Abstract
Background Numerous drugs prolong the QTc interval on the ECG and potentially increase the risk of cardiac arrhythmia. This risk is clinically relevant in patients with additional risk factors. Objective The objective was to develop and validate a risk model to predict QTc interval prolongation of eligible ECGs. Setting Spaarne Gasthuis (Haarlem/Hoofddorp, The Netherlands). Method A dataset was created from ECGs recorded in patients using one or more QTc prolonging drugs, in the period January 2013 and October 2016. In the development set, independent risk factors for QTc interval prolongation were determined using binary logistic regression. Risk scores were assigned based on the beta coefficient. In the risk-score validation set, the area under the ROC-curve, sensitivity and specificity were calculated. Main outcome measure QTc interval prolongation, defined as a QTc interval > 500 ms. Results In the development set 12,949 ECGs were included and in the risk-score validation set 6391 ECGs. The proportion of ECGs with a prolonged QTc interval in patients with no risk factors in the risk-score validation set was 2.7%, while in patients with a high risk score the proportion was 26.1%. The area under the ROC curve was 0.71 (95% CI 0.68–0.73). The sensitivity and specificity were 0.81 and 0.48, respectively. Conclusion A risk model was developed and validated for the prediction of QTc interval prolongation. This risk model can be implemented in a clinical decision support system, supporting the management of the risks involved with QTc interval prolonging drugs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Impacts on Practice
-
In clinical practice there is uncertainty about the management of the risks involved with QTc prolonging drugs.
-
The identified risk factors are applicable in managing the risks of QTc prolonging drugs used by individual patients.
-
This risk model can be implemented in a clinical decision support system.
Introduction
Numerous drugs prolong the QTc interval on the ECG [1, 2]. A prolonged QTc interval is a risk factor for Torsade de Pointes (TdP), a potentially life-threatening arrhythmia [3]. A QT interval corrected for the heart rate (QTc) is considered as prolonged if it exceeds 450 ms in men or 470 ms in women [4]. Arrhythmias are often associated with QTc intervals exceeding 500 ms [2, 5]. QTc prolonging drugs should be avoided if the use will likely result in QTc intervals above this threshold. Nowadays over 100 drugs are associated with QTc interval prolongation, and these drugs are enumerated on the list of QTc prolonging drugs, established by CredibleMeds (Arizona Center for Education and Research on Therapeutics) [6]. Among them are drugs that are frequently used in daily practice and prescribed for non-cardiac indications.
There is much debate about the management of the risks associated with drug-induced QTc prolongation and whether these drugs can be prescribed safely to patients. In addition to drug use, various other risk factors are associated with QTc prolongation, such as hypokalemia, older age and female gender [7, 8]. The risk of drug-induced QTc prolongation can frequently be circumvented by selecting an alternative drug that is not associated with QTc prolongation. Since QTc prolonging drug use itself will rarely result in QTc intervals exceeding 500 ms, other risk factors must be present. Therefore, in patients with a low baseline risk of QTc prolongation, the additional risk of QTc prolonging drugs is most likely negligible and the use acceptable in clinical practice [9]. However, in patients with a high baseline risk of QTc prolongation, QTc prolonging drugs should be either avoided or the QTc interval should be monitored closely [10]. If QTc prolongation is seen, the QTc prolonging drug should be reconsidered or risk factors, such as hypokalemia, should be intervened upon. Many healthcare information systems generate medication surveillance alerts if two or more QTc prolonging drugs are prescribed. In patients with no other risk factors for QTc prolongation, these alerts might be less clinically relevant and it could be considered to suppress these alerts. In patients with a high risk of QTc prolongation, the use of even one QTc prolonging drug may be undesirable.
Tisdale et al. [11] developed a risk model to predict QTc prolongation in patients admitted to cardiac critical care units, independent of the use of QTc prolonging drugs. It is questionable whether this model is also applicable to inpatients at non-cardiac departments and outpatients. Therefore, a risk model was developed in the present study to predict QTc prolongation in inpatients and outpatients of a general teaching hospital and included only patients using QTc prolonging drugs. Moreover, in the study by Tisdale et al. data were collected from both computerized and paper medical records, while in the present study the aim was to develop a model that does retrieve data automatically from healthcare information systems without manual review. Implementation of the risk model developed by Tisdale et al. at the cardiac critical care units resulted in a significant reduction of prescriptions for non-cardiac QTc prolonging drugs and a significant reduction of patients with a QTc interval prolongation (QTc > 500 ms) [12]. Similarly, a clinical decision support system warning physicians prescribing QTc prolonging drugs in patients who had an ECG with a QTc interval > 500 ms in the past, resulted in a higher proportion of physicians to take action [13]. These results emphasize the importance of a clinical decision support system to avoid QTc interval prolongation.
