed Chromatogr 23(8):865–872
Bai Y, Bi H, Zhuang Y et al (2014) Production of salidroside in metabolically engineered Escherichia coli. Sci Rep. doi:10.1038/srep06640
Booker A, Jalil B, Frommenwiler D et al (2015) The authenticity and quality of Rhodiola rosea products. Phytomedicine. doi:10.1016/j.phymed.2015.10.006
Brown R, Gerbarg P, Ramazanov Z (2002) Rhodiola rosea: a Phytomedicinal overview. HerbalGram 56:40–52
Buchwald W, Mordalski R, Kuchrski W et al (2015) Effect of fertilization on roseroot (Rhodiola rosea L.) yield and content of active compounds. Acta Sci Pol Hortorum Cultus 14(2):109–121
Buckley J, Lewis S (2009) The effects of an acute dose of Rhodiola rosea on exercise performance and cognitive function. J Int Soc Sports Nutr 6(1):P14
Cai L, Wang H, Li Q (2008) Salidroside inhibits H2O2-induced apoptosis in PC12 cells by preventing cytochrome c release and inactivating of caspase cascade. Acta Biochim Biophys Sin 40(9):796–802
Chen X, Liu J, Gu X et al (2008) Salidroside attenuates glutamate-induced apoptotic cell death in primary cultured hippocampal neurons of rats. Brain Res 1238:189–198
Chen X, Zhang Q, Cheng Q et al (2009) Protective effect of salidroside against H2O2-induced cell apoptosis in primary culture of rat hippocampal neurons. Mol Cell Biochem 332(1–2):85–93
Chiang H, Chen H, Wu C (2015) Rhodiola plants: chemistry and biological activity. J Food Drug Anal 23:359–369
Committee on Herbal Medicinal Products (2012a) Community herbal monograph on Rhodiola rosea L., rhizoma et radix. EMA/HMPC/232091/2011
Committee on Herbal Medicinal Products (2012b) Assessment report on Rhodiola rosea L., rhizoma et radix. EMA/HMPC/232100/2011
Cuerrier A, Archambault M, Rapinski M et al (2015) Taxonomy of Rhodiola rosea L., with special attention to molecular analyses of Nunavik (Québec) populations. In: Cuerrier A, Ampong-Nyarko K (eds) Rhodiola rosea. Traditional herbal medicines for modern times. CRC Press, Taylor & Francis Group, pp 1–34
Dayalan N, Kostov R, Dinkova-Kostova A (2015) Transcription factors Hsf1 and Nrf2 engage in crosstalk for cytoprotection. Trends Pharmacol Sci 36(1):6–14
Didukh YP (ed) (2009) Red Data Book of Ukraine: Flora. Ukrainian Scientific Publishers, Kyiv, p 900
Dneprovskii I, Kim E, Iumanova T (1975) Seasonal development and growth of Rhodiola rosea L. in relation to introduction [as drug plant]. Biull Gl Bot Sada 98:27–34
Dubichev A, Kurkin V, Zapesochnaya G et al (1991) Chemical composition of the rhizomes of the Rhodiola rosea by the HPLC method. Chem Nat Compd 27(2):161–164
Engler A, Melchior H (1964) Syllabus der Pflanzenfamilien. Gerbuder Borntraeger, Berlin
Evstatieva L, Todorova M, Antonova D (2010) Chemical composition of the essential oils of Rhodiola rosea L. of three different origins. Pharmacogn Mag 6(24):256–258
Fu K, Ohba H (2001) Rhodiola (Crassulaceae). In: Wu Z, Raven P (eds) Flora of China, vol 8. Science Press, Beijing, pp 251–268
Furmanowa M, Oledzka H, Michalska M et al (1995) Rhodiola rosea L. (Roseroot): in vitro regeneration and the biological activity of roots. In: Bajaj YPS (ed) Biotechnology in agriculture and forestry, vol 33. Medicinal and Aromatic Plants VIII. Springer, Berlin, pp 412–426
Furmanowa M, Skopińska-Rozewska E, Rogala E et al (1998) Rhodiola rosea in vitro culture-phytochemical analysis and antioxidant action. Acta Soc Bot Pol 67(1):69–73
