Abstract
Objective To avoid negative effects of drug treatment and need for additional medical care, drug treatment must be individualised. Our research group has developed a model for clinical pharmacy which improves several aspects of the patient’s drug treatment. This study describes the process behind these improvements, i.e. drug related problems identified by pharmacists within a clinical pharmacy service. Setting Three wards at a department of internal medicine. Method Pharmacists performed systematic interventions during the patient’s hospital stay, aiming to identify, solve and prevent drug related problems in the elderly. Identified drug related problems were put forward to the health care team and discussed. Information on identified problems, and their outcomes was collected and analysed. A questionnaire was used to evaluate the health care personnel’s attitudes towards the process. Main outcome measure The number of drug related problems identified by the clinical pharmacists, the proportion of problems discussed with the physicians, the proportion of problems adjusted by the physicians and whether pharmacists and physicians prioritised any subgroup of drug related problems when choosing which problems to address. Finally, we wanted to evaluate the health care personnel’s attitudes towards the model. Results In total, 1,227 problem were identified in 190 patients. The pharmacists discussed 685 (55.8%) of the identified problems with the physicians who accepted 438 (63.9%) of the suggestions. There was no significant difference in which subgroup to put forward and which to adjust. There was a high response rate (84%) to the questionnaire, and the health care personnel estimated the benefits to be very high, both for the patients and for themselves. Conclusion The process for identifying, solving and preventing drug related problems was good and the different types of problems were considered equally important. The addition of a clinical pharmacy service was considered very useful. This suggests that the addition of our clinical pharmacy service to the hospital setting add skills of great importance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Impact of findings on practice
-
Clinical pharmacist regard different types of drug related problems as equally important.
-
The LIMM-model and the pharmacist’s professional role are appreciated and important in the Swedish care team around the patient.
-
The process of communicating DRPs needs to be addressed for future clinical pharmacy services.
Introduction
Drug therapy in the elderly is an area commonly associated with problems. In Swedish nursing homes, 74% of the residents had one or more potentially inappropriate prescription [1] and 20% was prescribed medications with anticholinergic effects, risking side effects for the patient [2]. Inappropriate use of drugs is a common cause of health care contacts [3–6] and a Swedish study showed that 35% of admissions to geriatric wards were caused by adverse drug reactions (ADR) and that patients with severe ADR were older than average [7]. An ADR is defined by WHO as ‘a response to a medicine which is noxious and unintended, and which occurs at doses normally used in man’ [8]. A meta-analysis showed that as much as 90% of ADRs in the elderly could have been avoided [5].
In order to improve patient outcomes of drug therapy and reduce negative side effects, Pharmaceutical care has been developed. Pharmaceutical care is defined as ‘the responsible provision of drug therapy for the purpose of achieving definite outcomes that improve a patient’s quality of life’ [9]. The model is based on identifying, resolving and preventing drug related problems (DRPs) for the patient [9]. A DRP has been defined as ‘an undesirable patient experience that involves drug therapy and that actually or potentially interferes with the desired patient outcome’ [10]. DRPs can then be divided into subgroups, of which ADR is one. Clinical pharmacy describes ‘a practice of pharmacy that would contribute within a larger Pharmaceutical care system, to achieving therapeutic objectives’ and is performed by clinical pharmacists [11].
When clinical pharmacists interviewed hospitalised patients, significantly more DRPs were identified than with usual care [12]. Clinical pharmacy services such as participation on medical rounds and providing an admission drug history reduced ADR and hospital mortality rates in United States hospitals [13, 14]. An increase in staffing of clinical pharmacist at United States hospitals reduced hospital drug costs [15]. In Sweden, a clinical pharmacy service resulted in a decrease in drug related hospital readmissions and health care costs [16].
