Abstract
The guaianolide group of sesquiterpene lactones contains a large number of compounds with biological activity. One of these guaianolides, thapsigargin from the genus Thapsia (Apiaceae), has been a subject of particular interest in recent years because of its ability to induce apoptosis, as the active part of a pro-drug, has produced promising results for the targeted treatment of prostate cancer. In this review, recent advances in understanding the biosynthetic pathway of sesquiterpenes in plants is described with a special emphasis on guaianolides, and a hypothetical pathway for the biosynthesis of thapsigargin is presented. Eighty-seven guaianolides from Apiaceae are presented. These compounds provide clues to possible enzymatic mechanisms generating the guaianolides in Apiaceae. Some of these 87 compounds have proven or might prove interesting with regards to their biological activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants containing natural products have been used worldwide in traditional medicine since at least 4000 BC (Barton Sir et al. 1999). The pharmacological activity exhibited by some of these plants has been linked to one or more specific metabolites, and a biosynthetic understanding of how these metabolites are synthesized in vivo has been reported. Recent discoveries have led to an increased interest in the biological activity of the sesquiterpene class of metabolites, and particularly in sesquiterpene lactones such as the anti-malaria drug artemisinin, and thapsigargin, which constitutes the active part of a targeted pro-drug for the treatment of prostate cancer (GenSpera 2009). Sesquiterpenes are part of the large terpenoid group of metabolites, which are all biosynthetically produced from the precursor isopentenyl diphosphate (Fig. 1). Terpenoids, sometimes referred to as isoprenoids, can be divided into monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), triterpenes (C30) and so on. Within the group of sesquiterpenes several different carbon skeletons form the many subgroups of sesquiterpenes (guaianes, germacranes, eudesmanes etc.). Sesquiterpene lactones are derived by oxidation of the methyl moiety of the isopropyl group attached to these basic sesquiterpene carbon skeletons and are named accordingly.
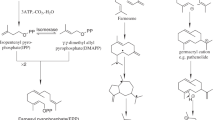
Overall sketch of the two IPP/DMAPP producing pathways and their intracellular localization in plants. Arrows indicate the overall pathway; many intermediates and additional substrates such as ATP and NADPH are omitted. The possible crosstalk between pathways is also marked with arrows. GPP, Geranyl diphosphate (C10), FPP, Farnesyl diphosphate (C15), GGPP, Geranylgeranyl diphosphate (C20) (Barton Sir et al. 1999 and Okada et al. 2008)
More than 5000 naturally occurring sesquiterpene lactones have been isolated and hundreds have been produced synthetically. The great majority of sesquiterpene lactones have been isolated from higher plants, mainly from those plants belonging to the large, species-rich families of Asteraceae and Apiaceae (Milosavljevic et al. 1999). The group of compounds called guaianolides is comprised of sesquiterpene lactones that are derived from germacrene A-D. In Apiaceae other groups of compounds have been isolated with the same skeleton structure as germacrene or with a methyl group transfer on the 10-carbon ring. These groups include the germacranoloids, eudesmanoloids, elemanoloids, eremophilanoloids and pseudoguaianolides. Their chemotaxonomic importance, together with various biological activities (antitumor, allergenic, phytotoxic, antimicrobial, insecticidal, etc.) is a major reason for the continuing interest in these compounds. However, despite their promising medicinal properties, difficulties exist with respect to the production of these compounds for use as therapeutic agents. Sesquiterpene lactones tend to be very complex, featuring many chiral centers that make them particularly difficult to synthesize using traditional organic chemistry. Hence, they are often extracted from the plant in which they were originally identified, where they are often only present in small amounts.
To increase the availability of these compounds and to obtain an economically viable supply, attempts have been made to transfer the sesquiterpene biosynthetic pathway into bacteria or fungi. These attempts have been somewhat successful. Recently, Chang and Keasling (2006) reported the successful generation of a yeast strain producing an artemisinin precursor. To achieve such success, an in-depth understanding of the original biosynthetic pathway of the molecule of interest is vital. Although this has been revealed in some detail, the complete biosynthesis of most sesquiterpenes has not yet been established.
The primary metabolism of terpenes, and hence sesquiterpenes, can be divided into several stages (Fig. 1). The first encompasses the biosynthesis of isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP). The next step encompasses the condensation of these two units into geranyl diphosphate (GDP) and the addition of a further IPP compound for the formation of farnesyl diphosphate (FPP). Further IPP compounds can be added to FPP to create larger isoprenoid precursors. The C15 unit, FPP, gives rise to sesquiterpenes through numerous rearrangement processes catalyzed by terpenoid synthases (cyclases) and these reactions are usually followed by various redox modifications producing a vast number of metabolites (Bohlmann et al. 1998). The issue of intracellular compartmentation has been observed to be of vital importance for distinguishing between the biosynthesis of mono and sesquiterpenes (Fig. 1; Bohlmann et al. 1998).
This review provides an overview of guaianolides identified in Apiaceae, and will describe the overall biosynthesis of isoprenoid structures, and particularly sesquiterpene lactones, in plants. Guaianolides are derived from germacrene with a unique ring closure forming 5 and 7 carbon rings. Among guaianolides the simplest form is called slovanolide, and guaianolides sharing this core structure form the group of slovanolides. Slovanolides have no or only one double bond within the carbon ring system (Fig. 2; Holub and Budesinsky 1986). Of other groups within the guaianolides, the most interesting pharmacologically are the thapsigargins. For thapsigargins and a few other guaianolides, one or more of the double bonds in the ring system are hydroxylated by later steps in the biosynthesis, and have thus increased the chirality of the molecule even further.
Main skeletal structures of (a) 6,12- and (b) 8,12-guaianolides. Note the lactone ring and the methyl group present as C-13 in both structures (Andrews et al. 2007)
The proposed hypothetical biosynthesis of the sesquiterpene lactone thapsigargin (1), described in this review, is based on results obtained from the family Asteraceae. This is necessary since the biosynthetic pathway of sesquiterpene lactones in Apiaceae has not yet been thoroughly investigated, and Asteraceae and Apiaceae are closely related with regards to phylogeny (Berenbaum 2001). The pharmacological activity of a number of guaianolides will also be discussed briefly, with special emphasis on the understanding of the pharmacological properties of thapsigargins.
The biosynthesis of isoprenoid molecules
The mevalonate pathway
As with other anabolic pathways, the classic biosynthesis of isoprenoid molecules involves using acetyl-CoA as a starting material and NADPH as an energy source (Berg et al. 2007). The first committed step in the mevalonate (MVA) pathway generates MVA, the precursor of the terpene building block IPP (Barton Sir et al. 1999). The first step in a series of reactions is catalyzed by the enzyme acetoacetyl-CoA synthase, producing acetoacetyl-CoA from two acetyl-CoA molecules (Fig. 1). A third acetyl moiety is added to form (S)-3-hydroxy-3-methylglutaryl-CoA (HMG-CoA), in a reaction catalyzed by HMG-CoA synthase. Evidence suggests that some organisms might harbor an enzyme system capable of generating HMG-CoA directly, without producing acetoacetyl-CoA as an intermediate. This hypothesis is based on the lack of acetoacetyl-CoA in mint leaves, and the purification of an enzyme system from radish seedlings that was capable of generating HMG-CoA directly from acetyl-CoA (Barton Sir et al. 1999).
