Abstract
Background The consumption of some psychotropic medicines has a negative effect on the fitness to drive. Pharmacists are expected to give useful advice to patients on their participation in traffic. However, almost no information is available on this topic. Objective To assess the effect of training and implementation of new dispensing guidelines with regard to driving-impairing medicines, in two types of dispensing support tools. User acceptance was measured as well as the effect on pharmacists’ attitudes & awareness, self-reported behaviour and knowledge. Setting Pharmacists from East Flanders in Belgium. Methods Two intervention groups and a control group participated. The intervention groups followed a training and were provided with a dispensing support tool containing information on the effect of medicines on driving ability, which was either stand-alone (USB stick) or integrated into the daily used software (ViaNova). The three groups filled out a questionnaire prior to and after the intervention period. Main outcome measure Answers to a pre/post-questionnaire on attitudes and awareness, self-reported behaviour, knowledge and user acceptance. Results Many pharmacists were already strongly interested in the topic at the beginning of the study. Positive changes in attitude, self-reported behaviour and knowledge were measured mostly in the group of pharmacists for which the information was integrated in their daily used software. These pharmacists asked significantly more about the patients’ driving experience, informed them more about driving-related risk and gave more detailed information on impairing effects of medicines. The knowledge of the participating pharmacists on the topic ‘medicines and driving’ remained generally low. The participants acknowledge the importance of being aware of the topic medicines and driving but they report a lack of information or education. They strongly prefer a tool that integrates the information in their daily used software. Conclusion Dispensing support tools with information on the potential impairing effect of a medicine on the fitness to drive increases awareness, reported risk communication behaviour as well as knowledge of pharmacists on this topic. Computerised dispensing support tools are most effective when the information is integrated into the daily used dispensing software.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Impact of findings on practice
-
Including risk information into the daily used dispensing software in a community pharmacy can change the counselling behaviour of pharmacists.
-
There are opportunities to improve the risk communication between pharmacists and patients with regard to medicines and driving.
Introduction
It is known since many years that the consumption of some psychotropic medicines has a negative effect on fitness to drive. Either taken alone or in combination with other substances such as alcohol, these medicines increase the risk of having a traffic accident [1–4]. The impairing medicinal effects not only depend on the active substance in the medicine but also on dose, time interval and indication or individual variations. Therefore it is important to inform drivers who use driving-impairing medicines on the risk of driving under the influence.
All psychotropic medicines are provided with a package information leaflet for the patient. However, the information it contains does not always provide proper advice for the patient on his/her participation in traffic. Healthcare professionals, like physicians and pharmacists, are expected to provide such information at the time of prescribing or dispensing a medicine [5]. In the past few years pharmacists started to play a more central role in providing information to patients when delivering a medicine [6–8]. The important role of pharmacists in the health information transfer to patients is also acknowledged in Belgium. In April 2010, a new system of remuneration for pharmacists came into force. Its objective is to reinforce the intellectual role of the pharmacist and to partly disconnect the remuneration based on the price of medicines [9].
Practitioners admit though that there is a need to use guidelines for safe prescribing and dispensing of medicines to patients who operate motor vehicles or other transportation vehicles but practical recommendations are not common [10]. With regard to pharmacists, such guidelines can help them to comply with their (risk communicating) role and allow them to provide more concrete information to the patient shortly before administration. Within the European project DRUID (driving under the influence of drugs, alcohol and medicines) prescribing/dispensing guidelines and a European medicinal categorisation system were developed. The emphasis of the DRUID dispensing guidelines lays on using safer medicines if available, and on improving the warning and counselling of patients about the risks and on how to act responsibly when using medicines that have the potential to impair driving [11]. In order to meet this goal, experts involved in DRUID proposed a four level classification and labelling system regarding the influence of a medicine on driving performance, from category 0 (no or negligible influence on fitness to drive) to category 3 (major influence on fitness to drive) [12]. The proposed categorisation system was made compatible with existing national labelling systems such as in Spain and France. About 1,500 medicines were categorised with regard to their influence on fitness to drive.