In order to optimise and individualise drug treatment, a systematic approach is necessary. Our research group has developed and studied such a model, Lund Integrated Medicines Management (LIMM)-model, which consists of medication reconciliation including a medication interview at admission, medication review during the hospital stay and a medication report as a medication reconciliation at discharge. Two articles have previously been published from the LIMM-study (Landskrona Integrated Medicines Management-study) in which we conclude that the LIMM-model improves the appropriateness in patient’s drug treatment [17] and reduces medication errors (‘any error in the process of prescribing, dispensing or administering a drug, whether there are adverse consequences or not’ [18]) at discharge from hospital [19]. In addition, the individual LIMM-tools have been shown to reduce unidentified DRPs during the patients’ hospital stay [20] and reduce the need for health care contacts caused by medication errors [21]. These findings describe outcomes for the patient, but we were also interested in how the model is appreciated by the health care personnel and how it affects the daily work regarding DRPs.
Aim of the study
The aim of this study was to describe the process regarding DRPs identified by pharmacists within the LIMM-model, which DRPs the pharmacists chose to discuss with the physicians as well as which suggestions the physician chose to accept. As the LIMM-model is a team approach, the health care personnel’s attitudes towards the LIMM-model was also included in the evaluation.
Method
Study design
This study is part of the LIMM-study, a prospective longitudinal study with an intervention group and a control group.
The ethics committee at Lund University had no objections to the study and it was performed in accordance with Swedish ethic legislation and the Declaration of Helsinki.
Setting and study population
Patients admitted to the department of internal medicine at Landskrona hospital in the southern Sweden was the source for inclusion. The department comprises three wards with 61 beds in total.
Patients eligible for inclusion were the intervention group in the LIMM study, inclusion criteria being patients 65 years or older and living in the towns of Landskrona or Svalöv. Inclusion was performed 1 March 2006 until 31 December 2006, with a break during summer.
At inclusion, the patient received oral and written information about the study and at acceptance, the patient was asked to give written consent. When it was not possible to communicate with the patient, a next of kin was asked instead. Patients in terminal stage of their disease were excluded for ethical reasons.
Interventions
During the LIMM-study four pharmacists took turns in taking part in the daily work at the wards, performing structured interventions aiming to identify, solve and prevent DRPs as described in Fig. 1.
The interview was performed at admission and included a medication reconciliation helping to identify a correct medication list. Medication reconciliation is a process that involves comparing the medications a patient is receiving to what he or she actually should be receiving and then resolving the discrepancies [22]. In addition, problems with compliance, handling, knowledge and attitudes to the drug therapy was also addressed during the interview using a structured checklist [23] and potential drug related symptoms were identified. A medication review, in order to further identify DRPs and inappropriate drug use, was performed according to a structured checklist. DRPs identified by the pharmacist were then put forward to the care team and discussed during the daily rounds (consisting of physicians, nurses, physiotherapists and ergotherapists). The pharmacists’ advice was noted as well as the response from the physician. A medication care plan was created in which all changes to the drug therapy were noted. The care plan was updated continuously and was decided on by the team. The pharmacists took active part in patient information and education, based on specifically developed drug information leaflets, with focus on new medications. At discharge the physician completed the discharge information, including the Medication Report and a medication list. The pharmacist then evaluated the document with focus on correctness of the Medication Report and the medication list. If information was lacking or was incorrect, the pharmacist discussed this with the physician who had a possibility to adjust the document before the patient was discharged. Identifying, preventing and solving DRPs were done continuously throughout the patient’s hospital stay.
Problems identified in the evaluation of the medication report are not presented here as they are described elsewhere [19].
Measures
Classification of DRPs
Several systems for classification of DRPs exist. In order to be consistent with earlier research performed by our research group, we chose the system published by Cipolle et al. [24] with following subgroups: unnecessary drug therapy, need for additional therapy, wrong drug, dosage too low, ADR, dose too high and non-compliance. To include all DRPs identified by the pharmacists in our study, an addition of two groups was made: “transferring errors” and “sub-optimal monitoring of drug treatment”.
One medication can introduce more than one DRP. For example, an unnecessary drug therapy can also lead to an ADR. Furthermore, a DRP can be either actual or potential, as prevention of illness also is of great importance. A DRP was considered to be actual if an event had occurred and potential if there was risk for an event to occur. In this study we have included both actual and potential DRPs.
Information on identified DRPs was collected, including type and description of the DRP, drugs involved, whether it was discussed with the physician as well as if the drug treatment was adjusted due to a DRP (will be termed ‘DRP adjusted’ further on). By using specific checklists, one of our researchers (ABC) performed the classification of DRPs into subgroups and whether a DRP was actual or potential.