Subsequently, HMG is converted to MVA, the precursor of cytosolic isoprenoids. MVA is then readily converted to IPP using ATP, and IPP is subsequently converted to its isomer dimethylallyl diphosphate (DMAPP); together, IPP and DMAPP are the primary precursors for terpenoid biosynthesis.
The DXP pathway
A second pathway that results in the production of IPP and DMAPP, and which does not involve the intermediate production of MVA, has been identified and partially described (Rohmer et al. 1993). The pathway is known as the DXP or MEP pathway, based on some of its intermediates, 1-deoxy-d-xylulose-5-phosphate (DXP) and 2-C-methyl-d-erythritol-4-phosphate (MEP). In this review, the abbreviation DXP will be used, although both DXP and MEP are used in the literature (Barton Sir et al. 1999; Hampel et al. 2005; Okada et al. 2008; Withers and Keasling 2007). Although some have claimed that the DXP pathway is mainly present in prokaryotes, other studies have verified the presence and importance of this pathway in higher plants (Barton Sir et al. 1999; Hampel et al. 2005; Okada et al. 2008; Rohdich et al. 2002).
The pathway is based on the generation of DXP from glyceraldehyde-3-phosphate and pyruvate, and in addition to having different biochemical intermediates; it differs from the MVA pathway in several other ways. The most important difference, in relation to plants, is the intracellular localization of the two pathways. The DXP pathway is found in plastids, where it generates precursors for carotenoids, chlorophylls, diterpenes and tocopherols (Okada et al. 2008). In contrast, the MVA pathway is found in the cytosol and produces IPP and DMAPP for the production of sesquiterpenes, sterols and ubiquinones. The MVA pathway also generates IPP that is converted into DMAPP by enzymatic conversion, whereas the last enzyme of the DXP pathway generates both IPP and DMAPP in a single step (Withers and Keasling 2007). An overall picture of the MVA and DXP pathways is shown in Fig. 1.
Cross talk has been observed between the two pathways (Fig. 1, Bick and Lange 2003; Hampel et al. 2005; Hemmerlin et al. 2003; Okada et al. 2008), and studies of this have revealed the presence of unidirectional proton symport of both IPP and GPP from plastids to the cytoplasm in several higher plants (Bick and Lange 2003). The same unidirectional flow was confirmed in Daucus carota, a member of Apiaceae (Hampel et al. 2005). In these studies, roots and leaves of two D. carota varieties were provided with the deuterium labeled precursors mevalonic acid lactone or 1-deoxy-d-xylulose. From here, two kinds of resulting labeled terpenoids were observed; terpineol, representing the monoterpenes (plastid biosynthesis), and β-caryophyllene, representing the sesquiterpenes (cytosolic biosynthesis). The results showed that the labeled MVA precursor was exclusively incorporated into β-caryophyllene, and not into terpineol. In contrast, the DXP precursor was incorporated into both terpineol and β-caryophyllene, confirming the unidirectional transport from plastids to the cytosol (Bick and Lange 2003).
Generation of farnesyl and larger terpenes
From the generation of IPP and DMAPP, the precursors of all terpenoids are formed. The reactions are catalyzed by a range of prenyl transferases, which are categorized into four groups based on their specificity regarding the length of isoprenoid molecules utilized (Barton Sir et al. 1999). Group I, short chain prenyl diphosphate synthases, are found in all living organisms and generate isoprenoid molecules of up to 20 carbon atoms in length. These enzymes require only Mg2+ or Mn2+ as cofactors (Barton Sir et al. 1999). Group II to IV are medium to long chain prenyl diphosphate synthases involved in the biosynthesis of terpenoids consisting of 20 or more carbons.
Group I prenyl transferases generate FPP, the precursor of all sesquiterpene structures. The enzyme generating FPP, FPP synthase, has been extensively studied in recent times (Barton Sir et al. 1999). The results have shown different product specificities between prokaryotic and eukaryotic synthases, and have also shown that particular phenyl groups on amino acid residues in the active site of these enzymes determine the length of the prenyl diphosphates generated.
Biosynthesis of sesquiterpene lactones
From FPP, several possible cyclization reactions can occur, all of which generate substances that act as starting materials for the more than 300 cyclic sesquiterpene skeletons known so far. These cyclizations commence with an isomerisation of trans-FPP (Fig. 3). This isomerisation and the following ionization dependent cyclization lead to a wide variety of cyclohexanoid, cyclodecanoid, cycloundecanoid or bicyclohexanoid structure elements. Internal additions to the remaining double bonds of the initially formed carbocations also occur. Methyl migrations, Wagner-Meerwein rearrangements, and hydride shifts lead to the generation of the great variety of structures found in nature, among which sesquiterpene lactones, including the guaianolides, are found (Bohlmann et al. 1998).
The formation of (+)-costunolide from FPP. Germacrene A production is catalyzed by Germacrene A synthase (I), which requires Mg2+ to function. The conversion of Germacrene A to (+)-costunolide is catalyzed by (+)-germacrene A hydroxylase (II), a cytochrome P450 enzyme using NADP+ in the process and costunolide synthase (IV), which facilitates the formation of the lactone ring (De Kraker et al. 2001 and Steele et al. 1998)
Of the sesquiterpene lactones identified so far, one of the best characterized is the drug artemisinin, which is used in the treatment of malaria. A second well-studied sesquiterpene lactone is the germacranoloide, costunolide, which exhibits various cytotoxic effects. Both artemisinin and costunolide were identified in Asteraceae and although they are not guaianolides, many of the reactions required to generate these molecules are believed to be similar to those generating thapsigargin (1). This assumption is also based on the phylogenetic relationship between Apiaceae and Asteraceae (Holub et al. 1987).
The initial step of artemisinin biosynthesis is the formation of amorpha-4,11-diene from FPP, a reaction catalyzed by amorphadiene synthase. Germacrene A was expected as an intermediate, but a single enzyme or an enzyme complex seems to be able to facilitate the complete reaction (Ro et al. 2006). Germacrene A synthases have also been isolated from Artemisia annua indicating that it is an enzyme complex that synthesizes amorpha-4-11-diene (Bertea et al. 2006). From amorpha-4,11-diene, a multifunctional sesquiterpene oxidase, CYP71AV1 catalyses the production of artemisinic acid and dihydroartemisinic acid (Teoh et al. 2006). The last steps in the biosynthesis responsible for converting dihydroartemisinic acid to artemisinin are less clear.
In a series of articles from 1998 to 2002, H. Bouwmeester and co-workers isolated several enzymes from chicory that are involved in the biosynthesis of the sesquiterpene lactone, costunolide. This group recently described the formation of germacrene A from FPP, and the subsequent conversion of germacrene A to (+)-costunolide in chicory (Fig. 3; De Kraker et al. 2002). The formation of costunolide is interesting when considering thapsigargin, in that the required lactone ring is present in costunolide, whereas the 5- and 7-carbon rings have not yet been formed.
