Abstract
Objective In Australia, accredited pharmacists perform medication reviews for patients to identify and resolve drug-related problems. We analysed the drug-related problems identified in reviews for both home-dwelling and residential care-facility patients. The objective of this study was to examine the number and nature of the drug-related problems identified and investigate differences between each type of review. Setting Australian patients living at home or in residential care-facilities. Method We collected a nation-wide sample of medication reviews conducted between 1998 and 2005. These reviews had been self-selected by pharmacists and submitted as part of the reaccreditation process to the primary body responsible for accrediting Australian pharmacists to perform medication reviews. The drug-related problems identified in each review were classified by type and drugs involved. Main outcome measure The number and nature of drug-related problems identified in pharmacist-conducted medication reviews. Results There were 1,038 drug-related problems identified in 234 medication reviews (mean 4.6 (±2.2) problems per review). The number of problems was higher (4.9 ± 2.0 vs. 3.9 ± 2.2; P < 0.001) in reviews for home-dwelling patients compared with care-facility residents. The number of clinically-significant problems was higher (2.1 ± 1.1 vs. 1.5 ± 0.7; P < 0.001) for home-dwelling patients. Oral hypoglycaemics and analgesics/antipyretics were significantly more likely to be associated with problems in home-dwelling patients than in residential care-facility patients. Conclusion These data illustrate the prevalence of drug-related problems and the ability of pharmacists to identify these problems in the Australian models of medication review. The nature and frequency of problems varied between reviews for home-dwelling and care-facility patients. Such information may be used to better focus the training of practitioners based on the most frequently encountered health problems and the nature of common drug-related problems in the two settings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Impacts of findings of practice
-
The nature of drug-related problems identified in community dwelling patients differs with regard to their living arrangements.
-
An awareness of these differences may assist pharmacists to more readily identify drug-related problems in different patient populations.
Introduction
Australia’s population is ageing, as is the world’s [1]. In Australia in 2004, persons aged 65 years and older made up 13% of the population; this proportion is projected to at least double by 2051. A number of studies have demonstrated that the elderly are at a greater risk of experiencing drug-related problems (DRPs) compared to the remainder of the population [2–4]. This is due to numerous factors, including changes in pharmacodynamics and pharmacokinetics, a higher incidence of multiple chronic diseases, polypharmacy and reduced cognitive capacity. In the United States, it is believed that as many as 200,000 people die annually as a consequence of DRPs. DRPs are estimated to result in 140,000 hospital admissions in Australia every year, and it has been suggested that between 32% and 69% of these admissions are preventable [5, 6].
There is ongoing evidence in the literature that pharmacist involvement in drug therapy review in older individuals can improve elderly health outcomes [7–9]. Pharmaceutical care services are now available in many parts of the world, including the United States, United Kingdom, Europe and South America [10]. The Australian government remunerates accredited pharmacists to formally review non-hospitalised patients in either Home Medicines Reviews (HMRs) or Residential Medication Management Reviews (RMMRs). HMRs are provided for patients living in their own home, whilst residential-care facility patients receive RMMRs. The aim of both types of review is the identification, resolution and prevention of DRPs [11, 12].
The DRPs experienced by the individual patient populations have been described by several authors [6, 10, 13–15]. There has been no direct comparison of the differences between the DRPs identified in HMRs and RMMRs.
The aim of this research was to examine the nature of the DRPs identified in RMMRs and HMRs, and investigate any differences between these two types of reviews.
Method
Pharmacists performing RMMRs and HMRs must be accredited with either the Society of Hospital Pharmacists of Australia or the Australian Association of Consultant Pharmacists [16]. Until 2005, as part of their annual reaccreditation with the Australian Association of Consultant Pharmacists, accredited pharmacists were required to submit a self-selected sample of their reviews to the association for assessment.