To describe drugs associated with DRPs, drugs involved were divided into different therapeutic classes according to the Anatomic Therapeutic Chemical Classification System published by the World Health Organization [25].
The health care personnel’s attitudes towards the LIMM-model
Physicians and nurses were asked to answer questions on the perceived benefits with the LIMM-model, both for themselves and for the patients. A questionnaire, using six-point ordinal scales from 1 (no benefit) to 6 (great benefit), was used. This questionnaire has previously been developed and used by our research group [20] and is shown in “Appendix”.
The questionnaire was sent out to all physicians and nurses employed at the department of internal medicine at Landskrona hospital during the study period. The administration and compilation of the questionnaire was handled by a person outside the LIMM study.
Statistical analysis
A χ2 test was used for the statistical analysis. Comparison were made to see if there was a difference in which DRP subgroups the pharmacist discussed with the physician or not and if there was a difference in which DRP subgroups the physicians chose to adjust or not.
Results are given as mean (standard deviation, SD) if not stated otherwise.
Results
A total of 190 patients were included in the study. Patient characteristics are shown in Table 1.
DRPs identified by the pharmacists
The pharmacists identified 1227 DRPs, ranging from no DRP at all in five patients to a maximum of 23 DRPs in one patient. On average, 6.5 (SD 4.3) DRPs were identified per patient. Of identified DRPs, 35.9% was considered actual and 64.1% potential. Unnecessary drug therapy was the DRP most frequently identified (17.2%) followed by transferring errors (15.9%), ADR (14.2%), need for additional therapy (13.0%), dose too high (11.2%), non-compliance (9.9%), wrong drug (7.2%), sub-optimal monitoring of drug treatment (5.8%) and dosage too low (5.7%).
The therapeutic class most often involved in unnecessary drug therapy was proton pump inhibitors (9.1% of all unnecessary drug therapy, ATC-code A02BC). The therapeutic classes most often involved in DRPs in general were antithrombotic agents (10.6% of all DRPs, ATC-code B01) followed by psycholeptics (10.0%, ATC-code N05) and diuretics (8.5%, ATC-code C03).
DRPs put forward to the physicians
Of 1227 identified DRPs, 685 (55.8%) were discussed with the physicians. Figure 2 describes the outcomes of the identified DRPs. The pharmacists could solve 54 (4.4%) DRPs directly, as it concerned information to the patient such as compliance issues. Adding these to DRPs put forward, 60.2% of the identified DRPs were addressed. The pharmacists chose not to put forward 14.4% of the DRPs. Of put forward DRPs, 39.0% were considered to be actual and 61.0% were considered to be potential.
DRPs adjusted by the physicians
When put forward to the physicians, 438 DRPs (63.9%) were adjusted, as seen in Fig. 2. In addition to this 35 DRPs were solved by the pharmacists after discussion with the physicians, resulting in 69.1% of DRPs being adjusted. The physicians chose not to adjust 16.6% of the DRPs. Of the adjusted DRPs, 39.3% were considered to be actual and 60.7% were considered to be potential.
Statistical analysis
Comparing the type of DRP subgroups put forward with those not put forward, there was no significant difference between the groups. This was also the result when comparing DRP subgroups adjusted by the physicians with those not adjusted. Thus, the pharmacists and the physicians did not prioritise a DRP subgroup for another but thought the different subgroups to be equally important. Identified, put forward and adjusted DRPs divided into DRP subgroups, are shown in Fig. 3.
The health care personnel’s attitudes towards the LIMM-model
The response rate to the questionnaire was high, in total 84.0% (68/81). For the physicians, 96.8% (30/31) answered the questionnaire and 74.5% (38/51) of the nurses participated. The majority of the respondents had repeatedly taken part in the clinical pharmacy service. The benefits for the patient and the health care personnel were estimated to be very high, as seen in Table 2. All claims in the questionnaire were given the highest score except for identification of problems in the handling, knowledge and compliance to the drug therapy and that the process was well documented that received a median of 5.