From here, it is hypothesized that guaianolides in chicory are generated via parthenolide (De Kraker et al. 2002; Dewick 1995) via a 4,5 epoxidation (Fig. 4). This reaction mechanism was already proposed in 1995 (Piet et al. 1995), but the enzymes catalyzing the production of costunolide were not identified until 2002 (De Kraker et al. 2002). Unfortunately, the enzymes involved in the last steps in guaianolide biosynthesis in Asteraceae have not yet been identified. However, studies in Lactuca floridana (Asteraceae) support the theory that parthenolide is the intermediate in guaianolide biosynthesis (Song et al. 1995). Close parallels can be drawn between the biosynthesis of guaianolides in Apiaceae and in Asteraceae (Holub et al. 1987).
As observed with thapsigargin (1) and other guaianolides, the presence of secondary modifications enhances the complexity of the stereochemistry, especially around the lactone ring. This is of particular interest with regards to the similarities and differences between Apiaceae and Asteraceae. Numerous sesquiterpene lactones have been isolated from both families (Ghisalberti 1994; Holub and Budesinsky 1986; Fraga 2006), but the guaianolides exhibit a peculiar difference between the two (Holub and Budesinsky 1986; Azarken et al. 2008). In Apiaceae, the conformation of all guaianolides identified so far is different from that of guaianolides that have been identified in Asteraceae (Fig. 5). In Apiaceae the lactone ring is either 6β, 8α or 6β, 8β, whereas in Asteraceae it has only been seen as 6α, 8β. Hence, even though the difference in guaianolide structure between the two families might not appear to be critical when looking at Fig. 5a. When taking panel b and c into account it becomes obvious that the orientation of both lactone ring and residues would be dramatically altered between the two conformations. Holub and Budesinsky (1986) proposed that these features arise through different enzymatic activity in the two families that cyclizes the trans,trans-farnesyl diphosphate in different conformations. Azarken et al. (2008) recently presented an alternative route based on the observation that (E,E)-germacradienes adopt several conformations of which UU, UD, DU and DD are the most stable ones. U (up) and D (down) refers to the orientation of the C-14 and C-15 methyl groups. Thus, it was hypothesized that it is possible to transform a germacrane in the dominant UU-conformation (as observed in Asteraceae) into a DD-conformation germacrane (as observed in Apiaceae) by adding a β-hydroxyl group at carbon C-6 (Fig. 5c, route b). This would lead to steric hindrance in the UU-conformation and induce the flipping to a DD-conformation. The confirmation of either of these hypotheses would explain the biogenetic origin of guaianolides (Holub and Budesinsky 1986; Azarken et al. 2008).
The difference of the conformations of guaianolides in Apiaceae and Asteraceae. a The guaianolide conformations showing different α/β positions between the two families. b A possible explanation for the difference–two alternative cyclization reactions from FPP to guaianolide (Holub and Budesinsky 1986). c Alternative explanation to the biosynthesis of guaianolides from Asteraceae and Apiaceae plants (Azarkan et al. 2008)
Proposed hypothetical biosynthesis of guaianolides in Apiaceae
No studies have yet been able to elucidate the biosynthetic pathway of guaianolides, including thapsigargin (1). Given its promising results against prostate cancer (Christensen et al. 1999), alternative production of the drug, thapsigargin, would be preferable, both to limit costs and also to ensure a steady supply of highly purified compound. The introduction of a plant derived pathway into another organism, however, might prove difficult. Based on the literature described above, a pathway for generating a thapsigargin precursor has been proposed (Fig. 6). The first step, I, is mediated by a germacrene B synthase. Germacrene B has been identified in several Apiaceae species, and could explain the opposite stereochemistry from that observed in guaianolides from Asteraceae. For step II the C(8) position is easily activated for allylic oxidations due to the positioning of the C(7) double bond (De Kraker et al. 1998). This C(8) position has been observed only to occur in its α conformation. In the next proposed step, a P450 acetyltransferase adds the acyloxy moiety, a reaction well known in the large scale production of the diterpene, taxol (Chang and Keasling 2006). In step III the lactone ring is formed by an enzyme similar to the P450 that synthesizes costunolide shown in Fig. 3 (De Kraker et al. 2002). Due to the presence of the C(8) butyloxy group, the formation will only generate the 6,12-lactone ring. The epoxidation in step IV initiates the last step of the guaianolide formation, step V, in which a P450 enzyme closes the 5 + 7 guaianolide structure. This should, in contrast to the proposed reaction in chicory (De Kraker et al. 2002), preferably proceed via 1,10-epoxidation in order to retain the 4,5-double bond that is present in thapsigargin (1) and found in many guaianolides from Apiaceae. The final step also insures the conformations found in most guaianolides in Apiaceae, namely 1βH, 10αCH3, and 11βCH3. Hydroxylation of C(7) is only rarely found in guaianolides, and the 7β-hydroxy group is unique to thapsigargins. A likely explanation for the unique 7β-hydroxy group is that a precursor possessing a C(7)-C(11) double bond is converted into an epoxide that is then opened into a trans-glycol (Christensen et al. 1997). The finding of guaiol and guaianes hydroxylated at position C(11) and C(8) could suggest that the guaiane structure is formed prior to any secondary modifications (Christensen et al. 1997). It has been observed in pine that sesquiterpene cyclases also perform the secondary modifications of the germacrene skeleton via hydride shifts, methyl migrations, and Wagner-Meerwien rearrangements (Bohlmann et al. 1998). It still needs to be seen whether this is true for enzymes found in Apiaceae, but this should be taken into considerations when elucidating the biosynthesis.
Since most of the enzymes needed in this pathway are P450’s, oxygen and NADPH are likely to be crucial to the efficiency of the process (Berg et al. 2007). One should also consider that although the reactions catalyzed in Asteraceae by the proposed enzymes might seem similar to those observed in Apiaceae, differences in the stereochemistry of substrates and desired products might result in difficulties generating the pathway described. Also, reports have mentioned the fact that slight alterations in the substrate processed may alter the mechanism of the enzyme (Rontein et al. 2008). However, these studies also demonstrated that one enzyme might facilitate a specific reaction on different substrates.
Guaianolides found in apiaceae
Structure and in planta activity
The particular interest in guaianolides has emerged from the observation that these compounds often exhibit some kind of biological activity. Thapsia garganica L. had long been known to irritate the skin. The isolation and characterization of the guaianolides thapsigargin and thapsigargicin, which have been identified as histamine releasing compounds, explained this activity (Rasmussen et al. 1978).
Thapsigargins
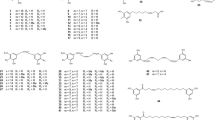
Being well described, thapsigargin and the many isolated thapsigargins (1–19) will comprise a large section of this review (Table 1). Furthermore, some of the studies carried out on thapsigargins have provided insight into the structure-activity relationship of the compounds described and also on their biogenesis. Although not strictly defined, the term thapsigargins in generally used for guaianolides that have a conformation of 1βH, 6αH, 3α, 7β, 8α, 10β, 11α-pentaoxygenated-6-12-guaianolides (Christensen et al. 1997).
