Abstract
Exosomes are secreted extracellular vesicles containing a wide array of biologically active components. Recent studies have demonstrated that exosomes serve as an important vehicle for extracellular communication and exert systemic effects on the physiology of organisms. Adipose tissues (ATs) play a key role in balancing systemic energy homeostasis as a central hub for fatty acid metabolism. At the same time, proper endocrine function of ATs has also been shown to be crucial for regulating physiological and metabolic health. The endocrine function of ATs is partially mediated by AT-derived exosomes that regulate metabolic homeostasis, such as insulin signaling, lipolysis, and inflammation. During the pathogenesis of obesity, metabolic syndrome, and cancer, exosomes shed by the resident cells in ATs may also have a role in regulating the progression of these diseases along with associated pathologies. In this review, we summarize the contents of AT-derived exosomes and their effects on various cell populations along with possible underlying molecular mechanisms. We further discuss the potential applications of exosomes as a drug delivery tool and therapeutic target.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adipose tissue (AT) was initially characterized as an energy storage organ but after the discovery of leptin, its endocrine function has become a focus of research. Many studies have demonstrated the critical role of AT-derived factors in regulating metabolic homeostasis and immune function. AT contains a highly diverse population of resident cells consisting of mature adipocytes, macrophages, endothelial cells, and a resident stem cell population named adipose-derived stem cells (ADSC), adipogenic progenitors, or preadipocytes (1). Also highly diverse is the AT secretome, dozens of cytokines designated as adipokines are secreted along with exosomes and extracellular vesicles(EVs) (2,3). Processes such as inflammation, apoptosis, differentiation, insulin resistance, and metabolism have been shown to be regulated by adipokines, highlighting the endocrine function of AT (4). Furthermore, alterations in adipokine secretion, most notably adiponectin, leptin, and TNFα have been associated with disease severity in cases of obesity, metabolic syndrome, and various cancers (2). Exosomes derived from AT also have a role in this organ’s extracellular signaling function (3). The AT-exosomes (AT-Exos) are known to contain various RNAs, proteins, and lipids that affect a host of processes in target cells (5,6,7). These effects range from regulating macrophage behavior and stimulating angiogenesis to modulating autophagy in hepatocytes (1,8,9). AT-Exos signaling contributes to the diverse extracellular signaling profile of ATs. In the clinical setting, the contents of these AT-Exos are being considered as candidates for disease markers due to the wide body of evidence showing the composition of these AT-Exos are altered under various physiological states (10,11). In addition, there is a growing interest in using AT-Exos as a therapeutic agent or modifying them to become drug delivery vehicles since they have been demonstrated to have low immunogenicity and cytotoxicity when administered in vivo (12).
Exosomes are a class of EVs that range in size from 30 to 150 nm and are formed from multivesicular bodies along the endocytic pathway (13). Exosomes were initially reported through two studies that explored EV secretion of reticulocytes (14,15). The membranes of these vesicles are characterized by their enrichment of certain lipids and proteins, including cholesterol, sphingomyelin, ceramide, and phosphatidylserine along with tetraspanins (CD9, CD63, CD81), integrins, and cell adhesion molecules (16,17). Exosomes can be distinguished from other EVs by their enrichment of endosomal components due to the nature of their biogenesis yet there is no universal marker that has been established to identify exosomes (18). The isolation of exosomes from various body fluids and cell culture media has been performed with a plethora of methods including immunoprecipitation targeting exosome-associated markers, various kits designed to precipitate EVs, flow cytometry, and differential ultracentrifugation, with the last being considered the most reliable for isolating pure exosome samples (19,20).
