Abstract
Background Vancomycin resistant enterococcal bloodstream infections are an important cause of morbidity and mortality in hospitalized patients. Aim of the Review A search of the literature was undertaken to determine the optimal antimicrobial therapy for the management of vancomycin resistant enterococcal bloodstream infections. Method MEDLINE, EMBASE, and the Cochrane Library (unrestricted to time or language) were searched for studies of vancomycin resistant enterococcal bloodstream infections in adults reporting outcomes of direct comparisons of linezolid versus daptomycin on November 26, 2012. Studies of basic science, reviews, commentaries, pharmacologic, epidemiologic, or pediatric studies, and those studies examining conditions other than enterococcal bacteremia, a single antimicrobial agent or other antimicrobials were excluded. Results 226 studies were screened for eligibility and yielded eight studies evaluating a total of 807 patients. Inter-rater agreement was 100 %. Qualitative evaluation of the studies was performed using the Newcastle–Ottawa scale. No randomized controlled trials were identified. All studies were retrospective cohorts and non-randomized. 458 (57 %) patients treated with linezolid and 349 (43 %) with daptomycin were analyzed. Variable comorbidities and severity of illness were described in the included studies and reported here for comparison. Conclusion The optimal treatment of vancomycin resistant enterococcal bloodstream infections is yet to be determined. Well-designed prospective studies are needed to lend more convincing evidence regarding choice of antimicrobial therapy for this important multidrug resistant organism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Impact of findings on practice statements
-
Given the quality and quantity of the available evidence, both linezolid and daptomycin remain alternatives for the treatment of VRE BSI.
-
Patient outcomes may differ with the antimicrobial choice depending on severity of illness and underlying comorbidities.
-
More robust studies are needed to determine which antimicrobial provides the best outcomes in patients critically ill patients with severe VRE BSIs.
Introduction
The evolution of antimicrobial resistance and the limited number of effective antimicrobials is challenging clinicians worldwide [1]. This is certainly the case with Vancomycin-resistant enterococci (VRE), which were first reported as clinically significant in 1988 [2] and have since been found globally [3–8]. In the United States, enterococci are among the most common organisms causing bloodstream infections (BSIs), representing 9 % of BSIs in the Surveillance and Control of Pathogens of Epidemiological Importance (SCOPE) study [7] and 10.2 % in the SENTRY program [8]. Clinically, VRE are associated with contamination of hospital environments and colonization of the gastrointestinal tracts of patients for prolonged periods of time [9] and can lead to a wide range of infections from intra-abdominal abscesses to infective endocarditis [10]. Despite a growing body of knowledge, these organisms continue to cause significant morbidity and mortality [11]. Vancomycin resistance has also been shown to have an independent association with mortality in VRE BSIs [12].
Enzymes that help synthesize alternative peptidoglycan precursors to those that would form complexes with vancomycin help Enterococcus sp. attain resistance [13]. In clinical practice, antimicrobial agents with alternate mechanisms of action against these bacteria have come into favor. For example, linezolid, tigecycline, and quinupristin-dalfopristin affect protein synthesis at the ribosomal level [14] while daptomycin is thought to act by formation of curved membrane patches that can cause slow leakage of ions and affect the cell wall synthesis mechanisms that ultimately lead to rupture of both the cell membrane and cell wall [15].
Linezolid belongs to the oxazolidiniones family and has bacteriostatic activity against most bacteria, including VRE [16]. Daptomycin is a cyclic lipopeptide and also has activity against clinical isolates of VRE [17] with demonstrated in vitro bactericidal activity at appropriate doses with and without coadministration of gentamicin, as demonstrated by pharmacodynamic modeling [18]. Clinically, daptomycin has been studied via the Cubicin Outcomes Registry and Experience (CORE) database by Mohr et al. [19] who found clinical cure in 87 % of VRE infections including Bacteremia. Cases of both linezolid [20, 21] and daptomycin resistance [22] have been reported in clinical isolates of enterococci confirmed by laboratory investigation.
Methods
Search strategy
The Cochrane Library, PubMed (http://www.ncbi.nlm.nih.gov/pubmed) and Scopus (http://www.scopus.com/home.url) databases were accessed on November 26, 2012. Literature in any language and any year was considered for inclusion to ensure a comprehensive search. Keywords searched included vancomycin, enterococcus, bacteremia, daptomycin, and linezolid. These were combined with the Boolean logic operator ‘AND’ to ensure results inclusive of all terms. The operator ‘OR’ was used between European brand names of medications as search terms and to account for spelling variations. Laboratory studies and studies of methicillin-resistant Staphylococcus aureus and children were excluded with the ‘NOT’ operator in the Scopus search to narrow the extensive results. Two independent reviewers screened study titles and abstracts (BS and TG) with 100 % inter-rater agreement.
