Abstract
Nowadays, new strategies for pest and disease control to be used in rotation with or replacement of conventional pesticides are required. Essential oils (EOs), as botanical pesticides, provide a potential resource to develop more environmentally friendly and less toxic means of control to be applied in different produces. Tomato crop is affected by many insects and fungal diseases, among which, the insects Trialeurodes vaporariorum and Tuta absoluta, and the fungi Alternaria spp. and Botrytis cinerea are of great incidence. In this work two EOs from Uruguayan specimens of the local species Eupatorium buniifolium and the world-wide distributed Artemisia absinthium (Asteraceae) were characterized in their chemical composition and insecticidal and antifungal activities. We found that the EO from local A. absinthium is rich in oxygenated monoterpenes and belongs to the thujone chemotype (β-Thujone abundance is 56 ± 2 %, and α-Thujone, 1.67 ± 0.07 %). On the other hand, monoterpene hydrocarbons (α-Pinene, 22 ± 2 %) and sesquiterpene hydrocarbons [(E)-β-Guaiene, 10 ± 1 %] are the most abundant components of E. buniifolium EO. Eventhough both EOs chemically differ, they exhibit insecticidal and antifungal activity not only by direct contact but also by contact with their vapors against the tested organisms. These results may indicate that these EOs could be raw material to develop control agents to manage some of the main pests and fungal diseases of tomato crops with only one kind of treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is well known that the intensive use of conventional synthetic pesticides present several drawbacks, including soil and groundwater contamination, disruption of natural biological control and pollination processes, mammalian toxicity and development of pest resistance (Isman 2006; Perry et al. 2011). Besides, there is a current global rise in the use of alternative production practices such as organic production and integrated pest management. These grounds have motivated a worldwide trend towards the development of new means for pest and disease control to be used as substitutes or alternatives to conventional pesticides (Isman et al. 2011; Regnault-Roger et al. 2012).
Cultivated tomato is the second most commonly consumed vegetable in the world, with an area devoted to its production higher than 5 million hectares. Although global figures on pesticide use are difficult to get, the situation in USA where 82 and 81 % of the total planted area receive applications of insecticides and fungicides respectively, is illustrative (Source: USDA, National Agricultural Statistics Service, Agricultural Chemical Usage: 2006 Vegetables Summary). In Uruguay the use of agrochemicals in conventional horticulture is higher than in other productions, being very important in apple and tomato crops. In the case of tomato, the plants are attacked mainly by two insects: the greenhouse whitefly, Trialeurodes vaporariorum Westwood (Hemiptera: Aleyrodidae) and the tomato leafminer, Tuta absoluta Meyrick (Lepidoptera: Gelechiidae) (DIGEGRA-MGAP 2009).
Trialeurodes vaporariorum is a generalist, highly polyphagous insect of great incidence in greenhouse crops (Choi et al. 2003; Anonymous 2005) distributed worldwide (Lourençao et al. 2008) being the most common and abundant whitefly in the Southern Cone (Lucatti et al. 2010). Not only affects tomato crops directly by phloem feeding, but also indirectly by transmission of plant viruses and honeydew deposition (Lourençao et al. 2008; Lucatti et al. 2010). Resistance to many active principles has been reported for this whitefly (Gorman et al. 2002; Karatolos et al. 2010); and various biological control agents, lures and traps are now commercially available (Anonymous 2012; Moreau and Isman 2012).
The tomato leafminer is a neotropical insect oligophagous on various solanaceous plants, widely distributed in America (Radcliffe and Lagnaoui 2007) where is considered the main tomato pest (Siqueira et al. 2000). Its control has been traditionally undertaken with different pesticides (including organophosphates, pyrethroids, thiocarbamates and acylurea growth regulators), however resistance to many of these active ingredients (Siqueira et al. 2000; Lietti et al. 2005; Reyes et al. 2012; Sparks et al. 2012), including cross-resistance (Siqueira et al. 2001) has already been reported. Since more recently, lures including the sexual pheromone (Svatoš et al. 1996; Filho et al. 2000) are commercially available (Benvenga et al. 2007). T. absoluta was added in 2004 to the list of quarantine pests by the EPPO (Anonymous 2004); and it has become a sanitary problem recently in Europe since its detection in various places from 2006 (Anonymous 2009).
Besides insect pests, among the various diseases that affect this crop, fungal infections by ascomycetes are of great incidence worldwide (Foolad and Panthee 2012). In Uruguay, Botrytis cinerea, and species from the genera Alternaria and Fusarium cause the most relevant pathologies (Bernal 2009; Granja 2009; Bernal 2010). Fusarium spp. are the cause of different soilborne diseases, occurring most frequently at mild and cold temperatures, that affect mainly leaves and stems (Foolad and Panthee 2012). Alternaria spp. are more common in high relative humidity areas, and can affect the fruit directly (Foolad and Panthee 2012). In turn, B. cinerea infests stems and petioles first, reaching eventually the whole plant and causing post-harvest problems (Zittner 1986).
In this context, products of botanical origin, and in particular essential oils (EOs), seem to be an attractive alternative given their effectiveness and low persistence in the environment. Insecticidal and antifungal activities of EOs from plants in different families, including Asteraceae, have already been reported (Choi et al. 2003; Isman 2006; Vázquez-Luna et al. 2007). In this work, we have explored the possibility of developing agents to control both, insect pests and fungal diseases of tomatoes. We here report on the chemical composition and activity against whiteflies and the tomato leafminer and to B. cinerea and Alternaria sp. of EOs from two Asteraceae: the nowadays worldwide distributed Artemisia absinthium, and the South-American endemic species Eupatorium buniifolium.
