Abstract
Background Chronic obstructive pulmonary disease (COPD) treatment goals are often not achieved despite the availability of many effective treatments. Furthermore, clinical pharmacist interventions to improve clinical and humanistic outcomes in COPD patients have not yet been explored and few randomized controlled trials have been reported to evaluate the impact of pharmaceutical care on health outcomes in patients with COPD. Objective The aim of the present study was to evaluate the impact of pharmaceutical care intervention, with a strong focus on self-management, on a range of clinical and humanistic outcomes in patients with COPD. Setting Outpatient COPD Clinic at the Royal Medical Services Hospital. Method In a randomised, controlled, prospective clinical trial, a total of 133 COPD patients were randomly assigned to intervention or control group. A structured education about COPD and management of its symptoms was delivered by the clinical pharmacist for patients in the intervention group. Patients were followed up at 6 months during a scheduled visit. Effectiveness of the intervention was assessed in terms of improvement in health-related quality of life, medication adherence, disease knowledge and healthcare utilization. Data collected at baseline and at the 6 month assessment was coded and entered into SPSS® software version 17 for statistical analysis. A P value of <0.05 was considered statistically significant. Main outcome measure The primary outcome measure was health-related quality of life improvement. All other data collected including healthcare utilization, COPD knowledge and medication adherence formed secondary outcome measures. Results A total of 66 patients were randomized to the intervention group and 67 patients were randomized to the control group. Although the current study failed to illustrate significant improvement in health-related quality of life parameters, the results indicated significant improvements in COPD knowledge (P < 0.001), medication adherence (P < 0.05), medication beliefs (P < 0.01) and significant reduction in hospital admission rates (P < 0.05) in intervention patients when compared with control group patients at the end of the study. Conclusion The enhanced patient outcomes as a result of the pharmaceutical care programme in the present study demonstrate the value of an enhanced clinical pharmacy service in achieving the desired health outcomes for patients with COPD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Impact of finding on practice
-
A structured Pharmaceutical care programme led by a clinical pharmacist for patients with COPD is associated with improved treatment outcomes.
-
There is a growing need to implement a comprehensive clinical pharmacy service for the purpose of achieving the desired health outcomes for patients with COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is primarily characterized by airflow limitation that is usually progressive and associated with abnormal inflammatory response of the lungs to noxious particles in addition to loss of lung elasticity or emphysema [1, 2]. Symptoms associated with COPD usually include cough, sputum production and shortness of breath associated with airflow obstruction. Besides smoking, other factors such as alpha1-antitrypsin deficiency, prolonged exposure to environmental pollutants and recurrent respiratory infections during childhood may precipitate COPD [3].
Currently, COPD causes approximately 2.7 million deaths annually and it is expected to be the third leading cause of death by disease worldwide by 2020 if successful strategies are not implemented to prevent it [1, 4–7]. It has been estimated that the annual death rate from the COPD exceeds death rates from lung cancer and breast cancer combined [8, 9].
Smoking has been defined as the leading cause of COPD and attributed to approximately 85–90% of all cases of COPD [3]. It has been estimated that the number of tobacco deaths will reach more than 8 million people worldwide per year by the year 2030, with 80% of these premature deaths occurring in low- and middle income developing countries including Jordan [10]. In a national survey conducted by the Jordanian Ministry of Health, the prevalence of cigarette use among adult males was estimated to be 43% in 2004. This figure increased to 62.7% in 2007 [11–13]. Beside smoking prevalence, the lack of knowledge of COPD among general population and the fact that management of this illness remains suboptimal, COPD is rapidly becoming one of the most challenging health problems worldwide that is particularly important in developing countries including Jordan.
Management of COPD is complex, with patients needing to perform self-management process which requires challenging behavioural and lifestyle changes such as smoking cessation, proper use of inhalation technique, adherence to exercise therapy along with optimal medication adherence [14]. Multiple co-morbidities are common among patients with COPD and they are often prescribed complex medication regimens to be administered by multiple routes for both respiratory and non respiratory conditions. All these factors predispose patients to risk of non-adherence which is considered the major reason behind emergency hospitalisation among COPD patients. Frequent hospital admissions due to acute exacerbation of airways disease have been found to have a negative impact on the quality of life of COPD patients, which is considered a vital issue to be targeted when implementing different interventions for patients with COPD [15–17].
