Abstract
The use of nanotechnology has been extensively explored for developing efficient drug delivery systems towards topical and transdermal applications. Ethosomes constitute a vesicular nanocarrier containing a relatively high concentration of ethanol (20–45%). Ethanol is a well-known permeation enhancer, which confers ethosomes unique features, including high elasticity and deformability, allowing them to penetrate deeply across the skin and enhance drug permeation and deposition. The improved composition of ethosomes offer, thereby, significant advantages in the delivery of therapeutic agents over particularly the conventional liposomes regarding different pathologies, including acne, psoriasis, alopecia, skin infections, hormonal deficiencies, among others. This review provides a comprehensive overview of the ethosomal system and an assessment of its potential as an efficient nanocarrier towards the skin delivery of active ingredients. Special attention is given to the composition of ethosomes and the mechanism of skin permeation, as well as their potential applications in different pathologies, particularly skin pathologies (acne, psoriasis, atopic dermatitis, skin cancer and skin infections). Some examples of ethosome-based formulations for the management of skin disorders are also highlighted. Besides the need for further studies, particularly in humans, ethosomal-based formulations hold great promise in the skin delivery of active ingredients, which increasingly asserts oneself as a viable alternative to the oral route.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the years, the skin delivery route has become more popular and convenient to efficient drug delivery and for overcoming the disadvantages associated with the oral and parenteral routes. Skin delivery constitutes a painless and non-invasive alternative to the parenteral route and provides several advantages, such as avoidance of the first-pass metabolism and consequent augmented plasma concentrations, reduced systemic side effects, and increased patient compliance. Skin delivery comprehends both topical and transdermal drug delivery. Topical drug delivery is intended to treat a local dermatological disorder without the need to target systemic circulation. Examples of topical formulations include anti-acne treatments, anti-inflammatory agents, local anaesthetics, and antifungal treatments (1). Contrastingly, transdermal drug delivery requires active ingredients to diffuse through the different skin layers into the systemic circulation, such as nicotine, fentanyl, and buprenorphine (1, 2). To fully understand the mechanism underneath topical and transdermal delivery, it is important to distinguish two different concepts: penetration and permeation. Penetration describes the passage of molecules/ non-fragmented vesicles through a semipermeable barrier with pores smaller than the vesicular diameter, and is caused by a driving force, whereas permeation occurs when this passage depends on a concentration gradient, which follows Fick’s law of diffusion (3). Active ingredients need to face a rate-limiting skin barrier, known as the stratum corneum (SC), in order to reach its target site of action and perform their therapeutic action. Thus, even though the SC constitutes an effective barrier against pathogens and xenobiotics, it can concurrently limit drug penetration and restrict the passage of many active ingredients (4).
Ongoing research has been developed to overcome this challenge and several approaches have been proposed, involving the use of physical and / or chemical methods. Physical methods apply an energy force (e.g. iontophoresis, electroporation, ultrasound, laser, radiofrequency ablation, and thermal poration) or a mechanical force (e.g. microneedles, microdermabrasion, needleless injections) to overcome the low permeability of the SC (1, 5, 6). On the other hand, chemical methods take advantage of permeation enhancers, such as fatty acids, surfactants, alcohols, phospholipids, cyclodextrins and terpenes (1, 6). However, these strategies are associated with several disadvantages, such as poor stability, complex preparation process and skin injury (7, 8). Another potential strategy is the encapsulation of active ingredients into nanocarriers as skin delivery systems. Nanocarriers offer multiple benefits: (1) enhanced skin permeation (9); (2) superior active ingredient retention in skin and consequent sustained drug release (1, 6, 10); (3) incorporation of both hydrophobic and hydrophilic active ingredients (1, 11); (4) optimal target delivery and bioavailability (1, 6); (5) increased stability and protection of active ingredients from degradation (1, 11).
There are various types of nanocarriers, including lipid-based, polymeric-based and surfactant-based nanocarriers (12). Among lipid-based nanocarriers, vesicular systems such as liposomes (composed of a phospholipid bilayer enclosing an aqueous cavity), niosomes (composed by non-ionic surfactants and amphipathic compounds, which impart a neutral charge), ethosomes (consisting of phospholipids and high concentrations of alcohol), transfersomes (containing an edge activator and double-chain lipids) and cubosomes (curved bicontinuous lipids bilayers arranged in three dimensions) are claimed to facilitate skin delivery, by improving both penetration and permeation through the SC in local and systemic treatments (2). In addition, vesicular systems are highly compatible with the skin, as they are prepared with lipids, which are also present in the skin. Liposomes have been extensively studied due to their advantageous characteristics, namely biodegradability, low cost and straightforward manufacturing process. However, it is widely recognized that liposomes are unable to penetrate effortlessly through the skin layers, and hence are generally restricted to the SC or the upper skin layers of the epidermis (4, 13). On the contrary, ethosomes are a vesicular carrier with enhanced skin delivery properties based on its singular attributes, such as high deformability and flexibility. Similarly to liposomes, ethosomes display a phospholipid bilayer and exceptional biocompatibility, but evidence a distinctive feature: a relatively high concentration of ethanol (20–45%) (8). Ethanol imparts unique characteristics to ethosomes, including (1) small vesicular size, which ranges from tens of nanometres to microns depending on its composition;(2) high deformability; (3) fluidity; and (4) stability (14, 15). Therefore, several studies suggest that ethosomes are more effective in improving the extension and efficiency of skin penetration, when compared to either classical liposomes or conventional hydroalcoholic solutions (16, 17).
The current review aims to assess the significance of ethosomes as efficient nanocarriers towards skin delivery of active ingredients, based on their characteristics and composition, as well as to understand the mechanism of permeation of these nanocarriers through the skin. Additionally, this paper will review the research conducted on potential applications of ethosomes in different pathologies, namely skin disorders, and provide examples of ethosome-based formulations.
Ethosomes
Types of Ethosomes
First introduced by Touitou et al. (2000), classical ethosomes are a modification of the classical liposomal formulation, and, overall, are composed of phospholipids, a relatively high concentration of ethanol, and water (16). These vesicle nanocarriers have demonstrated enhanced drug delivery on account of their (1) reduced particle size, (2) negative zeta-potential, (3) higher entrapment efficiency, and (4) improved stability, when compared to classical liposomes (18). However, in pursuit of a more efficient skin permeation system, a new generation of ethosomes was developed, namely binary ethosomes and transethosomes (Fig. 1).
In 2010, Zhou et al. added another type of alcohol to the classical ethosomes and, thus presented binary ethosomes. In addition to ethanol, binary ethosomes include in their formulation another alcohol, commonly propylene glycol (PG) and isopropyl alcohol (18,19,20). PG is a well-known penetration enhancer (18), and offers low toxicity, reduced skin irritation (21) and both higher viscosity and hygroscopicity when compared to ethanol, as well as conferring stability (21). This improves drug affinity to the dermis layer and increases drug retention in the deep layers of the skin (19, 22).
The combination of ethanol with other alcohols endows binary ethosomes with smaller vesicle size, higher skin permeation, higher entrapment efficiency and improved stability, as well as minimizing aggregation (19). Nonetheless, it is highly important to adjust the ratio of ethanol and PG in order to optimize drug permeation.
A new generation of ethosomes has been described by Song et al. (2012), with the objective of combining the advantages of both deformable liposomes and ethosomes (23). Transethosomes share the same composition of ethosomes, containing additionally a penetration enhancer or an edge activator (surfactant). Previous studies have reported enhanced results in comparison to classical ethosomes (23,24,25,26,27). Transethosomes evidenced smaller size, superior elasticity and deformability, and improved skin permeation, probably due to a synergistic effect between ethanol and the surfactant, that promotes a rearrangement in the lipid bilayer of these vesicles (27).
Formulation/ Composition
Ethosomes are typically composed of phospholipids-usually 2%–5% phosphatidylcholine (PC), 20%–45% ethanol and water to 100% (w/w) (3). Touitou et al. preliminary work revealed that high ethanol quantities enabled the formation of soft, flexible and highly fluid vesicles (16).
Ethanol is a well-known permeation enhancer that interacts with the hydrophilic head group of the SC lipid bilayer and enhances lipid fluidity (28, 29), besides playing a key role in determining ethosomes zeta-potential, stability, entrapment efficiency, and average size (18, 30). The vesicular charge is also recognised as an important factor to take into consideration during ethosomes formulation since it can impact stability and vesicle-skin interaction. Ethanol acts as a negative charge supplier for the surface of ethosomes, consequently the negative charge of ethosomes increases proportionately with increasing ethanol concentrations (18). Previous studies have reported that the addition of ethanol causes a modification of the ethosomes’ net surface charge and confers a certain degree of steric stabilization and electrostatic repulsion. Ultimately, this enables to prevent aggregation and results in smaller vesicle sizes (4, 11, 31), showing ethosomes’ vesicular size can easily be adjusted, directly as a result of the variation of ethanol content. For instance, a formulation with 2% PC has vesicles with approximately 200 nm diameter. However, if the proportion of ethanol is increased from 20% to 45%, the diameter is halved (3). Several reports claimed that 30–40% of ethanol is the optimal range to prepare stable and effective ethosomes, even though this range can vary from 20 to 45% (30). Conversely, increased levels of phospholipids lead to larger vesicle size, probably due to a thickening of the structure of the vesicles (7, 25, 32, 33). Hence, the combination of a low concentration of phospholipids with high levels of ethanol results in minimum vesicle size (7, 32). Yang et al. showed that the optimal design for both compounds was 2.45% (w/v) of phospholipids and 30% (v/v) of ethanol. The predicted value of the vesicle size was 132.4 nm (Fig. 2). However, optimum results are obtained with intermediate levels of both elements. On one hand, higher levels of phospholipids produce larger vesicles, which results in reduced skin permeation and deposition. On the other hand, increasing ethanol levels from intermediate to greater concentrations may lead to vesicle disruption (32). Thus, the association of intermediate levels of both elements is responsible for a synergistic effect, which contributes to deeper drug distribution and penetration into the skin (19, 32).
Three-dimensional response surface plots showing the effects of the independent variables (phospholipid and ethanol) on the vesicle size of ethosomes. Reprinted from (7)
Phospholipids can be obtained from different sources, such as natural, semisynthetic and synthetic materials, and can be incorporated in a range from 0.5% to 10% of the final ethosome concentration (27). Several phospholipids have been used in previous ethosomal formulations, such as PC, dipalmitoylphosphatidylcholine (DPPC), phosphatidylethanolamine (PE), Phospholipon®90, and Lipoid S100(18). Skin permeation also depends on the selected phospholipid, since it will merge with the skin lipid bilayer and allow the vesicle to create small openings in the SC (32). Therefore, during pharmaceutical development, the selection and concentration of the adequate phospholipid is a crucial step that will define the ethosomes’ successful permeation through the skin.
Cholesterol is a rigid steroid molecule and has been included in previous ethosomal systems. López-Pinto et al. study showed that the addition of cholesterol contributed to an increase in the mean particle size and stabilization of the lipid bilayer. Cholesterol regulates membrane fluidity, decreases its permeability and elasticity. Hence, when added to the formulation, ethosomes become more rigid, which limits the permeation of the active ingredient. Nonetheless, high amounts of cholesterol improved drug entrapment efficiency, due to its stabilizing effects (29). Similar results were verified in another study, in which cholesterol increased both particle size and entrapment efficiency (34). Moreover, Zhang et al. (2017) investigated the impact of the composition of nanocarriers on drug entrapment efficiency, in three different vesicles, namely liposomes, transfersomes and ethosomes. Cholesterol (30 mol%) was added into the ethosomes to convey an ordering arrangement of the phospholipids’ acyl chains and cause a condensing effect. Stearylamine (0–5 mol%) was also incorporated in order to assess the effect of cationic lipids in the formulation.
Stearylamine, a cationic molecule, conferred a significant improvement in drug entrapment efficiency, which reached 70.5% at a concentration of 2 mol% of stearylamine (35). This positive effect of stearylamine on entrapment efficiency was previously reported (34, 36). The presence of cationic lipids induces a positive charge to ethosomes, allowing them to establish more interactions with the negatively-charged skin membrane. Consequently, positively-charged vesicles have the ability to disrupt tight junctions and permeate deeper into the SC (37), despite the negative charge conferred by ethanol, which contributes to a decrease in its size. An improvement in permeation appears to occur when using ethosomes in comparison to the use of free ethanol, suggesting the occurrence of some kind of synergistic mechanism between ethanol, vesicles and skin lipids. In fact, authors have been suggesting an increase in thermodynamic activity, the so-called “push effect”, which results from the evaporation of ethanol, and allows for an increase in the penetration capacity of ethosomes (30).