Materials and methods
Plant material
The aerial parts of A. absinthium were collected from a plant located in Sauce-Canelones (34.65°S, 56.06°W), at different times during the years 2009–2011. The same plant was vegetatively propagated to obtain more material. In the case of E. buniifolium, plant material was collected in Las Brujas-Canelones (34.38°S, 56.20°W) in February 2010. Species were identified by Prof. Eduardo Alonso-Paz (Cátedra de Botánica, Chemistry School, Universidad de la República, Uruguay), and voucher specimens were deposited at the Herbarium of Facultad de Química, Montevideo, Uruguay (A. absinthium: Umpiérrez & Rossini s/n MVFQ 4382 and E. buniifolium: Santos s/n MVFQ 4391).
Essential oil extraction
All EOs were obtained from whole fresh material. The EO from A. absinthium was obtained by hydrodistillation with in situ steam generation in a Clevenger apparatus. The EO from E. buniifolium was obtained by exogenously generated steam distillation using a 200-l alembic connected to a 50-l plant material container. After drying with anhydrous magnesium sulfate, EOs were stored in amber glass vials under nitrogen at −4 °C. Extraction yields (EO weight/fresh plant material weight × 100) ranged from 0.3 to 0.7 % for A. absinthium and 0.2 to 0.3 % for E. buniifolium. EOs obtained in different distillations were batched before chemical characterization and activity studies.
Chemical characterization
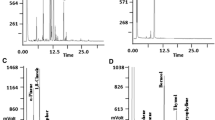
Gas chromatography (GC) was carried out using a Hewlett-Packard 5890 Series II instrument equipped with a flame ionization detector (FID) and an Elite-5 capillary column (30 m × 0.25 mm id × 0.25 μm film thickness; ALTECH INC.). The carrier gas was hydrogen at 1 ml/min and 9 psi inlet pressure. The oven temperature was initially held at 40 °C (2 min) and then increased first to 240 °C at 5 °C/min (1 min isotherm), and later to 310 °C at 10 °C/min (5 min isotherm). Temperatures of the injector and detector were 220 and 250 °C respectively; and injections were performed in the split mode (40:1). Analyses were conducted in triplicate, and the relative amount (uncorrected) of each component was estimated from the corresponding peak area expressed as the percentage of the total peak area on the chromatogram. For the identification of the individual components, a Shimadzu 2010 gas chromatograph coupled to a Shimadzu QP2010 plus mass spectrometer was used. Analyses were run with an OPTIMA-5-MS column (30 m × 0.25 mm id × 0.25 μm film thickness; Macherey–Nagel). The analytical conditions were as follows. Gas carrier: helium (1 ml/min); oven temperature: from 40 °C (isothermally held for 2 min) to 240 °C (5 °C/min, and held for 1 min), and then increased to 320 °C (10 °C/min, held for 5 min); injector and detector temperatures were 250 °C; injector mode was split (30:1); ionization potential 70 eV; scan range 40–350 m/z. The identification of the components of EOs was done by comparison of the retention indices (RI) calculated with those reported by Adams (2007) and Pherobase (El-Sayed 2011) and of fragmentation patterns with those contained in NIST 05 and SHIM 2205 mass spectrometer libraries (Table 1). In all cases injections were of 1 μl of EO diluted in dichloromethane (10 mg/ml).
Insects
Separate colonies of T. absoluta (tomato leafminer) and T. vaporariorum (whitefly) were established at the Cátedra de Entomología in the School of Agronomy (Universidad de la República, Uruguay). The colonies were initiated with immature-stages-infested leaves from tomato protected crops located nearby Montevideo city. For both insects, the leaves infested with juvenile stages were placed on healthy tomato plants (Solanum lycopersicum cv. Floradade, Solanaceae). For the whitefly, tomato plants were kept in a greenhouse under the natural photoperiod, humidity and temperature regime. In the case of the leafminer, tomato plants were kept in cages sealed with tulle at 25 ± 2 °C under natural photoperiod and humidity conditions.
Insecticidal activity
In all insect tests, but the one of direct contact with the whitefly, treatment and control assays were run using a filter paper piece (36 cm2) previously treated with 0.5 ml of different EO emulsions in water—Tween 20 (2 %) (treatment) or with 0.5 ml of a solution water—Tween 20 (2 %) (negative control) (N = 3 for each case).
Assay of exposure to EOs vapors (volatility)
To evaluate the activity of EOs’ vapors against the whitefly a two-chamber system [(made with two bases of plastic Petri dishes-9 cm × 1 cm-, separated by a holed lid (ca. 4 holes/cm2)] was used. Tomato leaflets (ca. 25 cm2) were placed on the agar-laden (2 %) top of the upper chamber with the adaxial side towards the agar. Ten to 20 whitefly adults were included in this upper chamber. In the lower, the filter paper piece (36 cm2) of treatment or the corresponding negative control was placed. After 24 h, whitefly mortality was determined, and lethal doses (LD) values were calculated.