Other guaianolides
Several other guaianolides have been identified in Apiaceae (20–87), and although their biological activities are not as well described, they could provide clues to the biosynthesis of guaianolides in general (Table 2). With the high degree of possible substitutions, it is plausible that the list of guaianolides in Apiaceae is much longer than the one presented here. This is supported by the observation that 19 different varieties of the specific group of thapsigargins have been detected thorough investigation following the initial identification of thapsigargin (1–19). It is most likely that the increased focus on natural products as medical drugs will result in the identification of numerous guaianolides that are presently unknown. An interesting observation was made by Serkerov (1980) that ferulidin (86) and ferulin (87) only occur in roots of Ferula oopoda that have become mouldy by storage. These results indicate that C(6)-C(7) lactone ring is the conformation formed by biosynthesis and that the C(7)-C(8) lactone ring in Apiaceae is formed as a result of moulding. By either plant breakdown enzymes or enzymes of bacterial and/or fungal origin, 49 is transformed into 87, which is then hydroxylated into 86 (Serkerov 1980). To our knowledge this observations has not been examined further by others.
Biological activity
As mentioned, thapsigargins have been systematically studied due to their interesting biological activities, leading to the identification of the importance of stereochemistry and functional groups (Christensen et al. 1997). The biological activity of guaianolides is generally accepted to be mediated by the lactone ring and its α,β- or α,β,γ-unsaturated carbonyl structures such as a α-mehtyl-γ-lactone, a α,β unsaturated cyclopentanone or a conjugated ester. These structural elements are thought to react with nucleophilic molecules by a Michael addition style reaction (Rüngeler et al. 1999).
It has been established that the biological activity of sesquiterpene lactones is often mediated by their reaction with thiol groups of cysteine residues (Rüngeler et al. 1999). The structure-activity relationships of sesquiterpene lactones have been studied in relation to cytotoxicity, anti-inflammatory activity and anti-tumor activity (Zhang et al. 2005). The DNA-fragmentation and apoptosis activity of sesquiterpene lactones is mediated by an increased level of glutathione release by cells and is related to the binding between the exo-methylene groups and thiols (Choi et al. 2002). This was also confirmed in a study of arteminolides (another group of sesquiterpenes) where structural changes in the lactone ring changed the activity of these compounds against various cancer cell lines (Lee et al. 2003). It was observed that costunolide lacking the exo-methylene group lost most of its ability to induce DNA-fragmentation and apoptosis.
Several structure-activity relationship studies have confirmed that the effect of sesquiterpene lactones on NF-κB related pathways and specific anti-cancer effects are closely related to the substitution pattern in the lactone ring, and that the α-methyl-γ-lactone and the α,β or α,β,γ unsaturated carbonyl group is essential for the activity (Beekman et al. 1997; Rüngeler et al. 1999; Siedle et al. 2004). Many specify the need for an unsaturated structure, such as a lactone ring with a methylene group present at C(11). Thapsigargins (1–19, Table 1), however, are known for their biological activities despite the fact that they do not have an unsaturated lactone ring. The reason for this discrepancy is probably that the biological activity of these compounds has been explained by their ability to react with the cysteine sulfurhydryl groups of proteins (Schall and Reiser 2008; Zhang et al. 2005). One of the main consequences of this ability is the depletion of intracellular glutathione of the targeted cell, which leads to apoptosis. Although this reaction mechanism facilitates apoptosis, it might be too toxic to actually be useful as an anti-cancer drug for use in vivo.
The anti-cancer activity of thapsigargins is mediated by a different mechanism than the one mentioned. They induce apoptosis by inhibiting the endo/sarcoplasmatic calcium ATPase (SERCA; Søhoel et al. 2006) resulting in an elevated cytoplasmic Ca2+ level, which eventually leads to the death of the cell. The crystal structure of the SERCA pump with 1 bound inside showed large conformational changes compared to the structure of SERCA with Ca2+ bound (Toyoshima and Nomura 2002), and also provided some of the clues used for later design of targeted pro-drug with thapsigargin as the active part. Much of the initial work on 1 involved changing the residues or stereochemistry at different positions (Christensen et al. 1997). These studies revealed the importance of several of the residues present in 1 (Fig. 7). An interesting discovery made from these trials was the dramatic effect of changing the stereochemistry of the butyloxy group at C(8) (Fig. 7, I). When changing this group from α to β conformation, almost all of the Ca2+-ATPase inhibiting properties of the molecule were lost, decreasing 3000-fold (Christensen et al. 1997). The reason for this dramatic effect was discovered when this residue was found to be situated in a cavity between helices when bound to the SERCA protein, in which it would not fit with the alternative configuration (Søhoel et al. 2006). Equally, the results showed that removing or simply changing the stereochemistry of the C(3)-anglelyoxy group (Fig. 7, II) resulted in a significant decrease of the Ca2+-ATPase inhibitory effect.
Replacement of the C(10)-acetoxy with a hydroxy moiety also decreased the biological activity of 1 (Fig. 7, III), as did the addition of residues to the lactone carbonyl group (Fig. 7, IV). This was also observed by dehydration of trilobolide (2) with the formation of an epoxide at C(7)-C(11), which significantly reduced the SERCA inhibition activity of 2 (Wictome et al. 1994). However, it is important to note that the decrease in biological activity of 1 was much more pronounced when changing stereochemistry of C(8) or C(3), emphasizing the importance of the conformation of the molecule and not necessarily its residues. This was further confirmed by Søhoel et al. (2006) as they showed that the moiety linked to the C(8)-O- position could actually be varied extensively, as long as the stereochemistry and flexibility of the residue was maintained (Christensen et al. 1999; Søhoel et al. 2006). These investigations laid the foundation of the subsequent design of thapsigargin analogues used for specific cell targeting.
The idea of specific cell targeting was first presented in 1996 (Denmeade et al. 1996), when it was suggested that the specific prostate cancer expression of a prostate-specific antigen (PSA) might facilitate targeting of thapsigargin. One of the remarkable features of 1 is its ability to induce apoptosis in both slowly and rapidly dividing cells (Denmeade and Isaacs 2005). This is in contrast to usual chemotherapeutic agents, which mainly target rapidly dividing cells, and are thus successful in cancer treatment. In contrast to many cancers, prostate cancer cells often proliferate slowly, and are therefore not effectively treated with traditional chemotherapy. Nevertheless, applying a drug that also facilitates the death of slowly dividing cells is not recommendable, since this would result in the death of healthy cells. Hence, targeting was essential for the possible success of a thapsigargin-based cancer pro-drug, and this is why PSA is of interest. PSA is a serine protease that cleaves at specific amino acid motifs. Therefore, it was proposed that the addition of a specific peptide to the C(8)-position, including both a flexible region and the amino acid sequence recognized by PSA, would result in a pro-drug that would be activated in the proximity of prostate cancer cells. The flexible region was determined by varying the length of the C(8)-positioned residue, and a candidate was chosen based on its preserved activity against prostate cancer cells (Jakobsen et al. 2001). This precursor was used in the development of a targeted prostate cancer drug, both by adding the specific amino acid motif, and by adding a macromolecular carrier, N-(2-hydroxypropyl) methacrylamide (HPMA), to enhance solubility and delivery of the pro-drug (Chandran et al. 2007).
