Abstract
Background
Augmented renal clearance is increasingly recognized in critically ill patients. This condition may lead to suboptimal dosing of renally excreted medications.
Aim
Our primary objective was to identify demographic and clinical factors associated with augmented renal clearance in a mixed critically ill population.
Method
This retrospective single center observational cohort study evaluated patients admitted in a mixed adult intensive care unit for augmented renal clearance, defined as a creatinine clearance of ≥ 130 ml/min/1.73m2, through weekly 24-h urine collection. Variables associated with augmented renal clearance were identified using univariate analysis, then served as covariates in a backward stepwise logistic regression. Goodness-of-fit of the model was assessed and receiver operating characteristic curve was generated.
Results
Augmented renal clearance was observed in 25.3% of the study cohort (n = 324). Age below 50 years (adjusted odds ratio 7.32; 95% CI 4.03–13.29, p < 0.001), lower serum creatinine at intensive care admission (adjusted odds ratio 0.97; 95% CI 0.96–0.99, p < 0.001) and trauma admission (adjusted odds ratio 2.26; 95% CI 1.12–4.54, p = 0.022) were identified as independent risk factors. Our model showed acceptable discrimination in predicting augmented renal clearance (Area under receiver operating characteristic curve (0.810; 95% CI 0.756–0.864, p < 0.001)).
Conclusion
We identified age below 50 years, lower serum creatinine upon intensive care admission and trauma as independent risk factors for augmented renal clearance, consistent with the literature suggesting that patients with low serum creatinine upon admission could have a higher risk of developing augmented renal clearance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Impact statements
-
Clinical pharmacists should recognize patients affected by augmented renal clearance to ensure adequate dosing of affected drugs. Empiric dosing adjustment may be required for renally cleared drugs to prevent suboptimal dosing.
-
Our study consolidated previous findings of risk factors for augmented renal clearance and added precision in terms of the timing of low serum creatinine as an independent risk factor. The results allow the application of risk factors in a broader ICU population (medical, surgical and trauma) and help clinical pharmacists to detect more rapidly potential at-risk patients admitted in a critically ill condition.
-
At present, there is limited data for dosing adjustment in augmented renal clearance. Pharmacists can contribute to optimal patient care with their knowledge of pharmacokinetic changes in augmented renal clearance.
-
Pharmacists should not rely on commonly used creatinine clearance estimations based on mathematical formulas to assess patients for augmented renal clearance as they tend to under-estimate renal function. A timed-measured creatinine clearance with urine collection has a better accuracy to screen for augmented renal clearance.
Introduction
Augmented renal clearance (ARC) has become increasingly recognized in critically ill patients. ARC is a physiological condition generally defined by creatinine clearance (CrCl) ≥ 130 mL/min/1.73m2 [1,2,3,4,5,6,7,8,9,10,11,12,13]. Although not fully understood, the mechanism underlying ARC likely involves the systemic inflammatory response syndrome during which inflammatory markers released in critical illness cause various physiological changes leading to development of ARC. These changes include increased cardiac output, systemic vasodilation, increased capillary permeability and renal blood flow [2, 14,15,16,17,18,19].
The prevalence of ARC in general intensive care unit (ICU) patients varies from 20 to 65% [1, 3,4,5, 7, 9, 11,12,13,14, 20,21,22,23,24,25,26]. Some data suggest it can reach 85% in patients with traumatic brain injury (TBI) and up to 100% in those with subarachnoid hemorrhage [27, 28]. ARC develops early on following ICU admission, generally within the first week, but may occur later [2, 3, 19, 20]. Its duration remains uncertain from lasting one day to persisting for 2–3 weeks [4, 7, 12, 21].
Several independent risk factors of ARC have been identified, which include younger age (≤ 50 years), male sex, trauma or polytrauma and lower severity of illness [1, 2, 6, 9, 11, 13, 21, 22, 29]. Other risk factors such as ethnicity, duration of mechanical ventilation, protein intake and use of diuretics or vasopressors are reported but their association with ARC is not well-established [3, 7, 20, 25,26,27, 30].