Discussion
In this study we showed that the LIMM-model identifies, solves and prevents problems in the patients’ drug therapy. The physicians acknowledged the pharmacists’ advice to a high extent and this, in addition to the positive outcomes of the evaluation questionnaire, suggests that the process is well-functioning and highly appreciated by the health care personnel.
To the best of our knowledge, no study exists that has compared DRPs put forward with not put forward as well as DRPs adjusted with not adjusted, in order to see if the pharmacists and the physicians select which DRP subgroups to address. We saw no selection in addressed DRP subgroups thus they were thought to be equally important.
However, some selection does take place, as 14.4% of the DRPs were not put forward and 16.6% were not adjusted. But this selection does not seem to be based on DRP subgroup. When looking at the distribution between actual and potential DRPs, it is similar in identified, put forward and adjusted DRPs. The fact that there is an actual DRP present is thus not always considered more important than the prevention of a potential DRP. Instead other factors must be involved. Possibly, it is the clinical significance of the DRP, actual or potential, that is the base of selection of which DRP to address or not. A Norwegian study showed that 80% of DRPs with extremely important clinical significance and 50% of DRPs with minor clinical significance were adjusted [26]. However, half of the DRPs put forward but not accepted by the physician were still considered to be clinically significant [26]. Maybe other factors such as the physicians’ views on clinical pharmacy, the relationship between the pharmacists and the physicians as well as how the communication takes place affect the physicians’ will to accept the pharmacists’ suggestions. Zillich et al. [27] have developed an instrument to measure physician-pharmacist collaboration from the physician perspective and identified trustworthiness, role specification and relationship initiation as important factors for the collaboration. It is also plausible that the pharmacists’ will to put forward DRPs change according to the status of the cooperation with the physicians. Is there a risk that you are more likely not to criticise someone you appreciate?
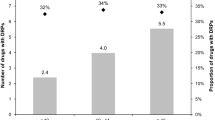
In this study 6.5 DPRs were identified per patient. Of the identified DRPs, 55.8% were put forward and 63.9% of these were adjusted, while 16.6% were rejected. In a study performed by our group at a similar department but at a University Hospital 9.9 DRPs were identified per patient, 65% of the DRPs was discussed with the physicians who adjusted 93.1% and rejected 6.9% of the suggestions [20]. Gillespie et al. [16] identified 2.6 DRPs per patient, all of which were put forward and the physicians made adjustments for 69% of the DRPs while 23% of the suggestions were rejected. Blix et al. [26] identified 3.2 DRPs per patient and 74% of the identified DRPs were put forward. The physicians’ immediate acceptance rate varied from 80 to 50%, while 8.2% were rejected [26]. Klopfer et al. [28] shows in a review article that physicians’ acceptance rate for DRPs put forward by pharmacists range from 58 to 98%, with an average rate of acceptance at 85.5%.
A high number of DRPs per patient were identified in our study, which could be due to the fact that we were looking at actual and potential DRPs. The rate of DRPs discussed with the physician were low in comparison with other studies, but when including the DRPs solved by the pharmacist directly, the rate of addressed DRPs increased to 60.2%. The acceptance rate from the physicians were lower than in other studies, but when including DRPs solved by the pharmacist after discussion with the physician, the acceptance rate increased to 69.1%, comparable to other studies.
In this study, it was not known whether 13.7% of the identified DRPs were put forward and whether 4.8% of the DRPs put forward were adjusted. This lack of information is a weakness in the study as it makes the results more uncertain.
Of the identified DRPs, 11.7% were not relevant to put forward to the physicians. This was the case when what at first had appeared to be a DRP turned out to no longer classify as such. For example, the pharmacist could have identified a DRP but before this was discussed with the physician, the physician could already have adjusted it.
The rate of DRPs put forward and DRPs adjusted differ between studies. Comparing studies on DRPs is difficult as different types of clinical pharmacy services, settings and study populations are being used. Furthermore, different classification systems of DRPs exist. A review study by van Mil et al. [29] shows that as much as 14 different systems are being used.