Preliminary trials showed that the pro-drug itself did not have any cytotoxic activity at 10 μmol l−1. However, the same dosage was able to completely inhibit the growth of a human prostate cancer cell line. Also, when tested in vivo in nude mice with xenografts from a human prostate cancer cell line, the level of cleaved active drug was much higher in tumor tissue than in the plasma or skeletal muscle of the mice, confirming effective drug targeting (Chandran et al. 2007). A pro-drug based on thapsigargin is currently being developed by GenSpera, Inc. They hope to market their drug in 2012 (GenSpera 2009).
Further results have shown that the C(2) positioned octyloxy moiety of 1 has almost no effect on its binding affinity to the SERCA pump. This is interesting because it suggests that compounds 2 and 3 might induce apoptosis just as effectively, and also because 2 has been isolated from T. villosa as well as L. trilobum. This might provide greater insight into the biosynthesis of thapsigargins, and make for easier access to plant material (Søhoel et al. 2006).
Thapsigargins 1–11, 14, 15, 17 and 18 have all been shown to have histamine releasing abilities to varying degrees (Christensen et al. 1984a; Liu et al. 2006; Norup et al. 1986; Rasmussen et al. 1981). Thus, the structure-activity relationship of thapsigargins seems rather flexible regarding the residues positioned at C(2) and C(8); as long as the correct stereochemistry is maintained, all are expected to bind to SERCA to some extent.
Biological activity of other guaianolides in Apiaceae
Of the remaining guaianolides presented (Table 2, 20–87), few have been described with respect to their biological activities. This is primarily due to the fact that many of them were described a number of years ago, at a time when effort and focus was placed on the elucidation of their structures rather than on the possible medical effects of the compounds. Furthermore, when purifying a minor plant constituent, it is not always possible to isolate sufficient amounts of the compound to be able to conduct relevant trials.
A few of the identified compounds have, however, been tested in different assays. The ability of compounds 40, 66, 72 and 76 to inhibit SERCA has been tested, and none of them had significant activity (Rubal et al. 2006). The lack of inhibitory effect might be explained by the absence of a C(8)-O-residue, the residue most important to the SERCA-inhibitory effect of thapsigargins. Compounds 72 and 66 even have an acetoxy group at C(3), but this is obviously insufficient to establish an effect. This also suggests that several of the other compounds isolated might, in fact, have SERCA inhibiting properties, since many have an ester bound residue at C(8). However, quite a few also have substituents at C(11) that might interfere with SERCA binding because of their proximity to one of the helices comprising the binding site (Søhoel et al. 2006). Furthermore, compounds 2, 23, 36, 39, 43, and 45 were found to have activity against adult insects and larvae (Nawrot et al. 1983). As mentioned, articles related to the activity of sesquiterpene lactones tend to focus on compounds with a methylene group at C(11) on the lactone ring.
Conclusion
In conclusion, this review aims at giving an overview of the guaianolides identified in Apiaceae to date. However, a few considerations should be taken into account when assessing the structures listed. Firstly, some of the compounds were identified when both NMR and MS were in their infancy compared to today’s standards. Although the data obtained might be valid, they were obtained by procedures that might be considered inadequate today. Furthermore, several of the compounds have only been identified in a single study, and it might be preferable to gain confirmation by a separate set of data. It should also be noted that since the focus on thapsigargins activity is on their effect on the SERCA protein, other interesting biological activities might be overlooked in the screening process.
Nonetheless, the data presented here shows the diverse range of guaianolides that are generated in vivo by different Apiaceae species. Possible therapeutic compounds and attractive targets are numerous, and since relatively few have been investigated, the potential for discovering sesquiterpene lactones with pharmacological activity remains enormous. Many of the compounds exhibit interesting biological activities, raising a compelling argument that the rest should also be investigated in depth.
Abbreviations
- DMAPP:
-
Dimethylallyl diphosphate
- DXP:
-
1-Deoxy-d-xylulose-5-phosphate
- FPP:
-
Farnesyl diphosphate
- GGPP:
-
Geranylgeranyl diphosphate
- GPP:
-
Geranyl diphosphate
- HMG-CoA:
-
(S)-3-Hydroxy-3-methylglutaryl-CoA
- HPMA:
-
N-(2-hydroxypropyl) methacrylamide
- IPP:
-
Isopentenyl diphosphate
- MEP:
-
2-C-methyl-d-erythritol-4-phosphate
- MVA:
-
Mevalonate
- PSA:
-
Prostate specific antigen
- SERCA:
-
Sarco/endoplasmatic reticulum calcium ATPase
References
Ahmed S (1998) Isolation and Structural elucidation of chemical constituents from Fumaria indica, Ferula oopoda and Withania somnifera. Ph.D. Thesis, University of Karachi
Andrews SP, Ball M, Wierschem F, Cleator E, Oliver S, Hogenauer K, Simic O, Antonello A, Hunger U, Smith MD, Ley SV (2007) Total synthesis of five thapsigargins: Guaianolide natural products exhibiting sub-nanomolar SERCA inhibition. Chem Eur J 13(20):5688–5712. doi:10.1002/chem.200700302
Appendino G, Valle MG, Caniato R, Cappelletti EM (1986) Sesquiterpene Lactones from Laserpitium garganicum. Phytochem 25(7):1747–1749. doi:10.1016/S0031-9422(00)81252-2
Azarken R, Guerra FM, Moreno-Dorado FJ, Jorge ZD, Massanet GM (2008) Substituent effects in the transannular cyclizations of germacranes. Synthesis of 6-epi-costunolide and five natural steiractinolides. Tetrahedron 64:10896–10905. doi:10.1016/j.tet.2008.09.017
