Abstract
Objective The therapeutic benefit of inhaled corticoids in bronchiectasis not due to cystic fibrosis is still not well documented. The aim of the present study was to assess the efficacy and safety of inhaled corticoids in this disease. Setting This study was conducted at a tertiary university hospital in the city of Barcelona, Catalonia, (Spain). Method A prospective, double-blind, parallel, placebo-masked study was conducted. Seventy-seven patients (40 women; mean age: 68 years) were randomly assigned to receive either 400 mcg budesonide twice daily or placebo and were regularly reviewed for six months. Results Differences in forced vital capacity and forced expiratory volume in the first second between the beginning and end of the study were not significantly lower in the budesonide group than in the placebo group, either in absolute values [−17.4 (386.9) versus −21.4 (375.5)] or in percentages [−1.9(9.5) versus −2.8 (11.6)]. Microbiological criteria applied to evaluate changes between the beginning and end of the study showed no worsening in the budesonide group compared with the control group, whereas a non-significant improvement was obtained in 8.1 % of cases in the budesonide group compared to 3 % in the placebo group. Although significance was only achieved for sputum eosinophils (p = 0.021), a consistent tendency towards improvement was also observed in secondary end-points (symptoms, number and duration of exacerbations, quality of life, sputum cytology and interleukin-8) in the budesonide group. Conclusion Although further studies are required, inhaled corticoid treatment may be efficacious and safe in bronchiectasis not due to cystic fibrosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Impact of findings on practice
-
Inhaled corticosteroids may improve the overall condition of patients with bronchiectasis not due to cystic fibrosis.
-
A 6-month inhaled corticoid treatment appears not to worsen chronic bronchial infection in patients with bronchiectasis not due to cystic fibrosis.
Introduction
Bronchiectasis constitutes the final stage in a series of pathological processes that cause dilatation and persistent inflammation of the bronchial tree. This inflammation stems from the maintained, uncontrolled migration of neutrophils to the area affected by the imbalance of cytokines released by epithelial cells and macrophages [1]. Patients with bronchiectasis suffer recurrent bronchial infections, probably facilitated by the existing chronic inflammation, a fact is reported by some authors as the vicious circle of bronchiectasis in which inflammation and infection are self-maintained [2].
Corticoid Inhalation delivers the drug directly to the airways where it acts locally and minimises the systemic side effects associated with oral or parenteral administration [3]. The anti-inflammatory efficacy of inhaled corticosteroids in the bronchial tree has been demonstrated in inflammatory airway diseases such as asthma [4] and chronic obstructive pulmonary disease (COPD) [5]. Patients with bronchiectasis could benefit from the anti-inflammatory effect of these drugs although, owing to their immunosuppressant effect [6], the frequency and severity of bronchial infections could be increased. Two recent reviews [7, 8] suggested there is still insufficient evidence to support the use of corticoids in patients with bronchiectasis secondary or not to cystic fibrosis. However, inhaled corticosteroids are frequently used in clinical practice in these patients with symptoms of bronchial hyperreactivity or because it is believed that these drugs could reduce inflammation in this disease.
Aim of the study
The present work aimed to evaluate the efficacy and safety of inhaled budesonide in patients with bronchiectasis not due to cystic fibrosis using a study which included follow-up of clinical-functional variables, quality of life, inflammatory parameters and bacteriological findings.
Method
A prospective, 6-month, randomised, double-blind, parallel, placebo-masked clinical trial was conducted. Randomisation was performed by hospital pharmacy personnel and the treatment assignment was not revealed to the study investigators until all participants had completed the study. Randomisation was performed to ensure that all treatment groups were balanced. Patients were divided into two groups: one received 400 mcg of inhaled budesonide administered by a metered-dose inhaler using a spacer chamber (Nebuhaler®) twice daily, and the other a placebo by a metered-dose inhaler with a spacer chamber. Eight hundred mg of budesonide daily were used since this is the dose usually recommended in other inflammatory airway diseases such as asthma or COPD [4, 5]. Patients were instructed in the inhalation technique by the same physician (MED) according to the recommended instructions [9]. The follow-up period was six months. Adherence and compliance were assessed following the count of returned (used and/or unused) medication.