Mechanism of Skin Permeation
The skin is the largest organ of the human body, with a total area of approximately 2 m2, and is considered the body’s first line of defense (32, 38). Therefore, the skin acts as a protection barrier from external factors (e.g., microorganisms, allergens, ultraviolet (UV) radiation, chemicals), prevents the loss of endogenous substances (e.g., body fluids and regulates body temperature (38).
The skin comprises three primary regions: epidermis, dermis and hypodermis (39). Epidermis is the outermost layer of the skin and can be further divided into four distinct layers: SC, stratum granulosum, stratum spinosum, and stratum basale (40, 41). The term “viable epidermis” is also applied to identify the SC underlying layers (38). The viable epidermis is composed of keratinocytes, melanocytes, Langerhans cells, migrant macrophages, and lymphocytes. Keratinocytes are formed in the stratum basale, undergo differentiation and maturation, until they reach the SC, where they transition into corneocytes (40). The SC is also described as a “brick and mortar” structure due to the disposition of corneocytes (flat dead keratinocytes), interconnected by desmosomes, and surrounded by a matrix of intercellular lipids (e.g., ceramides, cholesterol, and other fatty acids) (38, 40, 41). This peculiar structure and composition of the SC is fundamental to its barrier function. Therefore, the SC represents the limiting factor to drug penetration and permeation across the skin, after topical administration (38, 40, 42). Other defensive characteristics of the SC are low pH, presence of enzymes, and the transcutaneous concentration gradient (43). The dermis has a thickness of 1–4 mm and it is rich in collagen and elastin, which are embedded in a gel of glycosaminoglycans, salts and water (38). Unlike the epidermis, the dermis has a vast vascular network, as well as lymphatic vessels, and nerve endings. The skin appendages are located in the dermis and include hair follicles, sebaceous glands and sweat glands (40, 41). The hypodermis, also known as the subcutaneous tissue, acts as a support membrane for the epidermis and dermis (44). As well as the dermal layer, the hypodermis is related to several skin disorders (32).
Active ingredients (e.g. drugs) can cross the SC through three major pathways: intercellular, intracellular, and follicular pathways (Fig. 3). In the first pathway, the active ingredient penetrates through the skin by crossing the intercellular lipids, while in the intracellular route molecules pass through both the corneocytes and the intercellular lipids (5, 39). Recent attention has particularly focused on the contribution of the follicular pathway for active ingredients penetration, especially considering the administration of active ingredients-loaded nanocarriers. In this region, the barrier formed by the corneocytes is more vulnerable to penetration due to the presence of hair follicles and sebaceous glands that promote discontinuity to the SC (39, 45, 46). Also, hair follicles act as prolonged reservoirs for nanocarriers and enable sustained drug release (5, 6, 10). This route can be highly advantageous in the treatment of inflammatory hair diseases, hair growth, and improvement of wound healing or gene therapy (10).
Illustration of three major pathways for active ingredients across the SC: (a) intercellular, (b) intracellular and (c) follicular. The principal route is via a tortuous path through the lipids surrounding the corneocytes (intercellular route), while the intracellular route is more restrictive, as active ingredients need to pass through both corneocytes and the intercellular lipids. Hair shafts and sebaceous glands constitute an incoherence of the SC, hence facilitate the penetration of active ingredients through hair follicles openings (follicular route)
Research has consistently shown that classical liposomes are unable to penetrate the skin’s deeper layers. Therefore, Touitou et al. developed a novel vesicular system with improved release of active ingredients (16). Although ethanol is a widely known permeation enhancer, previous studies have shown increased permeation enhancement of active ingredients induced by ethosomes, when compared to hydroethanolic solutions (13). This suggests a synergistic effect caused by the interaction between ethanol and the lipid bilayer both present in the SC and in the ethosomes (16). Ethosomes first step to penetrate through skin is referred as the ‘ethanol effect’, in which ethanol disturbs the conformation of the SC lipids, which are extremely compacted and ordered under physiological circumstances. The interaction of ethanol with the polar headgroup region of lipids increases lipid fluidity and decreases the density of the lipid multilayer, thus providing the vesicles with soft flexible features that enable them to penetrate the deeper skin layers. This effect is followed by the ‘ethosome effect’ that involves the fusion between the phospholipids present in the SC and the ethosomal vesicle, leading to enhanced drug delivery (Fig. 4) (7, 14, 47).
Methods of Preparation
The preparation of different types of ethosomes consists of simple and easily scaled-up processes, without the need for complex and sophisticated equipment. Ethosomes can be formulated by two conventional methods (the cold method and the hot method), but other methods have been also reported, such as the classic mechanical-dispersion method and the transmembrane pH-gradient method.
Cold Method
The cold method represents the most commonly used method for preparing ethosomes and can be divided into two different steps. The first step involves the preparation of the organic phase, which is accomplished by dissolving the phospholipids and other lipid material in ethanol, at room temperature. The mixture is then stirred vigorously and heated to 30°C in a water bath. The second step involves the preparation of the aqueous phase, by heating water separately until 30°C. The previous organic phase is then added to the aqueous phase at a constant rate and stirred for five minutes, in a covered vessel (14, 18, 27, 30). The mixture is cooled at room temperature and may be sonicated or extruded, in order to achieve the desired vesicle size (14, 30, 45). Lastly, the final formulation is stored under refrigerated conditions (14, 30). The active ingredient can either be incorporated in the organic or aqueous phase, depending on its physicochemical properties (18, 45).
Hot Method
The hot method also requires the preparation of two different steps. Firstly, the phospholipid is dispersed in water and this mixture is heated in a water bath to 40°C, until a colloidal solution is obtained. In a different vessel, ethanol and PG are mixed, heated until 40°C, and then added to the aqueous phase. Vesicle size can be decreased using sonification or extrusion (14, 18, 30, 45).
Classic Mechanical-Dispersion Method
Ethosome preparation by the classic mechanical-dispersion method can be accomplished by dissolving soya PC in a mixture of chloroform: methanol (3:1), in a round bottom flask. The organic solvents are removed with the assistance of a rotary vacuum evaporator above the lipid transition temperature of 60°C, until a thin lipid film is formed on the wall of the flask. Traces of solvent are removed from the deposited lipid film by leaving the contents under vacuum overnight. This is followed by hydration with different concentrations of the hydroethanolic mixture, containing the active ingredient by rotating the flask at an appropriate temperature (14, 48, 49).
Transmembrane pH-Gradient Method
This method includes two separate steps: preparation of empty binary ethosomes and active loading of the drug. Firstly, the phospholipid (for example PC) is dissolved in an alcoholic phase, which comprises ethanol and PG. A citrate buffer solution is gradually added into the previous solution with constant stirring at 700 rpm. The system is kept approximately at 30 ± 1°C during this process and then cooled at room temperature. This completes the preparation of empty binary ethosomes. Subsequently, the drug is actively loaded into the ethosomes and the system is continuously stirred at 700 rpm, in order to effectively disperse and dissolve the drug. A pH gradient between the external phase (alkaline) and the internal phase (acid) of the ethosomal system can be established by adding a sodium hydroxide (NaOH) solution (0.5 M) to adjust the external pH. Afterwards, the system is incubated under an appropriate time and temperature, to allow the unionized drugs pass actively through the ethosomes’ lipid bilayer and get entrapped into the vesicles (20, 50).
Advantages of Ethosomal Active Ingredient Delivery
Skin delivery presents several therapeutic advantages, such as sustained drug release, improved patient compliance and access to local or systemic target sites (26). New strategies have been developed to overcome the poor skin permeability of nanocarriers, including liposomes. Indeed, in many cases ethosomes have shown significantly improved skin delivery, when compared to classical liposomes or any of the system components alone, as discussed before (16, 24). A key feature that differentiates ethosomes from other nanocarriers is their ability to carry active ingredients (hydrophilic or lipophilic) into deep skin layers, and their efficient application under occlusive and nonocclusive conditions (3, 38, 41, 51). Usually, hydrophilic active ingredients remain in the aqueous core, while amphiphilic and lipophilic active ingredients interact extensively with the vesicle’s lipid bilayer (27, 40).
Moreover, ethosomes exhibit a deformable character, high entrapment efficiency, stability at room temperature, and biocompatibility with the SC (7). The incorporation of higher concentrations of ethanol in ethosomes confers key attributes toward skin delivery of active ingredients: ethosomes become more deformable and elastic, which enhances their penetration through the skin (Fig. 4) (52,53,54). In addition, ethanol reduces the phase-transition temperature of SC lipid bilayers, increasing their fluidity and permeability, and allowing intercalation of ethosomes with the SC (19, 30). Lastly, ethanol confers a negative charge to ethosomes, leading to a decrease of vesicles size, and finally improves bioavailability of active ingredients (30).
Dermopharmaceutic Applications of Ethosomes
Ethosome-based formulations can be applied in a wide variety of pathologies, namely skin pathologies (acne vulgaris, psoriasis, atopic dermatitis, skin cancer and skin infections), but also inflammation, pain, erectile dysfunction, and hormonal deficiencies. For these skin applications in particular, the addition of functionalizing agents to the conventional ethosomes colloidal surface structure, including polymers, and targeting ligands, has shown to contribute to an improved skin performance of ethosomes, as it is going to be detailed below (Fig. 5).
Ethosome-Based Formulations for Inflammation and Analgesy
Inflammation is an essential defensive mechanism induced by the immune system, in response to any stimuli (e.g. tissue injury or pathogen), which involves the release of pro-inflammatory mediators, recruitment of innate immune cells, and tissue damage (55). In some cases, the inflammatory process is temporary, and the homeostasis is re-established, yet occasionally it can become persistent and evolve into a chronic disease. Non-steroidal anti-inflammatory drugs (NSAID) are one of the most commonly used anti-inflammatory agents in the treatment of chronic musculoskeletal disorders, osteoarthritis and acute or chronic rheumatoid arthritis (31). However, their oral administration is associated with severe side effects, including injury of the gastric epithelium, development of gastric ulcers and renal impairment, so their prolonged use is highly unadvised. (31, 55). Therefore, topical administration is a promising alternative to the delivery of active ingredients in the treatment of inflammation.
A preliminary study for assessing the suitability of ammonium glycyrrhizinate-loaded anti-inflammatory ethosomes was conducted by Paolino et al. (2005). Ammonium glycyrrhizinate is known for its anti-inflammatory activity, particularly in the treatment of inflammatory-based skin diseases. An ethosomal suspension was prepared, using ethanol 45% (v/v), lecithin 2% (w/v) and water. This suspension was then applied on human volunteers with methyl-nicotinate chemically induced erythema. Results showed a significant reduction in the intensity and duration of erythema, compared to 45% hydroethanolic solutions of the drug. Additionally, ethosomes showed positive skin tolerability in human volunteers, even when applied for 48 h, as well as increased percutaneous permeation, skin accumulation and sustained release of ammonium glycyrrhizinate (17).
Apigenin is an accessible flavonoid which has been reported to treat skin inflammation caused by free radicals generated by UV, X-ray, and γ-radiation. A possible mechanism suggests that apigenin inhibits cyclooxygenase-2 (COX-2), an essential enzyme responsible for the synthesis of prostaglandins. Hence, Shen et al. (2014) designed an ethosomal formulation with apigenin 0.02% (w/v), using different quantities of lipoid S75 and an alcohol mixture (ethanol and PG). Apigenin is a hydrophobic molecule and, consequently, is mainly entrapped in the vesicle’s lipid bilayer. Therefore, entrapment efficiency of apigenin improved when the PC content increased in the ethosomal formulation, as well as skin deposition and transdermal flux. On the other hand, excessive levels of PG increased viscosity and reduced fluidity of the vesicles, leading to decreased transdermal flux and skin deposition. The optimal proportion of PG and ethanol was 1:10 (v/v). Optimized ethosome formulation demonstrated higher skin deposition of apigenin in comparison to liposomes and deformable liposomes that were also evaluated. While liposomes remained limited to the SC upper layers, ethosomes permeated through the SC and reached blood flow. This formulation showed strong reduction of COX-2 levels in mouse skin inflammation induced by UV-B light (22).
A pre-clinical study evaluated the relevance of ethosomes encapsulated with ketoprofen in transdermal drug delivery. Ketoprofen is a NSAID with analgesic, anti-inflammatory and antipyretic properties, characterized by its hydrophobicity. Ethosomes were prepared with soya PC (1%–3%) and ethanol (20%–40%), showing small vesicular size, which ranged from 120.3 ± 6.1 nm to 410.2 ± 21.8 nm, depending on the concentration of ethanol and soya PC. The lowest vesicle size was obtained with 1% of soya PC and 40% of ethanol. Results also showed that entrapment efficiency increased when ethanol concentrations were increased. A formulation with 30% and 40% of ethanol reached an entrapment efficiency of 73.7 ± 2.9% and 78.7 ± 4.9%, respectively. In vitro skin permeation showed improved drug permeation and higher transdermal flux for the ethosomal formulation, when compared to the hydroethanolic solution. Confocal laser scanning microscopy (CLSM) also confirmed a significantly higher fluorescent intensity of rhodamine 123 entrapped in ethosomes in comparison to the same hydroethanolic solution. Furthermore, based on in vitro transdermal flux, the predicted in vivo plasma concentration revealed that the ethosomal formulation could generate a therapeutic response with a patch size of 50 cm2, as opposed to the hydroethanolic solution (56).