Aim of the study
This study aimed to develop and validate a risk model to predict QTc interval prolongation of eligible ECGs for patients using one or more QTc prolonging drugs. The risk factors included in this risk model are variables that are easily identifiable in a healthcare information systems, making this risk model suitable for use in a clinical decision support system. This risk model will alert healthcare providers in case multiple risk factors are present that may result in a QTc interval above the threshold of 500 ms.
Ethics approval
No approval of a Medical Ethical Committee was needed according to the Dutch Medical Research Involving Human Subjects Act, because this was a descriptive study. All patient data were processed anonymously, according to privacy legislation.
Method
Study design
The design of this study is a retrospective data collection and content analysis. This study was performed at the Spaarne Gasthuis hospital with locations in Haarlem and Hoofddorp, the Netherlands.
Patients and electrocardiograms
An analysis of ECGs recorded between January 2013 and October 2016 was performed in patients who had one or more prescriptions for QTc prolonging drugs with a known risk of TdP according to the CredibleMeds list (October 2016) at the time of ECG recording [6]. Prescriptions for QTc prolonging drugs with an ‘as needed’ frequency were excluded. ECGs of both inpatients and outpatients were included. ECGs were excluded if patients were younger than 18 years of age at the moment the ECG was recorded, had a QRS complex above 120 ms or if they had a QTc interval of less than 300 ms or more than 600 ms. ECGs with a deviant QTc interval were excluded, because most likely these are the result of incorrect interpretations of the ECG instead of strongly shortened or prolonged QTc intervals. If multiple ECGs were recorded within a time period of 4 h, only the last ECG was included.
Data collection
Data were extracted from the hospital information system Epic (Madison, WI, USA), using SAP Crystal Reports (Walldorf, Germany). For all ECGs, the RR, QRS and QTc interval were extracted and the patients gender and age at the time of the ECG recording. Subsequently, three data extractions were made, first all ECGs and the relevant medication orders at the time of the ECG, second all ECGs with the relevant laboratory values and third all ECGs with the relevant ECG information from the past. Data were processed using IBM SPSS Statistics (IBM Corporation, NY, USA) version 24. ECGs were standard 12-lead resting ECGs with automated analysis by the MUSE Cardiology Information System. The heart rate (RR), QT and QRS interval were calculated by the MUSE system and saved in the hospital information system Epic.
Study variables
The outcome measurement was a prolonged QTc interval, defined as a QTc interval above 500 ms. The Bazett’s formula [QTc interval (ms) = QT interval (ms)/√Cardiac cycle (s) = QT interval (ms)/√60/HR, where HR is heart rate (beats/min)] was used to correct the QTc interval for heart rate [14]. This correction is done, because at higher heart rates the QT interval is shorter. After correction with the Bazett’s formula, the QTc intervals are independent of the heart rate. For each ECG, the following variables were analyzed as possible risk factors for QTc prolongation at the time of the ECG recording to be included in the risk model: gender, age > 70 years, prescriptions for antidiabetic drugs, antiarrhythmics, acetylsalicylic acid, loop diuretics, thyroid hormones, beta-blockers and non-dihydropyridine calcium antagonists (verapamil/diltiazem), the number of prescriptions of QTc prolonging drugs, the result of the last laboratory value in the last 7 days before the moment of ECG recording for potassium (≤ 2.9, 3–3.5 mmol/l or > 5 vs 3.5–5 mmol/l), calcium (≤ 2.14 mmol/l or > 2.55 vs 2.15–2.55 mmol/l), magnesium (≤ 0.69 mmol/l or > 1.0 vs 0.7–1.0 mmol/l), ALAT (≥ 100 vs < 100 U/l) and eGFR (≤ 60 vs > 60 ml/min) calculated with the MDRD formula, and the maximum QTc time measured in the last 365 days before the moment of ECG recording. If laboratory values were missing, these values were categorized as the reference value, which is the normal value used by the laboratory of the hospital. Similarly, if no ECG was performed in the 365 days before the ECG, the maximum QTc time was categorized as not prolonged.