Presence of ARC may result in lowered serum concentrations of renally cleared medications, increasing the risk of treatment failure and development of antibiotic resistance [21, 31,32,33]. The role of clinical pharmacists is crucial to ensure optimal drug dosing in the critically ill population presenting ARC. Dosing adjustments should take into consideration drug characteristics, and pharmacokinetic alterations in ARC. In addition, close renal function and therapeutic drug monitoring, if available, are often necessary for adequate adjustments.
Aim
This retrospective cohort study aims to identify demographic and clinical factors associated with ARC in critically ill patients to better predict at-risk patients and document renally excreted medications likely impacted by this condition.
Ethics approval
The study obtained approval from the McGill University Health Center (MUHC) Research ethics board (Reference # 2021-7455).
Method
Study design and setting
This retrospective single center observational cohort study was undertaken in a mixed (medical, surgical and trauma) adult ICU of the Montreal General Hospital (MGH) affiliated to MUHC. Patient distribution over a 5-year period from 2016 to 2020 based on reasons of admission was medical (38%), surgical (27%) and trauma (35%). Patient consent was waived given its retrospective design.
Participants
Patients aged 18 years or older, admitted to ICU between January 1st, 2016 to December 31st, 2020 with a 24-h urine collection were eligible for this study. Patients were excluded if they had a baseline serum creatinine (SrCr) ≥ 120 μmol/L upon ICU admission. At the MGH, a 24-h once weekly urine collection is requested from the nutrition service for patients receiving enteral or parenteral nutrition and admitted for at least 3 days in the ICU. Collections are typically performed from Sunday 7:00 a.m. to Monday 7:00 a.m.
Procedures
All data were obtained from medical records. Demographics including age, sex, anthropometric measurements, comorbidities, reason for admission, ICU length of stay (LOS), duration of mechanical ventilation, number of days in the ICU at the time of 24-h urine collection, SrCr upon ICU admission, presence of co-treatments 24-h before and during urine collection period, renally excreted medications administered during urine collection, Acute Physiologic Assessment and Chronic Health Evaluation II (APACHE II) and Sequential Organ Failure Assessment (SOFA) scores were collected. Injury Severity Score (ISS) for patients admitted for trauma was obtained from our trauma registry. If the patient was readmitted in the ICU within the same hospitalization, only the first admission was considered. In case of multiple hospitalizations within the study time frame, we proceeded as follows: (1) If a 24-h urine sample was performed at each admission, data from the most recent admission was used. (2) If the patient had only one urine collection among the several admissions, the admission with the collection was recorded. (3) If the patient had no urine collection at each admission, the most recent episode was considered. In addition, age, sex, ICU LOS, duration of mechanical ventilation, APACHE II score and reason for admission were also collected for all excluded patients admitted within the study time frame. All anonymized data were entered into the REDCap® software [34, 35].
Presence of ARC was defined as CrCl ≥ 130 mL/min/1.73m2 and was calculated based on 24-h urine collection with the following formula:
CrCl = 24-h urine creatinine clearance (ml/s), converted in mL/min and adjusted for body surface area (BSA) using the Dubois and Dubois formula.
Quantitative determination of urine creatinine was performed by the local clinical laboratory using the Olympus AU5800 analyzer (Beckam Coulter Inc, Brea, CA). The Enzymatic Creatinine method was performed and the urine calibrator creatinine value is traceable to Isotope Dilution Mass Spectroscopy method via National Institute of Standards and Technology Standard Reference Material 967.
Missing data
Mean values for weight, height and SOFA score were calculated for all patients with urine collection. Single mean imputation was applied to missing data in order to determine the patient’s ARC status. This method was deemed acceptable and unlikely to lead to biased estimates as the percentage of missing data was less than 5% [36].