In an additional study, a meloxicam ethosomal formulation was prepared as a novel transdermal system. Meloxicam is a potent NSAID used in the treatment of arthritis, osteoarthritis and degenerative joint disease. Its oral administration is related to gastrointestinal (GI) tract disorders, such as stomach pain and indigestion, as well as to poor patient compliance. An optimized formulation was developed with ethanol (42.5%(v/v)) and Phospholipon®90G (3% (w/v)) and sonicated for 5 min, and subsequently converted into a gel, which contained Carbopol® 934 (1%, w/w). Skin inflammation was significantly favoured by this ethosomal gel, accomplishing a 65% inhibition of edema after 12 h when compared to the 25.23% registered during oral administration (57).
Other NSAID-loaded ethosomes were tested for its antipyretic and analgesic effects in animal models. Ibuprofen is a widely used antipyretic, analgesic and anti-inflammatory drug with high efficacy, but its oral administration is associated with GI ulceration and bleeding. In this study, ibuprofen was incorporated into an ethosomal gel and applied transdermally to avoid the mentioned side effects. The ethosomal ibuprofen gel was characterized by a soft unilamellar vesicle, with a mean particle size of 249.83 ± 44.3 nm, and entrapment efficiency of 97%. Pharmacokinetic studies showed a fast increase of ibuprofen plasma concentration, after the ethosomal gel application, as well as longer permanence in plasma, compared to the orally administered drug. The application of the ethosomal gel gradually decreased body temperature of fever induced rats, for at least 12 h, in comparison to 7 h after oral administration of ibuprofen. It was also revealed a systemic antinociceptive effect in rats. Moreover, the analgesic effect was achieved 30 min after application and persisted for at least 6 h. Data collected from this study suggests that the ethosomal gel should be further investigated in humans for its antipyretic and analgesic effect (58).
A recent study developed a transdermal drug delivery system composed of an ethosomal gel containing thymosin β-4 (Tβ-4). Tβ-4 is a macromolecular protein drug with numerous functions in the human body, such as the promotion of cell differentiation and maturation, tissue regeneration, repairment of blood vessels and hair follicles, among others. However, due to its protein nature, Tβ-4 faces several challenges, namely poor membrane permeability, inconstant physicochemical properties and susceptibility to GI tract destruction. The ethosomal formulation included soya PC, a surfactant (sodium deoxycholate), and cholesterol. The surfactant’s inclusion resulted in higher deformability, reduced particle size and improved negative charge, which favoured the system’s stability. This formulation revealed a superior drug transdermal rate, compared to the free drug group, and reduced wound healing time, as shown in Fig. 6 (8).
(a) In vitro drug-release study, Tβ-4 ethsomes pass through the stratum corneum into the deeper layers of the skin fixed in Franz diffusion cells and the cumulative permeation amount in unit area of the Tβ-4 ethsomes and Tβ-4 gel varies with time. Statistical significance was noted as follows: *p < 0.05. Kunming mice administrated with different gel for 15 days have different degrees of wound healing (b) and the photomicrographs of the healed skin structure of using different preparations (c). In Fig. 6C, the black arow indicated the degree of skin thickening, the red arow indicated new capillaries and the yellow arrow showed embolization and localized parakeratosis. Originally published by (8) and used with permission from Dove Medical Press Ltd.
Ethosome-Based Formulations for Skin Disorders
Acne Vulgaris
Acne is one of the most common skin diseases, especially affecting adolescents. It is considered a chronic inflammatory disease comprising four steps: follicular hyperproliferation, substantial secretion of sebum, inflammation and proliferation of Propionibacterium acnes (59). P. acnes is a Gram-positive anaerobic bacteria majorly involved in the pathophysiology of acne by inducing an inflammatory response in the sebaceous follicle (60). Over the years, new therapeutic options have been designed, including topical and systemic treatments. Topical therapy is usually the first choice and relies on antibacterial (e.g. clindamycin and erythromycin) and anti-keratinizing drugs (e.g. azelaic acid and tretinoin) (38).
Godin and Touitou (2004) investigated the dermal and intracellular delivery of bacitracin-loaded ethosomes. Bacitracin is a polypeptide antibiotic with bactericidal activity targeted to Gram-positive bacteria. Ethosomes were prepared with Phospholipon®90, 25% (w/w) of ethanol and the concentration of bacitracin varied from 1% to 3%. Dynamic light scattering (DLS) analysis revealed a homogenous vesicular size, ranging from 114.9 ± 3.5 nm and 96.4 ± 6.9 nm, for ethosomes with 1% and 3% of drug, respectively. Further data showed spherical vesicles with multilamellar appearance, which positively influenced the high entrapment efficiency (70%). According to in vitro and in vivo skin delivery studies, the researchers found that bacitracin was effectively delivered and equally distributed throughout the SC, viable epidermis and dermis, to a maximum depth of 200 μm. Additionally, data acquired in fluorescent-activated cell sorting experiments suggested that the fusion of bacitracin-loaded ethosomes with the outer membranes of fibroblasts allowed ethosomes to penetrate the cellular membrane, releasing bacitracin directly into the cell cytoplasm (61).
A clinical study involving 40 patients diagnosed with mild to moderate acne was carried out to test an ethosomal gel with clindamycin phosphate and salicylic acid (CLSA) for acne treatment. Patients were randomized to receive a gel containing either CLSA or a placebo to apply twice daily for eight weeks. The patients treated with the CLSA gel significantly improved their acne condition. A considerable decrease of comedones, pustules and a total number of lesions was verified, whereas in the placebo group 48% reported no improvement of the acne condition. A high tolerability and few side effects were also reported (62).
Tretinoin is a natural retinoid extensively employed in the treatment of skin disorders, such as acne, psoriasis, skin cancer and photoaging. However, its topical administration often causes desquamation and erythema of the skin, leading to poor patient acceptance (63, 64). Raza et al. (2013) prepared several tretinoin-loaded nanocarriers, including an ethosomal formulation with PC, tretinoin (5 mg), butylated hydroxytoluene and ethanol. The prepared ethosomes exhibited a mean particle size of 120 nm, a negatively charged vesicle as well as approximately 76% of entrapment efficiency and increased drug permeation. However, in comparison to other tested nanocarriers, ethosomes presented reduced skin retention and increased skin irritancy induced by alcohol. Thus, this study recommends the application of tretinoin-loaded ethosomes in skin disorders originated in deep skin layers (e.g. acne), whereas ethosomes have no benefit relatively to liposomes for superficial skin disorders (64).
Another potential acne treatment involves topical administration of azelaic acid. Azelaic acid has anti-keratinizing and bacteriostatic activity against P. acnes (38). Therapeutic action is achieved by inhibition of thioredoxin reductase enzymes, which is necessary to bacterial DNA synthesis (60). An azelaic acid ethosome-based cream was prepared with 35% of ethanol to assess its antibacterial activity. Ethosomes displayed a high percentage of entrapment efficiency (94.48 ± 0.1%) and an estimated particle size of 179 nm. The incorporation of ethanol and phospholipids played a key role in bacteria inhibition as both affected the integrity of the bacteria cell wall, thus allowing ethosomes access to the bacterial cytoplasmatic membrane, where they reached an optimum concentration. Antibacterial activity was assessed in vitro by determining the value of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) for both the ethosome-based cream and the commercially available cream (Zelface®). The ethosome-based cream obtained a MIC and MBC of 250 μg/ml, whereas the commercially available cream obtained a MIC and MBC of 250 μg/ml and 500 μg/ml, respectively. These results indicated that azelaic acid ethosomal cream improved antibacterial activity against P. acnes by the presence of ethanol, which disturbs the integrity of the bacterial wall and enables ethosomes to enter the bacterial cytoplasmic membrane (60).
Psoriasis and Atopic Dermatitis
Psoriasis is a chronic, inflammatory and autoimmune skin disorder (21, 65). The histopathology is characterized by skin surface inflammation, epidermal proliferation, hyperkeratosis, angiogenesis and anomalous keratinization. Psoriasis constitutes an important risk factor for certain diseases (e.g. diabetes type 2, hypertension and hypercholesterolemia) and is usually associated with other comorbidities (e.g. arthritis, depression, insomnia and obstructive pulmonary disease) (66). In the present day, since there is not any available cure, the only possibility is to manage the skin disorder signs and symptoms with topical and systemic therapies. Topical therapy is the first-line treatment and is advised in moderate disease, as opposed to systemic therapy which is recommended only in severe cases (65). Topical administration represents a valuable route to treat psoriasis, since the drug directly exerts its therapeutic action in the affected area and, consequently, avoids adverse systemic reactions that accompany the oral and intravenous route (21). However, conventional topical therapies have various limitations, such as limited drug penetration, regular dose administration, toxicity and reduced patient compliance (65).
Psoralen is a photosensitive coumarin used in the treatment of psoriasis. The Food and Drug Administration (FDA) has approved a psoralen UV-A therapy in the treatment of refractory psoriasis (67). Zhang et al. (2014) prepared psoralen-loaded ethosomes to assess drug release in the deep layers of animal skin. The optimized formulation contained PC from soybean lecithin (5.0%, w/v), ethanol (40%, v/v) and psoralen (2.0%, w/v), and was defined by a particle size of approximately 120 nm and a satisfactory level of entrapment efficiency (85.62 ± 0.8%). This formulation presented a 6.56-fold enhanced skin deposition of psoralen when compared to the psoralen ethanolic tincture. Also, compared to the tincture application, the ethosomal system induced a higher peak concentration and a topical area under the curve (AUC0–t) 2.34-fold superior. Researchers also reported enhanced permeation and skin deposition of psoralen, which may help reduce toxicity and increase efficacy in prolonged psoralen therapy (67). Zhang et al. (2014) developed another ethosomal formulation with psoralen, evaluated its skin permeation in vitro and compared it with a psoralen-loaded liposomal formulation. Transdermal flux and drug deposition significantly increased and was, respectively, 3.50 and 2.15 times greater than the liposomal system. The ethosomal system also showed improved biocompatibility and safety, thus suggesting that psoralen-loaded ethosomes constitute a potential transdermal and dermal delivery system of psoralen (68).
Photodynamic therapy (PDT) is a local, non-invasive medical treatment that requires a photosensitizer, a light source, and singlet oxygen. PDT involves two main steps: (1) the photosensitizer is incorporated in the target tissue and, (2) upon exposure to the light source with a specific wavelength, becomes activated. The activation of the light-sensitive molecule requires the presence of the oxygen molecule and results in the formation of cytotoxic reactive oxygen species (ROS), which leads to cell apoptosis or necrosis (50). Thereby, PDT is a highly selective therapy as it is possible to target specific regions of the skin where the light source is applied. 5-aminolevulinic acid (ALA) is a widely used photosensitizer in PDT. However, ALA is a prodrug and thus needs to be converted into the actual photosensitizer molecule, protoporphyrin IX (PpIX) (50, 69). Although the intravenous route is a viable option, topical administration would be preferable, since the systemic administration requires patients to avoid natural sunlight or interior light for 48 h (50). Yet, ALA is a highly hydrophilic drug and has notoriously limited skin penetration. Hence, Fang et al. (2009) prepared an ethosomal formulation in an attempt to overcome this issue in psoriatic skin. In inflamed psoriatic skin, PDT regulates cellular functions, which eventually results in the induction of transcription factors (e.g. activator protein 1 and nuclear factor- κB), without inducing cell death. The authors reported the major differences between human and mouse skin, which are responsible for higher skin penetration rates. For instance, while human SC and epidermises present 17 μm and 47 μm thick, mouse SC and epidermises have 9 μm and 29 μm, respectively. Aside from skin thickness, other key differences include composition and organization of intercellular SC lipids, as well as the density of hair follicles. Taking this into account, the ALA-loaded ethosomes strategy resulted in an enhanced cumulative amount of ALA across normal skin and hyperproliferative murine skin, when compared to ALA aqueous solution. The amount of ALA-induced PpIX was used to assess the therapeutic effect. The PpIX intensity in hyperproliferative murine skin increased about 3.64-fold in comparison to the ALA aqueous solution, and the maximum penetration depth ranged from 30 μm to 80 μm. Further results showed that the ethosomal formulation improved the delivery of ALA and subsequent formation of PpIX, in both murine skin samples. Psoriatic skin recovery was also confirmed by the reduction of tumor necrosis factor alpha (TNF-α) levels (approximately 20%), a key mediator in the inflammatory process of psoriasis (69). Since psoriasis is a T cell mediated disease, as soon as T lymphocytes reach lesioned skin, T-helper type-1 cytokines are released, including TNF-α, interleukin-1 and gamma interferon. These pro-inflammatory cytokines promote the massive production of cytokines from other cells and contribute to skin inflammation and epidermal hyper-proliferation (65).