Furmanowa M, Hartwich M, Alfermann A et al (1999) Rosavin as a product of glycosylation by Rhodiola rosea (roseroot) cell cultures. Plant Cell Tiss Org 56:105–110
Galambosi B (2006) Demand and availability of Rhodiola rosea L. raw material. In: Bogers R, Cracker L, Lange D (eds) Medicinal and aromatic plants. Springer, The Hague, pp 223–236
Galambosi B (2015) Cultivation of Rhodiola rosea in Europe. In: Cuerrier A, Ampong-Nyarko K (eds) Rhodiola rosea. Traditional herbal medicines for modern times. CRC Press, Taylor & Francis Group, pp 87–124
Georgiev M, Agostini E, Ludwig-Müller J et al (2012) Genetically transformed roots: from plant disease to biotechnological resource. Trends Biotechnol 30(10):528–537
Ghiorghită G, Hârtan M, Maftei D et al (2011) Some considerations regarding the in vitro culture of Rhodiola rosea L. Rom Biotechnol Lett 16(1):5902–5908
Grech-Baran M, Sykłowska-Baranek K, Giebułtowicz J et al (2013) Tyrosol glucosultransferase activity and salidroside production in natural and transformed root cultures of Rhodiola kirilowii (Regel) Regel et Maximowicz. Acta Biol Cracov Ser Bot 55(2):126–133
Grech-Baran M, Sykłowska-Baranek K, Krajewska-Patan A et al (2014) Biotransformation of cinnamyl alcohol to rosavins by non-transformed wild type and hairy root cultures of Rhodiola kirilowii. Biotechnol Lett 36:649–656
Grech-Baran M, Sykłowska-Baranek K, Pietrosiuk A (2015) Biotechnological approaches to enhance salidroside, rosin and its derivatives production in selected Rhodiola spp. in vitro cultures. Phytochem Rev 14:657–674
Gryszczyńska A, Krajewska-Patan A, Dreger M et al (2012) Proanthocyanidins in Rhodiola kirilowii and Rhodiola rosea callus tissues and transformed roots-determination with UPLC–MS/MS method. Herba Pol 58(4):52–61
Guan S, Feng H, Song B et al (2011a) Salidroside attenuates LPS-induced pro-inflammatory cytokine responses and improves survival in murine endotoxemia. Int Immunopharmacol 11(12):2194–2199
Guan S, Wang W, Lu J (2011b) Salidroside attenuates hydrogen peroxide-induced cell damage through a cAMP-dependent pathway. Molecules 16(4):3371–3379
György Z (2006) Glucoside production by in vitro Rhodiola rosea cultures. Dissertation, Acta Universitatis Ouluensis C Technica 244. Oulu University Press, Oulu
György Z, Hohtola A (2009) Production of cinnamyl glycosides in compact callus aggregate cultures of Rhodiola rosea through biotransformation of cinnamyl alcohol. In: Jain SM, Saxena P (eds) Protocols for in vitro cultures and secondary metabolite analysis of aromatic and medicinal plants. Methods in Molecular Biology, vol 547. Humana Press, New York, pp 305–312
György Z, Tolonen A, Pakonen M et al (2004) Enhancement of the production of cinnamyl glycosides in CCA cultures of Rhodiola rosea through biotransformation of cinnamyl alcohol. Plant Sci 166(1):229–236
György Z, Tolonen A, Neubauer P et al (2005) Enhanced biotransformation capacity of Rhodiola rosea callus cultures for glycosid production. Plant Cell Tiss Org Cult 83:129–135
György Z, Jaakola L, Neubauer P et al (2009) Isolation and genotype-dependent, organ-specific expression analysis of a Rhodiola rosea cDNA encoding tyrosinedecarboxylase. J Plant Physiol 166:1581–1586
Hauser G, Dayao E, Wasserloos K (1996) HSP induction inhibits iNOS mRNA expression and attenuates hypotension in endotoxin-challenged rats. Am J Physiol 271(6 Pt 2):H2529–H2535