Data abstraction: inclusion & exclusion criteria
Eligibility criteria for inclusion were for studies to have analyzed outcomes comparing treatment of VRE BSI with daptomycin or linezolid used as the primary intervention. To ensure consistency, only studies in which inclusion criteria mandated BSI as defined by positive blood cultures (regardless of infection source) were considered. With regard to outcomes, studies reporting an endpoint of mortality, clinical cure, or microbiologic cure were included and assessed. Any randomized control trials and cohort study designs were considered while review and opinion articles were excluded. Studies were also excluded if they were primarily pharmacologic, epidemiologic, pediatric, basic science, or did not directly investigate linezolid versus daptomycin. Articles with a primary focus of examining other specific conditions (e.g., skin and soft tissue infections, infective endocarditis, etc.) were excluded.
Data regarding characteristics and outcomes of each of the patient cohorts were manually abstracted for comparison to Microsoft Excel™ by two reviewers once article eligibility was determined (BS and TG). A spreadsheet was created from collected data based upon consistently reported baseline characteristics and comorbidities of the cohorts and three predetermined outcomes that were agreed upon by the reviewers, namely mortality, clinical and microbiologic cure. Further quantitative analysis by means of meta-analysis was also considered.
Quality assessment
Two independent reviewers (BS and RC) assessed the quality of each article using a five-step investigator-defined metric. Each metric was assessed as a 0, 1, or 2, with 0 signifying criterion not met, 1 signifying partially met, and 2 signifying completely met (Table 1). Assessed metrics included: culture diagnosis of VRE BSI and clear definition of outcomes, adjustment of outcomes for covariates, assessment of bias and confounding, and the authors’ conflict of interest.
Given that all of the included papers have cohort study designs, we further evaluated the quality of each article using the three-step metric of the Newcastle–Ottawa Quality Assessment Scale for Cohort Studies (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp). Although this scale has drawn criticism due to its unknown validity [31], we used it here to conduct a further assessment to the investigator-developed tool. Each metric was assessed as having 0–4 stars. Assessed metrics included selection, comparability, and outcomes with possible maximum scores of 4, 2, and 3 stars respectively.
Results
Search results
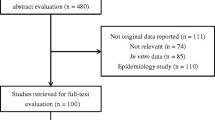
The literature search (Fig. 1) produced 18 studies from PubMed and 217 from Scopus. After removal of duplicates, 226 unique studies were identified. For the Cochrane Library, search of the keywords above eliminated any matches. Exclusion criteria eliminated 176 of the 226 articles after title review and another 42 after abstract review. Eight retrospective cohort studies evaluating a total of 807 patients and comparing outcomes of VRE BSIs treated with linezolid versus daptomycin were determined to meet all inclusion criteria and analyzed [23–30].
Outcomes
The common outcome assessed in all eight studies was mortality (Table 3). Except for the study by McKinnell et al. [27] which nearly reported statistical significance at p = 0.052, none of the studies reported significant p-values for mortality. Mortality was reported as higher in the linezolid group in two studies [23, 25], while the other five studies reported higher mortality in the daptomycin group [24, 26–30].
Microbiological cure rates were examined by three of the studies [23, 26, 30] and reported to be similar between patients receiving the two antimicrobials. Two authors also assessed clinical cure rates; while one [30] found similar cure rates between both antimicrobials, the other [23] reported a higher clinical cure rate with daptomycin (64.7 %) versus linezolid (50 %), (p = 0.19).
Other outcomes examined were unique to each particular study. For example, the study by Bio et al. [23] described duration of bacteremia, survival 7 days after end of therapy, length of stay after start of therapy, thrombocytopenia during therapy, and creatine kinase elevations during therapy. The study by Crank et al. [24] assessed duration of positive cultures and number of positive cultures and both the studies by Mave et al. [26] and Twilla et al. [30] assessed relapse of bacteremia. All of these findings were reported with no statistically significant differences, except the assessment of relapse of bacteremia in the study by Twilla et al. [30]. In this study, 12 % of those receiving daptomycin versus 3 % of those receiving linezolid (p = 0.0321) had relapse among patients who had follow-up cultures [30].