Over a 3-year period, 77 consecutive patients aged 18 or over (mean: 68.06; range: 40–85) previously seen at the Pneumology Outpatient Clinic of our centre and who fulfilled the study requirements were assessed. The diagnosis of bronchiectasis was made by the same pneumologist (RO) and confirmed by a radiologist based on a high-resolution chest CT. Sweat test and blood analysis for the most frequent cystic fibrosis mutations in Spain were negative [10]. Bronchiectasis aetiology was: idiopathic (budesonide group n = 31), post-tuberculous (n = 26), post-pneumonia (n = 10), IgG deficiency (n = 3) and others (n = 7). Patients had to be clinically stable of their basal disease. Exclusion criteria were corticosteroid treatment or acute exacerbations in the previous 30 days, allergy to budesonide, bronchial asthma, any other chronic disease, e.g. heart, kidney and liver failure, and any other illness with life expectancy under 2 years that could interfere with the study.
Symptom severity or frequency was measured every month on a scale of 0–3 similar to that reported by Portenoy et al. [11]. Cough and sputum production were measured as follows: 0: absent, 1: rarely, 2: frequently, 3: constantly, and dyspnoea as: 0: absent, 1: becoming short of breath when hurrying on the level or walking up a slight slope, becoming short of breath when walking at one’s own pace on the level, and 3: breathlessness on leaving home or when dressing or undressing. Fever was measured as follows: 0: <37 °C, 1: 37–37.5 °C, 2: 37.6–38 °C, 3: >38 °C. The symptom scale was explained by the same physician (MED). At each visit, this physician asked the patients to classify all symptoms observed throughout the month and checked whether the patient had followed the correct criteria. Exacerbation episodes were defined as worsening of more than 48 h’ duration of at least three of the four symptoms. The number of treatment days in cases of exacerbation, whether ambulatory or with hospitalisation, was also recorded. Patient quality of life was evaluated by the St. George’s Questionnaire [12] at baseline and at the end of the study. Forced vital capacity (FVC), forced respiratory volume in the first second (FEV1) and bronchodilator test were evaluated according to the American Thoracic and European Respiratory Societies criteria [13] at the beginning and end of the study. The bronchodilator test was considered positive if an increase of 200 ml and 12 % was recorded in FEV1 after salbutamol administration by a metered-dose inhaler.
Spontaneous sputum samples were collected monthly from all patients. Sputum was processed by the technique described by Pizzichini et al. [14]. Sputum was separated microscopically from contaminating saliva. The selected portion was placed in a test tube, weighed and mixed with four volumes of dithiotheitol solution. The preparation was vortexed for 15 s and then rocked gently for 15 min and 4 volumes of Dulbecco’s PBS were added. The suspension was filtered through a 48-μm nylon gauze (Millipore Corporate Headquarters, MA, USA) and centrifuged at 2,500 rpm for 10 min. The result obtained was a cell pellet and a supernatant solution. The supernatant was separated and stored at −80 °C for inflammatory marker analysis. The cell pellet material was resuspended in 500–1,000 μl (depending on macroscopic estimation of the sediment size) of Dulbecco’s PBS. Total cell count and cell viability were determined. The cell suspension was then mixed with Dulbecco’s PBS to obtain a quantity of 0.6 – 1 × 106 cells/ml of suspension. Cystospin cell preparations were then prepared using a cytocentrifuge. Cytospin slides with 400 cells per slide were stained with May-Grünwald-Giemsa to obtain the cell count. Sputum samples weighed more than 0.05 g, with 60 % viability and less than 20 % squamous cells. IL-8 levels in the supernatant of patient sputum and serum samples were determined by ELISA (Bender Medsystem, USA) at the beginning and end of the study.
A sputum sample was shipped to the laboratory after each monthly visit and also in cases of exacerbation or admission. A quantitative bacteriological culture was performed and tobramycin sensitivity was determined. The sputum sample, after determination of its quality by Gram staining [15], was studied microbiologically. All samples were cultured conventionally in blood agar, McConkey and Saboraud media and semiquantitatively cultured with calibrated loop, and over 106 colony-forming units per millilitre (CFU/ml) of sample were detected. Isolated micro-organisms were identified by standardised or automated means and the antibiogram performed using the Kirby-Bauer technique [16]. According to baseline microbiological findings, patients were classified as those with normal flora (Group A), presence of bacteria other than Pseudomonas aeruginosa (Group B) and presence of P. aeruginosa (Group C). Microbiological worsening was considered when any germ of groups B or C was isolated in a group A case, when any group C germ was isolated in a group B case or if, in a group C case, another non-fermenting gram-negative bacillus was isolated in the study. Improvement was considered if, during the study, a patient could not be included in his/her group and was changed to a better microbiological group (from C to B or A; from B to A).
The study was approved by the Ethics Committee of our centre. All patients gave their written informed consent prior to inclusion in the study.