Moreover, fact sheets were produced including information on indications, posology and method of administration, pharmacodynamic and pharmacokinetic properties, possible side-effects related to driving and the DRUID risk category of individual medicines. The aim of these fact sheets was to provide healthcare professionals with appropriate information on possible effects of medicines on driving that could be easily used in their (risk) communication with patients.
Even though some information is available, the problem of medicine-impaired driving remains complex. Physicians and pharmacists have to determine case-wise whether or not a particular patient will become an unsafe driver after using a specific psychotropic medicine. Practical guidelines with emphasis on prescribing and dispensing practices can assist the evaluation of the fitness to drive of patients undergoing medicinal treatment. Yet simple top-down dissemination of guidelines towards healthcare professionals alone has been proven ineffective [13–16]. For example, previous research concluded that purely disseminating guidelines (among physicians) had a minor impact on the prescribing behaviour [17, 18].
A more effective implementation strategy is the use of automatic computerised reminders in the daily used prescribing and dispensing software [19–21]. Computerised reminders and alerts are an increasingly common means of delivering support to physicians and other healthcare professionals, and their use is likely to increase as electronic medical records are used more widely. Furthermore, computerised prompts and reminders are effective in changing the behaviour of healthcare professionals in a variety of settings [5, 22, 23]. As pharmacists generally use a computer for dispensing, the potential of computerised decision-support for promoting clinical interventions that improve patient care and enhance pharmacists’ risk communication role is huge.
Since October 2008 the Dutch government funded the development of a software-oriented support in dispensing pharmacy practices in the Netherlands. A categorisation system was made available in all Dutch pharmacy dispensing systems. Health Base Foundation supports further use of counselling information while dispensing medicines. In Belgium, one company (ESCAPO) uses the information provided by the Health Base Foundation as input for their dispensing support tool ‘ViaNova’. Apart from the ViaNova software different other dispensing software tools/databases are available in Belgium (e.g. Delphi care, Sofie (Farm@doc)) and Pharmawin) but they don’t contain specific information on the possible influence of a medicine on the driving.
Aim of the study
We implemented and evaluated two different types of dispensing support tools, one integrated into the existing ViaNova dispensing software and one as a stand-alone tool (USB stick), containing the guidelines, fact sheets and categorisation system developed by DRUID [11]. The participating pharmacists received a training session and manual on how to use the provided tool as well as on the topic ‘medicines and driving’ in general [24]. The aim of the study was to evaluate the effectiveness of training and 6 month implementation of the support tools on pharmacists’ awareness and attitudes, self-reported behaviour and knowledge. Secondly, the two different types of dispensing support tools were evaluated on user acceptance to identify which tool was the most successful and appreciated by the pharmacists, and most realisable in practice.
Method
Study design
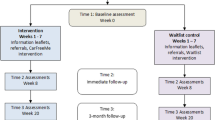
This study is a controlled prospective trial with 2 intervention groups and one control group. The first intervention group, further referred to as the integrated software group (IS), is a group of pharmacists using the ViaNova dispensing system in their daily practice (n = 68). The second intervention group, further referred to as stand alone (SA) group (n = 12), was a subgroup of the pharmacists from East Flanders (n = 636), that installed a program containing the DRUID information on their work computer [25]. In addition, a control group was added to evaluate the effectiveness of current practices with no DRUID-relevant information. Pharmacists in the IS and SA group were introduced to the tools/software through a training scheme. The participants filled in the pre-questionnaire at the start of their training and a post-questionnaire after 6 months of using the dispensing support tools in their practice.
For the IS group the DRUID dispensing guidelines, fact sheets and categorisation system were integrated into the ViaNova software. When a pharmacist dispensed for example a category 3 medicine for the first time, a warning sign (DRUID pictogram) automatically popped up indicating that driving is not allowed. If available, a safer alternative was proposed. When delivering the same medicine a second time the pharmacist was requested to ask about any side-effects encountered. The pharmacists had also the possibility to print out practical information letters for the patient.