Pharmacists can contribute to the care of all patients with COPD via implementing interventions that focus on patient education about disease, prescribed medications and proper use of inhalation technique in addition to ongoing assessments of patients’ willingness to adhere to treatment recommendations and to stop smoking and referring patients to smoking cessation programs when necessary [18]. To the best of our knowledge, this is the first research that investigates via a randomized, controlled, clinical trial the impact of pharmaceutical care on COPD patients, not only in Jordan, but within all the Middle Eastern countries.
Aim of the study
The aim of the present study was therefore to evaluate the impact of pharmaceutical care programme, with a strong emphasis on self-management, on clinical and humanistic outcomes in outpatients with COPD.
Method
Study design and subjects
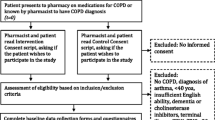
The effectiveness of the pharmaceutical care intervention was assessed through a randomised, controlled, prospective clinical trial with a 6 month follow-up. Study subjects were COPD patients attending an outpatient clinic at the Royal Medical Services Hospital in Jordan. The study received ethical approval of the Institutional Review Board, King Hussein Hospital, Royal Medical Services, Jordan. Patients had to meet the following inclusion criteria in order to take part in the clinical trial: patients only attend the outpatient COPD clinic at the Royal Medical Services, confirmed diagnosis of COPD by the hospital consultant for at least 1 year, over 35 years old, having a forced expiratory volume in 1 s (FEV1) of 30–80% of the predicted normal value and hospital consultant agreement that the patient is suitable for entering the trial. Patients were excluded from the study if they had moderate to severe learning difficulties, mobility problems, confusion, disorientation or terminal illness, congestive heart failure or if they attended a pulmonary rehabilitation programme or had consulted a pulmonary nurse or clinical pharmacist in the last 6 months. During an outpatient clinic visit, eligible patients were informed verbally about the study by the research pharmacist and were provided with an information sheet. The patients were asked to sign a consent form if they were willing to participate in the study. Study participants were randomly assigned to intervention and control groups via a minimisation technique using MINIM software [19]. The patients were recruited over a period of 3 months from January to April, 2011.
Sample size
Based on published data [20–22], it was estimated that to show a minimum clinically significant difference of four points improvements in the total St. George’s Respiratory Questionnaire (SGRQ) scores, which was considered the primary outcome measure in the study, with a significance level of 5% and a power of 80%, a sample size of 80 patients per group was required.
Baseline assessments
After randomisation, baseline data for each patient were collected by the researcher pharmacist using a custom-designed questionnaire, medical charts and hospital computers. The collected data included demographic measures, disease characteristics, respiratory and non-respiratory medications and medication regimen and healthcare utilization, i.e. emergency department (ED) visits and hospital admissions due to exacerbation 6 months preceding the study. The patients also completed a range of questionnaires which included: COPD knowledge questionnaire [23], medication adherence using Morisky scale [24] and disease-specific health-related quality of life using St George Respiratory Questionnaire (SGRQ) [25, 26].
Follow-up assessments
Baseline data collection measures (except demographic data) were repeated by the researcher at 6 months during scheduled clinic visits. The primary outcome measure was quality of life improvement. All other data collected including healthcare utilization, COPD knowledge and medication adherence, formed secondary outcome measures.
Study instruments
COPD knowledge questionnaire
This instrument [23] was developed to assess patient’s knowledge of COPD, breathing and exercise, energy conservation, medications, relaxation and stress control. The COPD knowledge scale consists of 16 true/false items in which correct responses are scored 1 and incorrect responses are scored 0, with unsure responses receiving no score. The range of possible scores is 0–16; the higher the score, the greater the knowledge level.
Self-reported adherence (Morisky scale)
This simple four-question survey [24] assesses the likelihood that patients take their medications as prescribed. On scoring of the questionnaire, each ‘yes’ response is given a score of 1 and each ‘no’ response is given a score of 0. Adherence scores can therefore range between 0 and 4. For the purpose of the present analysis, the patients were divided into two groups: those scoring 0 were considered adherent and those scoring 1–4 were considered non-adherent.