The health care personnel estimated the benefits of the LIMM-model to be very high, both for themselves and for the patients. All claims of the special benefits of the clinical pharmacy service and the addition of a clinical pharmacist to the health care team were also agreed on. This results shows that the introduction of the LIMM-model was successful. These results are in accordance with another study performed by members of our research group [20]. We believe that the high evaluations can be explained by several factors. At the clinic, there was a history of pharmacists being supportive and proactive in the development of better medication use, such as a more rational stock of medications. The LIMM-model was introduced with a scientific approach aiming to measure our interventions and their impact on the patient’s drug treatment. The clinical pharmacists spent some time on the wards before the intervention started, collecting information on patients in the control group, and during this time good relations were established. The different responsibilities were clearly divided between the professions, the interventions performed by the pharmacists had been clearly described and was performed in a structured and systematic way. The clinical pharmacists had had special training before attending the wards, both in pharmacotherapy and in how the health care system is organised and this ensured that the DRPs put forward and discussed were clinically relevant.
Limitations of the study
A study size calculation was not made in order to estimate the amount of DRPs needed to detect differences between identified DRPs, DRPs put forward and DRPs adjusted. Instead we used existing material to see if a difference could be detected.
When using a questionnaire, there is a risk that the most critical persons will not answer, making the results more positive then they actually are. The higher the response rate, the more accurate the results will be. In this study we had a high response rate and it is therefore likely that the results reflect the reality.
Future needs
If we could better understand how pharmacists and physicians prioritise among DRPs, it would be possible to target clinical pharmacy services in an even more efficient way. Therefore there is need for studies powered to further analyse how pharmacists and health care personnel prioritise between DRPs and to detect the factors influencing this. We hope that this descriptive study can be a base for that.
Conclusion
In general the process for identifying, solving and preventing the patients′ DRPs was good and the different DRP subgroups were considered equally important. The addition of the LIMM-model was very appreciated by the health care personnel and thought to be very useful for themselves and the patients. This suggests that the addition of the LIMM model with a clinical pharmacist and a clinical pharmacy service to the hospital setting ad skills of great importance that otherwise would be lacking.
References
Bergman A, Olsson J, Carlsten A, Waern M, Fastbom J. Evaluation of the quality of drug therapy among elderly patients in nursing homes. Scand J Prim Health Care. 2007;25(1):9–14.
Olsson J, Fastbom, J. The quality of the drug use in the elderly, the KÄLLA-project, an application of quality indicators to analyze the drug use in elderly with a medication dispensing system in nursing homes in a Swedish county (In Swedish: Kvaliteten i äldres läkemedelsanvändning KÄLLA-Projektet, en tillämpning av kvalitetsindikatorer för analys av läkemedelsanvändningen hos äldre med dosexpedition på kommunala äldreboenden i ett svenskt län) The National Board on Health and Welfare. http://www.socialstyrelsen.se/publikationer2004/2004-131-24. Accessed 12 August 2010.
Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279(15):1200–5.
Einarson TR. Drug-related hospital admissions. Ann Pharmacother. 1993;27(7–8):832–40.
Beijer HJ, de Blaey CJ. Hospitalisations caused by adverse drug reactions (ADR): a meta-analysis of observational studies. Pharm World Sci. 2002;24(2):46–54.
Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329(7456):15–9.
Paul E, End-Rodrigues T, Thylen P, Bergman U. Adverse drug reactions a common cause of hospitalization of the elderly. A clinical retrospective study. Läkartidningen. 2008;105(35):2338–42.
Organization WorldHealth. Safety of medicines. Geneva: A guide to detecting and reporting adverse drug reactions; 2002.
Hepler CD, Strand LM. Opportunities and responsibilities in pharmaceutical care. Am J Hosp Pharm. 1990;47(3):533–43.
Strand LM, Morley PC, Cipolle RJ, Ramsey R, Lamsam GD. Drug-related problems: their structure and function. DICP. 1990;24(11):1093–7.
Hepler CD. What is the relationship between clinical pharmacy and pharmaceutical care? ESCP Newsletter. 2001;105.
Viktil KK, Blix HS, Moger TA, Reikvam A. Interview of patients by pharmacists contributes significantly to the identification of drug-related problems (DRPs). Pharmacoepidemiol Drug Saf. 2006;15(9):667–74.