The second intervention group, SA group, had to install a program containing the DRUID information on their work computer [25]. The program was delivered by means of a USB stick and had access to a website where all DRUID information was posted. When pharmacists opened the program they could type in the generic name or ATC code of the medicine they wanted to deliver. This tool clearly differs from the integrated software as pharmacists have to look up the guidelines and information separately (no automatic pop-up and no link with the patient).
Thirdly, the control group was a group of pharmacists from East Flanders (n = 20). This group neither received training nor any dispensing support tool with access to the DRUID information.
Selection of pharmacists
About one hundred pharmacists use the ViaNova software in their daily practice. In collaboration with ESCAPO participation was asked through email. For the SA and control group, a letter was sent to all pharmacists of East Flanders (n = 636) including general study information, an invitation to follow a training session, the pre-questionnaire (prior to intervention), an informed consent form and a return envelope. These pharmacists were asked not to reply if they were ViaNova users. They were asked for their participation either in the SA group or the control group. These pharmacists could thus self-select the group in which they wanted to participate (SA or control), although the letter also indicated that only the first 30 respondents would be considered for following a training course (SA group) or would be selected for the control group.
Training and questionnaire survey
After the participants’ selection, the pharmacists in the IS and SA group were asked to complete the pre-questionnaire (baseline measurement) before the start of the training session. After that, the two intervention groups followed a training and received a manual [24] on the support tool and on the topic ‘medicines and driving’. During the training (3 h) the pharmacists were informed about the DRUID project and the aim of the study. The legal aspects of driving under the influence in Belgium and the role of pharmacists related to that were underlined. Finally the pharmacists were confronted with practical situations and examples. A step-by-step plan on how to activate and use the information integrated in the tools was provided. During the study newsletters with information on the topic ‘medicines and traffic’ were sent by email to all participants in order to keep the topic under the attention of the pharmacists. After a 6-month intervention period during which the DRUID information was available, the intervention as well as the control group participants were asked to complete the post-questionnaire. Table 1 gives an overview of the collected information in the pre- and post-questionnaire [26].
Ethical approval
The study was approved by the Ethics committee of Ghent University Hospital. No patient information was collected. The privacy of the patient was guaranteed throughout the whole study. The pharmacists were free to refuse participation in the study or could terminate their cooperation at any time. All participants signed an informed consent form. The participants received a voucher incentive with value of €100 for the IS and SA group and €25 for the control group.
Statistical analysis
SPSS version 19 was used for the data analysis. Due to sample size restrictions and variables’ scales, robust non-parametric analyses were used (significance level at p ≤ .05; 95 % confidence interval). The attitudes & awareness and the reported behaviour composite scores were calculated based on the median score. A composite score integrates all single responses and gives an overall evaluation of a cluster. The knowledge composite score was calculated based on the sum of correct answers. Pre-post significant differences were checked with the Wilcoxon matched pairs—signed-rank test. For the sum composite score of knowledge, paired samples t test was used for the IS group (not in the other groups because of sample size restrictions).
Results
Sample characteristics
Except for the number of inhabitants in the practice area the three groups did not differ significantly regarding personal or practice related background variables (see Table 2). The pharmacists in the IS group had their practice significantly more often in more populated areas (>10,000 inhabitants; p ≤ .05).
The participation rate was about 80 % in the IS group, 80.0 % in the SA group and 77.8 % in the control group (see Fig. 1). Twenty-eight pharmacists dropped out of the study (IS: 16, SA: 6, control: 6). There were no significant differences between participants and drop-outs in the SA and control group with regard to background variables and ICT familiarity. The IS dropped-out group seemed to be relatively younger (below 30), with less practicing years and more often working in a rural setting (p ≤ .05). Reasons for not participating in the study were time restraints, fear of incompatibility between their own software and the stand alone tool or not available on one of the evenings when the training sessions were given.