Hegi G (ed) (1963) Rhodiola, Rosenwurz. In: Illustrierte Flora von Mitteleuropa. Zweite völlig neubearbeitete Auflage. Band IV/2, Lieferung 2/3. Paul Parey, Hamburg, Berlin, pp 99–102
Hernández-Santana A, Pérez-López V, Zubeldia J (2014) A Rhodiola rosea root extract protects skeletal muscle cells against chemically induced oxidative stress by modulating heat shock protein 70 (HSP70) expression. Phytother Res 28(4):623–628
Héthelyi É, Korány K, Galambosi B et al (2005) Chemical composition of the essential oil from rhizomes of Rhodiola rosea L. grown in Finland. J Essent Oil Res 17(6):628–629
Hooker J, Jackson B (1895–1974) Index Kewensis. Plantarum phanerogamarum nomina et synonima generum et specium. Clarendron Press, Oxford
Hu X, Zhang X, Qiu S (2010) Salidroside induces cell-cycle arrest and apoptosis in human breast cancer cells. Biochem Biophys Res Commun 398(1):62–67
Huang X, Zou L, Yu X (2015) Salidroside attenuates chronic hypoxia-induced pulmonary hypertension via adenosine A2a receptor related mitochondria-dependent apoptosis pathway. J Mol Cell Cardiol 82:153–166
Hung S, Perry R, Ernst E (2011) The effectiveness and efficacy of Rhodiola rosea L.: a systematic review of randomized clinical trials. Phytomedicine 18:235–244
Jeong H, Ryu Y, Park S et al (2009) Neuraminidase inhibitory activities of flavonols isolated from Rhodiola rosea roots and their in vitro anti-influenza viral activities. Bioorg Med Chem 17(19):6816–6823
Joset K, Nyberg N, Van Diermen D et al (2011) Metabolic profiling of Rhodiola rosea rhizomes by 1H NMR spectroscopy. Phytochem Anal 22:158–165
Kenneth N, Rocha S (2008) Regulation of gene expression by hypoxia. Biochem J 414(1):19–29
Khanum F, Bawa A, Singh B (2005) Rhodiola rosea: a versatile adaptogen. Compr Rev Food Sci Food Saf 4:55–62
Kim J, Yenari M, Lee J (2015) Regulation of inflammatory transcription factors by heat shock protein 70 in primary cultured astrocytes exposed to oxygen–glucose deprivation. Neuroscience 286:272–280
Kirschke E, Goswami D, Southworth D (2014) Glucocorticoid receptor function regulated by coordinated action of the Hsp90 and Hsp70 chaperone cycles. Cell 157(7):1685–1697
Kotiranta H, Uotila P, Sulkava S et al (1998) Red data book of East Fennoscandia. Ministry of the environment, Finnish environment institute and botanical museum. Finnish museum of natural history, Helsinki, p 351
Krajewska-Patan A, Dreger M, Łowicka A et al (2007a) Chemical investigations of biotransformed Rhodiola rosea callus tissue. Herba Pol 53(4):77–87
Krajewska-Patan A, Furmanowa M, Dreger M (2007b) Enhancing the biosynthesis of salidroside by biotransformation of p-tyrosol in callus culture of Rhodiola rosea L. Herba Pol 53(1):55–64
Krajewska-Patan A, Dreger M, Łowicka A et al (2008) Preliminary pharmacological investigations of biotransformed roseroot (Rhodiola rosea L.) callus tissue. Herba Pol 53(4):50–58
Kudryavtseva O, Viracheva L (2006) Results of genus Rhodiola (Crassulaceae) species introduction in Polar–Alpine Botanical Garden (Kola Peninsula). Rastit Resur 42(4):28–34
Kurkin V, Zapesochanaya G, Shchavlinskii A (1984) Flavonoids of the rhizomes of Rhodiola rosea III. Chem Nat Compd 20(3):367–368
Kurkin V, Zapesochnaya G, Shchavlinskii A (1985) Flavonoids of the epigeal part of Rhodiola rosea I. Chem Nat Compd 20(5):623–624