St George Respiratory Questionnaire
The SGRQ [25, 26] is a self-administered 76-item instrument designed specifically for patients with chronic airways disease from which scores are calculated for three components: symptoms, activity and impact. The scoring range for each component is from 0 to 100, with the highest scores indicating the poorest level of the patient’s respiratory health and indicating maximum disability [27]. A change of 4 units in the mean total score has been validated as a clinically significant threshold [28].
The English version of both COPD knowledge [23] and medication adherence [24] questionnaires used in the present study was translated into Arabic as follows: (1) a forward translation of the original questionnaire from English into Arabic was carried out by two qualified independent, native linguistic expert translators. (2) A back translation from Arabic into English was carried out by two different translators. Finally, both translations were compared with the original English copy of the questionnaire and showed more than 95% match. Furthermore, a panel of experts in different specialties i.e. Clinical Pharmacy, Pharmacy Practice and Respiratory Medicine examined the research instrument for face and content validity. Pilot work was performed and questions were adjusted as appropriate before moving to the main study. Regarding the SGRQ, we used a validated Arabic version of SGRQ [29], the Arabic version was applied to 10 COPD patients enrolled to respiratory centre at King Hussein Hospital. Doubts and difficulties in answering the questions were investigated. Internal consistency of symptoms, activity and impact components was assessed using Cronbach’s alpha (a) reliability coefficient; they were 0.94, 0.91 and 0.90, respectively. The test retest reliability of components scores ranged from 0.70 to 0.87.
Pharmacist intervention
A structured patient education about COPD and management of its symptoms was delivered by the clinical pharmacist for the intervention patients in a separate room at the outpatient clinic. The clinical pharmacist also completed a medication table designed specifically to discuss types, indications, doses, frequency of administration, and possible side effects for each prescribed medication. Furthermore, the importance of simple exercises [30], symptoms control and the technique for expectoration [31] were discussed with the intervention patients. A booklet on these techniques [32] was prepared to assist in the education session and the patients were given a copy to take home with them. The clinical pharmacist used the motivational interviewing technique [33] with the aim of improving adherence to the prescribed treatment. Patients who still smoked were referred to a special smoking cessation programme within the hospital.
Data analysis
Data collected at baseline and at the 6 month assessments were coded and entered into SPSS® software version 17 for statistical analysis (data screening, descriptive statistics and univariate analysis). Data were examined using Chi-squared analysis for categorical variables. Regarding continuous variables, the Mann–Whitney U-test was performed for the non-normally distributed variables and the independent t test was performed for normally distributed variables. A P value of <0.05 was considered statistically significant.
Results
A total of 133 COPD patients (66 intervention, 67 control) attending an outpatient clinic were recruited into the study. As shown in Fig. 1 below, a total of 6 patients withdrew at the 6 month assessment; 3 patients from the intervention group and 3 patients from the control group. Accordingly, a total of 127 patients (63 intervention, 64 control) completed the 6 month study period.
Baseline assessments
Results indicated similar sociodemographic and clinical characteristics between the study participants at the baseline assessment point (Table 1). Most patients were female, elderly, married with low educational and occupational level. Most of the study participants were current smokers and more than half of the participants reported different co-morbidities which included depression, diabetes, hypertension, arthritis, osteoporosis, and other conditions. The use of respiratory medications was similar between the study groups. No difference in disease severity was reported between the intervention and control groups at the baseline assessment point and most of participants were found to have moderate to severe COPD with a mean FEV1 of approximately 50% of the predicted normal value (Table 1). No significant difference was also reported at baseline assessment point between both groups with regard to healthcare utilization represented by emergency department visits and hospital admissions for acute exacerbation of COPD (P > 0.05; Table 1).
Medications prescribed for study sample
The Mann–Whitney U-test revealed no significant differences (P > 0.05) in the total number of prescribed medications between the two groups. The intervention and the control patients were prescribed approximately the same number of total medications over the study period. Chi-squared analysis indicated no significant differences (P > 0.05) between the intervention group and the control group in the usage of key medications at both baseline and 6 month assessments (Table 2).