These isolated exosomes have been demonstrated to contain miRNA, lncRNA, mRNA, circRNA, proteins, and lipids that have been implicated in regulating various processes of their cellular targets (Fig. 1a) (5,21,22,23,24,25,26). MiRNAs are the most well-studied cargos found in exosomes, as they are a potent regulator of gene expression. MiRNAs such as miR-16, miR-27a, miR-146b, and miR-222 have all been demonstrated to be secreted in exosomes and have the effect of promoting adipogenesis (27). Exosomal lncRNAs display a wide range of regulatory functions. For example, GAS5 targets miR-223 to upregulate the PI3K/Akt pathway. RNCR3 found in HUVEC exosomes increases KLF2 expression to affect proliferation and migration, while TUC339/H19 from hepatocarcinoma exosomes affected M1 to M2 macrophage conversion and angiogenesis. In addition, the levels of exosomal lncRNAs are altered by aging and obesity, suggesting a physiological role of these exosomal lncRNAs (22). The presence of functional mRNAs has not only been demonstrated with sequencing of exosomal RNA content but also through studies utilizing CRE recombinase to “turn on” a reporter in remote tissues, showing long-distance transfer of translatable mRNA in vivo (28). Studies of exosomal proteins have been performed to identify cancer disease markers (17,29). In a proteomics analysis comparing exosomes secreted by breast cancer cells to those of non-cancer cells, it was shown that the cancer exosomes had significantly higher levels of metabolic enzymes, signaling, and chaperone proteins, suggesting that these proteins may have a role in tumor-healthy cell crosstalk (30). In this review, the contents of AT-Exos will be discussed along with their functions during healthy AT function, obesity/metabolic syndrome, and cancer.
Adipose tissue exosome contents and targets. (a) Various cells of adipose tissue have been demonstrated to secrete exosomes containing miRNA, mRNA, lncRNA, circRNA, proteins, lipids, and DNA with active biological function. (b) Reported targets of adipose tissue exosomes, which have been shown to regulate processes such as endothelial cell vascularization (via HIF-1α, Akt, ERK, and SDF-1), myocyte insulin resistance (through PPARG expression), macrophage inflammatory profile (through regulating gene expression and cell metabolism), etc. Furthermore, evidence suggests that systemic regulation by exosomes is common due to their ability to remain functional in vivo after traveling significant distances from donor cells. Created with BioRender.com.
AT Exosomal Secretion under Normal Physiological Conditions
In response to an increased need for lipid storage, there are two primary routes for AT expansion. These are adipocyte hypertrophy and hyperplasia, with the latter generally being correlated with healthy AT expansion. Efficient adipogenesis in AT is also necessary for the process of healthy lipid deposition, and ectopic deposition of lipids in non-adipocytes is heavily associated with organ injury and metabolic diseases (31). Because the expansion of preadipocytes and supporting cell populations is necessary for hyperplasia to occur, exosomal regulation of cell proliferation, apoptosis and adipogenesis can be an important area of research for AT health. AT derived exosomes have been shown to promote adipogenesis in ADSCs (32). Furthermore, ADSC-derived exosomes were shown to promote adipogenesis through miR-450a-5p mediated repression of WISP2 expression (33). These studies indicate that the crosstalk between ADSCs and mature adipocytes may regulate adipogenesis.
The population of AT resident stem cells directly contributes to the development and function of AT. In addition, ADSCs function through their extracellular communication dynamics with other cells in the AT. Many studies have been performed to analyze the content and effects of ADSC-derived exosomes on other cell types and populations (34). During healthy expansion of AT, proper vascularization is needed to facilitate blood flow to new cells; but in many cases of unhealthy AT expansion, local hypoxia leads to chronic inflammation and adipocyte dysfunction (31). ADSCs have been shown to secrete various miRNAs in exosomes that improve vascularization of endothelial cells (35). ADSC exosome derived miR-21 promotes angiogenesis by increasing the expression of HIF-1α, Akt, ERK, and SDF-1 (36). Interestingly, miR-21 is heavily associated with tumor angiogenesis (37). In a proteomics analysis exploring the secretome of ADSCs overexpressing telomerase and myocardin, exosomes from these cells improved the tube formation of HUVEC cells (38). MiR-125a, a miRNA that is expressed by ADSCs prior to differentiation, has also been found to promote angiogenesis in endothelial cells by repressing expression of DLL4 through targeting its 3’ UTR (9). Upon VEGF-C treatment, ADSC exosome secretion is enriched in miR-132 and promotes lympho-angiogenesis by targeting Smad-7 and regulating TGF-β/Smad signaling (39). This pro-angiogenic effect is not only observed in vitro but also in vivo; EVs from ADSCs have been shown to induce healthy vascularized AT formation when injected into mice (32). These represent several mechanisms by which ADSC exosomes regulate endothelial cell angiogenesis, a crucial process for healthy AT expansion.