These reviews were stored by the association alphabetically according to the reviewing pharmacist’s surname. To approximate a random sampling of the reviews, we collected an alphabetically sequential subset of them within a specified period of time in 2006. In the time available for data collection, 234 reports from medication reviews by 200 accredited pharmacists from various states in Australia were collected. Pharmacists were allocated a unique identifying number to link them to their reviews and their date of reaccreditation was recorded. Information concerning the patients and the problems identified in these reviews was entered into an electronic Microsoft Access 2000 database (Microsoft Inc, Redmond, Washington). Patient information included: gender; age at review; names of all current regular and when-required medications (coded according to World Health Organisation Anatomic Therapeutic Chemical classification index); and past and present medical conditions (classified according to the World Health Organisation International Classification of Primary Care 2 PLUS system). Information concerning the DRPs consisted of: the type and subtype of problem; and the clinical significance of the problem (classified according to the D.O.C.U.M.E.N.T. classification system [17]). This hierarchal DRP classification system was developed to satisfy the major requirements for a DRP classification system defined in a recent review of such systems [18]. The D.O.C.U.M.E.N.T. system allows DRPs to be classified as either actual (defined as moderate to high clinical significance, likely to either result in a medical consultation or hospitalisation unless resolved) or potential (of lower clinical significance-related to cost saving, information provision, or resolved without requiring medical attention).
All coding and classification was undertaken by two of the investigators (AH and CV). Each investigator coded approximately half of the data. As all data was classified using validated classification systems, it was not considered necessary to perform inter- or intra-rater variability analysis. Analysis of the frequencies and interrelationships of the data was performed using Microsoft Excel 2003 (Microsoft Inc, Redmond, Washington) and SPSS 14.0 (SPSS Inc., Chicago, Illinois). Demographic differences between the HMR and RMMR patients were determined with Student’s t-tests. Univariate correlation analysis between DRPs and patient characteristics was performed with the Pearson correlation coefficient (r). Standard multiple regression was then performed to assess the influence of number of diseases and number of medications on the number of DRPs identified. The χ2-test for categorical variables was used to compare the proportions of DRP types between HMRs and RMMRs. For all analyses, a probability value of less than 0.05 was considered statistically significant.
This research was undertaken with the permission of the Chief Executive Officer of the Australian Association of Consultant Pharmacists. The authors considered that formal ethics approval was not necessary as all information relating to patients and pharmacists was de-identified prior to data collection.
Results
The reports analysed were from medication reviews performed between 1998 and 2005, with 80% conducted between 2004 and 2005. Patient demographics and DRP frequencies are shown in Table 1. The percentage of females was greater in the RMMR group (67%) compared to the HMR group (54%) (χ2 = 3.9, df = 1; P = 0.049). The HMR patient group was taking a mean of 2.0 more medications than the RMMR group (95% CI 0.97–3.14, Student’s t = 3.73, P < 0.001). There was a mean of 0.9 (95% CI 0.3–1.4, Student’s t = 3.13, P = 0.002) more DRPs identified per HMR report compared to RMMR reports. The HMR reports contained a mean of 0.6 (95% CI 0.3–0.8, Student’s t = 3.74, P < 0.001) more moderately or highly significant [17] DRPs compared to RMMR reports.
Univariate analysis showed that the number of DRPs identified was weakly, but significantly, correlated with: the number of medications (r = 0.34, P < 0.001) and the number of medical conditions (r = 0.31, P < 0.001). Multiple regression found that number of medications (t = 3.73, P < 0.001) and number of medical conditions (t = 4.93, P < 0.001) independently predicted the number of DRPs. There was no relationship between age or gender and the number of DRPs identified.
The frequency of the DRP types and subtypes is shown in Table 2. The three most prevalent types of DRPs were Drug selection (24.9%), Toxicity, adverse reaction or side effect (19.7%) and Untreated indications (15.7%). Compliance problems were identified in HMRs significantly more frequently than with RMMRs (χ2 = 56.2, df = 1; P < 0.05). Over- and underdose (χ2 = 7.0, df = 1; P < 0.05), Monitoring (χ2 = 4.5, df = 1; P < 0.05) and Non-clinical (χ2 = 15.3, df = 1; P < 0.05) DRPs were seen significantly more frequently in the RMMR group. The number of DRPs of moderate and high clinical significance for each type of DRP is shown in Table 3. Again, the more clinically-significant Compliance-related DRPs were more common in HMRs than RMMRs (χ2 = 17.4, df = 1; P < 0.05).