Kurkin V, Zapesochnaya G, Gorbunov Y (1986) Chemical investigations on some species of Rhodiola L. and Sedum L. genera and problems of their chemotaxonomy. Rast Res 22(3):310–319
Kurkin V, Zapesochnaya G, Nukhimovsky E et al (1988) Chemical composition of rhizomes of Mongolian Rhodiola rosea population introduced into districts near Moscow. Khim Farm Zh 22(3):324–326
Kurkin V, Zapesochnaya G, Dubichev A (1991) Phenylpropanoids of a callus culture of Rhodiola rosea. Chem Nat Compd 27(4):419–425
Lan X, Chang K, Zheng L et al (2013) Engineering salidroside biosynthetic pathway in hairy root cultures of Rhodiola crenulata based on metabolic characterization of tyrosine decarboxylase. PLoS One 8(10):e75459
Li Q, Wang H, Wang Z (2010) Salidroside attenuates hypoxia-induced abnormal processing of amyloid precursor protein by decreasing BACE1 expression in SH-SY5Y cells. Neurosci Lett 481(3):154–158
Ling-ling S, Li W, Yan-xia Z et al (2007) Approaches to biosynthesis of salidroside and its key metabolic enzymes. For Stud China 9(4):295–299
Linh P, Kim Y, Hong S et al (2000) Quantitative determination of salidroside and tyrosol from the underground part of Rhodiola rosea by high performance liquid chromatography. Arch Pharm Res 23(4):349–352
Linnaeus C (1749) Materia Medica. Liber I. De Plantis. Holmiae-Laurentii Salvii
Lishmanov I, Naumova A, Afanus’ev S (1997) Contribution of the opioid system to realization of inotropic effects of Rhodiola rosea extracts in ischemic and reperfusion heart damage in vitro. Eksp Klin Farmakol 60:34–36
Ma G, Li W, Dou D et al (2006) Rhodiolosides A-E, monoterpene glycosides from Rhodiola rosea. Chem Pharm Bull 54(8):1229–1233
Ma L, Liu B, Gao D et al (2007) Molecular cloning and overexpression of a novel UDP-glucosyltransferase elevating salidroside levels in Rhodiola sachalinensis. Plant Cell Rep 26:989–999
Ma L, Gao D, Wang Y et al (2008) Effects of overexpression of endogenous phenylalanine ammonia-lyase (PALrs1) on accumulation of salidroside in Rhodiola sachalinensis. Plant Biol 10:323–333
Mao G, Wang Y, Qiu Q et al (2010) Salidroside protects human fibroblast cells from premature senescence induced by H(2)O(2) partly through modulating oxidative status. Mech Ageing Dev 131(11–12):723–731
Mao J, Xie S, Zee J et al (2015) Rhodiola rosea versus ertraline for major depressive disorder: a randomized placebo-controlled trial. Phytomedicine 22:394–399
Marchev A, Haas C, Schulz S et al (2014) Sage in vitro cultures: a promising tool for the production of bioactive terpenes and phenolic substances. Biotechnol Lett 36:211–221
Martin J, Pomahačová B, Dušek J et al (2010) In vitro culture establishment of Schizandra chinensis (Turz.) and Rhodiola rosea L., two adaptogenic compounds producing plants. J Phytol 2(11):80–87
Maslova L, Kondrat’ev B, Maslov L (1994) The cardioprotective and antiadrenergic activity of an extract of Rhodiola rosea in stress. Eksp Klin Farmakol 57(6):61–63
Mell C (1938) Dyes, tannins, perfumes, and medicines from Rhodiola rosea. Text Colorist 60(715):483–484
Mirmazloum I, György Z (2012) Review of the molecular genetics in higher plants towards salidrosid and cinnamyl alcohol glycosides biosynthesis in Rhodiola rosea L. Acta Aliment Hug 41:133–146
Mirmazloum I, Forgács I, Zok A et al (2014) Transgenic callus culture establishment, a tool for metabolic engineering of Rhodiola rosea L. Acta Sci Pol Hortorum Cultus 13(4):95–106
