Abstract
Background Vancomycin is a glycopeptide antibiotic of choice for the treatment of serious infections caused by multi-resistant Gram-positive bacteria. However, vancomycin-associated nephrotoxicity (VAN) often limits its use. Previous data suggested a few risk factors of VAN, including higher mean vancomycin trough level, higher daily doses, old age, long duration of vancomycin therapy, and concomitant nephrotoxins. Objective To evaluate the incidence and risk factors of VAN and determine whether higher vancomycin trough concentrations were associated with a greater risk for VAN. Settings A retrospective, observational, single-center study at the 1960-bed university-affiliated tertiary care hospital (Samsung Medical Center), Seoul, Korea. Method A retrospective analysis of adult patients who received vancomycin parenterally in a tertiary care medical center from March 1, 2013 to June 30, 2013 was performed. We excluded patients with a baseline serum creatinine level > 2 mg/dL and those who had a history of end-stage renal disease and dialysis at baseline. The clinical characteristics were compared between patients with nephrotoxicity and those without nephrotoxicity to identify the risk factors associated with VAN. Main outcome measure Incidence of VAN and VAN-associated risk factors were analyzed. Results Of the 315 vancomycin-treated patients, nephrotoxicity occurred in 15.2% of the patients. In multivariate analysis, higher vancomycin trough concentrations of > 20 mg∕L (OR 9.57, 95% CI 2.49–36.83, p < 0.01) and intensive care unit (ICU) residence (OR 2.86, 95% CI 1.41–5.82, p < 0.01) were independently associated with VAN. Conclusion Our findings suggest that higher vancomycin trough levels and ICU residence might be associated with a greater risk for VAN. More careful monitoring of vancomycin serum trough levels and patient status might facilitate the timely prevention of VAN.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Impacts on practice
-
Monitoring of serum vancomycin concentrations is necessary to ensure that the concentration does not exceed the upper limit of therapeutic concentration.
-
Nephrotoxicity of vancomyxin should be closely monitored, especially when it is used in critically ill patients.
Introduction
Vancomycin is a glycopeptide antibiotic used in the treatment of severe infections caused by multi-resistant Gram-positive bacteria, such as methicillin-resistant Staphylococcus aureus (MRSA) and for Gram-positive bacterial infections in patients who are allergic to penicillins [1, 2]. Despite its widespread use, vancomycin-associated nephrotoxicity (VAN) is the most serious adverse effect of parenteral vancomycin therapy. VAN can lead to discontinuation or modification of treatment. However, the incidence of VAN has not been well established yet [3], and the reported incidence ranges from 5 to 43% depending on the patient population [4,5,6,7]. Several conditions have been proposed as probable risk factors for VAN, including higher vancomycin daily dosages, longer duration of therapy, use of concomitant nephrotoxic agents, and history of kidney disease [8,9,10,11]. However, these predisposing factors differed between studies.
Correlations between serum vancomycin concentration and nephrotoxicity remain unclear [12]. Early suggestions have recommended a target vancomycin trough level ranging from 5 to 10 mg/L [13]. However, treatment failures were increasingly reported, particularly in patients infected with MRSA, with a minimal inhibitory concentration (MIC) of > 1 mg/L. Some studies have reported that serum vancomycin trough concentrations < 10 mg/L are more likely to produce MRSA with reduced vancomycin susceptibility [14, 15]. Based on available evidence, a consensus review from the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists published in 2009 recommended maintaining a vancomycin trough concentration above 10 mg/L in general. For complicated infections, 15–20 mg/L of vancomycin is recommended to improve tissue penetration and increase the probability of achieving target serum vancomycin concentrations [16]. After the publication of this guideline, preliminary studies have evaluated the safety of achieving higher serum trough concentrations and suggested that maintaining higher serum trough concentrations (i.e. ≥ 15 mg/L) can increase the risk of nephrotoxicity [17,18,19].
Aim of the study
The aim of the present study was to evaluate the incidence of VAN and VAN-associated risk factors and to determine whether higher vancomycin trough concentrations were associated with a greater risk for VAN.
Ethics approval
This single-center retrospective study was approved by the Institutional Review Board of Samsung Medical Center (approval number: 2013-06-121). The requirement of formal consent was waived owing to its retrospective nature.
Methods
Study design and setting
This single-center retrospective observational study was conducted at Samsung Medical Center (Seoul, Korea) between March 1, 2013 and June 30, 2013. Our hospital launched a computerized clinical decision support system (CDSS) for antibiotic prescription in 2008. The institutional policy states that the prescription of intravenous antimicrobials, including vancomycin, beyond 2 days requires prior authorization. The institution limits the duration of vancomycin use after the days approved by infectious disease clinicians. A prescribing physician can discuss antimicrobial treatment options with infection specialists and pharmacists for dosage, administration routes, and other patient-specific pharmacokinetic factors. Clinical pharmacists monitored vancomycin use and intervened via clinical pharmacokinetic services in multidisciplinary team round.