Forced expiratory volume in one second
Lung function did not change from baseline to the end of the study in either group. In the control group, the mean FEV1 was 1.08 L (CI 0.93–1.18) at the baseline assessment and 1.06 L (CI 0.94–1.21) at 6 months assessment. Corresponding data for the intervention group were 1.12 L (CI 0.97–1.26) and 1.15 L (CI 1.05–1.26) respectively. Accordingly, no significant differences (P > 0.05) in the mean FEV1 between the intervention and control groups was observed at baseline and over the study period as shown in Table 3.
Body mass index
There were no statistically significant differences in the BMI values between the intervention group and control group patients at the baseline and 6 month assessments in this study (P > 0.05; Table 3).
Knowledge of medication and disease management
As shown from the total score of COPD knowledge questionnaire, both control and intervention patients had poor knowledge about their medication and disease management at baseline with no statistical difference in the median scores between the two groups (P > 0.05). Compared with the control group, knowledge scores were significantly improved in the intervention group at the 6 month assessment time (P < 0.001) while it remained approximately constant in the control group (Table 3).
Adherence to prescribed medication
At the baseline assessment, intervention group and control group patients were found to have approximately the same proportion of patients who exhibited low adherence (P > 0.05). Chi-squared analysis revealed a significant decrease in the proportion of non-adherent patients in the intervention group when compared with the control group (28.6% vs. 48.4%) at the 6 month assessment (P < 0.05; Table 4).
Rating the effectiveness of COPD medications
Chi-squared and Fisher’s Exact tests indicated that there was no significant differences (intervention vs. control) in patient rating of the effectiveness of their COPD medications at baseline. There were, however, significant differences at 6 months (P < 0.01) with an increasing number of the intervention patients who rated their COPD medications as mostly or totally effective when compared with control patients (Table 5).
Health-related quality of life (SGRQ)
At the baseline assessment, intervention group and control group patients were found to have approximately the same scores in health related quality of life parameters including total SGRQ score and its subscales; symptoms, activity and impact (P > 0.05). Intervention patients showed some improvement in total SGRQ score and its subscales at the 6 assessment; however, this improvement in quality of life failed to reach statistical significance (P > 0.05) as a parallel improvement in such parameters was reported in patients assigned to control group (Table 6). Furthermore, the total score at 6 months was not clinically significant as it failed to reach the threshold of four units improvement.
Health resources utilization
Although the proportion of patients who had an emergency department visit for acute exacerbation of COPD decreased in the intervention group (from 16.7 to 15.2%) and increased in the control group (from 16.4 to 17.9%) over the study period, this change was not statistically significant. On the other hand, statistically significant reduction in hospital admission for acute exacerbation of COPD was illustrated in the intervention group when compared with the control group at the 6 month assessment point (P < 0.05; Table 7).
Discussion
Despite the development of effective treatments for patients with COPD, results still suboptimal. Literature indicates conflicting results regarding the effect of patient education and self-management programme on improving clinical and humanistic outcomes in patients with COPD. Furthermore, few data are available to support the role of clinical pharmacists in optimising therapy and improving health outcomes in patients with COPD.
A systemic review and Meta analysis indicated that only 1 of 7 studies reported a significant improvement in lung function in patients with COPD [34]. Consistent with this analysis, effects of the intervention on lung function tests in the present research were not statistically or clinically significant. This finding can be justified by the fact that COPD is a progressive disease characterized by irreversible damage, and hence FEV1 is difficult to change and is not expected to be sensitive to the intervention programme [35].
The pharmaceutical care group in the present study showed significant COPD knowledge improvement at the end of the study. This finding is consistent with the findings from Hill et al. [36] who reported a significant improvement in a disease-specific knowledge in patients received two 60 min face-to-face educational sessions at the primary care practice. Similar findings of improved COPD knowledge have also been reported by others [22, 37]. The significant improvement in COPD knowledge in the intervention patients in the present study was clearly attributed to the intensive education of intervention patients at baseline on all aspects of COPD self-management, combined with the regular reinforcement that those patients received during the study.