Assay of direct contact with EOs
This activity was evaluated against both insects. For the tomato leafminer contact activity was evaluated based on the bioassay previously reported by Verçosa de Magalhães et al. (2001) by placing 5 larvae per Petri dish (9 cm diameter) on the filter paper piece (36 cm2) and the number of dead larvae was recorded after 24 h. For the whitefly, the IRAC protocol (IRAC 2009) for the evaluation of resistance to conventional insecticides was used with minor modifications: the lids of plastic Petri dishes (5 cm × 1 cm) were covered by an agar film (2 %). Tomato leaflets (4 cm × 3 cm) were weighted and then immersed for 20 s in the treatment and control emulsions mentioned above. The leaves were allowed to dry on filter paper, and then weighted again and placed on the Petri dishes with their adaxial side toward the agar. Ten to 20 adult flies were placed per dish and to allow them to orient normally, the plates were incubated upside-down. The number of dead whiteflies was recorded after 24 h and lethal doses calculated.
Fungi
The strain of B. cinerea (isolated from tomato) was provided by the Cátedra de Microbiología, School of Chemistry (Universidad de la República, Uruguay). Alternaria sp. was isolated from leaves from an organic tomato crop maintained at INIA-Las Brujas, Canelones. Leaves showing symptoms of this disease (Bernal 2010) were collected, cut, washed with sodium hypochlorite and placed in plates with Potato Dextrose Agar (PDA, Becton, Dickinson and Company) culture medium. Successive replicas of the fungus were made to obtain a pure culture.
Antifungal activity
Inhibition of mycelium growth by exposure to EOs vapors
To evaluate the activity of the vapors of the EOs the bioassays were based on the work from Alvarez-Castellanos et al. (2001), with the following modifications. In PDA (5 ml) plastic Petri dishes (9 cm diameter) eight replicates of small media pieces inoculated with the appropriate fungal species were placed. Filter paper disks (1–5 disks depending on the dose to be applied) impregnated with different quantities of pure EO (treatment) or with sterile distillated water (control) were placed on the lids of the Petri dishes. The plates were sealed with Parafilm® M and incubated upside down at 28 °C in the dark. Mycelia growth in each replicate was measured after 48 h. For each replicate the inhibition percentage (I %) was calculated as I % = [(mean diameter of controls − diameter of the treatment replicate)/mean diameter of controls] × 100. From these data, inhibitory concentrations to produce a reduction of 50 or of 99 % of mycelia growth (IC50 and IC99 respectively) were calculated.
Inhibition of mycelia growth by direct contact with the EOs
This activity was analyzed by a modified agar-dilution method (Reina et al. 1997) at the laboratory of the Plant Pathology Unit of La Laguna University. Different doses of EOs were prepared in ethanol and incorporated into PDA culture media (2 % final concentration of solvent). Control experiments consisted of solvent-treated media. The plates were inoculated in 8 points with small media pieces loaded with the appropriate fungus and incubated at 24 °C in darkness. After 48 h the plates were digitalized and the diameter of the colony in each replicate was measured with the ImageJ software (Wayne Rasband. NIH, USA: http://imagej.nih.gov/ij) and I % were calculated for each one to calculate IC50 and IC99.
Statistical analyses
Lethal doses (LD50 and LD99) and inhibitory concentrations (IC50 and IC99) were calculated by Linear Regression Analyses using the Statgraphics Plus software with the concentration data logarithmically transformed. When needed, the Abbot (1925) correction was applied (T. vaporariorum and T. absoluta).
Results and discussion
Chemical characterization of the EOs assayed
Qualitative and quantitative compositions of the EOs tested are shown in Table 1. The main component of A. absinthium EO was β-thujone (56 ± 2 %). Several chemotypes have been recognized in regard to the EO composition of A. absinthium (Chialva et al. 1983; Sacco and Chialva 1988; Juteau et al. 2003; Bononi et al. 2006; Orava et al. 2006; Basta et al. 2007; Rezaeinodehi and Khangholi 2008; Judzentiene et al. 2009; Judzentiene and Budiene 2010; Martín et al. 2011), including the pure types rich in (Z)-epoxy-ocimene, sabinyl acetate and β-thujone; and the mixed types that contain mixtures of these terpenes and also (Z)-epoxy-ocimene and chrysanthenyl acetate (Chialva et al. 1983). The EO composition here reported indicates that the plants extracted would belong to the thujone chemotype. As a group, oxygen-containing monoterpenes accounted for 81 % of the total in this EO. In contrast, in E. buniifolium EO; hydrocarbons were the most abundant components, either as monoterpenes (47 ± 5 %) or as sesquiterpenes (45 ± 5 %). The monoterpene α-pinene (22 ± 2 %) was the principal constituent; besides other monoterpenes [β-pinene (6.1 ± 0.6 %), sabinene (5.9 ± 0.6 %)]; as well as sesquiterpenes [(E)-β-guaiene (10 ± 1 %), germacrene D (7.8 ± 0.8 %), β-elemene (6.7 ± 0.7 %), and β-caryophyllene (5.8 ± 0.6 %)] were also abundant. This composition resembles the one reported by Lorenzo et al. (2005), but is different from the results obtained by two other independent studies performed on plant material from Argentina (Ruffinengo et al. 2005; Lancelle et al. 2009). Even though α-pinene is the major constituent in these three studies and ours, being much higher in the reported by Ruffinengo et al. (2005) and by Lancelle et al. (2009) (68.8 and 50.98 % respectively), the three main compounds, (E)-β-guaiene, germacrene D and β-elemene, that share both Uruguayans EOs were either not detected or minor components in both Argentinean EOs. On the other hand, compounds as δ-2-carene or ocimene that were of medium abundance in the Argentinean oils, appear in much lower amounts in our sample (Table 1) and the one previously described obtained from Uruguayan plants (Lorenzo et al. 2005). Since the plant material used in these studies came from different locations and the plant used by us were geographically nearer to the ones used by Lorenzo et al. (2005), these results may point to the existence of different chemotypes also in E. buniifolium similarly to A. absinthium and other Asteraceae (Seaman 1982; Maia et al. 2002; Perez-Alonso et al. 2003; Gudaityta and Venskutonis 2007; Paolini et al. 2010).