Inclusion and exclusion criteria
Patients older than 18 years of age who received a minimum of 48 h of intravenous vancomycin were included. The exclusion criteria were baseline serum creatinine level > 2 mg/dL [8, 20,21,22], known history of kidney disease, hemodialysis or continuous renal replacement therapy at baseline, no serum creatinine recorded at baseline, or incomplete medical records.
Variables and outcome
Data were extracted from electronic medical records of Samsung Medical Information System. The endpoint was development of VAN. Data collected for each patient included sex, age, height, weight, serum creatinine levels, length of hospital stay, and intensive care unit (ICU) admission status during hospitalization. Baseline serum creatinine was defined as the value recorded within 24 h before the initiation of vancomycin therapy.
Acute kidney injury after vancomycin use was considered as VAN. Nephrotoxicity was defined as an increase in serum creatinine ≥ 0.5 mg/dL or > 50% from baseline in at least two consecutive measurements based on the 2009 consensus review of the American Society of Health-System Pharmacists [16].
Information regarding vancomycin therapy was collected to assess the relationship with VAN as a risk factor. Independent variables in statistical analysis included indication for use, total daily doses based on actual body weight, duration of therapy, vancomycin serum trough concentration, and concomitant nephrotoxic agents. The trough concentrations were measured in blood samples collected just prior to administration of the next dose. Receipt of nephrotoxic agents, including aminoglycosides, loop diuretics, angiotensin II receptor blockers, and angiotensin-converting enzyme (ACE) inhibitors, was recorded.
Subgroup analysis was performed for patients in the nephrotoxicity group. Relationship of vancomycin trough concentration with ICU stay and concomitant use of nephrotoxic agents in the nephrotoxicity group was also analyzed.
Statistical analyses
All statistical analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA). Nephrotoxicity was recorded as a binary variable to indicate whether nephrotoxicity developed in a patient. For univariate analyses, categorical variables were compared using χ2 test or Fisher’s exact test, whereas continuous variables were compared using Wilcoxon Rank-Sum test. All continuous variables were reported as median and interquartile range (IQR). We used multivariable logistic regression to analyze the risk factors for VAN. In the multivariable logistic regression, variables with p < 0.1 from Wald test in univariate analysis were included. However, variables with variance inflation factor (VIF) exceeding 3 were excluded to avoid multicollinearity issue. For comparisons of three groups in subgroup analysis, Fisher’s exact test and χ2 test were used for categorical variables, whereas Kruskal–Wallis test was used for continuous variables. For all analyses, p < 0.05 was considered statistically significant.
Results
In total, 409 patients treated with vancomycin from March 1, 2013 to June 30, 2013 at Samsung Medical Center were enrolled in this study. Ninety-four patients were excluded owing to baseline serum creatinine level > 2 mg/dL (n = 12), known history of kidney disease, hemodialysis or continuous renal replacement therapy at baseline (n = 19), no serum creatinine recorded at baseline or incomplete medical records (n = 41), and vancomycin therapy < 48 h (n = 22). Finally, 315 subjects were included for analysis.
Patient demographics are summarized in Table 1. Of the 315 patients, 210 (66.7%) were males. The median age of all patients was 58 years (IQR 45–69 years). Their median baseline serum creatinine level was 0.68 mg/dL (IQR 0.55–0.92 mg/L). The most common indication for vancomycin use was pneumonia (26.7%). The median vancomycin daily dose was 30.70 mg/kg/day (IQR 24.37–37.44 mg/kg/day). The median duration of vancomycin therapy was 8 days (IQR 5–13 days). Nephrotoxic agents were used in 137 (43.5%) patients. The most frequently used drug was furosemide (34.0%). Nephrotoxicity occurred in 48 (15.2%) of 315 vancomycin-treated patients. The median time from the start of vancomycin to onset of VAN was 5 days (IQR 3–8 days).
Renal function results are presented in Table 2. The median change in serum creatinine level at the time of occurrence of VAN from baseline serum creatinine was 0.63 mg/dL (IQR 0.51–0.98 mg/L). Of 48 episodes of VAN, 9 (18.8%) patients had subsequent reductions in serum creatinine levels toward baseline values before hospital discharge.