Of the 1,038 DRPs identified, 170 were either associated with no specific drug or more than two drugs. The majority of these (113, 66.5%) were either Untreated indications (72, 42.4%) or Monitoring (41, 24.1%). Those associated with more than two drugs involved examples such as St John’s Wort interacting with a number of medications or the combination of non-steroidal anti-inflammatory drug (NSAID), angiotensin converting enzyme (ACE) inhibitor and diuretic combinations potentially affecting renal function. A wide range of different medications (350) were associated with DRPs, with no single medication accounting for more than 5.4% of the DRPs.
The twenty drug classes (grouped at ATC Level 3 code) most commonly involved in the DRPs are shown in Table 4. Drug groups commonly involved in DRPs were NSAIDs (ATC Level 3 code M01A) (61 of 838 DRPs identified involving drugs, 7.3%), antidepressants (N06A) (57 DRPs, 6.8%), antithrombotic agents (B01A) (48 DRPs, 5.7%), ACE inhibitors (C09A) (38 DRPs, 4.5%) and oral hypoglycaemic agents (A10B) (38 DRPs, 4.5%).
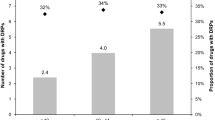
To assess the frequency with which a particular group of medications was implicated in a DRP, a “DRP frequency” was calculated by dividing the number of DRPs involving a drug group by the number of patients for whom the drug group was prescribed. This data is presented in Table 4. Six groups of drugs were associated with DRPs in at least 45% of the patients for whom they were prescribed. These were NSAIDs (M01A) (75%), antidepressants (N06A) (63%), inhaled corticosteroids (D07A) (53%), dihydropyridine calcium channel blockers (C08C) (49%), oral hypoglycaemics (A10B) (46%), and class I and III antiarrhythmics (C01B) (45%). When the medications involved in each of the two review types were compared, both oral hypoglycaemics (A10B) (χ2 = 8.9, df = 1; P = 0.003) and other analgesics and antipyretics (N02B) (χ2 = 8.5, df = 1; P = 0.004) were associated with DRPs more frequently in the HMR group than the RMMRs.
For six groups of medications, over 45% of the DRPs they were associated with were classified as either moderately or highly significant. These included selective calcium channel blockers (C08D) (60%), ACE inhibitors (C09A) (55%), oral hypoglycaemics (A10B) (50%), beta blocking agents (C07B) (48%), dihydropyridine calcium channel blockers (C08C) (45%) and inhaled adrenergics (R03A) (45%). No differences in the proportion of more clinically-significant DRPs were observed between the review types, although based on a relatively small number of DRPs.
Discussion
This study provides insight into the nature of DRPs identified by pharmacists performing medication reviews for Australian patients. The major findings included an indication of several factors associated with DRPs in RMMR and HMR reports. The number of medical conditions and the number of medications prescribed showed significant positive correlation with the number of DRPs. This implies that polypharmacy and comorbidities are major risk factors for experiencing DRPs, which is consistent with other studies [15, 19].
The demographics of the patients in this study are similar to other studies involving pharmacist-conducted medication reviews in both Australia and internationally. The numbers of DRPs identified per patient are also consistent with these studies. Studies of home-dwelling residents have reported between 2.2 [6] and 4.1 [20] DRPs identified per person, and studies of residential care-facility residents have reported between 2.4 [15] and 3.5 [21] DRPs identified per patient.
The HMR patients were taking a mean of two additional drugs compared to their RMMR counterparts. This may be due to the difference in criteria identifying patients as potential recipients of HMRs compared with patients who receive RMMRs. Any resident of a residential care facility may undergo an RMMR, however there are recommendations for identifying patients for HMRs, such as the patient taking at least five different medications or at least 12 medication doses per day [11].