Psoriasis systemic therapy consists of oral or intravenous administration of methotrexate, cyclosporine, hydroxycarbamide, among others, usually accompanied by immunosuppression, subtherapeutic effects and severe toxicity (66). Previously, Dubey et al. (2007) analysed methotrexate-loaded ethosomes in vitro and found that the ethosomal formulation provided superior values of transdermal flux (57.2 ± 4.3 μg/cm2/h) when compared to classical liposomes and a 45% hydroethanolic solution. The authors attributed the enhancement in skin permeation to the fusion of both the vesicular lipid bilayer and SC lipids caused by the combined effect of ethanol and phospholipids. Enhanced skin drug deposition (31.24 ± 1.34%) and better stability profile were also reported (49). More recently, a similar effect was reported in a methotrexate-salicylic acid ethosomal gel. Methotrexate-loaded ethosomes were prepared with soya lecithin, ethanol, and methotrexate (0.25%). An optimized formulation, with the highest efficiency of entrapment (91.77 ± 0.02%), was selected to prepare a gel with salicylic acid. The prepared gel exhibited an occlusive nature, which promotes methotrexate penetration and efficacy as well as improves its release profile, in comparison to a simple methotrexate solution. Entrapment efficiency benefited from an increase in lipid concentration and particularly from high ethanol concentration. In the ex-vivo permeation study, the ethosomal gel containing salicylic acid demonstrated a better release profile compared to methotrexate gel without salicylic acid and the methotrexate-loaded ethosomal solution. A possible reason is concerned with the inclusion of a permeation enhancer (i.e. ethanol) combined with a decrease in pH by salicylic acid. Ultimately, the addition of salicylic acid resulted in sustained drug release, higher drug permeation, and in successful elimination of psoriatic manifestations (70).
A recent study developed a new strategy to target inflammatory skin diseases, namely psoriasis. CD44 receptor is a highly expressed protein in psoriatic inflamed skin, indicating that CD44 is a potential target for topical nanocarriers. Hyaluronic acid, an extensively used molecule in targeted drug delivery systems, is a biological ligand to this protein. Therefore, a modified formulation of ethosomes was prepared with PG (in replacement of ethanol), a combination of phospholipids, cholesterol, curcumin, and hyaluronic acid. Hyaluronic acid was also added to the surface of ethosomes, as a functionalization surface agent, via covalent bond arrangement between this hydrophilic polymer and dioleoyl phosphoethanolamine (DOPE). Curcumin-loaded ethosomes functionalized with hyaluronic acid enhanced topical and transdermal drug delivery. In fact, the cumulative transdermal amount of curcumin and skin retention of curcumin were significantly higher than the other tested formulations without hyaluronic acid. This may be explained by the hydrating effect caused by the hyaluronic acid on the SC barrier, which expands and relaxes with increased water amounts and thus improves drug permeability and accumulation on the skin. Topical delivery experiments were conducted in vivo in imiquimod-induced psoriasis-like inflamed skin. The CD44-targeted hyaluronic acid-ethosomes formulation produced a strong inhibitory effect on psoriatic inflamed skin and a substantial downregulation of several cytokines (21).
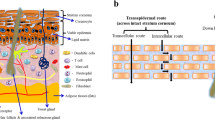
Tacrolimus is an immunosuppressor agent widely used in the treatment of psoriasis and atopic dermatitis. Atopic dermatitis is the most common chronic skin disorder that encompasses severe itching, desquamation, and redness. Dysfunction of the SC is accountable for this disease, which results in excessive transepidermal water loss and infiltration of allergens into the skin (3). Treatment of atopic dermatitis is centred on symptom’s control and reduction of inflammation and glucocorticoids are often employed as effective anti-inflammatory drugs. However, long-term treatment leads to severe adverse drug reactions, such as thinning of the skin and suppression of epidermal cell proliferation (71). Li et al. (2012) developed an ethosomal formulation of tacrolimus for topical delivery in atopic dermatitis induced skin. Three types of ethosomes were prepared, using ethanol and/or PG. The tacrolimus-loaded ethosomes displayed size homogeneity, unilamellar structure and a spherical shape. The incorporation of PG was also reported to decrease vesicular size. For instance, the raise of PG concentration from 0% to 20% induced a significant decrease in ethosomal size. Additionally, the entrapment efficiency reported varied from 76.6% to 79.8%, suggesting that it was not particularly affected by the different ratios of ethanol or PG on the ethosomes composition. The formulation with 30% (v/v) ethanol showed a significant increase in tacrolimus permeation through the epidermis compared with the 30% (v/v) PG ethosomal system (see Fig. 7), indicating different permeation-enhancing effects for ethanol and PG. Furthermore, the lower amount of drug in the epidermis observed for the ethosomal delivery system with a relatively high concentration of PG suggests that ethanol has stronger enhancing effects on SC penetration. This enhancing effect of ethanol appears to be concentration-dependent, showing that greater amounts of ethanol increase the amount of drug permeated in the epidermis. Therefore, all ethosomal systems established superior drug penetration in the epidermis, as well as superior therapeutic efficacy when compared to a commercial tacrolimus ointment (Protopic®) (Fig. 7) (71).
The amount of drug at the stratum corneum and epidermis for tacrolimus formulations. ns p > 0.05 between any of two group. Reprinted from (71)
Ethosome-Based Formulations for Alopecia
Minoxidil was firstly marketed, around 1970, for the treatment of hypertension as a potent vasodilator. However, in several clinical trials, hair growth and hypertrichosis were reported in patients, and a topical formulation was developed. Nowadays, topical minoxidil is the only FDA-approved topical treatment for androgenetic alopecia. Its pharmacological mechanism is not entirely clarified, but several studies demonstrated increased scalp blood circulation and activation of the potassium channels, leading to enhanced transition of hair follicles from telogen phase to anagen phase (72). Topical treatment can often cause pruritus, scaling scalp, contact dermatitis and intensification of seborrheic dermatitis. López-Pinto et al. (2005) prepared a minoxidil-loaded ethosomal system with α-DPPC, cholesterol and ethanol, following the classic mechanical-dispersion method. Spherical-shaped and multilamellar vesicles were obtained. Ethosomes mean particle size ranged from 136 to 230 nm, whereas concurrently prepared liposomes displayed superior values, which ranged from 412 to 520 nm. The addition of ethanol was probably responsible for this effect, even though an increase in the particle size proportional to an increase in cholesterol concentration was reported by the authors. Concerning entrapment efficiency, the maximum value obtained was approximately 84%, with an ethanol concentration of 40% (v/v). The entrapment efficiency was determined by two methods: ultracentrifugation and dialysis method. A reduction of the entrapment efficiency was verified when using the ultracentrifugation method, probably due to the high speed required, which resulted in the disruption of vesicles. Subsequently, studies of penetration and permeation of minoxidil into the skin in an animal skin model were performed. Depth of skin penetration and the main permeation pathway were analysed by using β-carotene as a fluorescent probe. Different formulations were tested, and different intensities of fluorescence were observed. The presence of cholesterol and ethanol generated higher fluorescence intensity and increased penetration depth. Additionally, increased concentrations of cholesterol and ethanol allowed the drug to reach deeper skin structures, including the pilosebaceous follicles (29).
Ethosome-Based Formulations for Skin Cancer
Nowadays, skin cancer is the most common form of human cancer. There are three major types of skin cancer: basal cell carcinoma (BCC), squamous cell carcinoma (SCC) and malignant melanoma. The BCC and SCC are the most common type of skin cancers worldwide, while melanoma is potentially more dangerous and is believed to be one of the most chemotherapy-resistant malignancies (53). Whereas BCC and SCC are originated in epidermal keratinocytes and usually remain confined to the same location, malignant melanoma develops in epidermal melanocytes and can expand to the dermis and even systemic circulation (42, 73). Currently, treatment requires surgery, chemotherapy, or topical treatment with chemotherapeutics. The skin route would be a preferable alternative since it is non-invasive, effective doses can be reduced, systemic side effects minimized, and patient’s compliance improved (3, 15). Particularly, transdermal drug delivery allows active ingredients to be delivered to systemic circulation without the drawbacks of first pass metabolism and risk of infection associated with oral and intravenous route of administration, respectively. Furthermore, transdermal delivery is claimed to favour the treatment of superficial cancers, as well as assist drug delivery to the lymphatic system and stimulate transcutaneous immunization (74).
The efficacy of fisetin-loaded binary ethosomes was also examined for the management of skin cancer. Fisetin is a naturally occurring flavonoid that appears to be involved in the inhibition of inflammatory cytokines, including TNF-α, Interleukin-1β, and Interleukin-6. In this study, binary ethosomes were prepared with phospholipid 90G, ethanol, and PG. Then, the optimized ethosomes were incorporated into a gel formulation. Data obtained from CLSM showed deeper skin penetration (70 μm) of rhodamine B loaded binary ethosomes formulation on rat abdominal skin fixed on Franz diffusion cell. A sustained drug release was also verified with the binary ethosomes fused to skin lipids. Accordingly, the binary ethosomal gel significantly enhanced the concentration of fisetin in both epidermis and dermis, as well as increased AUC values, in comparison with rat skin treated with conventional gel. Additionally, in vivo studies revealed a pronounced reduction in the levels of TNF-α and Interleukin-1β, which suggests that the treatment with fisetin binary ethosomes gel could improve the chronic inflammation caused by these pro-inflammatory cytokines, hence reducing the incidence of skin carcinogenesis. The pre-treatment of mice skin with binary ethosomes gel before UV exposure prevented lipid peroxidation and reduced it by 45%, probably due to an increase in the quantities of antioxidants. Concerning the preventive effect of skin cancer, tumour incidence in the fisetin binary ethosomes gel pre-treated group was 49%, whereas the UV only treated group achieved 96% tumour incidence. As shown in Fig. 8, the average number of tumours and its average volume was also reduced by the tested formula (54). In addition, concerning to histopathological evaluation, the fisetin binary ethosomes gel treated group had shown significant reduction in epidermal cell invasion with reduction of epidermal thickness (Fig. 8).
(a) Effect of fisetin binary ethosomes gel on tumor incidence. The fisetin binary ethosomes gel prolonged the tumor latency period compared to control. (b) Photographs of mice at the end of experiment (A) control (B) UV only (C) UV + fisetin binary ethosomes gel treated. (c) Microscopic image of mice skin cross section stained with hematoxyline and Eosin (A) control (B) UV only (C) UV + fisetin binary ethosomes gel treated. Reprinted from (54)
Mitoxantrone is an antineoplastic agent that intercalates with DNA and prevents its synthesis and transcription. Yu X. et al. (2015) prepared a mitoxantrone ethosome gel for the transdermal treatment of melanoma. In vitro studies, performed on Franz-type diffusion cells, revealed enhanced permeability of ethosomes, which the authors related to its high deformability. On the contrary, the mitoxantrone solution barely penetrated across rat skin, since no mitoxantrone was found in the receptor compartment. Different mitoxantrone formulations were daily applied on melanoma-bearing animals, divided into three groups: the first group represented the control group; the second group included the tumour-bearing mice treated with a solution of mitoxantrone; the third group included the tumour-bearing mice treated with the mitoxantrone ethosome gel. The first and third group displayed marked differences in tumour size, while the first and second group exhibited minimum differences. These results propose that the mitoxantrone solution lacked any therapeutic effect on melanoma-bearing mice. More importantly, the therapeutic effect is clearly dependent on the transdermal formulation. Finally, the mitoxantrone ethosome gel accomplished a tumour inhibitory rate of 68.44% (53).