Study size
A convenience sample was used for this study. It was estimated that weekly, 4 patients out of 24 available beds in MGH ICU would have a 24-h urine collection, corresponding to around 200 patients per year, which translates into 900–1000 patients over a 5-year period. Assuming a conservative prevalence of 20% of ARC stated in the literature, we estimated that around 150–200 patients would present ARC.
Statistical methods
Continuous data were expressed as means with standard deviation (SD) or as medians with interquartile range (IQR) for non-normally distributed variables and categorical data as frequencies (%). All measured variables were compared between patients with and without ARC. Comparison of normally distributed continuous data was performed using Student t test. Mann–Whitney U test was used for skewed data. Categorical data was compared using Pearson’s chi-squared test or Fisher’s exact test, where appropriate.
Based on current literature, age, sex, reason for admission and SOFA score were directly included in the logistic regression model as independent risk factors. A comorbidity score was computed for each patient by assigning a value of 0.25 to each of the four comorbidities assessed. Univariate analysis was used to identify other variables potentially associated with the development of ARC. Variables with a p-value < 0.10 in the univariate analysis served as covariates in the logistic regression analysis using a backward stepwise selection to identify independent risk factors of ARC. Correlation tables were used to assess collinearity between variables. Goodness-of-fit of the model was assessed using the Hosmer–Lemeshow test. A receiver operating characteristic (ROC) curve was generated to examine the accuracy of the model to distinguish between patients with and without ARC. All data analysis were performed using IBM SPSS Statistics version 27 (IBM Corporation, Chicago, IL).
Results
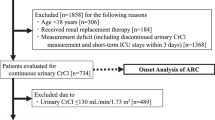
A total of 2670 patients admitted to the MGH ICU were retrospectively screened for eligibility and 324 patients met inclusion criteria. The remaining 2346 patients were excluded and reasons for exclusion included lack of urine collection during the ICU stay (n = 2241), a SrCr ≥ 120 umol/L (n = 103) and missing urine collection results (n = 2) (Fig. 1).
Baseline characteristics of included and excluded patients are shown in Supplemental material 1. Significant differences were observed between included and excluded patients for median ICU LOS and median duration of mechanical ventilation (16 vs. 3 days and 11 vs. 2 days, respectively) despite similar severity of illness as expressed by the APACHE II score (19.7 ± 6.7 points vs. 18.5 ± 8.3 points). More patients were admitted for trauma in the included group (52.8% vs. 32.3%).
Demographic and clinical data of ARC and non-ARC patients are presented in Table 1. Patients with ARC were younger (42.5 ± 13.9 years old vs. 60.9 ± 18.1 years old; p < 0.001), had a lower mean SrCr (69.9 ± 19.7 umol/L vs. 78.1 ± 20.9 umol/L, p = 0.002), a significantly lower SOFA score (7.3 ± 2.5 points vs. 8.1 ± 2.9 points; p = 0.028) and a lower APACHE II score (16.9 ± 6.3 points vs. 20.7 ± 6.5 points; p < 0.001). The number of days in ICU at the time of 24-h urine collection was comparable between groups with an overall mean of 7.4 ± 5.8 days. Mean adjusted CrCl was 161.5 ± 37.8 ml/min/1.73m2 in the ARC group versus 78.7 ± 29.4 ml/min/1.73m2 in the group without ARC (p < 0.001). Median ICU LOS was also significantly shorter for patients presenting ARC (14 vs. 17 days; p = 0.05). With respect to presence of comorbidities (hypertension, diabetes, coronary artery disease and active cancer), patients with ARC had less comorbidities, shown by a significantly lower comorbidity score (0.09 ± 0.17 points vs. 0.21 ± 0.24 points, p < 0.001). Most patients with ARC were admitted for trauma (70.7%) and appeared to have received less co-treatments in the 24 h prior and during urine collection than patients without ARC.
Weight and height data were missing for 1 and 8 patients, respectively. Additionally, SOFA score could not be calculated (n = 6) because of missing data. Information on co-treatments and renally excreted medication were not available for 1 patient.