Statistical analysis
Data were entered into IBM SPSS Statistics (IBM Corporation, NY, USA) version 24 and analysed using descriptive statistics and logistic regression. The significance level (α) was determined at 0.05. Of all ECGs included in the study a random sample of two-third of all ECGs was analyzed as a development set and the remainder one-third of ECGs was analyzed as a risk-score validation set. In the development set, the association between the variable and the QTc interval was assessed and cut-off values were determined for continuous variables. These cut-off values were used to dichotomize or categorize these variables for analysis. Binary logistic regression was performed for the associations between the risk factors and QTc interval prolongation, using a backwards conditional stepwise method in the development set. If the variables were significant (P < 0.05) they were considered as an independent risk factor. Risk scores were assigned to the risk factors based on their beta coefficient, by dividing the beta coefficient through 0.2 and rounding it to the nearest number. We have chosen to develop a risk model with whole numbers instead of a complex formula, because a risk model with whole numbers is much easier to use in clinical practice and easier to interpret. The value of 0.2 was chosen to have enough discrimination between the effect size of various risk factors. The total risk scores were calculated for each ECG. In the risk-score validation set, sensitivity [true positive/(true positive + false negative)], specificity [true negative/(true negative + false positive)], positive predictive value [true positive/(true positive + false positive)], Youden’s J statistic (sensitivity + specificity-1), negative predictive value [true negative/(true negative + false negative)] and accuracy (true positive + true negative/all) were calculated for the prediction of QTc interval prolongation using various cut-off values for the risk score. A ROC-curve was made by plotting the sensitivity versus one minus specificity for each cut-off point and the area under the curve was calculated. This study developed both a model in which all variables were analyzed and a simplified model in which calcium and magnesium levels and past QTc values were excluded, because these values are not available in all settings.
Results
In the study period, 19,340 ECGs were included that met the inclusion criteria, recorded in 6927 patients (Table 1). The average age of the patients per ECG was 71.7 years and 52.0% was male. Two or more QTc prolonging drugs were used in 8.8% of all ECGs. The QTc interval was prolonged in 1343 ECGs (6.9%). The ECGs were divided in a development set of 12,949 ECGs, recorded in 5685 patients, and a risk-score validation set of 6391 ECGs, recorded in 3721 patients.
The following risk factors with the accompanying risk score were included in the risk model with binary logistic regression; hypokalemia ≤ 2.9 mmol/l (7), the longest QTc interval in the last 365 days above 500 ms (7), the longest QTc interval in the last 365 days between 480 and 500 ms (3), hypokalemia between 3.0 and 3.4 mmol/l (3), hypocalcemia ≤ 2.14 mmol/l (3), use of loop diuretics (3), eGFR below 60 ml/min (2), use of antiarrhythmics (1) and age above 70 years (1). The beta coefficients are given in Table 2. The maximum risk score, if all risk factors would be present, is 24. In the analyses for the simplified model, calcium and magnesium levels and past QTc results were excluded. The following risk factors with the accompanying risk score were included in the simplified risk model with binary logistic regression: hypokalemia ≤ 2.9 mmol/l (8), hypokalemia between 3.0 and 3.4 mmol/l (4), use of loop diuretics (4), eGFR below 60 ml/min (2), use of antiarrhythmics (2), age above 70 years (1) and the use of beta-blockers (1). In this model, the use of beta blockers was a statistically significant additional risk factor. In the simplified model the maximum risk score is 18.
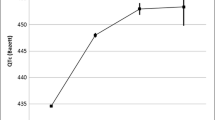
The quality of the predictability of the risk model was analyzed in the risk-score validation set. The mean risk score was 4.0. The proportion of ECGs with a prolonged QTc interval in patients with a risk score of zero was 2.7%, while in patients with a risk score of 13 or higher the proportion of ECGs with a prolonged QTc interval was 26.1% (Fig. 1). The area under the ROC curve was 0.71 (95% CI 0.68–0.73) (Fig. 2). The Youden’s J statistic was at maximum with a cut-off value of ≥ 5. At this cut-off value the sensitivity was 0.63 and the specificity was 0.69. In Table 3 the performance per cut-off value is given.