Regardless of accommodation status, the most commonly identified DRP types were Drug selection, Toxicity, adverse reaction or side effect and Untreated indication. The Drug selection problems included identification of agents with precautions or contraindications in renal failure, such as metformin. Toxicity, adverse reaction or side effect-related DRPs included antihypertensives causing postural hypotension and ACE inhibitor-induced cough. Untreated indications included patients using no analgesics for arthritic pain and diabetic patients at high risk of cardiovascular events not prescribed antiplatelet agents. A high incidence of Drug selection and Toxicity, adverse reaction or side effect DRPs is expected with the pharmacist’s expertise involving medications. That Untreated indication is a common DRP illustrates a pharmacist’s ability to individually assess a patient’s overall health status in these reviews, and alert prescribers to diagnosed conditions or symptoms that are potentially sub-optimally treated, or not treated at all. This result is consistent with other literature that indicates that underprescribing may even be a more prevalent issue than overprescribing or polypharmacy [22].
The type of both total and more clinically-significant DRPs varied with the form of medication review. As institutionalised patients are generally administered their medications by nursing staff, it is unsurprising that this group rarely experienced the compliance related issues of their self-administering home-dwelling counterparts. A low incidence of Compliance issues was seen in another study of institutionalised patients [15]. The high incidence of Monitoring and Over- and underdose DRPs in RMMR patients is potentially due to their more advanced age, with a corresponding increase in susceptibility to adverse effects and age-related physiological changes in response to drugs.
Analysis of the drugs most commonly associated with DRPs provides useful guidance regarding medications highly prone to involvement in DRPs. The presence of an NSAID (M01A), for example, caused at least one DRP in over 75% of patients for whom it was prescribed, and 36% of these DRPs were of at least moderate clinical significance. Common DRPs associated with NSAIDs were Drug interaction (21, 34.4%), Other drug selection problem (15, 24.6%) and Toxicity caused by drug interaction (13, 21.3%). Roughead et al. found that 60.5% of people taking NSAIDs in the community setting were at risk of DRPs [6]. Becker et al. identified NSAIDs as a major contributor to drug–drug interactions resulting in hospitalisations and emergency department visits [23].
When the more clinically-significant DRPs were examined as a proportion of all DRPs associated with a particular drug group, cardioselective calcium channel blockers (C08D), ACE inhibitors (C09A), oral anti-diabetic agents (A10B) and beta blockers (C07A) were each associated with a high proportion (>45% of DRPs more clinically-significant). DRPs associated with cardiovascular drugs are likely to result in more significant consequences (e.g. haemodynamic), accounting for the higher rating of significance in this drug group. Cardiovascular medications have frequently been implicated in DRPs in studies of home-dwelling patients [6, 15].
More DRPs were reported with oral blood glucose lowering drugs (A10B) and other analgesics and antipyretics (N02B) in the HMR group compared to the RMMR group. DRPs in HMRs associated with oral blood glucose lowering agents were commonly Toxicity, adverse reaction or side effect (8, 24.2%), Untreated indication (7, 21.2%) or Drug selection (7, 21.2%) types. This suggests that patients in the general community either required more assistance with management of their diabetes, or had more issues identified related to hypo- or hyperglycaemia compared to those in residential care facilities. Residential care facility patients have their blood glucose levels checked regularly by care staff, as well as predictable daily routines with fewer unexpected stressors, which probably accounts for this difference. DRPs associated with other analgesics and antipyretics were also commonly of the Untreated indication (9, 40.9%) or the Compliance (7, 31.8%) type. This implies that home-dwelling patients were less likely to be adequately managing their pain. Patients in residential care-facilities may be monitored more closely by carers and offered “when required” analgesics on a more regular basis than their home-dwelling counterparts.
With regard to the RMMR reports, DRPs involving calcium channel blockers (C08D and C08C) and antipsychotics (N05A) were reported frequently. Of the 20 RMMR reports where calcium channel blockers were prescribed, 12 (60%) had a DRP associated with this drug group. The most common DRP type involving calcium channel blockers was Toxicity, adverse reaction or side effect (5, 41.7%). Examples included the drugs being associated with potentially or actually causing constipation and/or peripheral oedema. Similarly, 13 of 32 (40.6%) RMMR reports where antipsychotics were prescribed identified a DRP associated with this drug group. DRPs involving antipsychotics were of a range of different types, including Toxicity, adverse reaction or side effect (4, 31%) and Untreated indication (3, 23%). Examples included patients taking combinations of psychoactive substances with an associated increase in risk of falls or patients with behavioural disorders requiring increased doses of antipsychotics.