5-fluorouracil is another widely used antineoplastic agent. Due to its polar nature, 5-fluorouracil skin penetration is precluded by the lipids of SC, and hence its retention is limited to the surface of the skin. Consequently, as far as the topical treatment of malignant melanoma is concerned, its therapeutic efficacy might be compromised. Khan, N.R. and T.W. Wong (2016) carried out an investigational study in which the effect of both 5-fluorouracil-loaded ethosomes and microwave technology was combined to promote skin drug deposition, as well as to reduce excessive transdermal drug permeation. The single application of 5- fluorouracil-loaded ethosomes facilitated transdermal 5-fluorouracil transport. These ethosomes contained low ethanol content and displayed a negative charge, which repelled the anionic lipids of the skin and prevented excessive permeation into deep skin layers. Moreover, data analysis suggested that ethanol acted as a permeation enhancer by fluidizing the lipid domain of epidermis, forming enlarged aqueous pores. Nonetheless, pre-treated skin with 2450 MHz microwave for 2.5 min, followed by the application of 5% ethanol loaded ethosomes, resulted in further increased skin drug retention and adequate transdermal drug permeation for local skin cancer treatment. The synergistic effect between ethosomes and microwave increased the lipid domain fluidization and decreased the protein domain fluidization, which improved intercellular pathways (42). In 2018 another study was published with new insights by the same scientific group. Likewise, the application of microwave, under the same conditions, increased skin penetration and skin retention of the drug (395.4 ± 7.9 μg), when compared to the single application of ethosomes (281.3 ± 13.9 μg). However, skin permeation was not significantly impacted by the microwave pre-treatment, probably due to the fluidization of specific extracellular proteins, which helped in the formation of a dermal barrier and, ultimately, narrowed the intercellular pathway. The in vivo bioavailability of 5-fluorouracil was also evaluated using an animal model in a pharmacokinetic study. Interestingly, permeation of the drug was considerably decreased in microwave pre-treated rats. Low systemic absorption associated with increased skin drug retention is rather advantageous in the treatment of local malignant melanoma. Hence, the combination of microwave with a vesicular nanocarriers is a promising strategy for skin malignant melanoma treatment (73).
The combination of two therapies in skin cancer treatment was also assessed by Nasr S. et al. (2019). PDT is a non-invasive procedure approved in the treatment of skin diseases, including several skin cancers. In this study, ferrous chlorophyllin (Fe-CHL) acted as the photosensitizer, loaded in ethosomes and lipid-coated chitosan nanocarriers. The skin retention of Fe-CHL was analysed by high performance liquid chromatography (HPLC). Results showed a significant increase in Fe-CHL skin retention with ethosomes and chitosan nanocarriers, especially after 24 h, in comparison to a Fe-CHL solution. CLSM was used to evaluate the penetration depth of both nanocarriers through mouse skin. Ethosomes demonstrated deeper skin penetration into the epidermis and dermis, evidenced by the presence of fluorescence in both layers, while the chitosan nanocarriers were restricted to the epidermis. This was attributed to the presence of ethanol and edge activator, which provides ethosomes increased fluidity and superior capacities to penetrate deep into skin layers. Contrastingly, even though chitosan nanocarriers displayed smaller vesicle size, their rigid structure limited skin penetration. In addition, the photodynamic effect of both nanocarriers was evaluated in vitro for human skin SCC monolayer and 3-D spheroids. Despite ethosomes increased retention and skin penetration, lipid-coated chitosan nanocarriers showed superior cellular uptake and cytotoxicity. Therefore, the authors concluded that the chitosan nanocarriers would be suitable carriers in superficial SCC, whereas ethosomes would be more adequate for invasive SCC (9).
Ethosome-Based Formulations for Skin Infections
Skin infections are highly prevalent in the global population and can affect a wide range of ages. Antimicrobial agents have numerous limitations, including frequent drug resistance, and deficient drug targeting and drug release (75). Regarding antibiotics, the lack of adequate and effective treatment can be fatal. Therefore, this field requires new and efficient methods to effectively improve antimicrobial therapy with well-known drugs or potential new ones. Accordingly, nanocarriers have been investigated as potential drug delivery systems in the treatment of several skin infections.
In an early study carried out by Godin et al. (2005), erythromycin loaded-ethosomes demonstrated improved antibacterial activity in vitro and in vivo when compared to a hydroethanolic solution. Bacterial growth inhibition was confirmed in three bacterial strains, including a strain of Bacillus subtilis, Staphylococcus aureus, and a strain of S. aureus resistant to erythromycin. In addition, the ethosomal formulation reduced the MIC against S. aureus. Specifically, in the S. aureus strain resistant to erythromycin, the MIC was 2.5 lower when the erythromycin loaded-ethosomes were administered. The animals treated with erythromycin loaded-ethosomes exhibited no sign of skin necrosis or damage, while the untreated group and the group treated with a hydroethanolic erythromycin solution manifested continuous bacterial growth, leading to necrosis, damaged skin structures and a considerable infiltrate of neutrophils and macrophages (76).
Regarding antiviral ethosomes, an ethosomal system with acyclovir was produced and investigated in a randomized double-blind clinical study. Acyclovir is a poorly soluble antiviral drug, which is mostly used in the treatment of recurrent herpes labialis infection. This formulation with 5% acyclovir was compared to a commercial 5% acyclovir cream (Zovirax®, GlaxoSmithKline). Results showed notable improvements regarding therapeutic efficiency, leading to the commercially available topical ethosomal-based acyclovir cream (Supravir®,Trima, Israel) (77).
Skin fungal infections are also widely present in the worldwide population, especially in immunocompromised patients (78, 79). The most representative fungous agent is Candida albicans, which is responsible for human cutaneous candidiasis. C. albicans penetrates the SC, and often reaches the systemic circulation, causing ulceration or infiltration of the affected area (79). Treatment of skin fungal infections usually comprises topical formulations with antifungal agents, whose effectiveness depends on the ability to penetrate and to remain in the SC and C. albicans biofilm’s matrix (80). Clotrimazole is an imidazole with a broad-spectrum antifungal activity that inhibits the biosynthesis of ergosterol, a fundamental element of the fungal membrane (81). A study, aimed at comparing the transdermal potential of ethosomes and ultradeformable liposomes containing clotrimazole, was conducted by Maheshwari et al. (2012). Transdermal flux of clotrimazole was assessed to determine skin permeation rate in rat abdominal skin. The ethosomal formulation revealed superior transdermal flux, as well as lower lag time (56.25 ± 5.5 μg/h/cm2 and 0.9 h, respectively), in comparison to the ultraderformable liposomes, a 45% hydroethanolic solution and a clotrimazole solution. Additionally, clotrimazole-loaded ethosomes exhibited greater in vitro antifungal activity against C. albicans, with an inhibition zone of 34.6 mm, as opposed to ultradeformable liposomes (29.6 mm) and marketed cream formulation (19.0 mm). This may be attributed to ethanol that causes fungal proteins denaturation and lipids dissolving. Overall, the authors considered the ethosomal formulation the most adequate carrier for clotrimazole transdermal delivery (81).
Recently, hybrid vesicles composed of phospholipids, ethanol, clotrimazole (10 mg/mL), low content of water (≤10%) and glycerol were prepared. In vitro permeation studies showed accumulation of clotrimazole in the epidermis and dermis (± 12%), and notably in the SC (± 22%), contrary to a marketed cream (Canesten®), which displayed a low accumulation of the drug (± 2%). Antimycotic activity was evaluated in vivo, using C. albicans infected skin. Results showed that the hybrid vesicles reduced the count of C. albicans colonies in greater extent than the marketed cream. A possible mechanism described by the authors suggests an interaction between the vesicles and the components of the SC and the biological fluids, facilitating the penetration through the SC and the biofilm matrix (80).
Sertaconazole nitrate is an additional imidazole antifungal agent with hydrophobic characteristics. Abdellatif, M. et al. (2017) conducted a study in which several sertaconazole-loaded vesicular systems were developed, including ethosomes, to optimize antifungal activity at deep skin layers. The study aimed to investigate the different vesicular systems’ skin permeation and accumulation properties to target dermal fungal infections, in an animal model. Data obtained from differential scanning calorimetry revealed that the gel-based ethosomal formulation showed a phase transition temperature of 85.8°C, suggesting that bellow this temperature the lipid bilayer displayed an ordered arrangement, whereas for temperatures above, skin permeability was facilitated due to the disturbance of the lipid bilayer. These findings are in accordance with previous studies which suggest that ethanol disturbs and increases the fluidity and flexibility of the lipid bilayer, even at low concentrations, hence enabling drug penetration into the skin, since other nanocarriers (ethanol free) had higher transition temperatures (16, 78). During the ex-vivo skin permeation experiments, the authors also verified increased cumulative sertaconazol permeation from the ethosomal gel, when compared to the other vesicular formulations and a commercial cream (Dermofix®). This may be explained by the presence of ethanol, which confers flexibility and fluidizes the lipid bilayers present both in the vesicles and the SC (78).
Furthermore, griseofulvin (GRF) is an alternative drug in the treatment of dermatophytosis, particularly in the paediatric population. GRF is an antibiotic drug with antifungal activity defined by its low bioavailability and high hydrophobicity. Thus, Marto et al. (2016) developed a GRF ethosomal formulation intended to enhance permeation through the SC barrier. The ethosomes were prepared with soybean PC 5% (w/v), 45% (v/v) ethanol and several concentrations of GRF (0.01–0.5%). Permeation and penetration studies were performed in new-born pigs using Franz diffusion cells to assess and predict the action of the ethosomal system on the skin. A hydroethanolic 0.1% GRF solution was used as control. The obtained results showed a significantly improved drug retention (± 40%) in the SC, 24 h after the formulation was applied. On the other hand, drug retention in the viable epidermis and dermis was not significantly higher than the GRF solution (see Fig. 9). These results are favourable to the targeting of dermatophytosis since the ethosomal formulation remains in its site of action and extends its therapeutic effect even 24 h after application. The authors concluded that both the phospholipid concentration and the GRF solubilization in the ethosomal structure played a vital role in improving drug retention in the SC. A fluorescence assay with Nile red-loaded ethosomes confirmed these results and revealed ethosomes accumulation in hair follicles (Fig. 9), an additional dermatophytes target, where they degrade keratin to remain alive. On the contrary, the hydroethanolic solution evidenced no therapeutic advantage since this formulation permeated mainly through the epidermis to the dermis layers, where dermatophytes usually do not disseminate. Additionally, the in vitro antifungal activity was assessed by the disc diffusion method. Results confirmed the fungicidal effect and the total drug released exceeded the MIC against the tested fungus (Fig. 9) (82).
(a) GRF retention in different skin layers, SC (stratum corneum); E + D (viable epidermis and dermis) and GRF permeated within 24 h solution and ethosomes (results expressed as mean ± standard deviation; n = 3, independent batches). (b) Penetration of Nile red from ethosomes, after 24 h. (c) Penetration of Nile red from ethanol:water solution 45:55 (v/v), after 24 h. The sections depict the SC (a), the epidermis (b), and the dermis (c). Pictures are obtained by superposing normal light and fluorescence images of the same area (magnification, 100×). Black arrows are pointing to hair follicles were retention also occurred. (d) In vitro antifungal activity of 0.1% GRF solution, 0.1% GRF ethosomes and empty ethosomes. Reprinted from (82)
An investigation with a terbinafine-loaded ethosomal gel, containing Carbopol® 934P, was carried out to assess its potential to deliver terbinafine to its targeted site for a prolonged period. In the ex vivo diffusion study, the ethosomal gel-based formulation exhibited a drug diffusion of 74.01 ± 0.62%, a transdermal flux of 144.61 ± 1.3 μg/cm2/h, and a longer permanence of the drug in the targeted site. Results also showed that the dissolution profile of the ethosomal formulation followed a zero order release, which turns out to be the most adequate dissolution profile for transdermal drug release (83).
Ethosome-Based Formulations for Erectile Dysfunction
Erectile dysfunction is a common sexual dysfunction in men, affecting mainly ageing men, characterized by the inability to develop or maintain a penile erection. Risk factors involve age, obesity, smoking, dyslipidaemia, diabetes mellitus, and a sedentary lifestyle (84). Moreover, damage to the cavernous nerve may be subjacent to the route of this condition. When an injury of the cavernous nerve is implied, oral treatment with Phosphodiesterase-5 (PDE5) inhibitors is compromised (85). Among the available PDE5 inhibitors, vardenafil is considered more selective than sildenafil and tadalafil, and more potent than sildenafil. Consequently, reduced doses can be administered, and fewer side effects will appear. However, vardenafil is classified as a class II drug of the Biopharmaceutical Classification System and hence exhibits low oral bioavailability. So, patients would benefit from a transdermal formulation that could, not only improve bioavailability and duration of action of vardenafil, but also avoid first-pass metabolism and reduce the required dose to maintain an erection. In a study conducted by Fahmy et al. (2015), ethosomes incorporated with vardenafil were examined for the transdermal treatment of erectile dysfunction. The optimized ethosomal formulation displayed a homogenous unilamellar arrangement with an average size of 128 nm and entrapment efficiency of 76.23%. An automated Franz diffusion cell apparatus was used to evaluate the diffusion of vardenafil from the ethosomal formulation. Additionally, the optimized formulation was analysed in vitro by CLSM to confirm enhanced diffusion of vardenafil in rat skin. Results showed a rapid diffusion of vardenafil from the encapsulated ethosomes. The CLSM revealed a notably high deposition of the ethosomal formulation, especially in the dermis and hypodermis skin layers. The pharmacokinetic study conducted in rats showed an approximately twofold increase of vardenafil bioavailability from the transdermal ethosomal formulation when compared to an oral drug suspension. The authors attributed this enhanced bioavailability to several factors: firstly, ethosomes’ vesicular structure acts as a vardenafil transporting vessel into the different skin layers; secondly, the vardenafil-loaded ethosomes exhibited smaller particle size, with consequently increased surface area, compared to the oral suspension; lastly, the ethosomal vesicles increased the adhesion surface contact with the absorption area. The pharmacokinetic study also allowed the authors to conclude that the vardenafil-loaded ethosomes remained in the skin layers for long periods of time (mean residency time of 16.84 ± 3.54 h), forming a drug reservoir for prolonged drug release into the viable epidermis. Therefore, it was concluded that ethosomes comprised a potential vardenafil drug delivery system in the treatment of erectile dysfunction (84).