In the simplified model, the mean risk score was 3.5 and the proportion of ECGs with a prolonged QTc interval varied from 4.5% in patients with a risk score of zero to 19.1% in patients with a risk score of 11 or higher (Fig. 1). The area under the ROC curve was 0.62 (95% CI 0.60–0.65) (Fig. 2, Table 3). The Youden’s J statistic was at maximum with a cut-off value of ≥ 5. At this cut-off value the sensitivity was 0.48 and the specificity was 0.73.
Discussion
A risk model was developed to predict QTc prolongation in patients using one or more QTc prolonging drugs, defined as a QTc interval of more than 500 ms. The variables that were analyzed are automatically available in healthcare information systems. Therefore, this risk model can be implemented in a clinical decision support system, to improve the management of the risks associated with QTc prolonging drugs. The variables included have been described in the literature as risk factors for QTc prolongation [3, 7, 11, 15,16,17,18], and were therefore analyzed. The maximum QTc interval measured in the last 365 days was selected, because these patients have proven to be at risk for QTc interval prolongation. A threshold of 500 ms was chosen, because QTc intervals above this threshold are clinically relevant and have an increased risk of arrhythmias [2, 5]. Many healthcare information systems do not document diagnoses in such a way that they are assessable for clinical decision support systems. Therefore, drug use associated with the diagnosis was included in the risk model. For example, antidiabetic drug use was included as a proxy for the diagnosis diabetes mellitus. In this study, also a model excluding the variables calcium level, magnesium level and maximum QTc interval measured in the past 365 days was developed, because these variables are not always available, for example in the setting of general practitioners and community pharmacies. This simplified model may therefore be of value in such settings.
In the risk-score validation set, the area under the ROC curve was 0.71. A perfect model that will predict all QTc prolongations correctly will have an area under the ROC curve of one. There are several reasons why prediction of QTc prolongation is substantially below one. First, even in patients with a high risk score, the risk of QTc prolongation is rather low and the majority of ECGs in these patients do not have a prolonged QTc interval. Second, there is variation over time in the QTc interval independent of risk factors. This intra-individual variation will hamper prediction models. It is the authors’ opinion that this model can predict QTc prolongation to a clinically relevant degree. The proportion of ECGs with a prolonged QTc interval showed a gradual increase from 2.7% in patients with a risk score of zero to 26.1% in patients with a risk score of 13 or higher. The area under the ROC curve for the simplified model, excluding magnesium and calcium levels and the maximum QTc interval in the past 365 days, was 0.62. This model predicted the presence of QTc interval prolongation to a lesser extent than the full model. Nevertheless, the proportion of ECGs with a prolonged QTc interval increased from 4.5% in patients with a risk score of zero to 19.1% in patients with a risk score of 11 or higher. In both models, the performance was best and the specificity plus sensitivity highest, if a cut-off value of ≥ 5 was used.
The management of drug-induced QTc prolongation includes a balance between the small risk of TdP and sudden cardiac death, and the risk of withholding first-line therapies and switching to non QTc prolonging alternatives. In patients with no risk factors for QTc prolongation, the risk of drug-induced TdP will be minor and withholding these therapies will result in a higher risk of adverse outcomes [9, 10]. Moreover, more frequent recording of ECGs due to the QTc prolonging effect will be of no added value. To identify these patients, the healthcare provider has to evaluate the risk factors in the medical file of the patient. With a clinical decision support system, this process can be automated which reduces this time-consuming manual evaluation. Many healthcare information systems do alert the healthcare provider for drug–drug interactions between two or more QTc prolonging drugs. In patients with a low risk for QTc prolongation, the clinical relevancy of these alerts can be questioned. Presenting too many alerts to a healthcare provider holds the risk that all generated alerts are overridden including the relevant ones, so called alert fatigue [19, 20]. Implementing a clinical decision support system in the medication surveillance can reduce the number of alerts for patients with a low risk of QTc prolongation. Before implementation of this risk model in medication surveillance, a cut-off value should be set. In patients with a risk score under this cut-off value, filtering of the alert could be considered. A cut-off value of three or above will result in correct identification of patients with a prolonged QTc interval in 81% of cases (sensitivity), and in patients without a prolonged QTc interval in 48% of cases (specificity). A higher cut-off value will result in an increase in patients with a prolonged QTc interval who are not identified by the risk model (lower sensitivity) and an increase in patients with a prolonged QTc interval who are identified by the risk model (higher specificity) and vice versa. In the limited model, a cut-off value of two or above will result in a sensitivity of 83% and a specificity of 27%. Further studies must reveal whether implementation of this risk model does result in better medication surveillance and whether this is cost-effective.