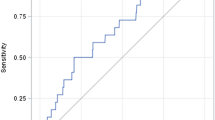
The results of comparison between group with nephrotoxicity and group without nephrotoxicity are shown in Table 3. The following variables showed significant associations with the occurrence of nephrotoxicity: longer duration of therapy, higher vancomycin trough concentration, concomitant use of nephrotoxic agents, and ICU residence. The proportion of patients who received furosemide was significantly higher in the group with nephrotoxicity than in the group without nephrotoxicity (50.0 vs. 31.1%, p = 0.01). The group with nephrotoxicity received a higher median furosemide daily dose than the group without nephrotoxicity (58.40 vs. 28.00 mg/day, p = 0.01). Portions of higher vancomycin trough concentrations were significantly larger in the group with nephrotoxicity than in the group without nephrotoxicity. Vancomycin trough concentrations of 15–20 mg/L were achieved in a higher percentage of patients in the group with nephrotoxicity than in the group without nephrotoxicity (45.8 vs. 25.8%, p < 0.01). Trough concentrations > 20 mg/L were also achieved in a higher percentage of patients in the group with nephrotoxicity than in the group without nephrotoxicity (14.6 vs. 4.5%, p < 0.01) (Fig. 1). Two variables (concomitant use of nephrotoxic agents and in particular, concomitant furosemide use) were excluded owing to high VIF (> 3) in multivariable logistic regression.
Vancomycin trough concentration and ICU residence were independently associated with nephrotoxicity (Table 4). Both vancomycin trough concentration of 15–20 mg/L (odds ratio [OR] 4.01, p < 0.01) and > 20 mg/L (OR 9.57, p < 0.01) were associated with significantly greater risk of nephrotoxicity than vancomycin trough concentration < 15 mg/L. Baseline serum creatinine level was also a risk factor of VAN. According to our analysis, lower baseline serum creatinine level was associated with nephrotoxicity. In the subgroup analysis for group with nephrotoxicity, ICU residence or concomitant use of nephrotoxic agents showed no significant association with vancomycin trough concentration.
Discussion
This study evaluated the incidence and associated risk factors of VAN in adult patients. Previous studies have reported a wide range of VAN incidence and risk factors. The true prevalence of VAN remains unclear. In this study, nephrotoxicity occurred in 15.2% of 315 patients who received vancomycin. VAN occurred more frequently in patients who had higher vancomycin trough concentration, ICU residence, and low baseline serum creatinine level.
Vancomycin trough concentration has been commonly evaluated as a potential predictor of nephrotoxicity in different patient populations. A prospective multicenter trial has reported that the incidence of VAN in patients with initial trough concentrations ≥ 20 mg/L, ≥ 25 mg/L, and ≥ 30 mg/L is 32, 45, and 50%, respectively [17]. We previously conducted a multivariate logistic regression analysis and found that the initial vancomycin trough concentration expressed as a continuous variable is independently associated with VAN (adjusted OR 1.13, 95.0% CI 1.05–1.21, p = 0.001) [21]. In addition, many other studies have reported that the rate of nephrotoxicity is significantly increased in patients who received higher initial and steady-state trough concentrations [18, 20, 22, 23]. Two recent studies reported conflicting results concerning vancomycin serum trough concentrations and clinical outcomes [24, 25]. However, in both the studies, renal adverse events occurred more frequently in the group receiving higher trough concentration [24, 25]. The results of our study support this finding. In the present study, multiple logistic regression analysis revealed that patients with vancomycin trough concentrations of 15–20 mg/L were four times more likely to develop VAN, while patients with vancomycin trough concentrations > 20 mg/L were 9.6 times more likely to develop VAN than patients with vancomycin trough concentrations < 15 mg/L. According to the 2009 consensus statement, S. aureus exposure to vancomycin trough concentrations < 10 mg/L can produce resistant strains, which are associated with a higher likelihood of vancomycin therapeutic failures [16]. It is recommend that vancomycin trough concentrations are maintained at 15–20 mg/L enough to penetrate into the infected tissue and to achieve more effective overall antibiotic exposure in severe infectious diseases. Therefore, when health caregivers target higher vancomycin concentrations, ongoing drug level monitoring accompanied by proper dosage adjustment should be performed to ensure that vancomycin levels do not exceed 20 mg/L. Moreover, the patients’ clinical condition should be carefully evaluated and a decision should be taken on whether the vancomycin trough level has to be maintained at a high level within the therapeutic range.