Mirmazloum I, Ladányi M, György Z (2015a) Changes in the content of the glycosides, aglycones and their possible precursors of Rhodiola rosea during the vegetation period. Nat Prod Commun 10(8):1413–1416
Mirmazloum I, Pedryc A, György Z, Komáromi B, Ladányi M (2015b) Glycoside content in Rhodiola rosea L.: dynamics and expression pattern of genes involved in the synthesis of rosavins. Acta Hortic 1098:81–89
Mirmazloum I, Radácsi P, Pedryc A et al (2015c) Hormonal effects of carbenicillin and cefotaxime on Rhodiola rosea callus culture. Planta Med 16(81):PM-243
Morimoto R (2011) The heat shock response: systems biology of proteotoxic stress in aging and disease. Cold Spring Harb Symp Quant Biol 76:91–99
Mossberg B, Stenberg L (2003) Den nya nordiska floran. Stockholm, Wahlström and Widstrand, p 928
Mosser D, Caron A, Bourget L et al (1997) Role of the human heat shock protein hsp70 in protection against stress-induced apoptosis. Mol Cell Biol 17(9):5317–5327
Mudge E, Lopes-Lutz D, Brown P (2013) Purification of phenylalkanoids and monoterpene glycosides from Rhodiola rosea L. roots by high-speed counter-current chromatography. Phytochem Anal 24(2):129–134
Olsson E, Schéele B, Panossian A (2009) A randomised, double-blind, placebo-controlled, parallel-group study of the standardised extract SHR-5 of the roots of Rhodiola rosea in the treatment of subjects with stress-related fatigue. Planta Med 75:105–112
Palumbo D, Occhiuto F, Spadaro F (2012) Rhodiola rosea extract protects human cortical neurons against glutamate and hydrogen peroxide-induced cell death through reduction in the accumulation of intracellular calcium. Phytother Res 26(6):878–883
Panossian A (2013) Adaptogens in mental and behavioral disorders. Psychiatr Clin North Am 36(1):49–64
Panossian A, Wagner H (2005) Stimulating effects of adaptogens: an overview of clinical trials of adaptogens with particular reference to their efficacy on single dose administration. Phytother Res 19(10):819–838
Panossian A, Wikman G (2009) Evidence-based efficacy of adaptogens in fatigue, and molecular mechanisms related to their stress-protective activity. Curr Clin Pharmacol 4(3):198–219
Panossian A, Wikman G (2010) Effects of adaptogens on the central nervous system and the molecular mechanisms associated with their stress-protective activity. Pharmaceuticals 3:188–224
Panossian A, Wikman G (2015) Evidence-based efficacy and effectiveness of Rhodiola SHR-5 extract in treating stress- and age-associated disorders. In: Cuerrier A, Ampong-Nyarko K (eds) Rhodiola rosea. Traditional herbal medicines for modern times. CRC Press, Taylor & Francis Group, pp 205–224
Panossian A, Wikman G, Kaur P (2009) Adaptogens exert a stress-protective effect by modulation of expression of molecular chaperones. Phytomedicine 16(6–7):617–622
Panossian A, Wikman G, Sarris J (2010) Rosenroot (Rhodiola rosea): traditional use, chemical composition, pharmacology and clinical efficacy. Phytomedicine 17(7):481–493
Panossian A, Wikman G, Kaur P et al (2012) Adaptogens stimulate neuropeptide y and Hsp72 expression and release in neuroglia cells. Front Neurosci 6:6. doi:10.3389/fnins.2012.00006
Panossian A, Hamm R, Wikman G et al (2014) Mechanism of action of Rhodiola, salidroside, tyrosol and triandrin in isolated neuroglial cells: an interactive pathway analysis of the downstream effects using RNA microarray data. Phytomedicine 21(11):1325–1348