Statistical analysis
Statistical analysis of efficacy was made only in patients who completed the treatment period. Initially, a confirmatory analysis was made to assess the comparability of the two study groups. For descriptive purposes, conventional mean, standard deviation and frequency tables were used, regardless of the distribution pattern.
No sample size was measured in this study, since the outcome of therapeutic interventions in bronchiectasic patients is not well established. Thus, the nature of the study was essentially exploratory and no sample-size calculations were made.
Functional parameters (FVC and FEV1) and microbiological changes between baseline and the end of the study were the primary end-points for evaluating efficacy and safety, respectively. Safety was evaluated under intention-to-treat conditions. Student’s t test was used to compare means (Mann–Whitney U test, when necessary). Chi-square test (Fisher’s exact test, when necessary) was used to compare proportions.
The potential influence of other variables, such as age, smoking or initial bacteriological group, was also studied by multiple regression and analysis of variance (Krushkal-Wallis test, when necessary). Statistical analysis was made using the SPSS v.12.0 (SPSS Inc. USA) software package. For single outcome comparisons, the treatment effect was considered if p values were 0.05 or less.
Results
Characteristics at the start of the study
Initially, seventy-seven patients were included in the clinical trial. Seven did not complete the study: three died from respiratory failure (all of the placebo group) and four voluntarily decided to abandon the study (three of the placebo group and one of the budesonide group). Patients were distributed in two study groups. No statistically-significant differences were found in any of the clinical or demographic characteristics at the start of the study (Table 1). Except for cough after inhalation (two patients in the placebo group and three in the budesonide group), no other adverse effects were observed.
Clinical-functional criteria and quality of life
At the end of the study, greater preservation, though without statistical significance, of all the functional parameters studied was observed in the budesonide-treated group (Table 2).
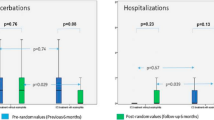
Mean total score of pulmonary symptoms between the beginning and end of the study showed no significant differences, although a tendency (p = 0.105) towards improvement was observed in the budesonide group (−0.70 ± 2.08) compared with the placebo group (−0.18 ± 2.5 %). Similarly, a tendency towards improvement in the remaining clinical variables analysed was verified in the budesonide group. The number of patients with exacerbations and admissions was lower in the budesonide group. Furthermore, the number of exacerbations and days of hospitalisation per patient were also lower in the budesonide group (Table 2). When quality of life was analysed, no significant differences were observed between groups. However, improvement in the quality of life of patients treated with budesonide compared with the placebo group was also observed (Table 2).
Analysis among groups A, B and C showed no significant differences in any of the clinical-functional variables.
Inflammatory criteria
Serum IL-8 values showed a non-significant decrease between the beginning and end of the study in the budesonide group compared with the placebo group. Eosinophil number in sputum fell significantly (p = 0.021) in the budesonide group compared with the placebo group. No significant differences were observed in the remaining cell types or in the analysis among groups A, B and C (Table 2).
Microbiological criteria
No worsening was observed in budesonide-treated patients compared with controls. Furthermore, 8.1 % of patients treated with budesonide showed an improvement in the established microbiological criteria, while only 3.0 % did so in the placebo group (Table 3).
No influence of other variables, such as age, smoking or initial bacteriological group, was observed on results (Tables 2 and 3).
Discussion
In the present study, a tendency was observed towards improvement in clinical symptoms, quality of life, inflammatory parameters and lung function in patients with bronchiectases not due to cystic fibrosis treated with inhaled budesonide compared with those receiving placebo. Moreover, inhaled corticoids were not found to provoke worsening of the chronic bronchial infection in patients with bronchiectases not due to cystic fibrosis.
The main limitation of this exploratory study was the difficulty in drawing clear and definite conclusions owing to the limited number of patients involved. Nevertheless, our report adds information on the efficacy and safety of inhaled corticoids in bronchiectasis not due to cystic fibrosis, aspects for which a recent systematic review concluded there was insufficient evidence [17].
A bronchial inflammatory response exists in bronchiectases not due to cystic fibrosis even when the patient is not in an acute phase [18]. In other bronchial diseases, inhaled corticoid treatment has produced a reduction in bronchial inflammation and improvement in clinical function parameters [19–21]. Thus, it has been postulated that a decrease in that inflammation might also be a beneficial factor in bronchiectases not due to cystic fibrosis.