Quality assessment
All eight studies met full criteria for inclusion of patients only with culture diagnosis of VRE BSI with a clear definition of mortality and other outcomes that were identified as a primary or secondary from study outset. None achieved statistical significance for mortality data, but three of the studies adjusted these results for covariates [23, 24, 26]. Bias and confounding were discussed by all studies, but many important elements of selection bias inherent to cohort study design were not assessed even though none of the studies were prospective or randomized. As a result, no included studies completely fulfilled the metric of ‘bias & confounding’ in our quality assessment tool (Table 1). Finally, with the exception of three [26, 28, 29], each study reported at least one author with conflict of interest as defined by present or previous funding or employment by the manufacturing companies of at minimum one of the antimicrobials studied [23–25, 27, 30].
For Newcastle–Ottawa scores, all studies received three stars for the selection metric with the exception of two articles that drew data derived from selected hematology and stem cell transplant patients [25, 29]. All articles received stars for representativeness, selection of the linezolid versus daptomycin-exposed cohorts, and secure record use for attainment of data, and lost a star as they did not investigate whether there were any prior incidences of VRE BSI in their patient groups (as they were limited by the predetermined timeline of their studies). For comparability, two stars were given to each of the studies except for one which made no direct comparisons, although this was not their stated intention [29]. Finally, for assessment of our principle outcome of mortality, all three stars were awarded for record linkage, adequate length of follow-up (7 days after completion of treatment was considered adequate by reviewers) and for all subjects accounted for. Microbiologic cure and clinical cure were not reported by all studies, so these outcomes were not assessed for quality.
Baseline characteristics
The number of patients who received each antimicrobial agent varied both within and between each study. Notably, the percentage of patients receiving linezolid therapy was larger in all but two of the studies [24, 25]. Each study included a variety of patient comorbidities and all of them attempted to correlate the illness severity before initiation of antimicrobial treatment. Table 2 summarizes the characteristics consistently reported among all included studies. A few of the characteristics were noteworthy. For ICU patients there were statistically significant differences in two studies: the study by Bio et al. [23], reported treatment with linezolid in 87.2 % of the patients compared to daptomycin in 64.9 % (p = 0.02); while the study by Mave et al. [26] reported the use linezolid in 29.4 % and daptomycin in 53.3 % of ICU patients (p = 0.03). A larger proportion of patients in the linezolid group was significantly older in two studies [26, 30] and more male patients were treated with daptomycin overall [23–28, 30].
While not explicitly mentioned by some of the other studies, the study by Crank et al. [24] noted that their cohort contained a sizeable portion (31.8 %) of daptomycin-treated patients that initially received linezolid. Five patients were changed due to treatment failure, four due to intolerance, and twelve due to physician preference [24]. Furthermore, 19.4 % of the same group was also on concurrent treatment with another antimicrobial, such as gentamicin (14.9 %) and rifampin (4.5 %). The study by Weinstock et al. [29] also reported patients whose antibiotics were changed. Finally, we found differences in the comorbidity scoring systems utilized by most studies. The Charlson Comorbidity Index [32] was most commonly used [23, 26–28]. Two of the studies [23, 26] reported APACHE II scores [33].
Discussion
Summary of evidence
The purpose of this review is to uncover whether choice of antibiotic for treatment of VRE BSIs can affect patient outcomes. Our results show that most but not all studies reported a lower mortality in patients treated with linezolid compared to those treated with daptomycin; however, these differences as well as all of the other outcomes evaluated in this review were not statistically significant. Clinical cure was examined by only two of the studies [23, 30] favoring daptomycin, while microbiological cure is less convincing in favor of an antibiotic over the other. Importantly, these differences are likely multifactorial considering that all of the studies published to date and included in this review are retrospective cohorts (Table 3).
Limitations
The most notable limitation of investigating this topic may appear to be the lack of meta-analysis, as given the common assessment of mortality there is potential. Due to the inherent weaknesses found within the retrospective study design of available studies, numerous differences among the cohorts, suspected heterogeneity, and differing definitions of mortality, a meta-analysis was determined to be of little value and not included here. Furthermore, inconsistency in reporting of patient baseline characteristics, illness severity scores, and outcomes was noted. Thus, we could not effectively compare the cohorts and outcomes using current statistical methods.
The cohorts themselves hold many limitations. The data reported by these studies is largely dependent on the assumed accurate record keeping of the clinicians involved with each patient’s care. There can be limitations in identifying institutional prescribing practices and susceptibility reporting by microbiology laboratories on the selection of antimicrobials, none of which are evaluated in the studies. Furthermore, there are multiple sources of bias such as: possible selective use of daptomycin in patients who are severely ill to avoid linezolid toxicities, hospital formulary of choice, drug costs or previous linezolid failures.