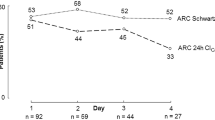
ARC was identified in 25.3% (n = 82) of the cohort. As shown in Fig. 2, most patients presented ARC within the first 10 days following ICU admission. Only one patient with ARC had a first 24-h urine collection performed 36 days after ICU admission, which was a 76-year-old patient with an active cancer with a SrCr of 52 umol/L and a CrCl of 169.03 ml/min/1.73m2.
Age, sex, reason for admission and SOFA score were included in the logistic regression model as defined a priori. Other variables identified with a p-value < 0.10 in univariate analysis included height, ICU LOS, APACHE II score, SrCr upon admission, hypertension, coronary artery disease, active cancer, use of vasopressors and use of diuretics 24-h before urine collection. Height was not included in the model considering the difference between groups is unlikely to be clinically significant. ICU LOS was excluded because of the lack of value as a predictive risk factor. APACHE II score was not included in the multivariable analysis due to collinearity with SOFA score, an approach also used by Udy et al. [22]. Age was dichotomized because it was found to be non-linear with the linearity of the logit. A cut-off of 50 years old was chosen based on visual inspection of a box plot of ARC status versus age (Supplemental material 2). As performed by Barletta et al. comorbidities were inputted in the model as a single continuous variable defined by the comorbidity score [9].
Eight variables were entered into a backward stepwise regression model: age < 50 years old, sex, SrCr upon ICU admission, SOFA score, reason for admission, comorbidity score, use of vasopressors and diuretics 24-h prior urine collection. Multivariate logistic regression analysis identified risk factors for ARC, namely age < 50 years old (Adjusted Odds Ratio (AOR) 7.315; 95% CI 4.028–13.286, p < 0.001), lower SrCr upon ICU admission (AOR 0.970; 95% CI 0.955–0.985, p < 0.001) and admission for trauma (AOR 2.257; 95% CI 1.122–4.540, p = 0.022). Although included in the model, male sex (AOR 1.946; 95% CI 0.90–3.945, p = 0.065) and admission for surgery (AOR 0.644; 95% CI 0.226–1.833, p = 0.410) were not significantly associated with ARC (Table 2). The Hosmer–Lemeshow test was non-significant (χ2(8) = 9.536, p = 0.299), suggesting an acceptable fit between the regression model and observed data. Likewise, the area under the ROC curve (AUC 0.810; 95% CI 0.756–0.864, p < 0.001) also suggested that the model was able to discriminate well patients with ARC from those without (Fig. 3).
Data on renally excreted medications administered to patients are provided in Table 3. The three most common medications given to patients with ARC were dalteparin, piperacillin-tazobactam and meropenem (75.3%, 42.0% and 29.6% respectively).
Discussion
In our study, age < 50 years old, trauma and lower SrCr at ICU admission were identified as independent risk factors for ARC, consistent with current literature using multivariate analysis. Age has been consistently identified as a risk factor of ARC among studies [6, 8, 9, 21, 22, 25, 26, 37, 38]. In addition, trauma is also strongly associated with ARC. Recently, Dickerson et al. found that in ICU patients receiving enteral or parenteral nutrition, severe TBI was a predictor of ARC [26]. Minville et al. reported that polytrauma (odds ratio (OR) 3.33; 95% CI 1.8–6, p = 0.0001) was independently correlated to a CrCl ≥ 120 ml/min/1.73m2 when compared to a medical/surgical population [37]. In contrast to these studies, our univariate results did not show a statistically significant difference between trauma subgroups (i.e. isolated TBI, polytrauma and polytrauma with TBI) and therefore subcategories were not included in our regression model. In a larger (n = 442) mixed ICU population (i.e. trauma, medical and surgical patients), Baptista et al. identified trauma as an independent risk factor (AOR of 2.0; 95% CI 1.1–3.7, p < 0.05) [6]. Udy et al. showed that trauma was a predictor of ARC in a population of septic and traumatized critically ill patients (AOR 28.6; 95% CI: 4.4–187.2). The high OR reported is likely explained by the specificity of their included population [22].