Effects on pharmacists’ attitudes/awareness, reported behaviour and knowledge
Attitudes and awareness
The majority of the pharmacists in all groups reported similar attitudes and awareness concerning driving under the influence of medicines before and after the 6 month intervention (see Table 3). Only one significant positive pre-post change was measured, in the IS group. On the question ‘I feel that I am well aware of the effects of medicines on driving skills’, 25 % of the pharmacists changed their answer in the positive sense (p = .048), which indicates that they felt more aware of the effect of medicines on driving skills after the training and using the DRUID information integrated in their software. No significant changes were found within the SA and control group.
Reported behaviour
A significant positive change was found on 7 of the 8 reported behaviour questions after the training and intervention phase of the IS participants (see Table 3). When medicines with impairing effects on driving were to be dispensed, significantly more pharmacists using the IS reported in the post-questionnaire asking patients about their driving experience (Z = −5,207; p < .001), informing them about the driving related risks (Z = −5.443; p < .001) and discussing the medicine consumption and driving-related responsibilities (Z = −5.231; p < .001). After the intervention, pharmacists also indicated that they provided detailed information on impairing effects of medicines more frequently (Z = −5.733; p < .001), and to keep records when dispensing such medicines (Z = −4.611; p < .001), when giving advice to patients (Z = −5.198, p = 0), and about patients’ traffic participation (Z = −3.589; p < .001). The proportion of pharmacists informing patients regularly or always about risks increased up to almost the maximum. Furthermore, most answers shifted from pre-level ‘seldom to sometimes’ to post-level ‘sometimes to regular’ (asking about driving exposure, discussing responsibilities, frequency of detailed informing). With regard to record keeping when dispensing risky medicines or when giving advice, this clearly seemed to be done more often, but still quite a big group of pharmacists indicated doing this never or seldom.
Only one significant positive change after the intervention was found in the SA group. Significantly more pharmacists (50 %) reported to sometimes or regularly discuss medicine consumption and driving-related responsibility issues with the patient (Z = −2.333; p = .02).
Knowledge
With regard to pharmacists’ obligations and patients’ responsibilities no big changes were found. The answers in the pre-questionnaire were already predominantly correct. Rather limited pre-post change was found on knowledge of individual medicinal risks on driving (see Table 3). This knowledge remained at a low level even after 6 months intervention. No significant changes were found in the control group. In the SA group only one significant positive change was found, related to the risk of amitriptyline (Z = −2.00; p = .046). On this question there were fewer ‘don’t know’ answers (−25 %) and an increase of 33 % correct answers. Almost 60 % (compared to 25 %) answered this question correctly in the post-questionnaire.
The best results were found in the IS group. Significantly more pharmacists in this group gave correct answers on the question about diazepam (Z = −2.200; p = .028) and amitriptyline (60 % correct; Z = −2.744; p = .006). For both questions there were also fewer ‘don’t know’ answers; this shift was the case in all medicinal risk related questions. One third of the pharmacists gave more correct answers (50 % correct) on the question on codeine after the intervention (Z = −1,859; p = .063). Although the pre-post change was mainly directed as expected, the number of incorrect or don’t know answers in the post-questionnaire remained quite high overall and for some questions this was the majority: paroxetine (70 %) and diazepam (67 %).
Composite scores
Table 4 gives an overview of the changes found in the three clusters on composite score level. Significant pre-post changes were only found in the IS group. This group reported significantly more behavioural consideration of medicinal driving risk in their practice (risk communication and record keeping; Z = −6.143; p < .001) and had a significant increase in knowledge of specific medicinal risk and legal responsibilities (Z = −2.511; p = .012). For the cluster ‘attitudes and awareness’ no significant change was found in any of the groups.