Conclusion
Differences in outcomes may exist between patients treated with daptomycin or linezolid for VRE BSI, but they cannot be ascertained from currently available literature. Thus, both antibiotics remain feasible options. Clinical insight including patient-specific comorbidities, side-effect profiles, convenience, availability, and costs will continue to be primary determinants of antibiotic choice. Well-controlled prospective studies assessing clinical outcomes are very clearly needed for further guidance.
While this conclusion is unable to help inform clinical judgment, there is much that we can ascertain from these studies to inform future study design. Among these is the need for larger sample sizes, clearer definitions of outcomes, accountability for medication toxicities and contraindications, and the effects of cost and formularies. Collaborations to maximize sample size with use of standardized definitions and appropriate comorbidity scoring systems could better help uncover the optimal antimicrobial therapy for the management of VRE BSIs.
References
Society for Healthcare Epidemiology of America, Infectious Diseases Society of America, Pediatric Infectious Diseases Society. Policy statement on antimicrobial stewardship by the society for healthcare epidemiology of America (SHEA), the infectious diseases society of America (IDSA), and the pediatric infectious diseases society (PIDS). Infect Control Hosp Epidemiol. 2012;33(4):322–327.
Uttley AHC, Collins CH, Naidoo J, George RC. Vancomycin-resistant enterococci. Lancet. 1988;1(8575–8576):57–8.
Zhao C, Sun H, Wang H, Liu Y, Hu B, Yu Y, et al. Antimicrobial resistance trends among 5608 clinical gram-positive isolates in China: results from the gram-positive cocci resistance surveillance program (2005–2010). Diagn Microbiol Infect Dis. 2012;73(2):174–81.
Adam HJ, DeCorby M, Rennie R, Karlowsky JA, Hoban DJ, Zhanel GG, Canadian Antimicrobial Resistance Alliance (CARA). Prevalence of antimicrobial resistant pathogens from blood cultures from Canadian hospitals: results of the CANWARD 2007–2009 study. Diagn Microbiol Infect Dis. 2011;69(3):307–13.
Biedenbach DJ, Bell JM, Sader HS, Fritsche TR, Jones RN, Turnidge JD. Antimicrobial susceptibility of gram-positive bacterial isolates from the Asia-Pacific region and an in vitro evaluation of the bactericidal activity of daptomycin, vancomycin, and teicoplanin: a SENTRY program report (2003–2004). Int J Antimicrob Agents. 2007;30(2):143–9.
Gales AC, Sader HS, Ribeiro J, Zoccoli C, Barth A, Pignatari AC. Antimicrobial susceptibility of gram-positive bacteria isolated in Brazilian hospitals participating in the SENTRY program (2005–2008). Braz J Infect Dis. 2009;13(2):90–8.
Cattoir V, Leclercq R. Twenty-five years of shared life with vancomycin-resistant enterococi: is it time to divorce? J Antimicrob Chemother. 2013;68(4):731–42.
Deshpande LM, Fritsche TR, Moet GJ, Biedenbach DJ, Jones RN. Antimicrobial resistance and molecular epidemiology of vancomycin-resistant enterococci from North America and Europe: a report from the SENTRY antimicrobial surveillance program. Diagn Microbiol Infect Dis. 2007;58(2):163–70.
Arias CA, Murray BE. The rise of the enterococcus: beyond vancomycin resistance. Nat Rev Microbiol. 2012;10(4):266–78.
Murray BE. Vancomycin-resistant enterococcal infections. N Engl J Med. 2000;342(10):710–21.
Salgado CD, Farr BM. Outcomes associated with vancomycin-resistant enterococci: a meta-analysis. Infect Control Hosp Epidemiol. 2003;24(9):690–8.
DiazGranados CA, Zimmer SM, Klein M, Jernigan JA. Comparison of mortality associated with vancomycin-resistant and vancomycin-susceptible enterococcal bloodstream infections: a meta-analysis. Clin Infect Dis. 2005;41(3):327–33.
Courvalin P. Vancomycin resistance in gram-positive cocci. Clin Infect Dis. 2006;42(SUPPL. 1):S25–34.
Nailor MD, Sobel JD. Antibiotics for gram-positive bacterial infections: vancomycin, teicoplanin, Quinupristin/Dalfopristin, oxazolidinones, daptomycin, dalbavancin, and telavancin. Infect Dis Clin North Am. 2009;23(4):965–82.