The proper regulation of inflammation is also a major factor in AT health. AT is highly heterogeneous with a resident macrophage population that not only regulates local inflammatory responses but also influences systemic inflammation (40). Regulation of these resident macrophages by neighboring cells can occur through a variety of mechanisms with exosomes having a major role. A study demonstrated that miR-34a containing exosomes from adipocytes affected resident macrophages by repressing KLF4, which in turn promoted high fat diet-induced insulin resistance, glucose intolerance, and systemic inflammation (41). The inflammatory profile of macrophages can also be modified via metabolic reprogramming, with M1 (pro-inflammatory) macrophages being more glycolytic and M2 (anti-inflammatory) having a fatty acid oxidation dependent metabolism (42). Adipocytes have been demonstrated to secrete exosomes containing lipids that serve as a major energy source for resident macrophages. Adipocytes from lean mice secreted exosomes with higher lipid content compared to those from obese mice, suggesting exosomal lipids and miRNA from healthy adipocytes are regulators of inflammation (43).
A role of the ADSC population being actively researched is in regulating inflammation. A study demonstrated that the immunosuppressive potential of ADSCs-secreted exosomes was increased in the presence of pro-inflammatory cytokines. Higher production of exosomal IL-10, IL-8, IL-6, and CCL-2 was observed along with upregulation of miR-34a-5p, miR-156a-5p, miR-21-5p, miR-135b-5p, miR-127-3p, and miR-155-5p (44). MiR-30d-5p from ADSC exosomes reversed ischemic stroke-induced, autophagy-mediated injury by promoting M2 macrophage polarization and resolution of the inflammatory response (45). ADSC exosomes have also been demonstrated to promote M1 to M2 macrophage polarization via STAT3 activation and upregulation of ARG1 (46). In a rat model of sepsis-induced overwhelming systemic inflammation, ADSCs cultured in hypoxic conditions were able to reduce the level of inflammation and improved outcomes (47). Another study demonstrated 3T3-L1 pre-adipocytes secrete exosomes enriched in sonic hedgehog (SHH) when cultured in a high glucose/insulin environment (48). Macrophages exposed to these exosomes exhibited M1 polarization through the Ptch/PI3K pathway. ADSC derived exosomes were also given to neutrophils, leading to a decrease in apoptosis and increased functional capacity (49). Although ADSC regulation of macrophages is not fully understood, preliminary evidence suggests that exosomal communication between the two cell types is an important component of the inflammatory response.
Likewise, resident macrophage exosomal secretion is an important regulatory function for other cell populations within AT. Obese AT derived macrophages secreted miR-29 enriched exosomes that were taken up by adipocytes from lean mice to cause insulin resistance through targeting of PPARδ (50). Song et al. demonstrated that macrophages treated with 3T3-L1 exosomes enriched in SHH reciprocally secreted exosomes that reduced resistant-substrate-1 (IRS1) and hormone-sensitive lipase (HSL) in adipocytes (48). The exosomal crosstalk between resident AT macrophages and neighboring cells shows that exosomes have a key role in regulating local AT inflammation, which in turn can regulate systemic inflammation.