Insecticidal activity
Biological activities are shown in Table 2. In the case of the direct contact on insects, the whitefly seems to be more susceptible to both EOs than the tomato leafminer, as lethal doses are lower by about one order of magnitude. On the other hand no straightforward trend seems to appear when comparing both EOs: while E. buniifolium seems to be more active than A. absinthium on T. vaporariorum, both insect pests showed a similar susceptibility to both EOs. When considering the effect of the EO vapors on T. vaporariorum, the results showed that greater amounts of both EOs must be applied in order to get the same effect. Indeed, almost complete mortality (LD99) is reached at 0.29 ± 0.03 mg/cm3 of the vapors compared to 0.19 ± 0.02 mg/cm2 of direct contact with A. absinthium EO. In the hypothetic scenario that all the EO applied by contact would have volatilized, the concentration reached in the volume of the assay dish (19.6 cm3) would be 0.12 mg/cm3 which is lower than the LD99 found for the effect of vapors, indicating that the contact effect is more effective. The same kind of calculations on the amounts of E. buniifolium EO applied led to the same conclusion.
Various natural products from the genus Artemisia have been characterized in their anti-insect activity against coleopteran plant feeders (Saleh 1984; Maggi et al. 2005), stored-products pests (Tripathi et al. 2000, 2001; Negahban et al. 2006; Wang et al. 2006), piercing hemipterans (Rao et al. 1999; Soliman, 2007; Dancewicz and Gabrys 2008; Mohamed et al. 2010; Zibaee and Bandani 2010) and lepidopteran chewers (Maggi et al. 2005; Gonzalez-Coloma et al. 2011). Specifically, the essential oil from A. absinthium aerial parts has been tested in their antifeedant activity against lepidoptera and hemiptera (Martín et al. 2011), and insecticide effect on stored-product pests (Derwich et al. 2009). However, in the former case the EO tested was not from the thujone chemotype, and in the later, α-thujone was the main component instead of β-thujone (the main component of the EO in our study). These differences in chemical composition of the tested products and the fact that the assay methodology used in the present study was different make difficult to make comparisons. Similar considerations can be done in regard to natural products from the genus Eupatorium. Activity against mosquito larvae (Ciccia et al. 2000), the groundnut seed beetles (Delobel and Malonga 1987), mustard aphids (Dey et al. 2005) and a chewer coleopteran (Palacios et al. 2007) as well as ant-repellent activity (Okijanade and Wiemer 1985) have been described from non-volatile constituents from different plant extracts. Concerning EOs, Eupatorium spp. have also yield some products with activity against store-product pests (T. castaneum) (Albuquerque et al. 2004; Lancelle et al. 2009); the mosquito Aedes aegypti (Gleiser et al. 2010; Tabanca et al. 2010); aphids (Sosa et al. 2012); and the acari Varroa destructor (Ruffinengo et al. 2005). Specifically, with respect to the anti-insect capacity of the EO from E. buniifolium, two activities were previously described: inhibition of aphid settling (Sosa et al. 2012), and mosquito repellency (Gleiser et al. 2010). Once again, none of these activities comprises toxicity by direct contact or vapors, as the results described here (Table 2).
Fungicide activity
Both EOs showed some degree of toxicity against Alternaria sp. and B. cinerea (Table 2). However, against Alternaria sp. the vapors from E. buniifolium EO are not enough to produce any growth inhibition; and direct contact is needed to get activity. Despite this, by contact, E. buniifolium EO is more effective than A. absinthium EO against this fungus. On the other hand, EO vapors from A. absinthium exhibited greater inhibition potency against both fungi. These observations may indicate different cellular targets in both effects. Previous works had documented fungicide activity of extracts from species in both Asteraceae genera here studied. In this way, various Artemisia EOs display activity against not only fungi (Graven et al. 1992; Ramezani et al. 2004; Kordali et al. 2005a, b; Lopes-Lutz et al. 2008) but also yeasts (Mangena and Muyima 1999; Juteau et al. 2003; Lopes-Lutz et al. 2008). However, when A. absinthium EOs were studied in their anti-fungal properties, the plants extracted also belonged to chemotypes different from ours: mixtures with (Z)-epoxy ocimene (Juteau et al. 2003); mixtures of myrcene, β-thujone and trans-sabinyl acetate (Lopes-Lutz et al. 2008), and with chamazulene as the main component (Kordali et al. 2005a). Some Eupatorium spp. yield non-volatile products that exhibited a great activity against hialohyphomycetes as well as dermatophytes (Muschietti et al. 2005), and EOs that also are fungicide (Dellacassa et al. 2003; Ei-Seedi 2006; Dubey et al. 2007; Tripathi et al. 2008). However, as far as we know, the EO from E. buniifolium has not been previously typified in this activity.
Conclusions
The EO from the aerial parts of Uruguayan A. absinthium has been characterized and belongs into the thujone chemotype. The EO from another Asteraceae, the regionally found E. buniifolium has also been typified. Its composition resembles a previous report and differs from others suggesting the existence of chemotypes also in this plant.