Barton Sir D, Nakanishi K, Meth-Cohn O (1999) Comprehensive natural products chemistry. Elsevier Science Ltd, London
Beekman AC, Woerdenbag WV, Pras N, Konings AWT, Wikström HV, Schmidt TJ (1997) Structure-cytotoxicity relationship of some helenanolide-type sesquiterpene lactones. J Nat Prod 60:252–257. doi:10.1021/np960517h
Berenbaum MR (2001) Chemical mediation of coevolution: phylogenetic evidence for apiaceae and associates. Ann Mo Bot Gard 88:45–59. doi:10.2307/2666131
Berg JM, Tymoczkom JL, Stryer L (2007) Biochemistry. W.H. Freeman and company, New York
Bertea CM, Voster A, Verstappen FWA, Maffei M, Beekwilder J, Bouwmeester HJ (2006) Isoprenoid biosynthesis in Artemisia annua: Cloning and heterologous expression of a germacrene A synthase from a glandular trichome cDNA library. Arch Biochem Biophys 448(1–2):3–12. doi:10.1016/j.abb.2006.02.026
Bick JA, Lange BM (2003) Metabolic cross talk between cytosolic and plastidial pathways of isoprenoid biosynthesis: unidirectional transport of intermediates across the chloroplast envelope membrane. Arch Biochem Biophys 415(2):146–154. doi:10.1016/S0003-9861(03)00233-9
Bohlmann J, Meyer-Gauen G, Croteau R (1998) Plant terpenoid synthases: molecular biology and phylogenetic analysis. Proc Natl Acad Sci USA 95(8):4126–4133. doi:10.1073/pnas.95.8.4126
Chandran SS, Nan A, Rosen DM, Ghandehari H, Denmeade SR (2007) A prostate-specific antigen activated N-(2-hydroxypropyl) methacrylamide copolymer prodrug as dual-targeted therapy for prostate cancer. Mol Cancer Ther 6(11):2928–2937. doi:10.1158/1535-7163.MCT-07-0392
Chang MCY, Keasling JD (2006) Production of isoprenoid pharmaceuticals by engineered microbes. Nat Chem Biol 2(12):674–681. doi:10.1038/nchembio836
Choi JH, Ha J, Park JH, Lee JY, Lee YS, Park HJ, Choi JW, Masuda Y, Nakaya K, Lee KT (2002) Costunolide triggers apoptosis in human leukemia U937 cells by depleting intracellular thiols. Jpn J Cancer Res 93:1327–1333
Christensen SB, Norup E, Rasmussen U (1984a) Chemistry and structure-activity relationship of the histamine secretagogue thapsigargin and related compounds. In: Krogsgaard-Larsen P, Christensen SB, Kofod H (eds) Natural products and drug development. Munksgaard, Copenhagen
Christensen SB, Norup E, Rasmussen U, Madsen JO (1984b) Structure of histamine releasing guaianolides from Thapsia species. Phytochem 23(8):1659–1663. doi:10.1016/S0031-9422(00)83463-9
Christensen SB, Andersen A, Smitt UW (1997) Sesquiterpenoids from Thapsia species and medicinal chemistry of the thapsigargins. Fortschr Chem Org Naturst 71:129–167
Christensen SB, Andersen A, Kromann H, Treiman M, Tombal B, Denmeade S, Isaacs JT (1999) Thapsigargin analogues for targeting programmed death of androgen-independent prostate cancer cells. Bioorg Med Chem 7(7):1273–1280. doi:10.1016/S0968-0896(99)00074-7
Christiansen AV, Paalum H, Andersen SM, Pujadas A, Smitt UW (1997) Quantitative determination of thapsigargins in roots and fruits from Thapsia gymnesica. Planta Med 63:565–567. doi:10.1055/s-2006-957769
De Kraker JW, Franssen MCR, de Groot A, Konig WA, Bouwmeester HJ (1998) (+)-Germacrene A biosynthesis—The committed step in the biosynthesis of bitter sesquiterpene lactones in chicory. Plant Physiol 117(4):1381–1392. doi:10.1104/pp.117.4.1381
De Kraker JW, Franssen MCR, Dalm MCF, de Groot A, Bouwmeester HJ (2001) Biosynthesis of germacrene A carboxylic acid in chicory roots. Demonstration of a cytochrome P450 (+)-germacrene A hydroxylase and NADP(+)-dependent sesquiterpenoid dehydrogenase(s) involved in sesquiterpene lactone biosynthesis. Plant Physiol 125(4):1930–1940. doi:10.1104/pp.125.4.1930
De Kraker JW, Franssen MCR, Joerink M, de Groot A, Bouwmeester HJ (2002) Biosynthesis of costunolide, dihydrocostunolide, and leucodin. Demonstration of cytochrome P450-catalyzed formation of the lactone ring present in sesquiterpene lactones of chicory. Plant Physiol 129(1):257–268. doi:10.1104/pp.010957
Denmeade SR, Isaacs JT (2005) The SERCA pump as a therapeutic target: making a “smart bomb” for prostate cancer. Cancer Biol Ther 4(1):14–22
Denmeade SR, Lin XS, Isaacs JT (1996) Role of programmed (apoptotic) cell death during the progression and therapy for prostate cancer. Prostate 28(4):251–265. doi:10.1002/(SICI)1097-0045(199604)28:4<251::AID-PROS6>3.0.CO;2-G
Dewick PM (1995) The Biosynthesis of C-5-C-20 Terpenoid Compounds. Nat Prod Rep 12:507–534. doi:10.1039/np9951200507
Djermanovic M, Stefanovic M, Djermanovic V, Milovanovic M (1995) Structure elucidation of the sesquiterpene lactones from plant species Laserpitium latifolium L. J Herbs Spices Med Plants 3(2):3–10. doi:10.1300/J044v03n02_02
Falsone G, Haddad H, Wendisch D (1986) Sesquiterpene lactone triesters with unusual structures from Thapsia garganica L (Umbelliferae). Arch Pharm (Weinheim) 319(4):372–379. doi:10.1002/ardp.19863190414
Fraga BM (2006) Natural sesquiterpenoids. Nat Prod Rep 23:943–972. doi:10.1039/b507870a
GenSpera (2009) Technology information, GenSpera, Inc, San Antonio, Texas, USA. http://www.genspera.com. Cited 23 Feb 2009
Ghisalberti EL (1994) The Daucane (Carotane) class of sesquiterpenes. Phytochem 37(3):597–623. doi:10.1016/S0031-9422(00)90327-3
Hampel D, Mosandl A, Wust M (2005) Biosynthesis of mono- and sesquiterpenes in carrot roots and leaves (Daucus carota L.): metabolic cross talk of cytosolic mevalonate and plastidial methylerythritol phosphate pathways. Phytochem 66(3):305–311. doi:10.1016/j.phytochem.2004.12.010
Hemmerlin A, Hoeffler JF, Meyer O, Tritsch D, Kagan IA, Grosdemange-Billiard C, Rohmer M, Bach TJ (2003) Cross-talk between the cytosolic mevalonate and the plastidial methylerythritol phosphate pathways in tobacco bright yellow-2 cells. J Biol Chem 278(29):26666–26676. doi:10.1074/jbc.M302526200
Holub M, Budesinsky M (1986) Review article number 20. Sesquiterpene lactones of the umbelliferae. Phytochem 25(9):2015–2026. doi:10.1016/0031-9422(86)80060-7