Evaluation of the dispensing support tools
The user acceptance of the dispensing guidelines (e.g. ‘do not drive 8 h after intake of zolpidem ≤10 mg’), and the information integrated in the ViaNova software were high (see Table 5). About 95 % of the pharmacists indicated to have used the guidelines in their communication to the patients, of which 84 % at least regularly. The clear majority found the guidelines helpful, useful and sufficient. On the other hand, the pharmacists noted a low use of the fact sheets and the pictogram system (10–20 %).
More than half of the pharmacists using the integrated software stated that the software with the guidelines changed the manner in which they dispensed medicines, and 60 % mentioned that the guidelines changed ‘quite a lot’ up to ‘very much’ their way to inform a patient. Up to 90 % of the pharmacists (strongly) agreed that they could find the information without difficulties and that the tool would fit well in their working routines. The texts and icons (pictograms) were easy to perceive. Some pharmacists mentioned that the tool should provide more thorough information on side-effects or less vague advice. The majority of the participants (80 %) were willing to use the tool in the future.
Analysis of the data extracted from the stand alone tool showed that the pharmacists seldom used the provided software because the tool was too time-consuming, not easy to use and contained information that remained too vague. All data taken together, only 1 search was made every 4 days (527 searches on 180 days*N = 12). In contrast with the negative evaluation of the tool itself, the provided dispensing guidelines were considered helpful, useful and sufficient by more than 80 %. They stated that they would have used the tool more often if it was integrated into their daily used software.
Discussion
The aim of the study was to evaluate the effectiveness of training and 6 months implementation of two dispensing support tools (integrated software and a stand alone tool) on pharmacists’ awareness and attitudes, self-reported behaviour and knowledge regarding driving impairing medicines. Most significant positive intervention effects were found within the IS group, who had the DRUID information integrated into their daily used software. In total 10 significant positive changes (on a total of 20 items) were found, compared to just 2 in the SA group. As expected there was no significant evolution in the control group, suggesting that the changes observed in the intervention groups are related to the intervention.
Little change on attitude was found in the intervention groups. The IS group did indicate a significant increase in awareness of the problem after the intervention and also in the SA group a positive evolution was observed in awareness although this was not statistically significant. Nevertheless, 43 % in the IS group and 50 % in the SA group still didn’t feel well-aware of the topic after the intervention. This small change on attitude is probably related to the already rather positive attitudes they had prior to the intervention. At the start of the study the participants indeed firmly underlined the importance of being well informed and aware of the possible risks of medicines on driving. The self-selection bias in the participant recruitment may have led to over-rated positive attitude and awareness prior to the intervention and training. Nevertheless, as this bias accounts for the three groups, and as no significant difference in prior attitude between the groups was found, the between-group differences can be considered valid.
The integrated information in the daily used software had an effect on the reported behaviour of the pharmacists using the integrated software. These participants were significantly more considerate of the problem on 7 of the 8 reported behaviour questions, as compared to only one significant positive change in the SA group. After the training and use of the tool for 6 months significantly more pharmacists were aware of the fact that their patients participate in traffic and informed them more about potential risks when taking a specific medicine.
The pharmacists recognised that they have an important role in advising patients, certainly in case of patients receiving prescriptions from different physicians, as they have a good overview of all medicines taken. The DRUID pictogram can help the pharmacists in their risk communication towards a patient. These pictograms were already positively evaluated by patients in another study [27]. The pharmacists noted that informing family members, who come to collect the prescribed medicines, can be difficult. A lot of information was lost when the communication was not directly with the patient. This problem may be partially overcome by introducing a pictogram on the medicine box.
The number of incorrect or ‘don’t know’ answers to the knowledge questions remained quite high after training and intervention. In contrast with the low knowledge on individual medicine risk, the basic knowledge on legal pharmacists’ obligations and patient responsibilities was already generally good prior to the intervention. Therefore, few pre-post changes were found on this aspect. In general, the highest increase in knowledge was found in the pharmacists from the IS group.