Both EOs exhibits comparable insecticidal properties against two of the major tomato pests, T. vaporariorum and T. absoluta. Although the activity improves by direct contact, vapors also are toxic. In the case of the tested fungi, both EOs increased their fungicide effect by direct contact.
No straightforward inference can be done between chemical composition and activity, as one of the EO is reach in oxygenated monoterpenes (A. absinthium) and the other in monoterpene hydrocarbons (E. buniifolium).
In sum, these results may indicate the potential of these Asteraceae EOs to develop control agents with dual activity against insects and fungi that affects tomato crops. The fact that the activity is found not only by direct exposure but also with vapors opens the possibility to study these plant products as fumigants avoiding in this way the direct contact with the plants which may cause effects in their development. In that direction, we are currently studying phytotoxic effects of these products on tomato.
References
Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Econ Ent 18:65–267
Adams R (2007) Identification of essential oils components by gas chromatography/mass spectrometry. Allured Publishing Corporation Illinois, Carol Stream
Albuquerque MRJR, Silveira ER, Uchôa DEDA, Lemos TLG, Souza EB, Santiago GMP, Pessoa ODL (2004) Chemical composition and larvicidal activity of the essential oils from Eupatorium betonicaeforme (U.C.) Baker (Asteraceae). J Agric Food Chem 52:6708–6711
Alvarez-Castellanos P, Bishop C, Pascual-Villalobos M (2001) Antifungal activity of the essential oil of flowerheads of garland chrysanthemum (Chrysanthemum coronarium) against agricultural pathogens. Phytochemistry 57:99–102
Anonymous (2004) Tuta absoluta: data sheets on quarantine pests. EPPO Bull 35:434–435
Anonymous (2005) Whitefly knowledgebase. United States Department of Agriculture, Knowledgebase Retrieved May 29, 2012 from http://entomology.ifas.ufl.edu/fasulo/whiteflies/wfly0082.htm
Anonymous (2009) First report of Tuta absoluta in Malta/First report of Tuta absoluta in Switzerland. EPPO Reporting Service: Pests Dis 10:2
Anonymous (2012) Commercially used biological control agents: EPPO database. Retrieved May 29, 2012 from http://archives.eppo.int/EPPOStandards/biocontrol_web/coleoptera.htm?utm_source=archives.eppo.org&utm_medium=int_redirect
Basta A, Tzakou O, Couladis M, Pavlovic M (2007) Chemical composition of Artemisia absinthium L. from Greece. J Essent Oil Res 19(4):316–318
Benvenga SR, Fernandes OA, Gravena S (2007) Decision making for integrated pest management of the South American tomato pinworm based on sexual pheromone traps. Hortic Brasileira 25(2):164–169
Bernal R (2009) Botrytis cinerea moho gris: importante patógeno en diferentes cultivos bajo protección. Revista INIA Uruguay 20:41–43
Bernal R (2010) Enfermedades de tomate (Lycopersicum esculentum Mill.) en invernadero en las zonas de Salto y Bella Unión. Serie Técnica. INIA. Montevideo, Editorial Hemisferio Sur S.R.L. 181:1–71
Bononi M, Giorgi A, Cocucci M, Tateo F (2006) Evaluation of productivity and volatile compound quality of Artemisia absinthium L. planted in Valle Camonica (Italy). J Sci Food Agric 86(15):2592–2596
Chialva F, Liddle P, Doglia G (1983) Chemotaxonomy of wormwood (Artemisia absinthum L.). I. Composition of the essential oil of several chemotypes. Eur Food Res Technol 176(5):363–366
Choi WI, Lee EH, Choi BR, Park HM, Ahn YJ (2003) Toxicity of plant essential oils to Trialeurodes vaporariorum (Homoptera:Aleyrodidae). J Econ Entomol 96(5):1479–1484
Ciccia G, Coussio J, Mongelli E (2000) Insecticidal activity against Aedes aegypti larvae of some medicinal South American plants. J Ethnopharmacol 72:185–189
Dancewicz K, Gabrys B (2008) Effect of extracts of garlic (Allium sativum L.), wormwood (Artemisia absinthium L.) and tansy (Tanaceum vulgare L.) on the behaviour of the peach potato aphid Myzus persicae (Sulz.) during the settling on plants. Pesticides 3–4:93–99
Dellacassa AD, Bailac PN, Ponzi MI, Ruffinengo SR, Eguaras MJ (2003) In vitro activity of essential oils from San Luis, Argentina against Ascosphaera apis. J Essent Oil Res 15(4):282–285
Delobel A, Malonga P (1987) Insecticidal properties of six plant materials against Caryecon serratus (OL.) (Coleoptera: Bruchidae). J Stored Prod Res 23(3):173–176
Derwich E, Benziane Z, Boukir A (2009) Chemical compositions and insecticidal activity of essential oils of three plants Artemisia sp: Artemisia herba-alba, Artemisia absinthium and Artemisia pontica (Morocco). Electron J Environ Agric Food Chem 8(11):1202–1211
Dey S, Sinha B, Kalita J (2005) Effect of Eupatorium adenophorum Spreng leaf extracts on the Mustard Aphid, Lipaphis erysimi Kalt: A scanning electron microscope study. Microsc Res Tech 66:31–36