Low SrCr has previously been identified as a significant risk factor for ARC [9, 26]. Although the specific pathophysiological mechanism is not well understood, ARC has been associated with systemic inflammatory process resulting in an increase of renal blood flow, that positively impacts glomerular filtration rate (GFR), and the activation of renal function reserve [1, 26, 39, 40]. As for creatinine, it is a waste product derived from creatine that is mostly cleared through glomerular filtration but also tubular secretion and is commonly used as a biomarker to estimate renal function. This suggests that GFR could be inversely associated to SrCr levels as there is a more rapid excretion by glomerular filtration. Thus, low SrCr could be correlated with ARC [26, 39, 40]. Barletta et al. observed that patients with a SrCr ≤ 62 μmol/L were at higher risk of developing ARC (OR 12.3; 95% CI 2.9–52.1, p = 0.001) [9]. In both studies, the population was limited to trauma patients and the timing of the measured SrCr was not well-defined. We found a significant correlation between SrCr upon ICU admission and ARC in our mixed ICU cohort. This observation is most likely driven by the higher proportion of trauma patients included in our study (71%). This finding adds further evidence that low SrCr upon ICU admission could be a predictor of ARC in a broader range of critically ill patients.
Although several studies identified male sex as an independent risk factor for ARC, we did not find a significant association between these two variables in our model, potentially due to our small sample size (n = 82) [6, 9, 21, 26].
ARC was identified in 25.3% of included patients which is within the lower range of prevalence of ARC reported [5,6,7, 12, 13, 22, 24, 26, 28, 41]. Most developed within 10 days of ICU admission, consistent with published data. One patient presented ARC on day 36 of ICU admission. He was admitted for pneumosepsis and had lung cancer with liver and bone metastases accompanied by severe weight loss. We hypothesize that the underlying hypermetabolism and inflammatory process may have contributed to persistent or late-onset ARC but cannot determine if it was a persistent phenomenon as only one urine collection was recorded. Besides, risk factors for sustained or late-onset ARC remain largely unknown. Nazer et al. reported an incidence of ARC ranging from 15.6 to 24% in critically ill patients with cancer over the first 5 days of ICU admission [38]. Recent studies have included the number of days since ICU admission as a predictive factor for ARC given the likelihood of its presence shortly after admission [13, 41]. However, this variable was not included in our model because 24-h weekly urine collection did not allow us to fully capture the time course of ARC. Including this time-related variable would mislead result interpretation and inaccurately suggest later onset of ARC.
Comparison between included and excluded patients revealed that included patients were mainly admitted for trauma, had a longer median ICU LOS and duration of mechanical ventilation (Supplemental material 1). It can be noted that 95.5% of subjects were excluded because of unavailable urine collection, which is explained predominantly by the short ICU LOS shown (Fig. 1). This once-weekly frequency selects patients with longer ICU stay and our results may not be generalizable to patients with lower illness severity and shorter ICU stay. Yet, our study aimed to detect ARC using a more precise tool of CrCl measurement and such differences were expected between included and excluded patients. Thus p-values were not calculated to demonstrate between-group differences.
Lastly, in patients displaying ARC, dalteparin, piperacillin-tazobactam and meropenem were the most frequently administered medications. Reports of lower serum concentrations support the importance of close monitoring of renal function and therapeutic drug monitoring to optimize antimicrobial and antithrombotic therapy in patients exhibiting ARC [5, 10, 11, 21, 23, 25, 31]. Clinical pharmacists play a significant role in assessing the appropriateness of pharmacotherapy in this population. With conventional dosing, enhanced renal clearance patients are more susceptible to experience subtherapeutic medication dosing and therapeutic failure, which highly correlates with clinical outcomes. They should be familiar with the pharmacological properties (e.g. hydrophilicity, molecular size, elimination routes, etc.) of commonly used renally cleared molecules in their respective center in order to estimate the impact of ARC on drug dosing. For these drugs, a shorter half-life, a lower peak serum concentration (Cmax) and smaller AUC are expected [1]. Pharmacists should conduct a risk assessment for ARC using risk factors to rapidly detect at-risk patients and should optimize dosing empirically based on clinical presentation. For those at risk, Hefny et al. recommended at least one measured CrCl on admission using urine collection for further evaluation as we know that mathematical estimations of CrCl may lose accuracy in ARC [42].