Petsalo A, Jalonen J, Tolonen D (2006) Identification of flavonoids of Rhodiola rosea by liquid chromatography-tandem mass spectrometry. J Chromatogr A 1112(1–2):224–231
Platikanov S, Evstatieva L (2008) Introduction of wild golden root (Rhodiola rosea L.) as a potential economic crop in Bulgaria. Econ Bot 62(4):621–627
Punja S, Shamseer L, Olson K et al (2014) Rhodiola rosea for mental and physical fatigue in nursing students: a randomized controlled trial. PLoS One 9(9):e108416
Rohloff J (2002) Volatiles from rhizomes of Rhodiola rosea L. Phytochemistry 59(6):655–661
Ross S (2014) Rhodiola rosea (SHR-5), Part I: a proprietary root extract of Rhodiola rosea is found to be effective in the treatment of stress-related fatigue. Holist Nurs Pract 28(2):149–154
Saratikov A, Krasnov E (2004) Rhodiola rosea (Golden root): a valuable medicinal plant. Tomsk University Press, Tomsk, pp 1–205
Saunders D, Poppleton D, Struchkov A et al (2013) Analysis of five bioactive compounds from naturally occurring Rhodiola rosea in eastern Canada. Can J Plant Sci 94(4):741–748
Schriner S, Avanesian A, Liu Y et al (2009) Protection of human cultured cells against oxidative stress by Rhodiola rosea without activation of antioxidant defenses. Free Radic Biol Med 47(5):577–584
Semenza G (2012) Hypoxia-inducible factors in physiology and medicine. Cell 148(3):399–408
Semple H (2010) Toxicology studies on Rhodiola rosea extract. Pharm Biol 48(S1):25–32
Shanely R, Nieman D, Zwetsloot K et al (2014) Evaluation of Rhodiola rosea supplementation on skeletal muscle damage and inflammation in runners following a competitive marathon. Brain Behav Immun 39:204–210
Shatar S, Adams R, Koenig W (2007) Comparative study of the essential oil of Rhodiola rosea L from Mongolia. J Essent Oil Res 19(3):215–217
Shi T, Feng S, Xing J et al (2012) Neuroprotective effects of salidroside and its analogue tyrosol galactoside against focal cerebral ischemia in vivo and H2O2-induced neurotoxicity in vitro. Neurotox Res 21(4):358–367
Sidjimova B, Valyovska-Popova N, Peev D (2014) Reproductive capacity of four medicinal plants in Nature Park “Rilsky Manastir”–West Bulgaria. J BioSci Biotech 177–180
Simar D, Jacques A, Caillaud C (2012) Heat shock proteins induction reduces stress kinases activation, potentially improving insulin signaling in monocytes from obese subjects. Cell Stress Chaperon 17(5):615–621
Simeonova V, Tasheva K, Kosturkova K et al (2013) A soft computing QSAR adapted model for improvement of golden root in vitro culture growth. Biotechnol Biotechnol Equip 27(3):3877–3884
Small E, Catling M (1999) Rhodiola rosea (L.) Scop. Roseroot. In: Cavers P (ed) Canadian medicinal crops. NRC Research Press, Ottawa, pp 134–139
Stancheva S, Mosharrof A (1987) Effect of the extract of Rhodiola rosea L. on the content of the brain biogenic monoamines. Proc Bulg Acad Sci Med 40:85–87
Stough C, Camfield D, Kure C et al (2011) Improving general intelligence with a nutrient-based pharmacological intervention. Intelligence 39:100–107
Talalay P (2000) Chemoprotection against cancer by induction of phase 2 enzymes. BioFactors 12:5–11
Tang Y, Vater C, Jacobi A (2014) Salidroside exerts angiogenic and cytoprotective effects on human bone marrow-derived endothelial progenitor cells via Akt/mTOR/p70S6K and MAPK signaling pathways. Br J Pharmacol 171(9):2440–2456