The HMR patients frequently experienced DRPs with antihypertensives, and over 50% of DRPs associated with ACE inhibitors, beta blockers and calcium channel blockers were of moderate or high clinical significance. DRPs involving antihypertensives were seen less frequently in RMMRs compared to HMRs (10.3% of DRPs vs. 12.1%), and were less likely to be of higher clinical significance (29.4% of DRPs related to antihypertensives in RMMR reports were classified as more clinically-significant, compared to 63.5% of DRPs in HMR reports).
The differences that we have identified in the nature and frequency of DRPs between the two review types may have applications in training pharmacists to perform reviews. By considering these differences, pharmacists may more readily identify the DRPs common to each review type. In addition, future studies of HMRs and RMMRs should consider the two review types independently, as the nature and frequency of the DRPs identified are different.
There are several limitations to our study. The data analysed was from medication reviews selected by the reviewing pharmacist for assessment purposes. These reviews may therefore not have accurately represented reviews being performed in practice. Another limitation is that information relating to the DRPs identified relied solely on documentation in the medication review reports. DRPs that were identified and resolved without involving the prescriber may therefore have not been documented. In addition, it is likely that the reviewing pharmacist did not have had the patient’s complete medical profile available, and may have identified some DRPs that were already being addressed. The number and type of DRPs in our study was similar to results of previous studies of pharmacist-conducted medication reviews, which support the findings in this study [20, 21]. Our results may also be influenced a lack of standardisation and completeness of the information available for the reviews that were analysed, as evidenced by the high proportion of RMMR patients with an unknown gender. We assumed that as the reviews were self-selected by pharmacists for assessment, sufficient information was provided to assess them.
Conclusion
A large number of DRPs involving a wide range of medications were identified through pharmacist-conducted medication reviews. Many of the DRPs and drug groups commonly identified in the RMMR and HMR services have been associated with adverse health outcomes and the potential for increased hospital admissions.
This is the first report that directly compares the nature of DRPs identified in the HMR and RMMR setting in Australia. The differences in the frequency and types of DRPs experienced by home-dwellers and residential care-facility patients indicate that they are two distinct groups with differing incidences and types of DRPs.
References
Population Projections: Australia 2004–2101 (Reissue). Australian Bureau of Statistics. http://www.ausstats.abs.gov.au/ausstats/subscriber.nsf/0/73D26920772F929ECA25718C001518FB/$File/32220_2004%20to%202101reissue.pdf. Accessed 2009 Jan 27.
Farris KB, Ganther-Urmie JM, Fang G, Doucette WR, Brooks JM, Klepser DG, et al. Population-based medication reviews: a descriptive analysis of the medication issues identified in a Medicare not-for-profit prescription discount program. Ann Pharmacother. 2004;38(11):1823–9. doi:10.1345/aph.1E204.
Routledge PA, O’Mahony MS, Woodhouse KW. Adverse drug reactions in elderly patients. Br J Clin Pharmacol. 2004;57(2):121–6. doi:10.1046/j.1365-2125.2003.01875.x.
Simonson W, Feinberg JL. Medication-related problems in the elderly: defining the issues and identifying solutions. Drugs Aging. 2005;22(7):559–69. doi:10.2165/00002512-200522070-00002.
Roughead EE. The nature and extent of drug-related hospitalisations in Australia. J Qual Clin Pract. 1999;19(1):19–22. doi:10.1046/j.1440-1762.1999.00288.x.
Roughead EE, Barratt JD, Gilbert AL. Medication-related problems commonly occurring in an Australian community setting. Pharmacoepidemiol Drug Saf. 2004;13(2):83–7. doi:10.1002/pds.912.
Christensen D, Trygstad T, Sullivan R, Garmise J, Wegner SE. A pharmacy management intervention for optimizing drug therapy for nursing home patients. Am J Geriatr Pharmacother. 2004;2(4):248–56. doi:10.1016/j.amjopharm.2004.12.002.