DIGEGRA-MGAP (2009) Principales problemas sanitarios de algunas hortalizas del Uruguay. Retrieved May, 2010 from http://www.mgap.gub.uy/direcciondelagranja/ElSectorGranjero/ProblemasSanitarios.htm
Dubey RK, Kumar R, Jaya T, Dubey NK (2007) Evaluation of Eupatorium cannabinum Linn. oil in enhancement of shelf life of mango fruits from fungal rotting. World J Microbiol Biotechnol 23(4):467–473
Ei-Seedi HR (2006) Antimicrobial activity and chemical composition of essential oil of Eupatorium glutinosum (Lam.). Nat Prod Commun 1(8):655–659
El-Sayed A (2011) The pherobase: database of pheromones and semiochemicals. In: The Pherobase, 2003–2011, Available from: http://www.pherobase.com
Filho MM, Vilela EF, Attygalle AB, Meinwald J, Svatoš A, Jham GN (2000) Field trapping of tomato moth, Tuta absoluta with pheromone traps. J Chem Ecol 26(4):875–881
Foolad MR, Panthee DR (2012) Marker-assisted selection in tomato breeding. Crit Rev Plant Sci 31(2):93–123
Gleiser RM, Bonino MA, Zygadlo JA (2010) Repellence of essential oils of aromatic plants growing in Argentina against Aedes aegypti (Diptera: Culicidae). Parasitol Res 108(1):69–78
Gonzalez-Coloma A, Bailen M, Diaz CE, Fraga BM, Martinez-Diaz R, Zuniga GE, Contreras RA, Cabrera R, Burillo J (2011) Major components of Spanish cultivated Artemisia absinthium populations: antifeedant, antiparasitic, and antioxidant effects. Ind Crops Prod 37(1):401–407
Gorman K, Hewitt F, Denholm I, Devine GJ (2002) New developments in insecticide resistance in the glasshouse whitefly (Trialeurodes vaporariorum) and the two-spotted spider mite (Tetranychus urticae) in the UK. Pest Manag Sci 58(2):123–130
Granja DGdl (2009) Principales problemas sanitarios de algunas hortalizas del Uruguay. Retrieved May 29, 2012 from http://www.mgap.gub.uy/direcciondelagranja/ElSectorGranjero/ProblemasSanitarios.htm
Graven EH, Deans SG, Svoboda KP, Mavi S, Gundidza MG (1992) Antimicrobial and antioxidative properties of the volatile essential oil of Artemisia afra Jacq. Flavour Fragr J 7(3):121–123
Gudaityta O, Venskutonis PR (2007) Chemotypes of Achillea millefolium transferred from 14 different locations in Lithuania to the controlled environment. Biochem Syst Ecol 35(9):582–592
IRAC (2009) Susceptibility test methods series (Method No: 12a). Retrieved May 29, 2012 from http://www.irac-online.org
Isman MB (2006) Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu Rev Entomol 51:45–66
Isman MB, Miresmailli S, Machial C (2011) Commercial opportunities for pesticides based on plant essential oils in agriculture, industry and consumer products. Phytochem Rev 10(2):197–204
Judzentiene A, Budiene J (2010) Compositional variation in essential oils of wild Artemisia absinthium from Lithuania. J Essent Oil-Bear Plants 13(3):275–285
Judzentiene A, Tomi F, Casanova J (2009) Analysis of essential oils of Artemisia absinthium L. from Lithuania by CC, GC(RI), GC-MS and C-13 NMR. Nat Prod Commun 4(8):1113–1118
Juteau F, Jerkovic I, Masotti V, Milos M, Mastelic J, Bessiere JM, Viano J (2003) Composition and antimicrobial activity of the essential oil of Artemisia absinthium from Croatia and France. Planta Med 69(2):158–161
Karatolos N, Denholm I, Williamson M, Nauen R, Gorman K (2010) Incidence and characterisation of resistance to neonicotinoid insecticides and pymetrozine in the greenhouse whitefly, Trialeurodes vaporariorum Westwood (Hemiptera: Aleyrodidae). Pest Manag Sci 66(12):1304–1307
Kordali S, Cakir A, Mavi A, Kilic H, Yildirim A (2005a) Screening of chemical composition and antifungal and antioxidant activities of the essential oils from three Turkish Artemisia species. J Agric Food Chem 53(5):1408–1416
Kordali S, Kotan R, Mavi A, Cakir A, Ala A, Yildirim A (2005b) Determination of the chemical composition and antioxidant activity of the essential oil of Artemisia dracunculus and of the antifungal and antibacterial activities of Turkish Artemisia absinthium, A. dracunculus, A. santonicum, and A. spicigera essential oils. J Agric Food Chem 53(24):9452–9458
Lancelle HG, Giordano OS, Sosa ME, Tonn CE (2009) Chemical composition of four essential oils from Eupatorium spp. biological activities toward Tribolium castaneum (Coleoptera: Tenebrionidae). Rev Soc Entomol Argent 68(3–4):329–338
Lietti MMM, Botto E, Alzogaray RA (2005) Insecticide resistance in Argentine populations of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Neotrop Entomol 34(1):113–119
Lopes-Lutz D, Alviano DS, Alviano CS, Kolodziejczyk PP (2008) Screening of chemical composition, antimicrobial and antioxidant activities of Artemisia essential oils. Phytochemistry 69(8):1732–1738