The significant difference in medication adherence between the two groups at the end of the study was most likely due to the fact that intervention patients received intensive education from the clinical pharmacist on the dosage, therapeutic effects, safe handling, possible side effects of their medications, in addition to an emphasis on that the patients were able and willing to use the inhaler devices as prescribed. Consistent with this finding, Khdour et al. [7] reported significant improvement in medication adherence over a 12-month study period as a result of clinical pharmacist-led intensive educational programme for COPD patients attending an outpatient clinic in Northern Ireland. Similar results also have been obtained by Steuten et al. [38] via implementing an integrated disease management programme for patients attending University Hospital and 16 general practices in Netherlands.
The positive medication beliefs which was manifested by the intervention patients was most likely due to the motivational interviewing technique implemented by the clinical pharmacist. This positive change might also be related to the improved knowledge reported by the intervention patients at the 6 month assessment.
Consistent with the findings from earlier research [22, 39–43], the pharmaceutical care intervention in the present study did not have positive impact on health related quality of life. This can be justified by the fact that the timeframe of our study may have been too short to detect clinically relevant changes for this parameter. Furthermore, the mean baseline SGRQ scores for the participants in this research was relatively low (45.2%), indicating generally good health status of the participants which in turn decrease the chance to detect significant improvements in health-related quality of life. Other studies [7, 9] illustrated significant improvement in health-related quality of life. However, the mean baseline SGRQ scores for the participants in the two latter studies were high (64.2%) and (54.0%) respectively, indicating generally poor quality of life of the participants, which in turn have increased the margin available for improvements and hence detect differences between groups. Furthermore, both studies [7, 9] recruited larger number of participants when compared with the study population in the current study. This may also have increased the possibility to detect improvements in health status in such earlier studies. However, it should be noted that the effect of the intervention on quality of life have diminished over time in these studies. This suggests the progressive nature of the disease and indicates that moving forward the clinical pharmacist intervention needs to include more robust patient follow-up.
Although no significant reductions in emergency department visits due to acute exacerbations have been shown over the study period, the clinical pharmacist intervention has demonstrated significant reduction in number of patients who had hospital admissions for acute exacerbation (from 9.8 to 5.0%) during the 6 month study period when compared with the increased number of control group patients (from 11.3 to 15%) who had hospital admission over the same study period (P < 0.05). Similar reductions in hospital admissions as a result of educational and self-management interventions have been reported from earlier studies [7, 43–46].
Study limitations
The study was limited in that the length of time required to complete the battery of questionnaires used in the present study may have encouraged bias in the responses gained from the participants, as in an effort to finish quickly, participants may have selected answers without giving due consideration to the questions posed. Furthermore, social desirability and recall bias associated with the use of a self-report method to assess medications adherence could have affected the results. Another limitation was that the target sample size was not attained because the capacity for inclusion was limited in this trial with a single investigator. However, increasing the sample size therefore may allow more robust conclusions to be drawn about the findings.
Conclusion
Enhanced patient outcomes as a result of the pharmaceutical care programme were obtained in the present study. This was illustrated by decreased hospital admission rates, significant improvement in medication adherence, improvement in disease and medication knowledge and enhanced positive attitudes toward medication effectiveness. The present study therefore clearly demonstrated the need to implement an integrated pharmaceutical care programmes by the clinical pharmacists in different hospital sites in Jordan for the purpose of improving health outcomes for patients with COPD. More comprehensive research is needed in this area, particularly the impact of such pharmaceutical care programmes on the health-related quality of life for patients with COPD in Jordan and other Middle Eastern countries.
References
Pauwels RA, Buist AS, Ma P, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: national heart, lung, and blood institute and world health organization global initiative for chronic obstructive lung disease (GOLD): executive summary. Resp Care. 2001;46:798–825.
Barnes PJ. Chronic obstructive pulmonary disease: effects beyond the lungs. PLoS Med. 2010;7(3):e1000220.
American Lung Association (ALA). Chronic obstructive pulmonary disease (COPD) fact sheet. 2011 Feb. Available from: http://www.lungusa.org/lungdisease/copd/resources/facts-figures/COPD-Fact-Sheet.html.
Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: global burden of disease. Lancet. 1997;349(9064):1498–504.
Taskin D, Cooper CB. The role of long acting bronchodilators in the management of stable COPD. Chest. 2004;125:249–59.
GOLD. global initiative for chronic obstructive lung disease. Updated 2008. www.goldcopd.com. Accessed 10 Nov 2009.