Acute kidney injury occurred in approximately 6% of critically ill patients staying in the ICU, with approximately two-thirds of these patients requiring renal replacement therapy [26]. A retrospective study indicated that the risk of nephrotoxicity is greater in ICU patients [21]. Our findings are consistent with the results of these studies. In our evaluation, ICU residence was associated with VAN in both univariate and multivariate analyses. Critically ill patients are known to have increased sickness severity associated with a vast array of pathophysiological changes, which can alter renal perfusion and antibiotic pharmacokinetics, thereby increasing the risk of acute kidney injury [27,28,29].
Baseline serum creatinine has been reported as a significant risk factor of VAN in a previous study [7]. However, our results indicated that lower baseline serum creatinine level might increase VAN risk. Although it was statistically significant in multivariate analysis, it may have no significant effect in clinical practice. As shown in Table 3, the baseline serum creatinine values in group without nephrotoxicity and group with nephrotoxicity were in the normal range (0.69 and 0.63 mg/mL), with no significant difference (p = 0.0693) between the two groups.
There were significant differences in median vancomycin daily dose and duration of therapy between patients with VAN and those without VAN. However, the daily dose of vancomycin therapy did not always increase the incidence of nephrotoxicity. It has been reported that a vancomycin dose of > 4 g/day can increase the incidence of VAN [8]. Meaney et al. [11] suggested that daily dose of vancomycin is not associated with nephrotoxicity status. Nevertheless, another study described an inverse relationship between the average daily vancomycin dose based on actual body weight and nephrotoxicity [30]. In a previous study, the nephrotoxicity group received a significantly lower average daily dose (mean ± SD 24.00 ± 15.22 mg/kg/day) than the no nephrotoxicity group (30.59 ± 15.56 mg/kg/day; p = 0.02) [30], similar to that in the present study. This might be because the vancomycin dose was reduced after development of nephrotoxicity. Regarding duration of therapy, prolonged duration of vancomycin therapy as a continuous variable or dichotomous variable has been found to be a significant independent risk factor of VAN [18, 20, 23]. Similarly, we found that the total duration of therapy was significantly longer in the group with nephrotoxicity than in the group without nephrotoxicity. However, differences in individual days of therapy between the two groups were not statistically significant. Thus, we could not exclude the possibility that longer duration of vancomycin therapy was due to, but not a source of occurrence of nephrotoxicity, considering that we defined duration as the total period from the start to the end of the treatment. Therefore, vancomycin daily dose and duration of therapy were not included in multivariable analysis.
Our study has a few limitations. First, this was a single-center and retrospective study. It is recommended that the blood samples for trough concentrations should be drawn within 60 min prior to the planned dosing. However, owing to the retrospective nature of this study, we could not strictly control the actual time of blood sample collection. There could be some time gap between the recommended time and actual sampling time. Second, the small sample size might have limited the statistical power to detect more significant findings. Finally, many factors that could affect nephrotoxicity, such as intravenous contrast dye and vasopressors, were not reviewed together because of the small number of such cases.
Conclusion
Higher vancomycin trough concentrations and ICU residence are statistically significant risk factors for VAN. Maintaining the vancomycin trough concentrations within the proper therapeutic range (not exceeding 20 mg/L) and early detection of mild changes in renal function of ICU patients could facilitate prompt intervention to prevent VAN. Further research is needed to develop useful strategies for safe vancomycin use.
References
Cook FV, Farrar WE Jr. Vancomycin revisited. Ann Intern Med. 1978;88:813–8.
Stevens DL. The role of vancomycin in the treatment paradigm. Clin Infect Dis. 2006;42(Suppl 1):S51–7.
Bailie GR, Neal D. Vancomycin ototoxicity and nephrotoxicity. A review. Med Toxicol Adverse Drug Exp. 1988;3:376–86.
Rybak MJ, Albrecht LM, Boike SC, Chandrasekar PH. Nephrotoxicity of vancomycin, alone and with an aminoglycoside. J Antimicrob Chemother. 1990;25:679–87.
Gupta A, Biyani M, Khaira A. Vancomycin nephrotoxicity: myths and facts. Neth J Med. 2011;69:379–83.
Carreno JJ, Kenney RM, Lomaestro B. Vancomycin-associated renal dysfunction: where are we now? Pharmacotherapy. 2014;34:1259–68.
Elyasi S, Khalili H, Dashti-Khavidaki S, Mohammadpour A. Vancomycin-induced nephrotoxicity: mechanism, incidence, risk factors and special populations. A literature review. Eur J Clin Pharmacol. 2012;68:1243–55.
Lodise TP, Lomaestro B, Graves J, Drusano GL. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob Agents Chemother. 2008;52:1330–6.
Hazlewood KA, Brouse SD, Pitcher WD, Hall RG. Vancomycin-associated nephrotoxicity: grave concern or death by character assassination? Am J Med. 2010;123(182):e1–7.