Elborn et al. [22] analysed a group of 20 patients with bronchiectases; of these, only 13 treated with 1,500 mcg of betametasone for 6 weeks completed the study. The authors observed a significant improvement in sputum volume reduction, FEV1, morning peak expiratory flow rate and symptom scores for cough. Tsang et al. [23] conducted a similar clinical trial with 24 patients treated with 1,000 mcg/day of fluticasone for 4 weeks. They observed a significant reduction in leukocyte density in sputum, though only a tendency towards improvement in pulmonary function. A study by Joshi [24], conducted in 20 patients treated with 800 mcg/day for 4 weeks, showed functional improvement; however, a postbronchodilator response could have biased the study in favour of response to inhaled corticoids. Later, Tsang et al. [25], studying 86 patients with bronchiectases treated with 1,000 mcg/day of fluticasone for 52 weeks, reported that fewer patients complained of cough and a significant reduction was observed in sputum volume in those treated with fluticasone. Moreover, a reduction in exacerbation frequency in the subgroup of patients with P. aeruginosa infection (12 patients) was also observed. However, in the whole group of patients studied and also in the subgroup with P. aeruginosa, no significant improvement was recorded in any of the other variables studied, including functional parameters. Finally, Martinez-Garcia et al. [26], in a study conducted in 86 patients with a duration of 6 months, observed an improvement in dyspnoea, days without cough, sputum production, short-acting β-2 agonist use and quality of life, though without changes in lung function, in the group treated with fluticasone 1,000 mcg/day compared with the other group treated with 500 mcg. In the present study, a constant tendency was found towards improvement in clinical, functional and inflammatory parameters. However, only a significant decrease in eosinophils in sputum was observed in the budesonide-treated group. Although a rise in eosinophil levels is a constant in bronchiectases not due to cystic fibrosis, its exact role in the pathogenic mechanism and a possible effect of inhaled corticoids remain unknown [18]. We consider that our findings point to a promising role of inhaled corticosteroids in bronchiectases not due to cystic fibrosis which should be confirmed in further studies.
Long-term systemic treatment with corticoids produces deregulation in cell immunity and a direct stimulatory effect on the growth of opportunistic germs, leading to an increase in susceptibility to opportunistic infections [27]. In this context, the use of inhaled corticoids, which permits high levels of the drug to reach the airways, could be considered counter-productive in patients with bronchiectases not due to cystic fibrosis, since a chronic bronchial infection was observed in 64 % of patients with this disease [28]. Tsang et al. [19] studied this hypothesis and found that no significant differences existed in the bacterial density of P. aeruginosa or in the total number of bacteria in sputum in a group treated with fluticasone compared with a control group. However, the small sample size (24 patients) and short exposure (4 weeks) limited the conclusions. Martinez-Garcia et al. [26] reported no significant changes in the number of chronic colonisations in groups treated with 0, 500 and 1,000 mcg/day of fluticasone during a 6-month study versus the pre-randomisation percentages. Concurring with the above-mentioned reports [23, 26], our study did not cause worsening of the bronchial infection or an increase in susceptibility to opportunistic infections. Thus, although understandable concern exists regarding overgrowth or the appearance of bacterial resistance with prolonged use of inhaled corticoids, and after an overall assessment of our study and the results published in the literature, we believe these drugs could be used if a patient requires them, e.g. as a consequence of associated symptoms of bronchial hyperreactivity.
Conclusion
Although several functional, clinical and inflammatory parameters showed a tendency to improve, except for the number of eosinophils in sputum, none of the clinical end-points reached statistical significance. The use of inhaled corticosteroids in bronchiectases not due to cystic fibrosis did not cause worsening of the bronchial infection, at least during the 6 months of our study. We believe further studies are required to definitively clarify whether long-term inhaled corticoids should be used in patients with bronchiectases not due to cystic fibrosis.
References
Levine SJ. Bronchial epithelial cell cytokine interactions in airway inflammation. J Investig Med. 1995;43:241–9.
Wilson R. The pathogenesis and management of bronchial infections: the vicious cycle of respiratory decline. Rev Contemp Pharmacother. 1992;3:103–12.
Derendorf H, Nave R, Drollmann A, Cerasoli F, Wurst W. Relevance of pharmacokinetics and pharmacodynamics of inhaled corticosteroids to asthma. Eur Respir J. 2006;28:1042–50.
From the Global Strategy for Asthma Management and Prevention. Global Initiative for Asthma (GINA) 2010. Available from: http://www.ginasthma.org.
From the Global Strategy for the Diagnosis, Management and Prevention of COPD. Global initiative for chronic obstructive lung disease (GOLD) 2010. Available from: http://www.goldcopd.org.
King P. Is there a role for inhaled corticosteroids and macrolide therapy in bronchiectasis? Drugs. 2007;67:965–74.
Inhaled corticosteroids for cystic fibrosis (Review). The Cochrane collaboration 2009.