The user acceptance of the provided information in the daily used software was high. The majority of the pharmacists using the integrated software found the dispensing guidelines helpful, useful and sufficient. The majority also reported though not to have used the fact sheets and the pictograms, but this was probably partly related to confusing terminology in the questionnaire with regard to what fact sheet and pictogram referred to. The term ‘fact sheet’ was not used in the integrated software, hence there is a possibility that pharmacists did read the text/fact sheet but did not mention this in the questionnaire. Moreover, the pictogram integrated in the software was rather small and could be easily ‘overlooked’. Consequently, the pharmacists did not recall to have seen ‘fact sheets’ or ‘pictograms’, resulting in low reported use.
The pharmacists who used the stand alone tool found it too time-consuming and not easy to use which resulted in limited use. The clear first choice of all the pharmacists was information integrated in their software, followed by a website. These findings are in line with previous research [5]. In order to be effective, the prompt should be part of the workflow, as close as possible to the decision moment and should be linked to supporting material to help increase the pharmacist’s knowledge [5, 23]. A recent review showed that the use of information is compromised if it could not be integrated with existing systems [28]. Finally, several studies describe the danger of desensitization to alerts. This effect is greatest when prompts are perceived as repetitious, time-consuming and not relevant to the decision at hand [5, 29, 30].
Based on pharmacists’ feedback and the experiences in this study, three critical points can be mentioned to increase knowledge and sustain a positive attitude and awareness of pharmacists: keep the topic under the attention of the pharmacists, education and collaboration with physicians. First, to keep the topic under the attention of the pharmacists, newsletters proved very useful in the present study. In general, newsletters or other direct media may help to keep the topic under the attention of pharmacists. Furthermore, collaboration between pharmacists and physicians is needed to optimise the risk communication to the patient. The participating pharmacists stressed the importance of a shared responsibility. However, in reality there is often a difficult cooperation between physicians and pharmacists in Belgium. Thirdly, some pharmacists were unsure if pharmacy assistants, who often work under supervision of a pharmacist in Belgium, are educated enough to give advice about the influence of medicines on the driving abilities. Other studies also underlined the importance of a good training or education in order to realise a change in attitude and behaviour in health professionals [28, 30, 31].
It is furthermore highly recommended that computerised alerts and reminders for selecting the safest medicine or for getting important risk information are presented automatically as part of the usual workflow. In order to optimise the risk communication between pharmacists and patients the information should be updated regularly and automatically, be easy to use, focus on first deliveries, be cost- and time-efficient, contain detailed information and (if possible) safer alternatives. The dispensing support tool should show suggestions on how to act at the time of dispensing and include practical advice for the patient on how to use the medicine at the start of treatment or in case of chronic use when driving is intended. A combination of tools, ideally integrated software and a manual or a website, is recommended.
Limitations of the study
It is essential to note that this was a small-scale and time-limited study with rather few participating pharmacists, especially in the SA and control group. This might have affected the results. The found positive effects of the dispensing support tools are suggestive and would need to be confirmed in larger study designs.
The study design initially took care that each participant had a unique identification code in order to link the individual questionnaire and software data. However, since many of the participants worked in pharmacies with several pharmacists using the same computer, the software data were not individual and could thus not be linked to the questionnaire data.
Approximately half of the participating pharmacists were older than 46 years. Younger pharmacists found it easier to install the stand alone tool. It is not unlikely that the older pharmacists were less inclined to use the stand alone tool when experiencing problems with installation or use, which could have resulted in a low use of this tool.
Conclusions
Almost all pharmacists involved in the present study underlined the importance of being informed on the potential risk of medicines on driving, yet they couldn’t readily access relevant information. The information developed by the DRUID project (medicinal risk categories for driving, individual medicine fact sheets, dispensing guidelines) could fill the gap. The present study indicated that making such information available within the daily used dispensing software, in an automatically prompting way, can lead to more considerate behaviour as well as increased awareness of the potential impairing effects of certain medicines.