The AT secretome is critical not only for local metabolic homeostasis, but also for regulation of many distant organs and tissues (Fig. 1b). ADSC-derived exosomes enriched in miR-20b-3p were successfully used to treat hyperoxaluria in rat kidneys by reducing oxalate-induced autophagy and inflammatory responses (51). Evidence suggests that liver tumorigenesis and fibrosis are regulated by miR-122, during the development of NAFLD, AT-Exos are suspected to be a significant source of compensatory miR-122 production for the failing liver (52). Another study showed that miRNA-181-5p from ADSC-derived exosomes downregulated STAT3/Bcl-2/Beclin1, a fibrosis associated pathway in liver cirrhosis (53). Exosomal resistin from AT was also shown to repress phosphorylation of pAMPKa-Thr-172 to trigger ER stress and led to liver damage (54). As obesity is associated with an increased risk of both kidney disease and non-alcoholic fatty liver disease, disruption of the exosomal regulatory functions in obese individuals may be a factor in both pathologies (55).
Brown adipose tissue (BAT) is a form of fat that is metabolically active and has a significant impact on regulating overall energy expenditure (56). The miRNAs of BAT derived exosomes, especially miR-99b, were shown to accumulate in hepatocytes and reduce hepatic FGF21 levels, a metabolic hormone that enhances BAT function (57,58). In another study, BAT derived exosomes of healthy mice were able to improve metabolism, hepatic and cardiac function in a mouse model of diet-induced obesity. These exosomes were also found to preferentially accumulate in the liver and were enriched in functional mitochondrial proteins that altered hepatocyte metabolism (59). These results together suggest that many of the beneficial effects associated with increased BAT mass and activity may be due to the secretion of exosomes and their systemic effects on organs such as the liver. Despite there being evidence that the secretomes of BAT and WAT adipocytes are distinct, a comprehensive analysis identifying differences in EVs or AT-exos secretion has not yet been conducted (60).
Neuronal cells may also be affected by exosomes from AT as ADSC-derived exosomes were used to reduce apoptosis in an in vitro model of ALS by increasing Bcl-2 expression (61,62). In addition, EVs from differentiated 3T3-L1 cells were shown to be taken up by POMC neurons in vitro and regulate mTOR signaling via MALAT1, a lncRNA upregulated in AT of ob/ob mice (63). MALAT1-containing exosomes from ob/ob mice injected into lean mice in the same study increased both appetite and weight correlated with hypothalamic mTOR signaling. In another study, differentiated ADSC exosomal RNA was able to trigger neurite outgrowth (64). These studies suggest that exosomal communication is an important method of regulation between AT resident cells and neurons.
Cardiac cells have been shown to respond to ADSC exosomal treatment in vivo with reduced apoptosis in a rat ischemia/reperfusion injury model through activation of the Wnt/β-catenin pathway (65). After a high-calorie meal, serum-derived CD36 exosomes contained free fatty acids that were taken up by cardiac cells (7). Interestingly, adiponectin associated T-cadherin increased exosome biogenesis of aorta cells, leading to a decrease in cellular ceramide (66). As cardiovascular disease is well-known to be associated with obesity, the impact of AT-Exos in the development of the disease warrants future exploration.