Holub M, Samek Z (1973) On Terpenes. 222. Structure of archangelolide, a sesquiterpenic lactone from Laserpitium archangelica wulf. Collect Czech Chem Commun 38(3):731–738
Holub M, Degroote R, Herout V, Sorm F (1968) Plant Substances. 28. Oxygen-containing components of light petroleum extract of Laser trilobum (L) Borkh root structure of laserine. Collect Czech Chem Commun 33(9):2911
Holub M, Herout V, Samek Z, Motl O (1972) Terpenes. 214. Structure of 2 sesquiterpenic lactones, isomontanolide and acetylisomontanolide from Laserpitium siler L. Collect Czech Chem Commun 37(4):1186
Holub M, Samek Z, Vasickova S, Masojidkova M (1978a) Terpenes. 251. 11-hydroxy-1-beta-H, 5-beta-H, 6-alpha-, 7-alpha-H-guaian-6, 12-olides—relative and absolute-configuration of sesquiterpenic lactones montanolide, isomontanolide, acetylisomontanolide and related substances. Collect Czech Chem Commun 43(9):2444–2470
Holub M, Motl O, Samek Z (1978b) Terpenes. 252. Structure and relative and absolute-configurations of sesquiterpenic lactones gradolide and polhovolide from Laserpitium Siler L. Collect Czech Chem Commun 43(9):2471–2477
Holub M, Toman J, Herout V (1987) The phylogenetic-relationships of the asteraceae and apiaceae based on phytochemical characters. Biochem Syst Ecol 15(3):321–326. doi:10.1016/0305-1978(87)90006-8
Iranshahi M, Hosseini ST, Shahverdi AR, Molazade K, Khan SS, Ahmad VU (2008) Diversolides A-G, guaianolides from the roots of Ferula diversivittata. Phytochem 69:2753–2757 Observe that retraction of claims on diversolides A, C–G are in the process of being printed
Jakobsen CM, Denmeade SR, Isaacs JT, Gady A, Olsen CE, Christensen SB (2001) Design, synthesis, and pharmacological evaluation of thapsigargin analogues for targeting apoptosis to prostatic cancer cells. J Med Chem 44(26):4696–4703. doi:10.1021/jm010985a
Kabilov MH, Saidkhodzhaev AI, Malikov VM, Melibaev S (1994) Sesquiterpene lactones of Ferula koso-poljanskyi. Chem Nat Compd 30(4):523. doi:10.1007/BF00630416
Lee SH, Lee MY, Kang HM, Han DC, Son KH, Yang DC, Sung ND, Lee CW, Kim HM, Kwon BM (2003) Anti-tumor activity of the farnesyl-protein transferase inhibitors arteminolides, isolated from artemisa. Bioorg Med Chem 11:4545–4549. doi:10.1016/j.bmc.2003.08.008
Li YS, Chen JJ, Zhou H, Luo SD, Wang HY, Zhu DY (2003) Two new guaianolides and a new daucene derivative from Sinodielsia yunnanensis. Planta Med 69(10):962–964. doi:10.1055/s-2003-45111
Liu HZ, Jensen KG, Tran LM, Chen M, Zhai L, Olsen CE, Sohoel H, Denmeade SR, Isaacs JT, Christensen SB (2006) Cytotoxic phenylpropanoids and an additional thapsigargin analogue isolated from Thapsia garganica. Phytochem 67(24):2651–2658. doi:10.1016/j.phytochem.2006.10.005
Milosavljevic S, Bulatovic V, Stefanovic M (1999) Sesquiterpene lactones from the Yugoslavian wild growing plant families asteraceae and apiaceae. J Serb Chem Soc 64(7–8):397–442
Muckensturm B, Diyani F, Reduron J-P (1995) Grilactone and other terpenoids from Anthriscus nitida. Biochem Syst Ecol 23(7/8):875–876. doi:10.1016/0305-1978(95)00058-5
Muckensturm B, Diyani F, Nouën DL, Fkih-Tetouani S, Reduron J-P (1997) Ammolactone, a guaianolide from a medicinal plant, Ammodaucus leucotrichus. Phytochem 44(5):907–910. doi:10.1016/S0031-9422(96)00621-8
Nawrot J, Smitalova Z, Holub M (1983) Terpenes. 273. Deterrent activity of sesquiterpene lactones from the umbelliferae against storage pests. Biochem Syst Ecol 11(3):243–245. doi:10.1016/0305-1978(83)90061-3
Norup E, Smitt UW, Christensen SB (1986) The potencies of Thapsigargin and analogs as activators of rat peritoneal mast-cells. Planta Med 52(4):251–255. doi:10.1055/s-2007-969144
Nurmukhamedova MR, Kasymov SZ, Melibaev S (1982) Grilactone from Ferula-Penninervis. Him Prir Soedin 2:261
Nurmukhamedova MR, Kasymov SZ, Sidyakin GP (1983) Ferolid—A new lactone from Ferula-Penninervis. Him Prir Soedin 4:533
Nurmukhamedova MR, Kasymov S, Abdullaev ND, Sidyakin GP (1985) Structure of fegolide. Him Prir Soedin 3:335–337
Okada K, Kasahara H, Yamaguchi S, Kawaide H, Kamiya Y, Nojiri H, Yamane H (2008) Genetic evidence for the role of isopentenyl diphosphate isomerases in the mevalonate pathway and plant development in arabidopsis. Plant Cell Physiol 49(4):604–616. doi:10.1093/pcp/pcn032
Piet DP, Schrijvers R, Franssen MCR, de Groot A (1995) Biotransformation of Germacrane epoxides by Cichorium intybus. Tetrahedron 51(22):6303–6314. doi:10.1016/0040-4020(95)00272-A
Pinar M, Rico M, Rodriguez B (1982) Desangeloylshairidin, a sesquiterpene lactone from Guillonea scabra. Phytochem 21:1802–1804
Pinar M, Rodriguez B, Rico M, Perales A, Fayos J (1983) Guillonein, An epoxyguaianolide from Guillonea scabra, X-Ray structure determination. Phytochem 22:987–990. doi:10.1016/0031-9422(83)85037-7
Rasmussen U, Christensen SB, Sandberg F (1978) Thapsigargine and thapsigargicine, two new histamine liberators from Thapsia garganica L. Acta Pharm Suec 15(2):133–140
Rasmussen U, Christensen SB, Sandberg F (1981) Phytochemistry of the genus Thapsia. Planta Med 43:336–341. doi:10.1055/s-2007-971521
Ro DK, Paradise EM, Ouellet M, Fisher KJ, Newman KL, Ndungu JM, Ho KA, Eachus RA, Ham TS, Kirby J, Chang MCY, Withers ST, Shiba Y, Sarpong R, Keasling JD (2006) Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 440(7086):940–943. doi:10.1038/nature04640
Rohdich F, Hecht S, Gartner K, Adam P, Krieger C, Amslinger S, Arigoni D, Bacher A, Eisenreich W (2002) Studies on the nonmevalonate terpene biosynthetic pathway: metabolic role of IspH (LytB) protein. Proc Natl Acad Sci USA 99(3):1158–1163. doi:10.1073/pnas.032658999
Rohmer M, Knani M, Simonin P, Sutter B, Sahm H (1993) Isoprenoid biosynthesis in bacteria: a novel pathway for the early steps leading to isopentenyl diphosphate. Biochem J 295:517–524
Rontein D, Onillon S, Herbette G, Lesot A, Werck-Reichhart D, Sallaud C, Tissier A (2008) CYP725A4 from yew catalyzes complex structural rearrangement of taxa-4(5), 11(12)-diene into the cyclic ether 5(12)-oxa-3(11)-cyclotaxane. J Biol Chem 283(10):6067–6075. doi:10.1074/jbc.M708950200