In this study, a risk model was developed for patients who were treated in a general teaching hospital using at least one QTc prolonging drug. Tisdale et al. developed a risk model to predict QTc prolongation in patients admitted to cardiac critical care units [11]. Notwithstanding the differences in methodology, there are similarities between the risk models. In both models, older age, use of loop diuretics and hypokalemia are risk factors. Differences between the models can be explained by the differences in study population and variables studied. In the study by Haugaa et al., a risk model was composed that could predict mortality in patients with an electrocardiographically isolated QTc interval of 500 ms or greater [21].
This study has some potential strengths and limitations. A set of ECGs in routine clinical practice in a general teaching hospital was used. Since both in and outpatients in all departments were included, the results can be extrapolated to many health care settings, treating patients with similar risks as in this population. However, ECGs will be especially recorded if heart rhythm disturbances are expected, and therefore patients with a prolonged QTc interval will be over represented in this study. The variables incorporated in this model can be extracted automatically from healthcare systems, making implementation in a clinical decision support system without manual review of the patient files possible.
A limitation is that the ECGs were not reviewed manually. In the literature, there is discussion whether manual or automatic assessment of the ECG interval is better [22]. The QT interval was adjusted using the Bazett’s formula. This formula was used, because this is the one most frequently used in clinical practice. Recent studies, however, have shown that other formula’s, such as the Fridericia and Framingham formula may perform better [23]. If multiple ECGs were recorded in the same patient, all ECGs were included. We choose to include multiple ECGs per patient, because patients in whom multiple ECGs are recorded are the patients with the highest number of risk factors. Excluding ECGs in these patients would result in a set of ECGs not representative for all ECGs recorded in the hospital. The potential disadvantage is that the actual confidence intervals are wider than the calculated confidence intervals. Not all laboratory values were available in the patients. Missing values were analyzed as being within the normal range, because if deviant values were suspected, these laboratory assessments would have been ordered. However, some deviant values might have been missed, resulting in a too low risk score. Calcium levels are corrected for the albumin levels in clinical practice. In patients with hypoalbuminemia, a too low calcium level may actually be within normal and the albumin adjusted calcium levels may have a stronger correlation with QTc prolongation. However, such a correction formula would be difficult to implement in an automated clinical decision support system, and therefore calcium levels were analyzed without correction. In a sensitivity analysis, we adjusted the calcium level for the albumin level in the risk model, if the albumin level was measured within the 48 h before the calcium level. This model did not have a higher area under the ROC curve in the risk-score validation set.
Conclusion
A risk model was developed and validated a for the prediction of QTc interval prolongation in patients using one or more QTc prolonging drug. This risk model is implementable in a clinical decision support system, evaluating automatically the information from the healthcare information systems. Implementation may result in a reduction of the number of alerts in patients with a low risk of QTc prolongation and improve patient safety by reducing alert fatigue.
References
Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350:1013–22.
Yap YG, Camm AJ. Drug induced QT prolongation and torsades de pointes. Heart. 2003;89:1363–72.
Straus SM, Kors JA, De Bruin ML, van der Hooft CS, Hofman A, Heeringa J, et al. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol. 2006;47:362–7.
The European Agency for the Evaluation of Medicinal Products. Committee for proprietary medicinal products. [internet] London: 1997. [cited 27 feb 2018] The assessment of the potential for QT interval prolongation by non-cardiovascular medicinal products; 1997. http://www.fda.gov/ohrms/dockets/ac/03/briefing/pubs/cpmp.pdf.
Beitland S, Platou ES, Sunde K. Drug-induced long QT syndrome and fatal arrhythmias in the intensive care unit. Acta Anaesthesiol Scand. 2014;58:266–72.
crediblemeds.org. Oro Valley, AZ: Arizona Center for Education and Research on Therapeutics. [cited 27 feb 2018]; 2015. http://www.Crediblemeds.org.