ADSCs and their exosomes have been explored as a potential route to enhance wound healing due to their low immunogenicity and ease of harvest (12,67). The exosomes of ADSCs are suspected to play a role in regulating many of the cells involved with the wound healing process. Fibroblasts are a crucial cell type mediating wound healing, their role being the creation of new extracellular matrix and secretion of collagen (68). Exosomes from ADSCs containing MALAT1 enhanced fibroblast migration and improved wound recovery in an ischemic rat model (69). Another study analyzing the effect of ADSC exosomes on fibroblasts saw an improvement in proliferation, migration, and collagen deposition via upregulation of the PI3K/Akt signaling pathway, leading to increased MMP1, bFGF, TGF-β1, collagen I and III expression (70). This result was corroborated in another study that analyzed the effect of ADSC exosomes on vaginal fibroblasts and found increased expression of collagen 1 and TIMP-1/3 (regulators of MMPs) (71). Keratinocytes treated with adipocyte-derived exosomes containing hsa-circ-0075932 displayed increased apoptosis and inflammation, mediated by the circRNA binding to PUM2, which upregulates Aurora A kinase and activates NF-κB (72). Notably, this circRNA is overexpressed in the burned skin of obese patients, suggesting another mechanism by which AT-Exos may influence tissue regeneration. Because ADSC exosomes have already been shown to facilitate wound and organ repair in clinical settings, research is being conducted to explore possible ways to optimize their effectiveness. In this regard, additives such as hyaluronic acid have been shown to retain exosomes in a target area for a longer time, improving re-epithelialization and vascularization of the wound area (35).
It is evident that AT is involved in complex extracellular communication dynamics locally and remotely with tissues throughout the body. Proper regulation of functions in these remote tissues is influenced by the AT secretome, and exosomes appear to be a notable component involved in these processes and general homeostasis. Further research into this communication method may be critical in understanding the complex endocrine functions of AT.
AT Exosomal Secretion during Obesity and Metabolic Syndrome
Obesity is a multifaceted disease continually growing in prevalence and is associated with many other morbidities (4,73). During the onset and progression of obesity, a phenomenon observed is the altered state of AT, displaying insulin resistance, a pro-inflammatory secretome, and dysregulated metabolic profile (40). Among the many aspects of AT altered during obesity, the exosomal secretion profile is modified (Fig. 2) (74). There is increasing interest in using exosomal contents as markers for associated diseases, some of which are suspected to have a major role in disease development. During obesity, the level of plasma EVs, including exosomes, increases by 10-fold and does not return to normal levels after sustained weight loss, suggesting that obesity causes permanent changes in the secretome of AT that may contribute to the development of obesity-related diseases (75).
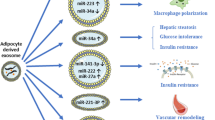
Proposed impact of AT-Exos on the progression of obesity, metabolic syndrome, and associated diseases. The dysfunctional state of adipose tissue leads to altered exosomal production that has systemic effects leading to altered pancreatic insulin secretion (via miR-146b/15b), hepatocyte insulin resistance (through regulation of AKT phosphorylation, skeletal muscle insulin resistance (downregulation of PPARγ) and macrophage activation. Combined, these effects may have an impact on the development of associated diabetes, metabolic dysregulation, and chronic inflammation. Created with BioRender.com.
One of the hallmarks of obesity is a chronic increase in inflammation, not only within AT marked by excessive macrophage accumulation, but also throughout the entire body (76). This inflammation is correlated with the development of obesity-related diseases such as cancer, diabetes, and heart disease (73,77,78). AT-Exos contained a high amount of neutral lipids that induce an AT macrophage-like phenotype in bone marrow cells, potentially contributing to the macrophage population number during obesity (79). Macrophages derived from the white AT of obese mice secrete exosomes that induce insulin resistance in lean mice, indicating that macrophage dysregulation during obesity may lead to the development of diabetes (50). Injection of ob/ob AT-Exos into WT mice caused TLR4-dependent macrophage-induced insulin resistance. Uptake of these exosomes from AT by peripheral blood monocytes caused differentiation into activated macrophages that secreted proinflammatory TNFα and IL-6 (8). Expression of miR-223 was found to be upregulated in the visceral AT of obese mice and patients, specifically in the stromal vascular fraction free of mature adipocytes (80). This microRNA is known to modulate macrophage phenotype and activation state via the TLR4/FBXW7 axis. Regulation of miR-223 expression by the exosomes of preadipocytes has been displayed, implying a possible communication dynamic between the two cell populations to regulate inflammation (81). Upregulated during obesity, miR-34a is found in adipocyte exosomes and suppresses M2 macrophage polarization through its repression of KLF4, leading to obesity induced adipose inflammation (41). MiR-155, another miRNA found in obese adipocyte exosomes, induced the proinflammatory M1-macrophage phenotype by activating STAT1 and repressing STAT6 expression (82). Intercellular communication via exosomes has been consistently demonstrated to be an important regulator of macrophage behavior and may have a role in causing the systemic inflammation heavily associated with obesity and metabolic syndrome.