Tang H, Gao L, Mao J et al (2015) Salidroside protects against bleomycin-induced pulmonary fibrosis: activation of Nrf2-antioxidant signaling, and inhibition of NF-κB and TGF-β1/Smad-2/-3 pathways. Cell Stress Chaperon. doi:10.1007/s12192-015-0654-4
Tasheva K, Kosturkova G (2010) Bulgarian golden root in vitro cultures for micropropagation and reintroduction. Cent Eur J Biol 5(6):853–863
Tasheva K, Kosturkova G (2012a) The role of biotechnology for conservation and biologically active substances production of Rhodiola rosea: endangered medicinal species. Sci World J. doi:10.1100/2012/274942
Tasheva K, Kosturkova G (2012b) Towards Agrobacterium-mediated transformation of the endangered medicinal plant golden root. AgroLife Sci J 1:132–138
Tasheva K, Kosturkova G (2014) The effect of sucrose concentration on in vitro callogenesis of golden root-endangered medicinal plant. Sci Bull Ser F Biotechnol 18:77–82
Taskaev A (1999) Red book of Komi Republic. Rare and endangered species of plants and animals. Design and Cartography, Moscow-Syktyvkar, p 528
Tolonen A, Pakonen M, Hohtola A et al (2003) Phenylpropanoid glycosides form Rhodiola rosea. Chem Pharm Bull 51(4):467–470
Tolonen A, György Z, Jalonen J et al (2004) LC/MS/MS identification of glycosides produced by biotransformation of cinnamyl alcohol in Rhodiola rosea compact callus aggregates. Biomed Chromatogr 18:550–558
Troshchenko A, Kutikova G (1967) Rhodioloside from Rhodiola rosea and Rh. quadrifida. I. Chem Nat Compd 3(4):204–207
Tutin T (1964) Flora europaea. Cambridge University Press, Cambridge, p 363
van Diermen D, Marston A, Bravo J (2009) Monoamine oxidase inhibition by Rhodiola rosea L. roots. J Ethnopharmacol 122(2):397–401
Volkova L, Urmantseva V, Burgutin A et al (2013) Adaptogenic action of the complex of phenylpropanoids on Dioscorea deltoidea cell culture under abiotic stress. Russ J Plant Physiol 60(2):235–243
Wang H, Ding Y, Zhou J (2009) The in vitro and in vivo antiviral effects of salidroside from Rhodiola rosea L. against coxsackievirus B3. Phytomedicine 16(2–3):146–155
Weglarz Z, Przybył J, Geszprych A (2008) Roseroot (Rhodiola rosea L.): effect of internal and external factors on accumulation of biologically active compounds. In: Ramawat K, Mérillon J (eds) Bioactive molecules and medicinal plants. Springer, Berlin Heilderberg, pp 297–315
Wu Y, Lian L, Jiang Y et al (2009) Hepatoprotective effects of salidroside on fulminant hepatic failure induced by d-galactosamine and lipopolysaccharide in mice. J Pharm Pharmacol 61(10):1375–1382
Xin T, Li X, Yao H, Lin Y, Ma X, Cheng R, Song J, Ni L, Fan C, Chen S (2015) Survey of commercial Rhodiola products revealed species diversity and potential safety issues. Sci Rep 9(5):8337
Xing S, Yang X, Li W (2014) Salidroside stimulates mitochondrial biogenesis and protects against H2O2-induced endothelial dysfunction. Oxid Med Cell Longev 2014:904834
Xu J, Su Z, Feng P (1998a) Activity of tyrosol glucosyltransferase and improved salidroside production through biotransformation of tyrosol in Rhodiola sachalinensis cell cultures. J Biotechnol 61:69–73
Xu J, Liu C, Han A et al (1998b) Strategies for the improvement of salidroside production in cell suspension cultures of Rhodiola sachalinensis. Plant Cell Rep 17(4):288–293
Xu M, Gong Y, Su M et al (2011) Absence of the adenosine A2A receptor confers pulmonary arterial hypertension and increased pulmonary vascular remodeling in mice. J Vasc Res 48(2):171–183