Ethosome-Based Formulations for Hormonal Deficiencies
Androgen deficiency is usually associated with inadequate levels of testosterone both in men and women. In men, testosterone is responsible for male sex organs development, whereas in women it improves vaginal blood flow and lubrification. However, testosterone’s effects are also extended to other nonreproductive systems and can affect calcium balance and bone health, as well as regulation of glucose and lipid metabolism, and even prostatic hyperplasia (25). Androgen replacement therapy is available in oral, intramuscular, and transdermal formulations (51). Transdermal administration is considered the most suitable delivery form, since it avoids first pass metabolism, low oral bioavailability and pain related to intramuscular injections.
A transdermal testosterone ethosomal patch (Testosome) was designed and compared to a commercially available testosterone patch (Testoderm®) in an early investigational study. Following application in rabbit pinna skin, authors reported 30 times higher skin permeation with the ethosomal patch. In an in vivo study, the daily application of the same patch for five consecutive days resulted in a significant increase of the AUC and peak plasma drug concentration (Cmax) values (16). In a further study, a transdermal testosterone ethosomal gel was designed and tested for enhanced transdermal absorption. In a pharmacokinetic study, systemic absorption was evaluated in an animal model and compared to a testosterone-based gel (AndroGel®). The ethosomal formulation achieved considerably higher Cmax and AUC values (1970 ± 251 ng/dL and 9313 ± 385 ng/dL/h, respectively), when compared to AndroGel® (601 ± 88 ng/dL and 5678 ± 719 ng/dL/h, respectively). Following ethosomal application in men, testosterone flux through human skin was assessed based on in vitro permeation experiments. The application of the ethosomal formulation resulted in a six-fold enhancement of transdermal testosterone permeated (51).
Moreover, transdermal testosterone propionate ethosomes were prepared and modified by adding two surfactants (hexadecyl trimethyl ammonium bromide and Cremophor EL-35). The authors highlighted the importance of an adequate receiver medium to deliver a drug and to maintain sink conditions, during in vitro experiments. Therefore, the inclusion of these surfactants was aimed at increasing testosterone’s solubility in water since testosterone is a lipophilic molecule. The ethosomes displayed a spherical, unilamellar structure, and entrapment efficiency of 92.7 ± 3.7%. Skin permeation experiments across male mouse skin demonstrated that ethosomes enhanced transdermal permeation by fivefold when compared to a liposomal formulation. Additionally, skin permeation was further assessed by CLSM, which confirmed enhanced permeation of testosterone ethosomes into deeper skin layers (260 μm), as opposed to the liposomal formulation (120 μm). Regarding in vivo studies, the ethosomal formulation also provided notably improved AUC and Cmax values and a considerably longer elimination half-life, as well as more stable plasma concentrations than the liposomal and a 40% hydroethanolic formulation. Thus, it was concluded that testosterone ethosomes offered enhanced drug absorption and bioavailability (25).
On a different subject, Shumilov et al. (2010) investigated the effects of buspirone in the management of menopausal syndrome, which often involves hot flushes and anxiety. Buspirone hydrochloride, a well-known anxiolytic, interacts with two main 5-hydroxytryptamine-1A receptor subtypes, which are thought to be engaged in body temperature control. When administered orally, buspirone is subjected to extensive first-pass metabolism and short elimination half-life, thus transdermal delivery is considered a potential alternative. A transdermal system with buspirone-loaded ethosomes was produced and tested in an animal model. After transdermal administration, the pharmacokinetic study revealed that buspirone reached a Cmax value of 120.07 ± 87 ng/ml after 2 h, whereas oral administration presented a Cmax of 93.44 ± 76.5 ng/ml after 1 h. Interestingly, despite these similar values, considerably higher values were reported in the following 12 h for the transdermal formulation. Concerning the pharmacodynamic study, changes in tail skin temperature of ovariectomized rats were monitored to evaluate buspirone’s effect on hot flushes. The application of the ethosomal formulation caused a significant temperature reduction 3 h following administration and this effect lasted for 6 h (86).
Cosmetic/ Skin Care Applications of Ethosomes
The history of cosmetics begins with Ancient Egyptians, where scented oils and ointments were especially used for their healing properties. Nowadays, the cosmetic industry has become increasingly popular and is now present worldwide. Currently, consumers are more knowledgeable and express more necessities, expectations and demands on a cosmetic product. This generates a growing pressure in the cosmetic industry manufacturers to develop more innovative products that meet consumer’s expectations. Therefore, nanocarriers are widely employed in the cosmetic field to provider antioxidants, UV filters, vitamins, anti-acne, and anti-ageing ingredients (87).
Antioxidants are molecules with the ability to delay or prevent cell oxidation by interacting with free radicals. ROS promote oxidative stress and, subsequently, accelerate the skin ageing process (45). In addition, oxidative stress is believed to be the primary cause of several skin disorders, such as pigmentation, psoriasis, atopic dermatitis, vitiligo, acne vulgaris, melanomas, etc. Hence, antioxidants are regarded as highly important molecules since they neutralize ROS produced during cellular metabolism (endogenous ROS) and during exposition to air pollutants and sunlight (exogenous ROS). However, the incorporation of antioxidants in cosmetics is confronted with several challenges. Firstly, they are highly unstable towards temperature, light, pH, and oxygen, which may compromise their activity and limit their shelf-life; secondly, antioxidants are poorly soluble, limiting their permeability to the upper layers of the skin (19).
Resveratrol is a non-flavonoid polyphenol, naturally found in a broad range of plants, particularly in red grapes (Vitis vinifera) (11, 88, 89). Several attractive properties have been identified in resveratrol, such as antioxidant, anti-inflammatory, anti-ageing, anti-acne and anti-cancer activities (11, 32, 90). However, due to its considerable instability, resveratrol trans form (trans-resveratrol) can easily be converted into its cis form, which is biologically less active (11, 90). In addition, oral administration of resveratrol is characterized by its poor water solubility and short in vivo half-life (91). Hence, there is a current need to develop a suitable formulation to deliver resveratrol in its active form into the different skin layers. In a study carried out by Scognamiglio et al. (2013), the ability of different nanocarriers to topically deliver trans-resveratrol was assessed and compared. Instead of the original preparation method (Touitou et al. 2000), ethosomes were prepared by hydration of a lipid film with an aqueous solution with ethanol, in an attempt to homogenize vesicle morphology. For this reason, the authors identified the prepared vesicles as ethanol-containing vesicles. Nonetheless, ethanol-containing vesicles with different amounts of soya PC and cholesterol were prepared. Vesicles displayed a mean diameter between 92.5 and 94.0 nm and 70% of encapsulation efficiency. The incubation of human keratinocytes with hydrogen peroxidase resulted in an increase of ROS production, which was significantly inhibited by both the soya PC and the soya PC/cholesterol formulation. Enhanced permeation of trans-resveratrol through porcine skin was only observed with the ethanol-containing vesicles, whereas an absence of permeation was verified for the correspondent transferosomes. In fact, with the soya PC formulation, it was possible to visualize trans-resveratrol permeation 1 h after application and accumulation of the total dosage after 6 h. Additionally, accumulation of trans-resveratrol into the dermis was also enhanced with the soya PC/cholesterol formulation. The authors hypothesize that ethanol and the vesicular bilayer are accountable for the obtained results since transferosomes were unable to accumulate and permeate as efficiently. The absence of cholesterol in the ethanol-containing vesicles also facilitated the major role of ethanol by increasing the vesicles fluidity (88).
Arora D. et al. (2019) conducted an innovative study in which a resveratrol-loaded ethosomal hydrogel is developed and continuously optimized based on a Quality by Design approach. Firstly, quality attributes which could impact the performance of the product were defined, taking into consideration the route of administration, dosage form, dosage type, ex vivo permeation, and stability. Hence, critical quality attributes included vesicle size, drug entrapment efficiency, permeation flux and skin deposition. Concerning vesicle size, data analysis showed that reduced lipid quantities associated with increased levels of ethanol produced vesicles with minimum size. The authors attributed this effect to a steric stabilization mechanism and edge activation as a consequence of high ethanol concentrations. Another possible explanation associates the reduced vesicle size with a reduction of the width of the membranes, on account of the establishment of a phase with an interpenetrating hydrocarbon chain. In addition, it was also observed enhanced values of permeation flux with increasing concentrations of ethanol, probably due to ethosomes ability to fluidize the lipids’ membrane and penetrate more easily into the skin. Also, resveratrol, as a lipophilic substance, is likely to solubilize in ethanol and improve permeation flux. The ex vivo permeation studies revealed a significant improvement of the skin permeation parameters and skin deposition (Fig. 10), following application of the ethosomal hydrogel, in comparison to a conventional resveratrol cream. Again, ethanol enables fluidization of both the vesicle lipid bilayer and the SC lipids which, combined with the formation of small openings in the SC by the phospholipids, renders ethosomes flexible and facilitate dermal permeability. Instead of drug, Coumarin 6 (C6) was used for histopathological studies. Data obtained from CLSM confirmed extensive distribution of the ethosomal hydrogel in the deeper skin layers (Fig. 11) (32).
(a) Permeation profile of different resveratrol formulations: optimized ethosomes (Opt-etho); ethosomal carbopol hydrogel (Etho-CBP-Gel); resveratrol hydroethanolic carbopol hydrogel (Res-CBP-Gel); conventional cream ofresveratrol (Res-Cream), resveratrol ethanolic solution (Res-Alc-Sol). (b) Skin deposition of resveratrol from various formulations. Reprinted from (32)
CLS micrographs (a) C6 loaded plain hydrogel (b) C6 loaded ethosomal hydrogel. Reprinted from (32)
Ethosomes with Fraxinus angustifolia bark and leaf extract were prepared. F. angustifolia is well-known for its antioxidant, anti-inflammatory, diuretic and digestive properties due to its highly enriched polyphenolic composition. Polyphenols are plant secondary metabolites known for their ability to scavenge free radicals, hence contributing to the prevention and protection of human cells from oxidative stress. In the study performed by Moulaoui K. et al. (2015), polyphenols were identified in F. angustifolia bark and leaf extract, including quercetin, rutin, tannic acid and catechin. The extract-loaded ethosomes notably inhibited hydrogen peroxidase stress damage in in vitro human keratinocytes, when compared to the other ethanolic solutions. After skin inflammation and ulceration being induced in mouse skin, a model of skin lesion and oedema was created to evaluate the formulations therapeutic efficacy. The bark extract was only noticeably effective when incorporated into ethosomes, leading to a reduction of myeloperoxidase activity. Regarding the leaf extract incorporated into ethosomes, the enzyme’s activity was also reduced, yet not as significantly. Overall, an improvement in the healing process was always visually observed in the skin treated with the leaf extract (92).
Furthermore, a cream formulation with curcumin-loaded ethosomes was developed in order to improve its delivery to the dermal layer. Curcumin is the primary bioactive in Curcuma longa, which is recognized for its antioxidant and anti-inflammatory properties. Similarly to other antioxidant substances, curcumin scavenges oxygen free radicals and superoxide radicals, which are responsible for the activation of metalloproteinases (e.g. collagenases). These group of enzymes catabolize the degradation of extracellular matrix proteins, such as collagen and elastin, and hence are implicated in the formation of skin wrinkles and premature ageing. Nonetheless, curcumin needs to reach the dermal layer, so that it can exert its therapeutic action. Skin permeation and deposition study were conducted to select the most suitable ethosomal formulation to be incorporated into the cream. The formulation prepared with an 85% ethanolic extract of C. longa, 2% (w/v) PC, and 40% (v/v) ethanol was selected for its high entrapment efficiency (58%), enhanced deposition (44%) in the dermal layer, and low cumulative skin permeation (6.0%). Subsequently, the curcumin-based ethosomal cream was applied on young female volunteers for two weeks. Noticeable enhancements were verified in terms of elasticity, viscoelasticity, recovery of deformed skin, and reduction of sagginess. The ethosomal cream increased the overall elasticity of the skin by 15.4%, while the curcumin cream without ethosomes increase was 10.7%. Additionally, the skin sagginess was considered the most suggestive parameter of the ethosomal cream hydration effect and was reported to decrease by 30% (93).