A common symptom that is found in patients with obesity and metabolic syndrome is systemic insulin resistance, which leads to further complications in metabolism and health (Fig. 2). The contents of exosomes are currently being studied as a possible mechanism by which this symptom develops, as evidence shows that exosomes derived from obese subjects can induce insulin resistance in lean subjects (8). Analysis of the serum exosomal miRNA of nondiabetic obese patients that underwent bariatric surgery identified nine surgery responsive miRNAs (miR-1246, −1290, −193b-5p, −378c/d/g, −424-5p, −4449, and − 6126), whose target genes are correlated with insulin signaling (83). Another study analyzing exosomal miRNA from obese children found that miR-146b, miR-15b, and miR-486 were upregulated in the obese group. MiR-486 had been shown to decrease myotube glucose tolerance, while miR–146b and miR–15b suppressed glucose-induced pancreas insulin secretion (84). Notably, another study found a similar result of miR-15b being upregulated in obese women (85). MiR-27a, which was found to be elevated in the exosomal RNA of obese children and mice, was able to induce insulin resistance in C2C12 myocytes through repression of PPARγ. Macrophages treated with obese AT extracellular vesicles were also able to induce insulin resistance in C2C12 myocytes, indicating that AT may have direct and indirect effects on the progression of skeletal muscle insulin resistance (86,87). Mir-29a and miR–122 were found to be upregulated in another analysis of obese children’s exosomes and these two are heavily correlated with insulin resistance and diabetes (50,88). The exosomes of obese AT when given to hepatocytes induced insulin resistance and glucose intolerance possibly due to reduced exosomal miR-141-3p content that affects Akt phosphorylation (89). A proteomic analysis of exosomes released by adipocytes of Otsuka Long-Evans Tokushima Fatty rats showed that one of the exosomal proteins, catalase, was downregulated in obese samples. This protein is associated with hepatocyte insulin resistance by protecting against oxidative stress (90). Lastly, analysis of the exosomal proteome of women with gestational diabetes mellitus (GDM), during which placental hormones cause insulin resistance, found that AT of patients with GDM display higher numbers of exosomes containing proteins that increased glycolysis and gluconeogenesis, implying that AT-Exos directly regulate glycemic levels (6). This body of evidence demonstrates AT-Exos can have a systemic effect on the development of insulin resistance during obesity.
Obesity and metabolic syndrome are heavily associated with dysfunctional AT, this dysfunction can manifest in several ways. Adipocytes display severe metabolic dysfunction and are unable to perform lipogenesis or lipolysis, leading to improper lipid accumulation in the body (91). The secretome of AT has been shown to be altered during obesity and metabolic syndrome and the contents of AT-Exos may have a paracrine role in the gradual decline of function in AT (75,92). Patients with metabolic syndrome displayed increased levels of miR-31 in exosomal RNA, which has been shown to reduce adipogenic markers: PPARγ, aP2, and CEBP-α (93). Obese patients had decreased levels of miR-100 in circulation and in visceral AT, miR-100 modulates adipogenesis by reducing mTOR and IGFR (94). In an analysis of circulating miRNAs from overweight patients that responded to exercise mediated weight loss, miR-140 was found to be decreased in responsive patients. This miRNA targets FNDC5 in muscle, therefore reducing the production of irisin, a cleavage product of FNDC5 that promotes lipolysis (95). This result suggests that miR-140 may be an important regulator of myocyte-adipocyte crosstalk. A panel of miRNAs differentially expressed in obese children showed miR-551a and − 501-5p were upregulated while miR-10b-5p and − 215-5p were downregulated, with the upregulated genes targeting BCAA-1A, typically upregulated in obesity, while downregulated miRNAs target genes were related to proper adipogenesis (96). Patients that underwent bariatric surgery saw a decline in exosomal FABP4, a fatty acid binding protein that is a key regulator of lipid metabolism and is associated with obesity-related diseases (97). Exosomes of obese women contain increased levels of miRNAs that modulated lipolysis and adipogenesis (miR-17 family and miR-15b, respectively) (98). Treatment of adipocytes with these exosomes led to reductions in lipid biosynthesis and increased IRS1. Together, these studies show that obese AT releases exosomes with cargo that deregulates adipogenesis and lipid metabolism, perhaps contributing significantly to AT dysfunction.