Hidayat LK, Hsu DI, Quist R, Shriner KA, Wong-Beringer A. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med. 2006;166:2138–44.
Meaney CJ, Hynicka LM, Tsoukleris MG. Vancomycin-associated nephrotoxicity in adult medicine patients: incidence, outcomes, and risk factors. Pharmacotherapy. 2014;34:653–61.
Hermsen ED, Hanson M, Sankaranarayanan J, Stoner JA, Florescu MC, Rupp ME. Clinical outcomes and nephrotoxicity associated with vancomycin trough concentrations during treatment of deep-seated infections. Expert Opin Drug Saf. 2010;9:9–14.
Geraci JE. Vancomycin. Mayo Clin Proc. 1977;52:631–4.
Soriano A, Marco F, Martinez JA, Pisos E, Almela M, Dimova VP, et al. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis. 2008;46:193–200.
Lodise TP, Graves J, Evans A, Graffunder E, Helmecke M, Lomaestro BM, et al. Relationship between vancomycin MIC and failure among patients with methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin. Antimicrob Agents Chemother. 2008;52:3315–20.
Rybak M, Lomaestro B, Rotschafer JC, Moellering R Jr, Craig W, Billeter M, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66:82–98.
Bosso JA, Nappi J, Rudisill C, Wellein M, Bookstaver PB, Swindler J, et al. Relationship between vancomycin trough concentrations and nephrotoxicity: a prospective multicenter trial. Antimicrob Agents Chemother. 2011;55:5475–9.
Jeffres MN, Isakow W, Doherty JA, Micek ST, Kollef MH. A retrospective analysis of possible renal toxicity associated with vancomycin in patients with health care-associated methicillin-resistant Staphylococcus aureus pneumonia. Clin Ther. 2007;29:1107–15.
Hal SJ, Paterson DL, Lodise TP. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother. 2013;57:734–44.
Cano EL, Haque NZ, Welch VL, Cely CM, Peyrani P, Scerpella EG, et al. Improving Medicine through Pathway Assessment of Critical Therapy of Hospital-Acquired Pneumonia(IMPACT-HAP) Study Group. Incidence of nephrotoxicity and association with vancomycin use in intensive care unit patients with pneumonia: retrospective analysis of the IMPACT-HAP Database. Clin Ther. 2012;34:149–57.
Lodise TP, Patel N, Lomaestro BM, Rodvold KA, Drusano GL. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin Infect Dis. 2009;49:507–14.
Prabaker KK, Tran TP, Pratummas T, Goetz MB, Graber CJ. Elevated vancomycin trough is not associated with nephrotoxicity among inpatient veterans. J Hosp Med. 2012;7:91–7.
Carreno JJ, Jaworski A, Kenney RM, Davis SL. Comparative incidence of nephrotoxicity by age group among adult patients receiving vancomycin. Infect Dis Ther. 2013;2:201–8.
Barriere SL, Stryjewski ME, Corey GR, Genter FC, Rubinstein E. Effect of vancomycin serum trough levels on outcomes in patients with nosocomial pneumonia due to Staphylococcus aureus: a retrospective, post hoc, subgroup analysis of the Phase 3 ATTAIN studies. BMC Infect Dis. 2014;14:183.
Steinmetz T, Eliakim-Raz N, Goldberg E, Leibovici L, Yahav D. Association of vancomycin serum concentrations with efficacy in patients with MRSA infections: a systematic review and meta-analysis. Clin Microbiol Infect. 2015;21:665–73.
Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Beginning, Ending Supportive Therapy for the Kidney I(BEST Kidney Investigators). Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–8.
Roberts JA, Abdul-Aziz MH, Lipman J, Mouton JW, Vinks AA, Felton TW, et al. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis. 2014;14:498–509.
Roberts JA, Lipman J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit Care Med. 2009;37:840–51.
Pannu N, Nadim MK. An overview of drug-induced acute kidney injury. Crit Care Med. 2008;36(Suppl 4):S216–23.
Pritchard L, Baker C, Leggett J, Sehdev P, Brown A, Bayley KB. Increasing vancomycin serum trough concentrations and incidence of nephrotoxicity. Am J Med. 2010;123:1143–9.
Funding
This study has no specific funding sources.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest with regard to this paper.
Rights and permissions
About this article
Cite this article
Park, S.J., Lim, N.R., Park, H.J. et al. Evaluation of risk factors for vancomycin-induced nephrotoxicity. Int J Clin Pharm 40, 1328–1334 (2018). https://doi.org/10.1007/s11096-018-0634-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-018-0634-8