Pogliano J, Pogliano N, Silverman JA. Daptomycin-mediated reorganization of membrane architecture causes mislocalization of essential cell division proteins. J Bacteriol. 2012;194(17):4494–504.
Diekema DI, Jones RN. Oxazolidinones: a review. Drugs. 2000;59(1):7–16.
Cantón R, Ruiz-Garbajosa P, Chaves RL, Johnson AP. A potential role for daptomycin in enterococcal infections: what is the evidence? J Antimicrob Chemother. 2010;65(6):1126–36.
DeRyke CA, Sutherland C, Zhang B, Nicolau DP, Kuti JL. Serum bactericidal activities of high-dose daptomycin with and without coadministration of gentamicin against isolates of staphylococcus aureus and enterococcus species. Antimicrob Agents Chemother. 2006;50(11):3529–34.
Mohr JF, Friedrich LV, Yankelev S, Lamp KC. Daptomycin for the treatment of enterococcal bacteraemia: results from the cubicin outcomes registry and experience (CORE). Int J Antimicrob Agents. 2009;33(6):543–8.
Dibo I, Pillai SK, Gold HS, Baer MR, Wetzler M, Slack JL, et al. Linezolid-resistant enterococcus faecalis isolated from a cord blood transplant recipient. J Clin Microbiol. 2004;42(4):1843–5.
Gonzales RD, Schreckenberger PC, Graham MB, Kelkar S, DenBesten K, Quinn JP. Infections due to vancomycin-resistant enterococcus faecium resistant to linezolid. Lancet. 2001;357(9263):1179.
Kelesidis T, Humphries R, Uslan DZ, Pegues DA. Daptomycin nonsusceptible enterococci: an emerging challenge for clinicians. Clin Infect Dis. 2011;52(2):228–34.
Bio LL, Perez ME, MacDougall C, Gallagher JC. Comparison of linezolid and daptomycin in the treatment of vancomycin-resistant enterococcal bacteremia. Infect Dis Clin Pract. 2011;19(5):343–7.
Crank CW, Scheetz MH, Brielmaier B, Rose WE, Patel GP, Ritchie DJ, et al. Comparison of outcomes from daptomycin or linezolid treatment for vancomycin-resistant enterococcal bloodstream infection: a retrospective, multicenter, cohort study. Clin Ther. 2010;32(10):1713–9.
Kraft S, Mackler E, Schlickman P, Welch K, DePestel DD. Outcomes of therapy vancomycin-resistant enterococcal bacteremia in hematology and bone marrow transplant patients. Support Care Cancer. 2012;20(9):1935–6.
Mave V, Garcia-Diaz J, Islam T, Hasbun R. Vancomycin-resistant enterococcal bacteraemia: is daptomycin as effective as linezolid? J Antimicrob Chemother. 2009;64(1):175–80.
McKinnell JA, Patel M, Shirley RM, Kunz DF, Moser SA, Baddley JW. Observational study of the epidemiology and outcomes of vancomycin-resistant enterococcus bacteraemia treated with newer antimicrobial agents. Epidemiol Infect. 2011;139(9):1342–50.
Chou CH, Lee NY, Lee HC, Chang CM, Lee CC, Ko WC. Emergence of vancomycin-resistant enterococcus bloodstream infections in Southern Taiwan. J Microbiol Immunol Infect. 2012;45(3):221–7.
Weinstock DM, Conlon M, Iovino C, Aubrey T, Gudiol C, Riedel E, et al. Colonization, bloodstream infection, and mortality caused by vancomycin-resistant enterococcus early after allogeneic hematopoietic stem cell transplant. Biol Blood Marrow Transpl. 2007;13(5):615–21.
Twilla JD, Finch CK, Usery JB, Gelfand MS, Hudson JQ, Broyles JE. Vancomycin-resistant enterococcus bacteremia: an evaluation of treatment with linezolid or daptomycin. J Hosp Med. 2012;7(3):243–8.
Stang A. Critical evaluation of the newcastle-ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83.
Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–29.
Acknowledgments
The authors would like to thank Dr. Leonardo Tamariz for guidance in data abstraction and preliminary analysis.
Funding
None.
Conflicts of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shukla, B.S., Gauthier, T.P., Correa, R. et al. Treatment considerations in vancomycin-resistant enterococcal bacteremia: daptomycin or linezolid? A review. Int J Clin Pharm 35, 697–703 (2013). https://doi.org/10.1007/s11096-013-9825-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-013-9825-5