Another characteristic of unhealthy AT is decreased vascular function that leads to hypoxia. Dysfunctional vasculature is commonly observed in obese individuals and those with metabolic syndrome. In women with metabolic syndrome, circulating miR-21-5p is downregulated and is predicted to affect endothelial cell migration (99). Morbidly obese patients treated for sleep apnea demonstrated a downregulation of exosomal miR-16-5p, miR-320a, miR-21-5p, miR-126-5p, miR-146a-5p, and miR-320a-3p, all of these having target genes related to cardiovascular disease progression and endothelial cell dysfunction (100). Downregulation of miR-126 was also observed in another study with obese adolescents successfully undergoing weight loss (101). In a study that analyzed the effects of obese perivascular AT-derived exosomes, exosomal miR-221-3p was shown to cause vascular smooth muscle cell dysfunction by suppressing contractile genes (102). In summary, the regulation of vascular function is compromised during obesity and metabolic syndrome and the exosomes secreted by AT during these conditions may have a role.
It is known that obesity and metabolic syndrome cause a plethora of physiological issues in affected patients with their AT often showing severe dysregulation. This dysregulation of AT affects the secretome leading to systemic effects on the body. One portion of the AT secretome that has been proven to be affected is exosomes, and their cargo have been shown to be implicated in many of the pathologies that are associated with obesity and metabolic syndrome. Therefore, AT-Exos and their targets may be important therapeutic pathways to help alleviate health complications associated with obesity and metabolic syndrome.
Exosomes, AT, and Cancer
In many cases of cancer, one of the phenomena commonly observed is cachexia or wasting of skeletal muscle and AT, often induced by secreted factors from cancer cells (103). Conversely, obesity and secreted factors from adipocytes have been shown to regulate cancer cell behavior in vitro and in vivo. One of the proposed mechanisms by which these phenomena occur is exosomal communication between tumor cells and AT (Fig. 3) (104). Cancer-associated adipocytes have altered behavior displaying smaller, dispersed lipid droplets and are derived from normal mature adipocytes possibly exposed to oncogenic miRNAs such as miR-144, miR-126, and miR-155. These cancer-associated adipocytes are suspected to have a significant role in the progression of tumors through their secretome (105). Adipocyte-derived exosomes increased MMP3 production in lung cancer while exosomal miR-21 inhibits cancer cell apoptosis by binding to APAF1 (106). Another study found that adipocyte exosomal circ-DB, upregulated during obesity, stimulated the growth of hepatocellular carcinoma by targeting USP7 deubiquitination (23). Fatty acid oxidation enzymes delivered by adipocyte exosomes to cancer led to a more aggressive phenotype (105). The ADSC population of AT may also have a role in cancer progression with their exosomal contents promoting proliferation, invasion, and migration of endothelial cells, possibly for the purpose of tumor supporting angiogenesis (34). A proteomic analysis of immortalized ADSC exosomes (overexpressing telomerase and myocardin) showed an increase of MMP2 and TIMP2, suggesting a possible mechanism by which this increased angiogenesis occurs (38). ADSC exosomes have also been shown to stimulate stem cell behavior of certain tumor cells, allowing for a more metastatic phenotype (107). However, other cancer cells treated with ADSC exosomes showed decreased proliferation via miR-503-3p, a stemness attenuating factor (34). As we can see, AT-Exos contain a variety of cargo that regulates various aspects of cancer cell biology and tumor progression (Fig. 3a), highlighting the complexity of this communication dynamic dependent upon cell type and context.