Lorenzo D, Paz D, Davies P, Villamil J, Vila R, Cañigueral S, Dellacassa E (2005) Application of multidimensional Gas Chromatography to the enantioselective characterisation of the essential oil of Eupatorium buniifolium Hooker et Arnott. Phytochem Anal 16:39–44
Lourençao AL, Alves AC, Fugi CGQ, Matos ES (2008) Outbreaks of Trialeurodes vaporariorum (West.) (Hemiptera: Aleyrodidae) under field conditions in the State of Sao Paulo, Brazil. Neotrop Entomol 37:89–91
Lucatti AF, Alvarez AE, Machado CR, Gilardon E (2010) Resistance of tomato genotypes to the greenhouse whitefly Trialeurodes vaporariorum (West.) (Hemiptera: Aleyrodidae). Neotrop Entomol 39(5):792–798
Maggi M, Mangeaud A, Carpinella M, Ferrayoli C, Valladares G, Palacios S (2005) Laboratory evaluation of Artemisia annua L. extract and artemisinin activity against Epilachna paenulata and Spodoptera eridania. J Chem Ecol 31(7):1527–1536
Maia JGS, MdGB Zoghbi, Andrade EHA, da Silva MHL, Luz AIR, da Silva JD (2002) Essential oils composition of Eupatorium species growing wild in the Amazon. Biochem Syst Ecol 30(11):1071–1077
Mangena T, Muyima NYO (1999) Comparative evaluation of the antimicrobial activities of essential oils of Artemisia afra, Pteronia incana and Rosmarinus officinalis on selected bacteria and yeast strains. Lett Appl Microbiol 28(4):291–296
Martín L, Julio LF, Burillo J, Sanz J, Mainar AM, González-Coloma A (2011) Comparative chemistry and insect antifeedant action of traditional (Clevenger and Soxhlet) and supercritical extracts (CO2) of two cultivated wormwood (Artemisia absinthium L.) populations. Ind Crops Prod 34(3):1615–1621
Mohamed A, El-Sayed MA, Hegazy ME, Helaly SE, Esmail AM, Mohamed NS (2010) Chemical constituents and biological activities of Artemisia herba-alba. Rec Nat Prod 4(1):1–25
Moreau TL, Isman MB (2012) Combining reduced-risk products, trap crops and yellow sticky traps for greenhouse whitefly (Trialeurodes vaporariorum) management on sweet peppers (Capsicum annum). Crop Prot 34:42–46
Muschietti L, Derita M, Salsen V, de Dios Muñoz J, Ferraro G, Zacchino S, Martino V (2005) In vitro antifungal assay of traditional Argentine medicinal plants. J Ethnopharmacol 102(2):233–238
Negahban M, Moharramipour S, Sefidkon F (2006) Chemical composition and insecticidal activity of Artemisia scoparia essential oil against three Coleopteran stored-product insects. J Asia Pac Entomol 9(4):1–8
Okijanade A, Wiemer D (1985) Ant-repellent sesquiterpene lactones from Eupatorium quadrangularae. Phytochemistry 6:1199–1201
Orava A, Arakb A, Müüriseppa M, Kailas T (2006) Composition of the essential oil of Artemisia absinthium L. of different geographical origin. Proc Estonian Acad Sci Chem 55(3):155–165
Palacios S, Maggi M, Bazán C, Carpinella M, Turco M, Munoz A, Alonso R, Nunez C, Cantero J, Defago M, Ferrayoli C, Valladares G (2007) Screening of Argentinian plants for pesticide activity. Fitoterapia 78:580–584
Paolini J, Barboni T, Desjobert J-M, Djabou N, Muselli A, Costa J (2010) Chemical composition, intraspecies variation and seasonal variation in essential oils of Calendula arvensis L. Biochem Syst Ecol 38(5):865–874
Perez-Alonso MJ, Velasco-Negueruela A, Palá-Paú J, Sanz J (2003) Variations in the essential oil composition of Artemisia pedemontana gathered in Spain: chemotype camphor-1,8-cineole and chemotype davanone. Biochem Syst Ecol 31(1):77–84
Perry T, Batterham P, Daborn PJ (2011) The biology of insecticidal activity and resistance. Insect Biochem Mol Biol 41(7):411–422
Radcliffe EB, Lagnaoui A (2007) Insect pests in potato. In: Vreugdenhil D, Bradshaw J, Gebhardt C, Govers F, Mackerron DKLATM, Ross HA (eds) Potato biology and biotechnology. Elsevier Science B.V, Amsterdam, pp 543–567
Ramezani M, Behravan J, Yazdinezhad A (2004) Chemical composition and antimicrobial activity of the volatile oil of Artemisia khorassanica from Iran. Pharm Biol 42(8):599–602
Rao P, Kumar K, Singh S, Subrahmanyam B (1999) Effect of Artemisia annua oil on development and reproduction of Dysdecus koenigii. J Appl Entomol 139:315–318
Regnault-Roger C, Vincent C, Arnason JT (2012) Essential oils in insect control: low-risk products in a high-stakes world. Annu Rev Entomol 57(1):405–424
Reina M, González-Coloma A, Gutiérrez C, Cabrera R, Henríquez J, Villarroel L (1997) Bioactive saturated pyrrolizidine alkaloids from Heliotropium floridum. Phytochemistry 46:845–853
Reyes M, Rocha K, Alarcón L, Siegwart M, Sauphanor B (2012) Metabolic mechanisms involved in the resistance of field populations of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) to spinosad. Pestic Biochem Physiol 102(1):45–50
Rezaeinodehi A, Khangholi S (2008) Chemical composition of the essential oil of Artemisia absinthium growing wild in Iran Pakistan. J Biol Sci 11:946–949
Ruffinengo S, Eguaras M, Floris I, Faverin C, Bailac P, Ponzi M (2005) LD50 and repellent effects of essential oils from Argentinian wild plant species on Varroa destructor. J Econ Entomol 98(3):651–655