Conclusions and Future Prospects
In recent years, the application of ethosomes in the fields of dermatology and cosmetics has gained growing interest among the scientific community, owing to their high deformability, high encapsulation efficiency and enhanced penetration through deeper skin layers. This review presents numerous ethosomal formulations developed and investigated so far for the treatment of several diseases, including acne, psoriasis, alopecia, skin infections, hormonal deficiencies, among others. Summing up the results, it can be concluded that ethosomes, as lipid-based vesicles, are efficient non-invasive nanocarriers for a wide range of therapeutic agents. In fact, ethosomes’ unique composition confers them high elasticity and flexibility, allowing them to penetrate deeply across the skin, and enhance drug permeation and deposition on the skin’s distinct layers. Semi-solid formulations, namely creams and gels, are the most suitable to incorporate ethosomes, attending to the adequate viscosity and adherence to the skin surface features, improving efficiently the skin delivery pattern. Although the exact underlying mechanism is not yet fully disclosed, the proposed mechanism suggests that ethanol’s fluidizing effect, both of the vesicles phospholipid bilayer and the SC lipid bilayer, generates disorganization of SC lipids, resulting in the effective drug delivery across the skin.
The results arising from the use of ethosomes are very encouraging, placings these biocompatible lipid-based nanostructures in advantage when compared with other vesicular nanocarriers, particularly regarding the efficiency of delivery of active ingredients. Permeation across the skin was invariably achieved, however additional studies are required to understand the attributes that compose ethosomes targeted to topical or systemic drug delivery. In addition, in vivo trials, especially on humans, should be performed to confirm the efficacy, safety and biocompatibility of ethosomes, which has already been proven in vitro and in other animal models, as well as to evaluate the transposition of doses of active ingredients to the human scale. Further research still features as a huge challenge when referring to skin formulations.
In this sense, future research should be focused in the elucidation of the mechanism of permeation of the ethosomes, the therapeutic efficacy, the dermal and systemic bioavailability of the encapsulated therapeutic active ingredients agents, in order to ultimately increase the translation of the ethosomal-based formulations into marketed products.
Due to their enhanced drug delivery system qualities, low toxicity, and manufacturing simplicity, ethosomes future holds promising opportunities for the development of new and improved therapies and care by using the skin as route of administration.
Abbreviations
- ALA :
-
5-aminolevulinic acid
- AUC :
-
Area under the curve
- BCC :
-
Basal cell carcinoma
- C6 :
-
Coumarin 6
- CLSA :
-
Clindamycin phosphate and salicylic acid
- CLSM :
-
Confocal laser scanning microscopy
- C max :
-
Peak plasma drug concentration
- COX-2 :
-
Cyclooxygenase-2
- DLS :
-
Dynamic light scattering
- DNA :
-
Deoxyribonucleic acid
- DOPE :
-
Dioleoyl phosphoethanolamine
- DPPC :
-
Dipalmitoylphosphatidylcholine
- FDA :
-
Food and Drug Administration
- Fe-CHL :
-
Ferrous chlorophyllin
- GI :
-
Gastrointestinal
- GRF :
-
Griseofulvin
- HPLC :
-
High performance liquid chromatography
- MBC :
-
Minimum bactericidal concentration
- MIC :
-
Minimum inhibitory concentration
- NaOH :
-
Sodium hydroxide
- NSAID :
-
Non-steroidal anti-inflammatory drugs
- PC :
-
Phosphatidylcholine
- PDE5 :
-
Phosphodiesterase-5
- PDT :
-
Photodynamic therapy
- PE :
-
Phosphatidylethanolamine
- PG :
-
Propylene glycol
- PpIX :
-
Protoporphyrin IX
- ROS :
-
Reactive oxygen species
- SC :
-
Stratum corneum
- SCC :
-
Squamous cell carcinoma
- Tβ-4 :
-
Thymosin β-4
- TNF-α :
-
Tumor necrosis factor alfa
- UV :
-
Ultraviolet
References
Goyal R, Macri LK, Kaplan HM, Kohn J. Nanoparticles and nanofibers for topical drug delivery. J Control Release. 2016;240:77–92.
Sinico C, Fadda AM. Vesicular carriers for dermal drug delivery. Expert Opin Drug Deliv. 2009;6(8):813–25.
Romero EL, Morilla MJ. Highly deformable and highly fluid vesicles as potential drug delivery systems: theoretical and practical considerations. Int J Nanomedicine. 2013;8:3171–86.
Bellefroid C, Lechanteur A, Evrard B, Mottet D, Debacq-Chainiaux F, Piel G. In vitro skin penetration enhancement techniques: a combined approach of ethosomes and microneedles. Int J Pharm. 2019;572:118793.
Roberts MS, Mohammed Y, Pastore MN, Namjoshi S, Yousef S, Alinaghi A, et al. Topical and cutaneous delivery using nanosystems. J Control Release. 2017;247:86–105.
Krishnan V, Mitragotri S. Nanoparticles for topical drug delivery: potential for skin cancer treatment. Adv Drug Deliv Rev. 2020.
Yang L, Wu LF, Wu DZ, Shi DS, Wang T, Zhu XL. Mechanism of transdermal permeation promotion of lipophilic drugs by ethosomes. Int J Nanomedicine. 2017;12:3357–64.
Fu XL, Shi YB, Wang H, Zhao XG, Sun QF, Huang Y, et al. Ethosomal gel for improving transdermal delivery of Thymosin beta-4. Int J Nanomedicine. 2019;14:9275–84.
Nasr S, Rady M, Gomaa I, Syrovets T, Simmet T, Fayad W, et al. Ethosomes and lipid-coated chitosan nanocarriers for skin delivery of a chlorophyll derivative: a potential treatment of squamous cell carcinoma by photodynamic therapy. Int J Pharm. 2019;568:11.
Vogt A, Wischke C, Neffe AT, Ma N, Alexiev U, Lendlein A. Nanocarriers for drug delivery into and through the skin — do existing technologies match clinical challenges? J Control Release. 2016;242:3–15.
Santos AC, Rodrigues D, Sequeira JAD, Pereira I, Simoes A, Costa D, et al. Nanotechnological breakthroughs in the development of topical phytocompounds-based formulations. Int J Pharm. 2019;572:16.
Dragicevic N, Maibach H. Combined use of nanocarriers and physical methods for percutaneous penetration enhancement. Adv Drug Deliv Rev. 2018;127:58–84.
Elsayed MMA, Abdallah OY, Naggar VF, Khalafallah NM. Deformable liposomes and ethosomes: mechanism of enhanced skin delivery. Int J Pharm. 2006;322(1–2):60–6.
Verma P, Pathak K. Therapeutic and cosmeceutical potential of ethosomes: an overview. J Adv Pharm Technol Res. 2010;1(3):274–82.
Cristiano MC, Froiio F, Spaccapelo R, Mancuso A, Nistico SP, Udongo BP, et al. Sulforaphane-loaded Ultradeformable vesicles as a potential natural nanomedicine for the treatment of skin Cancer diseases. Pharmaceutics. 2020;12(1):13.
Touitou E, Dayan N, Bergelson L, Godin B, Eliaz M. Ethosomes - novel vesicular carriers for enhanced delivery: characterization and skin penetration properties. J Control Release. 2000;65(3):403–18.
Paolino D, Lucania G, Mardente D, Alhaique F, Fresta M. Ethosomes for skin delivery of ammonium glycyrrhizinate: in vitro percutaneous permeation through human skin and in vivo anti-inflammatory activity on human volunteers. J Control Release. 2005;106(1–2):99–110.
Abdulbaqi IM, Darwis Y, Khan NAK, Abou Assi R, Khan AA. Ethosomal nanocarriers: the impact of constituents and formulation techniques on ethosomal properties, in vivo studies, and clinical trials. Int J Nanomedicine. 2016;11:26.
Tran VV, Moon JY, Lee YC. Liposomes for delivery of antioxidants in cosmeceuticals: challenges and development strategies. J Control Release. 2019;300:114–40.
Zhou Y, Wei YH, Liu HX, Zhang GQ, Wu XA. Preparation and in vitro evaluation of Ethosomal Total alkaloids of Sophora alopecuroides loaded by a transmembrane pH-gradient method. AAPS PharmSciTech. 2010;11(3):1350–8.
Zhang YT, Xia Q, Li YY, He ZH, Li Z, Guo T, et al. CD44 assists the topical anti-psoriatic efficacy of curcumin-loaded Hyaluronan-modified Ethosomes: a new strategy for clustering drug in inflammatory skin. Theranostics. 2019;9(1):48–64.
Shen LN, Zhang YT, Wang Q, Xu L, Feng NP. Enhanced in vitro and in vivo skin deposition of apigenin delivered using ethosomes. Int J Pharm. 2014;460(1–2):280–8.
Song CK, Balakrishnan P, Shim CK, Chung SJ, Chong S, Kim DD. A novel vesicular carrier, transethosome, for enhanced skin delivery of voriconazole: characterization and in vitro/in vivo evaluation. Colloid Surf B-Biointerfaces. 2012;92:299–304.
Bragagni M, Mennini N, Maestrelli F, Cirri M, Mura P. Comparative study of liposomes, transfersomes and ethosomes as carriers for improving topical delivery of celecoxib. Drug Deliv. 2012;19(7):354–61.
Meng S, Chen ZX, Yang LQ, Zhang W, Liu DH, Guo J, et al. Enhanced transdermal bioavailability of testosterone propionate via surfactant-modified ethosomes. Int J Nanomedicine. 2013;8:3051–60.
Chen M, Gupta V, Anselmo AC, Muraski JA, Mitragotri S. Topical delivery of hyaluronic acid into skin using SPACE-peptide carriers. J Control Release. 2014;173:67–74.
Ascenso A, Raposo S, Batista C, Cardoso P, Mendes T, Praca FG, et al. Development, characterization, and skin delivery studies of related ultradeformable vesicles: transfersomes, ethosomes, and transethosomes. Int J Nanomedicine. 2015;10:5837–51.
Xie J, Ji Y, Xue W, Ma D, Hu YF. Hyaluronic acid-containing ethosomes as a potential carrier for transdermal drug delivery. Colloid Surf B-Biointerfaces. 2018;172:323–9.
López-Pinto JM, González-Rodríguez ML, Rabasco AM. Effect of cholesterol and ethanol on dermal delivery from DPPC liposomes. Int J Pharm. 2005;298(1):1–12.
Pandey V, Golhani D, Shukla R. Ethosomes: versatile vesicular carriers for efficient transdermal delivery of therapeutic agents. Drug Deliv. 2015;22(8):988–1002.
Abd El-Alim SH, Kassem AA, Basha M, Salama A. Comparative study of liposomes, ethosomes and transfersomes as carriers for enhancing the transdermal delivery of diflunisal: in vitro and in vivo evaluation. Int J Pharm. 2019;563:293–303.
Arora D, Nanda S. Quality by design driven development of resveratrol loaded ethosomal hydrogel for improved dermatological benefits via enhanced skin permeation and retention. Int J Pharm. 2019;567:13.
Ainbinder D, Paolino D, Fresta M, Touitou E. Drug delivery applications with Ethosomes. J Biomed Nanotechnol. 2010;6(5):558–68.
Sakdiset P, Amnuaikit T, Pichayakorn W, Pinsuwan S. Formulation development of ethosomes containing indomethacin for transdermal delivery. J Drug Deliv Sci Technol. 2019;52:760–8.
Zhang YB, Ng WB, Feng X, Cao FY, Xu HX. Lipid vesicular nanocarrier: quick encapsulation efficiency determination and transcutaneous application. Int J Pharm. 2017;516(1–2):225–30.
Zhang YB, Ng W, Hu JG, Mussa SS, Ge YR, Xu HX. Formulation and in vitro stability evaluation of ethosomal carbomer hydrogel for transdermal vaccine delivery. Colloid Surf B-Biointerfaces. 2018;163:184–91.
Muzzalupo R, Perez L, Pinazo A, Tavano L. Pharmaceutical versatility of cationic niosomes derived from amino acid-based surfactants: skin penetration behavior and controlled drug release. Int J Pharm. 2017;529(1–2):245–52.