Roberts MS, Stokes JA, King MA, Lynne TA, Purdie DM, Glasziou PP, et al. Outcomes of a randomized controlled trial of a clinical pharmacy intervention in 52 nursing homes. Br J Clin Pharmacol. 2001;51:257–65. doi:10.1046/j.1365-2125.2001.00347.x.
Sorensen L, Stokes JA, Purdie DM, Woodward M, Elliott R, Roberts MS. Medication reviews in the community: results of a randomised, controlled effectiveness trial. Br J Clin Pharmacol. 2004;58(6):648–64. doi:10.1111/j.1365-2125.2004.02220.x.
Rao D, Gilbert A, Strand LM, Cipolle RJ. Drug therapy problems found in ambulatory patient populations in Minnesota and South Australia. Pharm World Sci. 2007;29(6):647–54. doi:10.1007/s11096-007-9123-1.
Framework Document for Domiciliary Medication Management Reviews. Medication Management Implementation Steering Group. http://www.psa.org.au/site.php?id=856. Accessed 2009 Jan 27.
Guidelines and Standards for the Collaborative and Pharmacist Residential Medication Management Review (RMMR) Program and Quality Use of Medicines (QUM) Services. Pharmaceutical Society of Australia. http://www.psa.org.au/site.php?id=1122. Accessed 2009 Jan 27.
Burkiewicz JS, Sweeney BL. Medication reviews in senior community housing centers. Consul Pharm. 2006;21(9):715–8.
Lau DT, Kasper JD, Potter DE, Lyles A, Bennett RG. Hospitalization and death associated with potentially inappropriate medication prescriptions among elderly nursing home residents. Arch Intern Med. 2005;165(1):68–74. doi:10.1001/archinte.165.1.68.
Ruths S, Straand J, Nygaard HA. Multidisciplinary medication review in nursing home residents: what are the most significant drug-related problems? The Bergen District Nursing Home (BEDNURS) study. Qual Saf Health Care. 2003;12(3):176–80. doi:10.1136/qhc.12.3.176.
Home Medicines Review (HMR). Medicare Australia. http://www.medicareaustralia.gov.au/provider/pbs/fourth-agreement/hmr.jsp. Accessed Jan 27 2009.
Peterson GM, Tenni PC. Identifying, prioritising and documenting drug-related problems. Aust Pharm. 2004;23(10):706–9.
van Mil JW, Westerlund LO, Hersberger KE, Schaefer MA. Drug-related problem classification systems. Ann Pharmacother. 2004;38(5):859–67. doi:10.1345/aph.1D182.
Krska J, Cromarty JA, Arris F, Jamieson D, Hansford D, Duffus PR, et al. Pharmacist-led medication review in patients over 65: a randomised, controlled trial in primary care. Age Ageing. 2001;30(3):205–11. doi:10.1093/ageing/30.3.205.
Haugbolle LS, Sorensen EW. Drug-related problems in patients with angina pectoris, type 2 diabetes and asthma—interviewing patients at home. Pharm World Sci. 2006;28(4):239–47. doi:10.1007/s11096-006-9023-9.
Finkers F, Maring JG, Boersma F, Taxis K. A study of medication reviews to identify drug-related problems of polypharmacy patients in the Dutch nursing home setting. J Clin Pharm Ther. 2007;32(5):469–76. doi:10.1111/j.1365-2710.2007.00849.x.
Elliott RA. Problems with medication use in the elderly: an Australian perspective. J Pharm Pract Res. 2006;36(1):58–66.
Becker ML, Kallewaard M, Caspers PW, Visser LE, Leufkens HG, Stricker BH. Hospitalisations and emergency department visits due to drug–drug interactions: a literature review. Pharmacoepidemiol Drug Saf. 2007;16(6):641–51. doi:10.1002/pds.1351.
Acknowledgement
The assistance with data collection provided by Mr. Bill Kelly, Chief Executive Officer of the Australian Association of Consultant Pharmacists, is acknowledged with gratitude.
Funding
None.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stafford, A.C., Tenni, P.C., Peterson, G.M. et al. Drug-related problems identified in medication reviews by Australian pharmacists. Pharm World Sci 31, 216–223 (2009). https://doi.org/10.1007/s11096-009-9287-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-009-9287-y