Khdour MR, Kidney JC, Smyth BM, McElnay JC. Clinical pharmacy-led disease and medicine management programme for patients with COPD. Br J Clin Pharmacol. 2009;68(4):588–98.
Ramsey SD. Suboptimal medical therapy in COPD: exploring the causes and consequences. Chest. 2000;117:33S–7S.
Bourbeau J, Sebaldt RJ, Day A, et al. Practice patterns in the management of chronic obstructive pulmonary disease in primary practice: the CAGE study. Can Respir J. 2008;15:13–9.
Haddad LG, Al-Zyoud S, Abu Baker N, Gharaibeh H, El Shahawy O, Alramadhani R. Secondhand smoking in Jordan: clearing the air for one of the highest tobacco prevalence countries in the Middle East. Tobacco use insights. 2011; 4. doi: 10.4137/TUI.S6802. Available from http://www.la-press.com.
World Health Organization. Report on the global tobacco epidemic, 2009: the MPOWER package. Geneva, Switzerland: WHO Press; 2009.
Belbeisi A, Zindah M, Walke H, Jarrar B, Mokdad AH. Assessing risk factors for chronic disease—Jordan, 2004. MMWR Morb Mortal Wkly Rep. 2006;55:653–5.
Nsour M, Mahfoud Z, Kanaan MN, Balbeissi A. Prevalence and predictors of non-fatal myocardial infarction in Jordan. East Mediterr Health J. 2008;14:818–30.
Johnson G, Kong D, Rambha K, Stewart K. Factors associated with medication nonadherence in patients with COPD. Chest. 2005;128:3198–204.
Rutschmann OT, Janssens JP, Vermeulen B, Sarasin FP. Knowledge of guidelines for the management of COPD: a survey of primary care physicians. Respir Med. 2004;98:932–7.
Glaab T, Banik N, Rutschmann OT, Wencker M. National survey of guideline-compliant COPD management among pneumologists and primary care physicians. COPD. 2006;3:141–8.
Miravitlles M, de la Roza C, Naberan K, Lamban M, Gobartt E, Martin A. Use of spirometry and patterns of prescribing in COPD in primary care. Respir Med. 2007;101:1753–60.
National Institute for Clinical Excellence (NICE). Guideline development process information for national collaborating centres and guideline development groups. London: NICE; 2004.
Evans S, Royston P, Day S. Minim allocation by minimisation in clinical trials. 2011 Sep. Available from: http://www-users.york.ac.uk/~mb55/guide/randsery.htm.
Watson PB, Town GI, Holbrook N, Dwan C, Toop LJ, Drennan CJ. Evaluation of a self-management plan for chronic obstructive pulmonary disease. Eur Respir J. 1997;10:1267–71.
Bourbeau J, Julien M, Maltais F, Rouleau M, Beaupre A, Begin R, et al. Reduction of hospital utilization in patients with chronic obstructive pulmonary disease: a disease specific self-management intervention. Arch Intern Med. 2003;163:585–91.
McGeoch RB, Willsman K, Dowson C, Town G, Frampton C, McCartin F, et al. Self-management plans in the primary care of patients with chronic obstructive pulmonary disease. Respirology. 2006;11:611–8.
Scherer YK, Schmieder LE, Shimmel S. The effects of education alone and in combination with pulmonary rehabilitation on self-efficacy in patients with COPD. Rehabil Nurs. 1998;23:71–7.
Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74.
Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145:1321–7.
Spencer S, Calverley PM, Sherwood Burge P, Jones PW, ISOLDE Study Group. Inhaled steroids in obstructive lung disease. Health status deterioration in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163:122–8.
Jones PW, Quirk FH, Baveystock CM. The St. George’s respiratory questionnaire. Respir Med. 1991;85(Suppl. B):25–31.
Quanjer PH, Lebowitz MD, Gregg I, Miller MR, Pedersen OF. Peak expiratory flow: conclusions and recommendations of a Working Party of the European Respiratory Society. Eur Respir J Suppl. 1997;24:2S–8S.