Xu M, Shi H, Wang H et al (2013) Salidroside protects against hydrogen peroxide-induced injury in HUVECs via the regulation of REDD1 and mTOR activation. Mol Med Rep 8(1):147–153
Yaglom J, Gabai V, Meriin A et al (1999) The function of HSP72 in suppression of c-Jun N-terminal kinase activation can be dissociated from its role in prevention of protein damage. J Biol Chem 274(29):20223–20228
Yousef G, Grace M, Cheng D (2006) Comparative phytochemical characterization of three Rhodiola species. Phytochemistry 67(21):2380–2391
Yu H, Ma L, Zhang J et al (2011) Characterization of glycosyltransferases responsible for salidroside biosynthesis in Rhodiola sachalinensis. Phytochemistry 72:862–870
Zapesochnaya G, Kurkin V (1982) Glycosides of cinnamyl alcohol from the rhizomes of Rhodiola rosea. Chem Nat Compd 18(6):685–688
Zapesochnaya G, Kurkin V (1983) The flavonoids of the rhizomes of Rhodiola rosea II A flavonolignan and glycosides of herbacetin. Chem Nat Compd 19(1):21–29
Zhang L, Yu H, Sun Y et al (2007) Protective effects of salidroside on hydrogen peroxide-induced apoptosis in SH-SY5Y human neuroblastoma cells. Eur J Pharmacol 564(1–3):18–25
Zhang J, Liu A, Hou R et al (2009) Salidroside protects cardiomyocyte against hypoxia-induced death: a HIF-1alpha-activated and VEGF-mediated pathway. Eur J Pharmacol 607(1–3):6–14
Zhang J, Ma L, Yu H et al (2011) A tyrosine decarboxylase catalyzes the initial reaction of the salidroside biosynthesis pathway in Rhodiola sachalinensis. Plant Cell Rep 30:1443–1453
Zhang H, Shen W, Gao C et al (2012) Protective effects of salidroside on epirubicin-induced early left ventricular regional systolic dysfunction in patients with breast cancer. Drugs R&D 12(2):101–106
Zhao X, Jin L, Shen N et al (2013) Salidroside inhibits endogenous hydrogen peroxide induced cytotoxicity of endothelial cells. Biol Pharm Bull 36(11):1773–1778
Zheng K, Zhang Z, Guo A et al (2012) Salidroside stimulates the accumulation of HIF-1α protein resulted in the induction of EPO expression: a signalling via blocking the degradation pathway in kidney and liver cells. Eur J Pharmacol 679(1–3):34–39
Zheng K, Sheng Z, Li Y et al (2014) Salidroside inhibits oxygen glucose deprivation (OGD)/re-oxygenation-induced H9c2 cell necrosis through activating of Akt-Nrf2 signalling. Biochem Biophys Res Commun 451(1):79–85
Zhong X, Lin R, Li Z et al (2014) Effects of salidroside on cobalt chloride-induced hypoxia damage and mTOR signaling repression in PC12 cells. Biol Pharm Bull 37(7):1199–1206
Zhou X, Wu Y, Wang X (2007) Salidroside production by hairy roots of Rhodiola sachalinensis obtained after transformation with Agrobacterium rhizogenes. Biol Pharm Bull 30(3):439–442
Zhu J, Wan X, Zhu Y et al (2010) Evaluation of salidroside in vitro and in vivo genotoxicity. Drug Chem Toxicol 33(2):220–226
Zhu Y, Shi Y, Wu D et al (2011) Salidroside protects against hydrogen peroxide-induced injury in cardiac H9c2 cells via PI3K-Akt dependent pathway. DNA Cell Biol 30(10):809–819
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Rights and permissions
About this article
Cite this article
Marchev, A.S., Dinkova-Kostova, A.T., György, Z. et al. Rhodiola rosea L.: from golden root to green cell factories. Phytochem Rev 15, 515–536 (2016). https://doi.org/10.1007/s11101-016-9453-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11101-016-9453-5