Sacco T, Chialva F (1988) Chemical characteristics of the oil from Artemisia absinthium collected in Patagony (Argentina). Planta Med 1:93
Saleh M (1984) An insecticidal diacetylene from Artemisia monosperma. Phytochemistry 23(11):2497–2498
Seaman FC (1982) Sesquiterpene lactones as taxonomic characters in the Asteraceae. Bot Rev 48(2):121–595
Siqueira HAA, Guedes RNC, Picanco MC (2000) Insecticide resistance in populations of Tuta absoluta (Lepidoptera: Gelechiidae). Agric Forest Entomol 2(2):147–153
Siqueira HAA, Guedes RNC, Fragoso DB, Magalhaes LC (2001) Abamectin resistance and synergism in Brazilian populations of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Int J Pest Manag 47(4):247–251
Soliman M (2007) Phytochemical and toxicological studies of Artemisia L. (Compositae) essential oil against some insect pests. Arch Pytopathol Plant Prot 40(2):128–138
Sosa ME, Lancelle HG, Tonn CE, Andres MF, Gonzalez-Coloma A (2012) Insecticidal and nematicidal essential oils from Argentinean Eupatorium and Baccharis spp. Biochem Syst Ecol 43:132–138
Sparks TC, Dripps JE, Watson GB, Paroonagian D (2012) Resistance and cross-resistance to the spinosyns: a review and analysis. Pestic Biochem Physiol 102(1):1–10
Svatoš A, Attygalle AB, Jham GN, Frighetto RTS, Vilela EF, Šaman D, Meinwald J (1996) Sex pheromone of tomato pest Scrobipalpuloides absoluta (Lepidoptera: Gelechiidae). J Chem Ecol 22(4):787–800
Tabanca N, Bernier UR, Tsikolia M, Becnel JJ, Sampson B, Werle C, Demirci B, Baser KHC, Blythe EK, Pounders C, Wedge DE (2010) Eupatorium capillifolium essential oil: chemical composition, antifungal activity, and insecticidal activity. Nat Prod Commun 5(9):1409–1415
Tripathi A, Prajapati V, Accarwal K, Khanuja S, Kumar A (2000) Repellency and toxicity of oil from Artemisia annua to certain stored-product beetles. J Econ Entomol 93:43–47
Tripathi A, Prajapati V, Accarwal K, Kumar A (2001) Toxicity, feeding deterrence, and effect of activity of 1,8-cineole from Artemisia annua on progeny production of Tribolium castanaeum (Coleoptera: Tenebrionidae). J Econ Entomol 94(4):959–964
Tripathi P, Dubey NK, Shukla AK (2008) Use of some essential oils as post-harvest botanical fungicides in the management of grey mould of grapes caused by Botrytis cinerea. World J Microbiol Biotechnol 24(1):39–46
Vázquez-Luna A, Pérez-Flores L, Díaz-Sobac R (2007) Biomolécules con actividad insecticida: Una alternativa para mejorar la seguridad alimentaria. Cienc Tecnol Aliment 5(4):306–313
Verçosa de Magalhães S, Jham G, Picanço M, Magalhães G (2001) Mortality of second-instar larvae of Tuta absoluta produced by the hexane extract of Lycopersicon hirsutum f. glabratum (PI 134417) leaves. Agric Forest Entomol 3:297–303
Wang J, Zhu F, Zhou L, Niu C, Lei C (2006) Repellent and fumigant activity of essential oil from Artemisia vulgaris to Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J Stored Prod Res 42:339–347
Zibaee A, Bandani A (2010) A study on the toxicity of a medicinal plant, Artemisia annua L. (Asteracea) extracts to the sunn pest, Eurygaster integriceps Puton (Hemiptera: Scutelleridae). J Plant Prot Res 50(1):89–96
Zittner TA (1986). botrytis gray mold of greenhouse and field tomato. Vegetable crops Retrieved May 31, 2012 from http://vegetablemdonline.ppath.cornell.edu/factsheets/Tomato_Botrytis.htm
Acknowledgments
Financial support from the Comisión Sectorial de Investigación Científica (CSIC, Universidad de la República –UDELAR-; Grant from I + D 2009 Program) is acknowledged. Graduate scholar ships from the Agencia Nacional de Investigación e Innovación (ANII), CSIC and the Laboratorio Tecnológico de Uruguay (LATU)—School of Chemistry, UDELAR were granted to MLU. We also acknowledge the Instituto Nacional de Investigaciones Agropecuarias (INIA, Uruguay) for EO distillation (I.A. José Villamil); for help with the bioassay designs (I.A. Roberto Zoppolo); and isolation of Alternaria (I.A. Gustavo Giménez). The Cátedra de Microbiología, School of Chemistry, UDELAR (Dra. Silvana Vero) provided B. cinerea culture. Prof. Eduardo Alonso-Paz (Cátedra de Botánica, School of Chemistry, UDELAR) identified plant material. Lic. Estela Santos collected the original plant material. The Laboratorio de Biocatálisis y Biotransformaciones from the School of Chemistry, UDELAR, allowed to use their installations for microbiology work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Umpiérrez, M.L., Lagreca, M.E., Cabrera, R. et al. Essential oils from Asteraceae as potential biocontrol tools for tomato pests and diseases. Phytochem Rev 11, 339–350 (2012). https://doi.org/10.1007/s11101-012-9253-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11101-012-9253-5