References
Orriols L, Salmi LR, Philip P, Moore N, Delorme B, Castot A, et al. The impact of medicinal drugs on traffic safety: a systematic review of epidemiological studies. Pharmacoepidemiol Drug Saf. 2009;18(8):647–58.
England A, Skurtveit S, Morland J. Risk of road traffic accidents associated with the prescription of drugs: a registry-based cohort study. Ann Epidemiol. 2007;17(8):597–602.
Movig KL, Mathijssen MP, Nagel PH, van Egmond T, de Gier JJ, Leufkens HG, et al. Psychoactive substance use and the risk of motor vehicle accidents. Accid Anal Prev. 2004;36(4):631–6.
Verster JC, Mets MA. Psychoactive medication and traffic safety. Int J Environ Res Public Health. 2009;6(3):1041–54.
Reeve J, Tenni P, Peterson G. An electronic prompt in dispensing software to promote clinical interventions by community pharmacists: a randomized controlled trial. Br J Clin Pharmacol. 2007;65(3):377–85.
Wheeler A, Crump K, Lee M, Li L, Patel A, Yang R, Zhao J, Jensen M, et al. Collaborative prescribing: a qualitative exploration of a role for pharmacists in mental health. Res Soc Adm Pharmac. 2012;8(38):179–92. doi:10.1016/j.sapharm.2011.04.003.
Nkansah N, Mostovetsky O, Yu C, Chheng T, Beney J, Bond C, et al. Effect of outpatient pharmacists’ non-dispensing roles on patient outcomes and prescribing patterns. Cochrane Database Syst Rev. 2010;7(7):CD000336.
Beney J, Bero L, Bond C. Expanding the roles of outpatient pharmacists: effects on health services utilisation, costs, and patient outcomes. Cochrane Database Syst Rev. 2000;3:CD000336.
Royal Decree of 21 January 2009 regarding the remuneration of pharmacists (K.B. van 21 januari 2009 houdende onderrichtingen voor de apothekers).
de Gier JJ, Alvarez JF, Mercier-Guyon C, Verstraete AG. Prescribing and dispensing guidelines for medicinal drugs affecting driving performance. In: Verster JC, Pandi-Perumal SD, Ramaekers JG, de Gier JJ, editor. Drugs, driving and traffic safety. Basel: Birkhauser Verlag AG; 2009. p. 121–34.
Monteiro SP, de Gier JJ. Guidelines and professional standards. Report and CD with examples of ICT supported tools for prescribing and dispensing of medicines affecting driving performance, and for informing patients who use other psychoactive substances than medicines (D 7.2.2) Available at www.druid-project.eu 2011.
Ravera S, Monteiro S, de Gier J, van der Linden T, Gómez-Talegón T, Alvarez F, et al. A European approach to categorising medicines for fitness to drive: outcomes of the DRUID project. Br J Clin Pharmacol. 2012. doi:10.1111/j.1365-2125.2012.04279.x.
Grimshaw J, Eccles M, Tetroe J. Implementing clinical guidelines: current evidence and future implications. J Contin Educ Health Prof. 2004;24(1):31–7.
Grol R. Implementation of evidence and guidelines in clinical practice: a new field of research? Int J Qual Health Care. 2000;6:455–6.
Jamtvedt G, Young JM, Kristoffersen DT, Thomson O’Brien MA, Oxman AD. Audit and feedback: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2003;3:CD000259.
Bero LA, Grilli R, Grimshaw JM, Harvey E, Oxman AD, Thomson MA. Closing the gap between research and practice: an overview of systematic reviews of interventions to promote the implementation of research findings. BMJ. 1998;317(7156):465–8.
Martens JD, Winkens RAG, van der Weijden T, de Bruyn D, Severens JL. Does a joint development and dissemination of multidisciplinary guidelines improve prescribing behaviour: a pre/post study with concurrent control group and a randomised trial. BMC Health Serv Res. 2006;2(6):145–51.
Lagerløv P, Loeb M, Andrew M, Hjortdahl P. Improving doctors’ prescribing behaviour through reflection on guidelines and prescription feedback: a randomised controlled study. Qual Health Care. 2000;9(3):159–65.