This study has several limitations. First, due to its retrospective design, some variables could not be studied (e.g. ethnicity, cardiac index). Information bias is possible because some variables required clinical interpretation (e.g. reason for admission). Our method can also lead to a potential selection bias as more severely ill patients with a longer stay in the ICU were selected (Supplemental material 1) due to the lack of systematic urine collection for every admitted patient. Urine collections are conducted on those in a more critical condition requiring longer ICU length of stay as the test is ordered for nutritional support consults, suggesting a prioritization of the procedure by illness severity. Therefore, it may be less likely for patients with favorable clinical outcomes and short ICU stay to get a urine collection. Although prospectively gathered data is generally associated with fewer bias, both retrospective and prospective studies provide consistent and complementary results [3, 5,6,7,8,9, 11, 12, 21, 24, 25, 27, 31, 43]. Second, being a single-center study, our results may not apply to other settings. Third, convenience sampling using weekly urine collection, and not daily, may under-represent ARC patients and dilute our results. Our sampling method can be explained by the unavailability of 24 h urine collection for all patients, but we chose the preferred method for measuring CrCl given its clinical feasibility and greater accuracy over CrCl estimations with SrCr [14, 18, 42]. It also prevented assessment of onset and duration of ARC. Although recently, Dickerson et al. found that African American ethnicity and protein intake were significantly associated with ARC, neither were assessed in our study [26]. Further investigations should aim to identify risk factors for ARC in older patients as they are not well-elucidated.
Conclusion
In our mixed ICU cohort with a weekly 24-h urine collection, 25.3% of patients developed ARC within 10 days of ICU admission. Age < 50 years old, lower SrCr upon ICU admission and trauma were identified as independent risk factors for the development of ARC. To our knowledge, this is the first study identifying SrCr upon admission as an independent risk factor for ARC in a mixed critically ill population. Antimicrobials and antithrombotics are likely affected by ARC and require therapeutic drug monitoring.
References
Bilbao-Meseguer I, Rodriguez-Gascon A, Barrasa H, et al. Augmented renal clearance in critically Ill patients: a systematic review. Clin Pharmacokinet. 2018;57(9):1107–21.
Mahmoud SH, Shen C. Augmented renal clearance in critical illness: an important consideration in drug dosing. Pharmaceutics. 2017;9(3):36.
Udy AA, Baptista JP, Lim NL, et al. Augmented renal clearance in the ICU: results of a Multicenter observational study of renal function in critically ill patients with normal plasma Creatinine concentrations. Crit Care Med. 2014;42(3):520–7.
Huttner A, Von Dach E, Renzoni A, et al. Augmented renal clearance, low beta-lactam concentrations and clinical outcomes in the critically ill: an observational prospective cohort study. Int J Antimicrob Agents. 2015;45(4):385–92.
Baptista JP, Roberts JA, Sousa E, et al. Decreasing the time to achieve therapeutic vancomycin concentrations in critically ill patients: developing and testing of a dosing nomogram. Crit Care. 2014;18(1):654.
Baptista JP, Martins PJ, Marques M, et al. Prevalence and risk factors for augmented renal clearance in a population of critically Ill patients. J Intensive Care Med. 2020;35(10):1044–52.
De Waele JJ, Dumoulin A, Janssen A, et al. Epidemiology of augmented renal clearance in mixed ICU patients. Minerva Anestesiol. 2015;81(10):1079–85.