Bond CA, Raehl CL. Clinical pharmacy services, pharmacy staffing, and hospital mortality rates. Pharmacotherapy. 2007;27(4):481–93.
Bond CA, Raehl CL. Clinical pharmacy services, pharmacy staffing, and adverse drug reactions in United States hospitals. Pharmacotherapy. 2006;26(6):735–47.
Bond CA, Raehl CL, Franke T. Clinical pharmacy services, pharmacist staffing, and drug costs in United States hospitals. Pharmacotherapy. 1999;19(12):1354–62.
Gillespie U, Alassaad A, Henrohn D, Garmo H, Hammarlund-Udenaes M, Toss H, et al. A comprehensive pharmacist intervention to reduce morbidity in patients 80 years or older: a randomized controlled trial. Arch Intern Med. 2009;169(9):894–900.
Bergkvist A, Midlov P, Hoglund P, Larsson L, Eriksson T. A multi-intervention approach on drug therapy can lead to a more appropriate drug use in the elderly. LIMM-Landskrona Integrated Medicines Management. J Eval Clin Pract. 2009;15(4):660–7.
Leape LL. Preventing adverse drug events. Am J Health Syst Pharm. 1995;52(4):379–82.
Bergkvist A, Midlov P, Hoglund P, Larsson L, Bondesson A, Eriksson T. Improved quality in the hospital discharge summary reduces medication errors–LIMM: Landskrona Integrated Medicines Management. Eur J Clin Pharmacol. 2009;65(10):1037–46.
Bondesson A, Eriksson T, Kragh A, Holmdahl L, Midlöv P, Höglund P. In-hospital medication reviews reduce the number of unidentified drug-related problems. In: Bondesson Å, editor. Aspects on optimisation of drug therapy in the elderly. Dissertation, Lund University.
Midlov P, Deierborg E, Holmdahl L, Hoglund P, Eriksson T. Clinical outcomes from the use of Medication Report when elderly patients are discharged from hospital. Pharm World Sci. 2008;30(6):840–5.
Institute for HealthCare Improvement. Prevent Adverse Drug Events (Medication Reconciliation) http://www.ihi.org/IHI/Topics/PatientSafety/MedicationSystems/Tools/Medication+Reconciliation+Review.htm. Accessed 10 Oct 2010.
Bondesson A, Hellström L, Eriksson T, Höglund P. A structured questionnaire to assess patient compliance and beliefs about medicines taking into account the ordered categorical structure of data. J Eval Clin Pract. 2009;15(4):713–23.
Cipolle R, Strand LM, Morley PC. Pharmaceutical Care Practice. New York: The McGraw-Hill Companies Inc; 1998.
ATC classification index with DDDs. WHO collaborating centre for drug statistics methodology. Oslo; 2006.
Blix HS, Viktil KK, Moger TA, Reikvam A. Characteristics of drug-related problems discussed by hospital pharmacists in multidisciplinary teams. Pharm World Sci. 2006;28(3):152–8.
Zillich AJ, Doucette WR, Carter BL, Kreiter CD. Development and initial validation of an instrument to measure physician-pharmacist collaboration from the physician perspective. Value Health. 2005;8(1):59–66.
Klopfer JD, Einarson TR. Acceptance of pharmacists’ suggestions by prescribers: a literature review. Hosp Pharm 1990;25(9):830–2, 4–6.
van Mil JW, Westerlund LO, Hersberger KE, Schaefer MA. Drug-related problem classification systems. Ann Pharmacother. 2004;38(5):859–67.
Acknowledgments
We would like to thank the staff at the department of internal medicine at Landskrona Hospital, especially Dr Per Löfdahl, and the clinical pharmacists Sofia Jönsson and Emma Olsson for excellent cooperation and work.
Funding
We are grateful to the National Board of Health and Welfare, the Swedish Academy of Pharmaceutical Sciences, the County of Skåne and Apoteket Farmaci AB for funding the study.
Conflicts of interests
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Bergkvist Christensen, A., Holmbjer, L., Midlöv, P. et al. The process of identifying, solving and preventing drug related problems in the LIMM-study. Int J Clin Pharm 33, 1010–1018 (2011). https://doi.org/10.1007/s11096-011-9575-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-011-9575-1