Rubal JJ, Guerra FM, Moreno-Dorado FJ, Jorge ZD, Massanet GM, Sohoel H, Smitt UW, Frydenvang K, Christensen SB, Nielsen C, Erikson M (2006) Sesquiterpenes from Thapsia nitida var. meridionalis and Thapsia nitida var. nitida. J Nat Prod 69(11):1566–1571. doi:10.1021/np0603065
Rubal JJ, Guerra FM, Moreno-Dorado FJ, Akssira M, Mellouki F, Pujadas AJ, Jorge ZD, Massanet GM (2007a) Sulfur-containing sesquiterpenes from Thapsia villosa. Tetrahedron 60:159–164. doi:10.1016/j.tet.2003.10.079
Rubal JJ, Moreno-Dorado FJ, Guerra FM, Jorge ZD, Saouf A, Akssira M, Mellouki F, Romero-Garrido R, Massanet GM (2007b) A pyran-2-one and four meroterpenoids from Thapsia transtagana and their implication in the biosynthesis of transtaganolides. Phytochem 68:2480–2486. doi:10.1016/j.phytochem.2007.06.023
Rüngeler P, Castro V, Mora G, Gören N, Vichnewski W, Pahl HL, Merfort I, Schmidt TJ (1999) Inhibition of transcription factor NF-κB by sesquiterpene lactones: a proposed molecular mechanism of action. Bioorg Med Chem 7:2343–2352. doi:10.1016/S0968-0896(99)00195-9
Rychlewska U, Hodgson DJ, Holub M, Budesinsky M, Smitalova Z (1985) On Terpenes 288 The Structure of 2-Oxo-8-alpha-angeloyloxy-11-alpha-acetoxy-5-beta-H,6-alpha-H,7-alpha-H-guai-1(10),3-dien-6,12-olide, a sesquiterpene lactone from Laserpitium prutenicum L. Revision of the stereostructures of native 2-oxoguai-1(10),3-dien-6,12-olides from the species of the Umbelliferae family. Collect Czech Chem Commun 50(11):2607–2624
Sagitdinova GV, Saidkhodzhaev AI, Malikov VM, Pimenov MG, Melibeav S (1990) Sesquiterpene lactones of Ferula clematidifolia and Ligularia alpigena. Him Prir Soedin 4:553–555
Schall A, Reiser O (2008) Synthesis of biologically active guaianolides with a trans-annulated lactone moiety. Eur J Org Chem 2353–2364. doi:10.1002/ejoc.200700880
Serkerov SV (1980) Stereochemistry of Guaianolides of Ferula oopoda. Him Prir Soedin 5:629–633
Serkerov SV, Rikhlevska U, Aleskerova AN, Mirbabaev NF (1991) New guanolide—Opofersin from Ferula Oopoda roots. Him Prir Soedin 3:318–319
Siedle B, García-Piñeres AJ, Murillo R, Schulte-Mönting J, Castro V, Rüngeler P, Klaas CA, Da Costa FB, Kisiel W, Merfort I (2004) Quantitative structure-activity relationship of sesquiterpene lactones as inhibitors of the transcription factor NF-κB. J Med Chem 47:6042–6054. doi:10.1021/jm049937r
Smitalova Z, Budesinsky M, Saman D, Vasickova S, Holub M (1984) On Terpenes. 279. Components of the extract from the underground parts of Laserpitium siler L of Slovenian origin, mainly sesquiterpenic lactones. Collect Czech Chem Commun 49(4):852–870
Smitalova Z, Budesinsky M, Saman D, Holub M (1986) On Terpenes. 291. Minor sesquiterpenic lactones of Laser trilobum (L) Borkh species. Collect Czech Chem Commun 51(6):1323–1339
Smitt UW, Cornett C, Andersen A, Christensen SB, Avato P (1990) New proazulene guaianolides from Thapsia villosa. J Nat Prod 53(6):1479–1484. doi:10.1021/np50072a012
Smitt UW, Jager AK, Adsersen A, Gudiksen L (1995) Comparative studies in phytochemistry and fruit anatomy of Thapsia garganica and T. transtagana, Apiaceae (Umbelliferae). Bot J Linn Soc 117(4):281–292. doi:10.1006/bojl.1995.0019
Søhoel H, Jensen AM, Moller JV, Nissen P, Denmeade SR, Isaacs JT, Olsen CE, Christensen SB (2006) Natural products as starting materials for development of second-generation SERCA inhibitors targeted towards prostate cancer cells. Bioorg Med Chem 14(8):2810–2815. doi:10.1016/j.bmc.2005.12.001
Song Q, Gomezbarrios ML, Hopper EL, Hjortso MA, Fischer NH (1995) Biosynthetic-studies of lactucin derivatives in hairy root cultures of Lactuca floridana. Phytochem 40(6):1659–1665. doi:10.1016/0031-9422(95)00478-P
Steele CL, Crock J, Bohlmann J, Croteau R (1998) Sesquiterpene synthases from grand fir (Abies grandis)—Comparison of constitutive and wound-induced activities, and cDNA isolation, characterization and bacterial expression of delta-selinene synthase and gamma-humulene synthase. J Biol Chem 273(4):2078–2089. doi:10.1074/jbc.273.4.2078
Suzuki K, Okasaka M, Kashiwada Y, Takaishi Y, Honda G, Ito M, Takeda Y, Kodzhimatov OK, Ashurmetov O, Sekiya M, Ikeshiro Y (2007) Sesquiterpene lactones from the roots of Ferula varia and their cytotoxic activity. J Nat Prod 70(12):1915–1918. doi:10.1021/np0703996
Teoh KH, Polichuk DR, Reed DW, Nowak G, Covello PS (2006) Artemisia annua L (Asteraceae) trichome-specific cDNAs reveal CYP71AV1, a cytochrome P450 with a key role in the biosynthesis of the antimalarial sesquiterpene lactone artemisinin. FEBS Lett 580(5):1411–1416. doi:10.1016/j.febslet.2006.01.065
Toyoshima C, Nomura H (2002) Structural changes in the calcium pump accompanying the dissociation of calcium. Nature 418(6898):605–611. doi:10.1038/nature00944
Wang NH, Taniguchi M, Tsuji D, Doi M, Ohishi H, Yoza K, Baba K (2003) Four guaianolides from Sinodielsia yunnanensis. Chem Pharm Bull (Tokyo) 51(1):68–70. doi:10.1248/cpb.51.68
Wictome M, Holub M, East JM, Lee AG (1994) The importance of the hydroxyl moieties for inhibition of Ca2+-ATPases by trilobolide and 2, 5-di(tert-butyl)-1, 4-benzohydroquinone. Biochem Biophys Res Commun 199(2):916–921. doi:10.1006/bbrc.1994.1316
Withers ST, Keasling JD (2007) Biosynthesis and engineering of isoprenoid small molecules. Appl Microbiol Biotechnol 73(5):980–990. doi:10.1007/s00253-006-0593-1
Zhang S, Won YK, Ong CN, Shen HM (2005) Anti-cancer potential of sesquiterpene lactones: bioactivity and molecular mechanisms. Curr Med Chem Anticancer Agents 5(3):239–249. doi:10.2174/1568011053765976
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Drew, D.P., Krichau, N., Reichwald, K. et al. Guaianolides in apiaceae: perspectives on pharmacology and biosynthesis. Phytochem Rev 8, 581–599 (2009). https://doi.org/10.1007/s11101-009-9130-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11101-009-9130-z