Inhaled corticosteroids for bronchiectasis (Review). The Cochrane collaboration 2009.
Giner J, Basualdo LV, Casan P, Hernandez C, Macián V, Martínez I, et al. Normativa sobre la utilización de fármacos inhalados. Recomendaciones SEPAR. Arch Bronconeumol. 2000;36:34–43.
Casals T, Nunes V, Palacio A, Giménez J, Gaona A, Ibáñez N, et al. Cystic fibrosis in Spain: high frequency of mutation G542X in the Mediterranean coastal area. Hum Genet. 1993;91:66–70.
Portenoy RK, Thaler HT, Kornblith AB, Lepore JM, Friedlander-Klar H, Kiyasu E, et al. The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer. 1994;30:1326–36.
Ferrer M, Alonso J, Prieto L, Plaza V, Monso E, Marrades R, et al. Validity and reliability of the St George’s Respiratory Questionnaire alter adaptation to a different language and culture: the Spanish example. Eur Respir J. 1996;9:1160–6.
Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38.
Pizzichini E, Pizzichini MM, Efthimiadis A, Hargreave FE, Dolovich J. Measurement of inflammatory indices in induced sputum: effects of selection of sputum to minimize salivary contamination. Eur Respir J. 1996;9(6):1174–80.
Murray PR, Washington JA. Microscopic and bacteriologic analysis of expectorated sputum. Mayo Clinic Proc. 1975;50:339–44.
Bauer AW, Kirby WMM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45:493–6.
Kapur N, Bell S. Kolbe J, Chang AB. Inhaled steroids for bronchiectasis. Cochrane Database Syst Rev. 2009; 1. Art. no.: CD000996. doi:10.1002/14651858.CD000996.pub2.
Fuschillo S, De Felice A, Balzano G. Mucosal inflammation in idiopathic bronchiectasis: cellular and molecular mechanisms. Eur Respir J. 2008;31:396–406.
Phua GC, Macintyre NR. Inhaled corticosteroids in obstructive airway disease. Respir Care. 2007;52:852–8.
Sutherland R, Allmers H, Ayas NT, Venn J, Martin RJ. Inhaled corticosteroids reduce progression of airflow limitation in chronic obstructive pulmonary disease: a meta-analysis. Thorax. 2003;58:937–41.
Escotte S, Tabary O, Dusser D, Majer-Teboul C, Puchelle E, Jacquot J. Fluticasone reduces IL-6 and IL-8 production of cystic fibrosis bronchial epithelial cells via IKK-β kinase pathway. Eur Respir J. 2003;21:574–81.
Elborn JS, Johnston B, Allen F, Clarke J, McGarry J, Varguese G. Inhaled steroids in patients with bronchiectasis. Respir Med. 1992;86:121–4.
Tsang KWT, Ho PL, Lam WK, Ip MSM, Chan KN, Ho CS, et al. Inhaled fluticasone reduces sputum inflammatory indices in severe bronchiectasis. Am J Respir Crit Care Med. 1998;158:723–7.
Joshi JM, Sundaram P. Role of inhaled steroids in stable bronchiectasis. Indian Pract. 2004;57(4):243–5.
Tsang KW, Tan KC, Ho PL, Ooi GC, Ho JC, Mak J, et al. Inhaled fluticasone in bronchiectasis: a 12 month study. Thorax. 2005;60:239–43.
Martínez-García MA, Perpiñá-Tordera M, Román-Sánchez P, Soler-Cataluña JJ, Carratalá A, Pastor M. Inhaled steroids improve quality of life in patients with steady-state bronchiectasis. Respir Med. 2006;100:1623–32.
Lionakis MS, Kontoyiannis DP. Glucocorticoids and invasive fungal infections. Lancet. 2003;362:1828–38.
Angrill J, Agustí C, de Celis R, Rañó A, Gozalez J, Solé T, et al. Bacterial colonisation in patients with bronchiectasis: microbiological pattern and risk factors. Thorax. 2002;57:15–9.
Acknowledgments
The authors wish to thank Christine O’Hara for her help with the English translation of the manuscript and Rosa Llòria for editorial assistance.
Funding
This work was supported by the Catalan Foundation of Pneumology (FUCAP) through a grant attributed to RH, MED and RO.
Conflicts of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hernando, R., Drobnic, M.E., Cruz, M.J. et al. Budesonide efficacy and safety in patients with bronchiectasis not due to cystic fibrosis. Int J Clin Pharm 34, 644–650 (2012). https://doi.org/10.1007/s11096-012-9659-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-012-9659-6