Benoit SR, Mendelsohn AB, Nourjah P, Staffa JA, Graham DJ. Risk factors for prolonged QTc among US adults: Third National Health and Nutrition Examination Survey. Eur J Cardiovasc Prev Rehabil. 2005;12:363–8.
Sohaib SM, Papacosta O, Morris RW, Macfarlane PW, Whincup PH. Length of the QT interval: determinants and prognostic implications in a population-based prospective study of older men. J Electrocardiol. 2008;41:704–10.
Schwartz PJ, Woosley RL. Predicting the unpredictable: drug-Induced QT prolongation and Torsades de Pointes. J Am Coll Cardiol. 2016;67:1639–50.
Nachimuthu S, Assar MD, Schussler JM. Drug-induced QT interval prolongation: mechanisms and clinical management. Ther Adv Drug Saf. 2012;3:241–53.
Tisdale JE, Jaynes HA, Kingery JR, Mourad NA, Trujillo TN, Overholser BR, et al. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2013;6:479–87.
Tisdale JE, Jaynes HA, Kingery JR, Overholser BR, Mourad NA, Trujillo TN, et al. Effectiveness of a clinical decision support system for reducing the risk of QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2014;7:381–90.
Sharma S, Martijn Bos J, Tarrell RF, Simon GJ, Morlan BW, Ackerman MJ, et al. Providers’ response to clinical decision support for QT prolonging drugs. J Med Syst. 2017;41(10):161.
Bazett HC. An analysis of the time-relations of the electrocardiograms. Heart. 1920;7:353–70.
Pickham D, Helfenbein E, Shinn JA, Chan G, Funk M, Weinacker A, et al. High prevalence of corrected QT interval prolongation in acutely ill patients is associated with mortality: results of the QT in practice (QTIP) study. Crit Care Med. 2012;40:394–9.
Trojak B, Astruc K, Pinoit JM, Chauvet-Gelinier JC, Ponavoy E, Bonin B, et al. Hypokalemia is associated with lengthening of QT interval in psychiatric patients on admission. Psychiatry Res. 2009;169:257–60.
Mangoni AA, Kinirons MT, Swift CG, Jackson SH. Impact of age on QT interval and QT dispersion in healthy subjects: a regression analysis. Age Ageing. 2003;32:326–31.
Jardin CG, Putney D, Michaud S. Assessment of drug-induced torsade de pointes risk for hospitalized high-risk patients receiving QT-prolonging agents. Ann Pharmacother. 2014;48:196–202.
van der Sijs H, Mulder A, van Gelder T, Aarts J, Berg M, Vulto A. Drug safety alert generation and overriding in a large Dutch university medical centre. Pharmacoepidemiol Drug Saf. 2009;18:941–7.
Nanji KC, Slight SP, Seger DL, Cho I, Fiskio JM, Redden LM, et al. Overrides of medication-related clinical decision support alerts in outpatients. J Am Med Inform Assoc. 2014;21:487–91.
Haugaa KH, Bos JM, Tarrell RF, Morlan BW, Caraballo PJ, Ackerman MJ. Institution-wide QT alert system identifies patients with a high risk of mortality. Mayo Clin Proc. 2013;88:315–25.
Postema PG, De Jong JS, Van der Bilt IA, Wilde AA. Accurate electrocardiographic assessment of the QT interval: teach the tangent. Heart Rhythm. 2008;5:1015–8.
Vandenberk B, Vandael E, Robyns T, Vandenberghe J, Garweg C, Foulon V, et al. Which QT Correction Formulae to Use for QT Monitoring? J Am Heart Assoc. 2016;5(6):e003264.
Acknowledgements
We would like to thank Karlijn van Stralen for her help in methodological and statistical issues.
Funding
No special funding was received for performing this study.
Conflicts of interest
All authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bindraban, A.N., Rolvink, J., Berger, F.A. et al. Development of a risk model for predicting QTc interval prolongation in patients using QTc-prolonging drugs. Int J Clin Pharm 40, 1372–1379 (2018). https://doi.org/10.1007/s11096-018-0692-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-018-0692-y