Sala M, Diab R, Elaissari A, Fessi H. Lipid nanocarriers as skin drug delivery systems: properties, mechanisms of skin interactions and medical applications. Int J Pharm. 2018;535(1–2):1–17.
Bolzinger MA, Briancon S, Pelletier J, Chevalier Y. Penetration of drugs through skin, a complex rate-controlling membrane. Curr Opin Colloid Interface Sci. 2012;17(3):156–65.
Wu X, Guy RH. Applications of nanoparticles in topical drug delivery and in cosmetics. J Drug Deliv Sci Technol. 2009;19(6):371–84.
Carter P, Narasimhan B, Wang Q. Biocompatible nanoparticles and vesicular systems in transdermal drug delivery for various skin diseases. Int J Pharm. 2019;555:49–62.
Khan NR, Wong TW. Microwave-aided skin drug penetration and retention of 5-fluorouracil-loaded ethosomes. Expert Opin Drug Deliv. 2016;13(9):1209–19.
Hua S. Lipid-based nano-delivery systems for skin delivery of drugs and bioactives. Front Pharmacol. 2015;6:5.
Marwah H, Garg T, Goyal AK, Rath G. Permeation enhancer strategies in transdermal drug delivery. Drug Deliv. 2016;23(2):564–78.
Costa R, Santos L. Delivery systems for cosmetics - from manufacturing to the skin of natural antioxidants. Powder Technol. 2017;322:402–16.
Santos AC, Pereira-Silva M, Guerra C, Costa D, Peixoto D, Pereira I, et al. Topical Minoxidil-loaded nanotechnology strategies for alopecia. Cosmetics. 2020;7:21.
Zylberberg C, Matosevic S. Pharmaceutical liposomal drug delivery: a review of new delivery systems and a look at the regulatory landscape. Drug Deliv. 2016;23(9):3319–29.
Mishra D, Mishra PK, Dabadghao S, Dubey V, Nahar M, Jain NK. Comparative evaluation of hepatitis B surface antigen-loaded elastic liposomes and ethosomes for human dendritic cell uptake and immune response. Nanomed-Nanotechnol Biol Med. 2010;6(1):110–8.
Dubey V, Mishra D, Dutta T, Nahar M, Saraf DK, Jain NK. Dennal and transdermal delivery of an anti-psoriatic agent via ethanolic liposomes. J Control Release. 2007;123(2):148–54.
Zhang Z, Chen YS, Xu H, Wo Y, Zhang Z, Liu Y, et al. 5-Aminolevulinic acid loaded ethosomal vesicles with high entrapment efficiency for in vitro topical transdermal delivery and photodynamic therapy of hypertrophic scars. Nanoscale. 2016;8(46):19270–9.
Ainbinder D, Touitou E. Testosterone Ethosomes for enhanced transdermal delivery. Drug Deliv. 2005;12(5):297–303.
Ashtikar M, Nagarsekar K, Fahr A. Transdermal delivery from liposomal formulations - evolution of the technology over the last three decades. J Control Release. 2016;242:126–40.
Yu X, Du LN, Li Y, Fu GY, Jin YG. Improved anti-melanoma effect of a transdermal mitoxantrone ethosome gel. Biomed Pharmacother. 2015;73:6–11.
Moolakkadath T, Aqil M, Ahad A, Imam SS, Praveen A, Sultana Y, et al. Fisetin loaded binary ethosomes for management of skin cancer by dermal application on UV exposed mice. Int J Pharm. 2019;560:78–91.
Sharma S, Kumar D, Singh G, Monga V, Kumar B. Recent advancements in the development of heterocyclic anti-inflammatory agents. Eur J Med Chem. 2020;200:25.
Chourasia MK, Kang L, Chan SY. Nanosized ethosomes bearing ketoprofen for improved transdermal delivery. Results in pharma sciences. 2011;1(1):60–7.
Ahad A, Raish M, Al-Mohizea AM, Al-Jenoobi FI, Alam MA. Enhanced anti-inflammatory activity of carbopol loaded meloxicam nanoethosomes gel. Int J Biol Macromol. 2014;67:99–104.
Shumilov M, Bercovich R, Duchi S, Ainbinder D, Touitou E. Ibuprofen transdermal Ethosomal gel: characterization and efficiency in animal models. J Biomed Nanotechnol. 2010;6(5):569–76.
Elmowafy M, Shalaby K, Ali HM, Alruwaili NK, Salama A, Ibrahim MF, et al. Impact of nanostructured lipid carriers on dapsone delivery to the skin: in vitro and in vivo studies. Int J Pharm. 2019;572:11.
Apriani EF, Rosana Y, Iskandarsyah I. Formulation, characterization, and in vitro testing of azelaic acid ethosome-based cream against Propionibacterium acnes for the treatment of acne. J Adv Pharm Technol Res. 2019;10(2):75–80.
Godin B, Touitou E. Mechanism of bacitracin permeation enhancement through the skin and cellular membranes from an ethosomal carrier. J Control Release. 2004;94(2–3):365–79.
Touitou E, Godin B, Shumilov M, Bishouty N, Ainbinder D, Shouval R, et al. Efficacy and tolerability of clindamycin phosphate and salicylic acid gel in the treatment of mild to moderate acne vulgaris. J Eur Acad Dermatol Venereol. 2008;22(5):629–31.
Manconi M, Sinico C, Caddeo C, Vila AO, Valenti D, Fadda AM. Penetration enhancer containing vesicles as carriers for dermal delivery of tretinoin. Int J Pharm. 2011;412(1):37–46.
Raza K, Singh B, Lohan S, Sharma G, Negi P, Yachha Y, et al. Nano-lipoidal carriers of tretinoin with enhanced percutaneous absorption, photostability, biocompatibility and anti-psoriatic activity. Int J Pharm. 2013;456(1):65–72.
Pradhan M, Alexander A, Singh MR, Singh D, Saraf S, Saraf S, et al. Understanding the prospective of nano-formulations towards the treatment of psoriasis. Biomed Pharmacother. 2018;107:447–63.
Rahman M, Akhter S, Ahmad J, Ahmad MZ, Beg S, Ahmad FJ. Nanomedicine-based drug targeting for psoriasis: potentials and emerging trends in nanoscale pharmacotherapy. Expert Opin Drug Deliv. 2015;12(4):635–52.
Zhang YT, Shen LIN, Zhao JH, Feng NP. Evaluation of psoralen ethosomes for topical delivery in rats by using in vivo microdialysis. Int J Nanomedicine. 2014;9:669–78.
Zhang YT, Shen LN, Wu ZH, Zhao JH, Feng NP. Comparison of ethosomes and liposomes for skin delivery of psoralen for psoriasis therapy. Int J Pharm. 2014;471(1–2):449–52.
Fang YP, Huang YB, Wu PC, Tsai YH. Topical delivery of 5-aminolevulinic acid-encapsulated ethosomes in a hyperproliferative skin animal model using the CLSM technique to evaluate the penetration behavior. Eur J Pharm Biopharm. 2009;73(3):391–8.
Chandra A, Aggarwal G, Manchanda S, Narula A. Development of topical gel of methotrexate incorporated Ethosomes and salicylic acid for the treatment of psoriasis. Pharmaceutical nanotechnology. 2019;7(5):362–74.
Li GL, Fan YT, Fan C, Li XR, Wang XN, Li M, et al. Tacrolimus-loaded ethosomes: physicochemical characterization and in vivo evaluation. Eur J Pharm Biopharm. 2012;82(1):49–57.
Stoehr JR, Choi JN, Colavincenzo M, Vanderweil S. Off-label use of topical Minoxidil in alopecia: a review. Am J Clin Dermatol. 2019;20(2):237–50.
Khan NR, Wong TW. 5-fluorouracil ethosomes - skin deposition and melanoma permeation synergism with microwave. Artif Cell Nanomed Biotechnol. 2018;46:S568–S77.
Ma LL, Wang XY, Wu JL, Zhang DD, Zhang L, Song XR, et al. Polyethylenimine and sodium cholate-modified ethosomes complex as multidrug carriers for the treatment of melanoma through transdermal delivery. Nanomedicine. 2019;14(18):2395–408.
Singh S, Hussain A, Shakeel F, Ahsan MJ, Alshehri S, Webster TJ, et al. Recent insights on nanomedicine for augmented infection control. Int J Nanomedicine. 2019;14:2301–25.
Godin B, Touitou E. Erythromycin ethosomal systems: physicochemical characterization and enhanced antibacterial activity. Current drug delivery. 2005;2(3):269–75.
Horwitz E, Pisanty S, Czerninski R, Helser M, Eliav E, Touitou E. A clinical evaluation of a novel liposomal carrier for acyclovir in the topical treatment of recurrent herpes labialis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87(6):700–5.
Abdellatif MM, Khalil IA, Khalir MAF. Sertaconazole nitrate loaded nanovesicular systems for targeting skin fungal infection: in-vitro, ex-vivo and in-vivo evaluation. Int J Pharm. 2017;527(1–2):1–11.
Verma S, Utreja P. Vesicular nanocarrier based treatment of skin fungal infections: potential and emerging trends in nanoscale pharmacotherapy. Asian J Pharm Sci. 2019;14(2):117–29.
Manca ML, Usach I, Peris JE, Ibba A, Orru G, Valenti D, et al. Optimization of innovative three-dimensionally-structured hybrid vesicles to improve the cutaneous delivery of Clotrimazole for the treatment of topical candidiasis. Pharmaceutics. 2019;11(6):18.
Maheshwari RGS, Tekade RK, Sharma PA, Darwhekar G, Tyagi A, Patel RP, et al. Ethosomes and ultradeformable liposomes for transdermal delivery of clotrimazole: a comparative assessment. Saudi Pharm J. 2012;20(2):161–70.
Marto J, Vitor C, Guerreiro A, Severino C, Eleuterio C, Ascenso A, et al. Ethosomes for enhanced skin delivery of griseofulvin. Colloid Surf B-Biointerfaces. 2016;146:616–23.
Iizhar SA, Syed IA, Satar R, Ansari SA. In vitro assessment of pharmaceutical potential of ethosomes entrapped with terbinafine hydrochloride. J Adv Res. 2016;7(3):453–61.
Fahmy UA. Nanoethosomal transdermal delivery of vardenafil for treatment of erectile dysfunction: optimization, characterization, and in vivo evaluation. Drug Des Dev Ther. 2015;9:6129–37.
Wang AY, Podlasek CA. `role of nanotechnology in erectile dysfunction treatment. J Sex Med. 2017;14(1):36–43.
Shumilov M, Touitou E. Buspirone transdermal administration for menopausal syndromes, in vitro and in animal model studies. Int J Pharm. 2010;387(1–2):26–33.
Santos AC, Morais F, Simoes A, Pereira I, Sequeira JAD, Pereira-Silva M, et al. Nanotechnology for the development of new cosmetic formulations. Expert Opin Drug Deliv. 2019;16(4):313–30.
Scognamiglio I, De Stefano D, Campani V, Mayol L, Carnuccio R, Fabbrocini G, et al. Nanocarriers for topical administration of resveratrol: a comparative study. Int J Pharm. 2013;440(2):179–87.
Santos AC, Pereira I, Pereira-Silva M, Ferreira L, Caldas M, Collado-González M, et al. Nanotechnology-based formulations for resveratrol delivery: effects on resveratrol in vivo bioavailability and bioactivity. Colloids Surf B: Biointerfaces. 2019;180:127–40.
Santos AC, Veiga F, Ribeiro A. New delivery systems to improve the bioavailability of resveratrol. Expert Opin Drug Deliv. 2011;8:973–90.
Santos AC, Pereira I, Pereira-Silva M, Ferreira L, Caldas M, Magalhães M, et al. Nanocarriers for resveratrol delivery: impact on stability and solubility concerns. Trends Food Sci Technol. 2019;91:483–97.
Moulaoui K, Caddeo C, Manca ML, Castangia I, Valenti D, Escribano E, et al. Identification and nanoentrapment of polyphenolic phytocomplex from Fraxinus angustifolia: in vitro and in vivo wound healing potential. Eur J Med Chem. 2015;89:179–88.
Jeswani G. Topical delivery of Curcuma longa extract loaded Nanosized Ethosomes to combat facial wrinkles research article. Journal of Pharmaceutics & Drug Delivery Research. 2014;03.
Acknowledgments
MP-S acknowledges the PhD research grant SFRH/BD/148771/2019 funded by Fundação para a Ciência e a Tecnologia (FCT) and Programa Operacional Capital Humano (POCH).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Paiva-Santos, A.C., Silva, A.L., Guerra, C. et al. Ethosomes as Nanocarriers for the Development of Skin Delivery Formulations. Pharm Res 38, 947–970 (2021). https://doi.org/10.1007/s11095-021-03053-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-021-03053-5