Kawano Y, Maruyama J, Hokama R, et al. Outcomes in patients with infections and augmented renal clearance: a multicenter retrospective study. PLoS ONE. 2018;13(12):e0208742.
Barletta JF, Mangram AJ, Byrne M, et al. Identifying augmented renal clearance in trauma patients: validation of the Augmented Renal Clearance in Trauma Intensive Care scoring system. J Trauma Acute Care Surg. 2017;82(4):665–71.
Udy AA, Varghese JM, Altukroni M, et al. Subtherapeutic initial beta-lactam concentrations in select critically ill patients: association between augmented renal clearance and low trough drug concentrations. Chest. 2012;142(1):30–9.
Baptista JP, Sousa E, Martins PJ, et al. Augmented renal clearance in septic patients and implications for vancomycin optimisation. Int J Antimicrob Agents. 2012;39(5):420–3.
Tomasa-Irriguible TM, Sabater-Riera J, Perez-Carrasco M, et al. Augmented renal clearance. An unnoticed relevant event. Sci Prog. 2021;104(2):368504211018580.
Johnston BW, Perry D, Habgood M, et al. Augmented renal clearance: a retrospective, cohort study of urinary creatinine clearance in critically ill patients in the United Kingdom. J Int Med Res. 2021;49(5):3000605211015573.
Hobbs ALV, Shea KM, Roberts KM, et al. Implications of augmented renal clearance on drug dosing in critically ill patients: a focus on antibiotics. Pharmacotherapy. 2015;35(11):1063–75.
Atkinson AJ. Augmented renal clearance. Transl Clin Pharmacol. 2018;26(3):111–4.
Sime FB, Udy AA, Roberts JA. Augmented renal clearance in critically ill patients: etiology, definition and implications for beta-lactam dose optimization. Curr Opin Pharmacol. 2015;24:1–6.
Udy AA, Putt MT, Boots RJ, et al. ARC–augmented renal clearance. Curr Pharm Biotechnol. 2011;12(12):2020–9.
Cook AM, Hatton-Kolpek J. Augmented renal clearance. Pharmacotherapy. 2019;39(3):346–54.
Udy AA, Roberts JA, Boots RJ, et al. Augmented renal clearance: implications for antibacterial dosing in the critically ill. Clin Pharmacokinet. 2010;49(1):1–16.
Fuster-Lluch O, Gerónimo-Pardo M, Peyró-García R, et al. Glomerular hyperfiltration and albuminuria in critically ill patients. Anaesth Intensive Care. 2008;36(5):674–80.
Claus BOM, Hoste EA, Colpaert K, et al. Augmented renal clearance is a common finding with worse clinical outcome in critically ill patients receiving antimicrobial therapy. J Crit Care. 2013;28(5):695–700.
Udy AA, Roberts JA, Shorr AF, et al. Augmented renal clearance in septic and traumatized patients with normal plasma creatinine concentrations: identifying at-risk patients. Crit Care. 2013;17(1):R35.
Abdel El Naeem HEM, Abdelhamid MHE, Atteya DAM. Impact of augmented renal clearance on enoxaparin therapy in critically ill patients. Egypt J Anaesth. 2017;33(1):113–7.
Barletta JF, Mangram AJ, Byrne M, et al. The importance of empiric antibiotic dosing in critically ill trauma patients: are we under-dosing based on augmented renal clearance and inaccurate renal clearance estimates? J Trauma Acute Care Surg. 2016;81(6):1115–21.
Campassi ML, Gonzalez MC, Masevicius FD, et al. Augmented renal clearance in critically ill patients: incidence, associated factors and effects on vancomycin treatment. Rev Bras Ter Intensiva. 2014;26(1):13–20.
Dickerson RN, Crawford CN, Tsiu MK, et al. Augmented renal clearance following traumatic injury in critically Ill patients requiring nutrition therapy. Nutrients. 2021. https://doi.org/10.3390/nu13051681.