El Rhazi K, Nejjari C, Benjelloun MC, Bourkadi J, Afif H, Serhier Z, Tachfouti N, Berraho M, Barberger-Gateau P. Validation of the St George’s Respiratory Questionnaire in patients with COPD or asthma in Morocco. Int J Tuberc Lung Dis. 2006;10(11):1273–8.
Lolak S, Connors GL, Sheridan MJ, Wise TN. Effects of progressive muscle relaxation training on anxiety and depression in patients enrolled in an outpatient pulmonary rehabilitation program. Psychother Psychosom. 2008;77:119–25.
Ambrosino N, Di Giorgio M, Di Paco A. Strategies to improve breathlessness and exercise tolerance in chronic obstructive pulmonary disease. Respir Med COPD Update. 2006;2:2–8.
Treasure J. Motivational interviewing. Adv Psychiatr Treat. 2004;10:331–7.
Miller W. Motivational interviewing with problem drinkers. Behav Psychother. 1983;11:147–72.
Rea H, McAuley S, Stewart A. A chronic disease management programme can reduce days in hospital for patients with chronic obstructive pulmonary disease. Intern Med J. 2004;34:608–14.
Fletcher C, Peto R. The natural history of chronic airflow obstruction. BMJ. 1977;1:1645–8.
Hill K, Mangovski-Alzamora S, Blouin M, Guyatt G, Heels-Ansdell D, Bragaglia P, et al. Disease-specific education in the primary care setting increases the knowledge of people with chronic obstructive pulmonary disease: a randomized controlled trial. Patient Educ Couns. 2010;81(1):14–8.
Hesselink AE, Penninx BW, van der Windt DA, van Duin BJ, de Vries P, Twisk JW, et al. Effectiveness of an education programme by a general practice assistant for asthma and COPD patients: results from a randomised controlled trial. Patient Educ Couns. 2004;55:121–8.
Steuten L, Vrijhoef B, Van MF. Evaluation of a regional disease management programme for patients with asthma or chronic obstructive pulmonary disease. Int J Qual Health Care. 2006;18:429–36.
Monninkhof E, van der Valk P, van der Palen J, van Herwaarden C, Partridge MR, Zielhuis G. Self-management education for patients with chronic obstructive pulmonary disease: a systematic review. Thorax. 2003;58:394–8.
Gallefoss F, Bakke PS, Rsgaard PK. Quality of life assessment after patient education in a randomized controlled study on asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159:812–7.
Engstrom CP, Persson LO, Larsson S, Sullivan M. Long-term effects of a pulmonary rehabilitation programme in outpatients with chronic obstructive pulmonary disease: A randomized controlled study. Scand J Rehabil Med Suppl. 1999;31:207–13.
Ries AL, Kaplan RM, Myers R, Prewitt LM. Maintenance after pulmonary rehabilitation in chronic lung disease: a randomized trial. Am J Respir Crit Care Med. 2003;167:880–8.
Soler JJ, Martinez-Garcia MA, Roman P. Effectiveness of a specific program for patients with chronic obstructive pulmonary disease and frequent exacerbations. Arch Bronconeumol. 2006;42:501–8.
Tinkelman D, Corsello P. One year otucomes from a disaese managment program for COPD. Dis Manag Health Outcomes. 2003;11:49–59.
Bourbeau J, Julien M, Maltais F, Rouleau M, Beaupre A, Begin R, et al. Reduction of hospital utilization in patients with chronic obstructive pulmonary disease: a disease-specific self-management intervention. Arch Intern Med. 2003;163:585–91.
Casas A, Troosters T, Garcia-Aymerich J. Integrated care prevents hospitalisations for exacerbations in COPD patients. Eur Respir J. 2006;28:123–130.
Acknowledgments
The authors wish to thank Dr. Imad Aldoghim (Lieutenant Colonel Pharmacist), Department of Pharmacy, Royal Medical Services Hospital for his assistance to obtain the ethical approval for the present study.
Funding
The authors wish to express their sincere appreciation to Alzaytoonah University of Jordan for the financial support.
Conflicts of interest
None to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jarab, A.S., AlQudah, S.G., Khdour, M. et al. Impact of pharmaceutical care on health outcomes in patients with COPD. Int J Clin Pharm 34, 53–62 (2012). https://doi.org/10.1007/s11096-011-9585-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-011-9585-z