Hunt DL, Haynes RB, Hanna SE, Smith K. Effects of computer-based clinical decision support systems on physician performance and patient outcomes. A systematic Review. JAMA. 1998;280(15):1339–46.
Johnston ME, Langton KB, Haynes B, Mathieu A. Effects of computer-based clinical decision support systems on clinician performance and patient outcome: a critical appraisal of research. Ann Intern Med. 1994;120(2):135–42.
De Clercq PA, Blom JA, Korsten HM, Hasman A. Approaches for creating computer interpretable guidelines that facilitate decision support. Artif Intell Med. 2004;31(1):1–27.
Garg A, Adhikari N, McDonald H, Rosas-Arellano MP, Devereaux P, Beyene J, et al. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. JAMA. 2005;293(10):1223–38.
Martens JD, van der Weijden T, Severens JL, de Clercq PA, de Bruijn DP, Kester ADM, et al. The effect of computer reminders on GPs’ prescribing behaviour: a cluster-randomised trial. Int J Med Inf. 2007;76(3):403–16.
Margaretis D, Touliou K, Ravera S, Monteiro S, de Gier JJ, Boets S, et al. Training manual for physicians and pharmacists on medicinal drugs and driving (D 7.4.1). Available at www.druid-project.eu2009.
Touliou K, Margaritis D, Spandidis P, Monteiro S, Ravera S, de Gier JJ, et al. Report on the implementation, evaluation and new technologies of practice guidelines and information materials (D 7.4.2). Available at www.druid-project.eu 2011.
Legrand SA, Boets S, Meesmann U, Van der Linden T, Verstraete A. Belgian country report on the implementation, evaluation and new technologies of practice guidelines and information materials for pharmacists. Section of the EU project DRUID D7.4.2. Available at: www.druid-project.eu 2011.
Monteiro S, de Gier JJ. Dutch Country report: pharmacists’ intervention study: implementation an evaluation of DRUID information materials regarding the influence of medicines on driving fitness. DRUID Deliverable 7.4.2. Available at: www.druid-project.eu 2011.
Moxey A, Robertson J, Newby D, Hains I, Williamson M, Sallie-Anne Pearson SA. Computerized clinical decision support for prescribing: provision does not guarantee uptake. J Am Med Inform Assoc. 2010;17(1):25–33.
Ahearn M, Kerr S. General practitioners’ perceptions of the pharmaceutical decision-support tools in their prescribing software. Med J Aust. 2003;179(1):34–7.
Berner ES. Ethical and legal issues in the use of health information technology to improve patient safety. HEC Forum. 2008;20(3):243–58.
Smith WR. Evidence for the effectiveness of techniques to change physician behaviour. Chest J. 2009;118(2):8–17.
Acknowledgments
The research team would like to thank all pharmacists involved in the study. A special thanks to Ms Chantal Leirs and Ms Anneleen Janssen from ESCAPO. We would also like to thank Dr. Hilka Wolschrijn for guiding and leading the training sessions. The authors would also like to express their gratitude to the Hellenic Institute of Transport (Greece) for developing the program on the stand alone tool (USB stick) to support the pharmacists in this study.
Funding
The present study was part of the European DRUID project and financed by the European Commission.
Conflicts of interest
This article has been produced under the project “Driving Under the Influence of Drugs, Alcohol and Medicines” (DRUID) financed by the European Community within the framework of the EU 6th Framework Program (Contract No TREN-05-FP6TR-S07.61320-518404-DRUID). This document reflects only the author’s view. The European Commission is not liable for any use that may be made of the information contained therein.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Legrand, SA., Boets, S., Meesmann, U. et al. Medicines and driving: evaluation of training and software support for patient counselling by pharmacists. Int J Clin Pharm 34, 633–643 (2012). https://doi.org/10.1007/s11096-012-9658-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-012-9658-7