Udy A, Boots R, Senthuran S, et al. Augmented creatinine clearance in traumatic brain injury. Anesth Analg. 2010;111(6):1505–10.
May CC, Arora S, Parli SE, et al. Augmented renal clearance in patients with subarachnoid hemorrhage. Neurocrit Care. 2015;23(3):374–9.
Chen IH, Nicolau DP. Augmented renal clearance and how to augment antibiotic dosing. Antibiotics (Basel). 2020;9(7):393.
Burnham JP, Micek ST, Kollef MH. Augmented renal clearance is not a risk factor for mortality in Enterobacteriaceae bloodstream infections treated with appropriate empiric antimicrobials. PLoS ONE. 2017;12(7):e0180247.
Carrie C, Chadefaux G, Sauvage N, et al. Increased beta-Lactams dosing regimens improve clinical outcome in critically ill patients with augmented renal clearance treated for a first episode of hospital or ventilator-acquired pneumonia: a before and after study. Crit Care. 2019;23(1):379.
Carlier M, Carrette S, Roberts JA, et al. Meropenem and piperacillin/tazobactam prescribing in critically ill patients: does augmented renal clearance affect pharmacokinetic/pharmacodynamic target attainment when extended infusions are used? Crit Care. 2013;17(3):R84.
Cucci M, Wooten C, Fowler M, et al. Incidence and risk factors associated with multi-drug–resistant pathogens in a critically Ill trauma population: a retrospective cohort study. Surg Infect (Larchmt). 2020;21(1):15–22.
Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81.
Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208.
Tabachnick BG, Fidell LS. Using multivariate statistics. 6th ed. Upper Saddle River, NJ: Pearson Education; 2013. (ISBN 13: 978-0205849574).
Minville V, Asehnoune K, Ruiz S, et al. Increased creatinine clearance in polytrauma patients with normal serum creatinine: a retrospective observational study. Crit Care. 2011;15(1):R49.
Nazer LH, AbuSara AK, Kamal Y. Augmented renal clearance in critically ill patients with cancer (ARCCAN Study): a prospective observational study evaluating prevalence and risk factors. Pharmacol Res Perspect. 2021;9(2):e00747.
Silva CM, Baptista JP, Santos I, et al. Recommended antibiotic dosage regimens in critically Ill patients with augmented renal clearance: a systematic review. Int J Antimicrob Agents. 2022;59:106569.
Xiao Q, Zhang H, Wu X, et al. Augmented renal clearance in severe infections-an important consideration in vancomycin dosing: a narrative review. Front Pharmacol. 2022;13:835557.
Gijsen M, Huang CY, Flechet M, et al. Development and external validation of an online clinical prediction model for augmented renal clearance in adult mixed critically Ill patients: the augmented renal clearance predictor. Crit Care Med. 2020;48(12):e1260–8.
Hefny F, Stuart A, Kung JY, et al. Prevalence and risk factors of augmented renal clearance: a systematic review and meta-analysis. Pharmaceutics. 2022. https://doi.org/10.3390/pharmaceutics14020445.
Grootaert V, Willems L, Debaveye Y, et al. Augmented renal clearance in the critically ill: how to assess kidney function. Ann Pharmacother. 2012;46(7–8):952–9.
Acknowledgements
We would like to thank Omar Husainalamoodi, data clerk at ICU of MGH affiliated with MUHC, for his contribution to the data collection for this study.
Funding
No specific funding was received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: “Authors’ contributions, Data availability, Ethical approval and Consent for publication” are removed. In reference 36, bold is removed for “ISBN”. The text “Statements and Declarations” is removed from Table 3 footnote
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bing, E., Archambault, K., Sananikone, A. et al. Risk factors associated with augmented renal clearance in a mixed intensive care unit population: a retrospective study. Int J Clin Pharm 44, 1277–1286 (2022). https://doi.org/10.1007/s11096-022-01458-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-022-01458-9