Adipose and tumor tissue have both been demonstrated to secrete exosomes that affect each other mutually, as well as other tissue and cell types. (a) Tumor cells exposed to AT-Exos display increased proliferation and a more aggressive phenotype (possibly through delivery of fatty acid oxidation enzymes). Tumor angiogenesis, a vital factor for tumor growth, is theorized to be enhanced by ADSC that affects endothelial cells in the tumor microenvironment (via miR-21). (b) Resident cells of adipose tissue treated with cancer cell-derived exosomes display significant changes in behavior. Adipocytes undergo increased lipolysis (via delivery of adrenomedullin), display increased beiging (activation of PRDM16). This adipose-tumor crosstalk via exosomes may be important for regulating cancer disease progression and associated fat-mass loss during cachexia. Created with BioRender.com.
On the tumor side of cancer-AT crosstalk, the exosomes from tumors have been shown to have a profound impact on the resident cells of AT (Fig. 3b), playing a role in perpetuating the fat-mass loss observed during cachexia due to increased lipolysis (103). Much of this fat-mass loss is suspected to be caused by browning of white adipose tissue (WAT). During WAT browning, increased UCP-1 expression shifts mitochondrial respiration towards thermogenesis, resulting in increased lipolysis, metabolic activity, and contributing to reduction in adipocyte mass (103,104). Pancreatic cancer exosomes contain adrenomedullin (AM), which activates ERK1/2 and p38 MAPK pathways while also increasing the expression of phosphorylated hormone-sensitive lipase – effects that can be ameliorated with AM receptor blockade (108). Tumor cell exosomes contained miR-144, which induced browning through downregulation of MAP3K8, ERK1/2, and PPARγ, while miR-126 remodeled adipocyte metabolism by disrupting IRS/GLUT-4 to activate autophagy and stabilizing HIF-1A expression (104). Gastric tumor exosomes were able to promote the differentiation of pre-adipocytes into brown adipocyte-like cells by activating PRDM16 and sponging miR-133 through circRNA-133 (24). Dedifferentiated liposarcoma secretes MDM2 DNA copies that were taken up by preadipocytes to increase proliferation and migration through the production of MMP2 (109). Multiple studies have shown that exosomes of cancer cells have a variety of effects on AT cell populations by inducing phenotypes related to cachexia or reprogramming the behavior of adipocytes and ADSCs. The exosomal crosstalk between tumor and AT cells may be an important aspect of tumor progression, with the purpose being to provide tumor cells with metabolites and reprogramming surrounding cells to be more supportive of tumor growth. These results imply therapeutic targeting of exosomal communication between tumor cells and niche supporting cells may be a promising area of research.
Exosomal Therapeutics
Although analysis of endogenous exosomal contents may provide crucial information about extracellular communication dynamics, the use of exogenous exosomes is a promising avenue for drug delivery. Studies have shown therapeutics (miRNA, protein, drugs) packaged into exosomes may be more effective than drugs delivered by traditional delivery methods. The improved effectiveness of therapeutics packed in exosomes is due to the low immunogenic response and higher bioavailability of exosomes (110). Similarly, in the area of stem cell therapy research, stem cell-derived exosomes not only provide the positive effects of direct cell transplantation but have the benefit of being a cell-free therapy. This aspect is of great interest as there is a concern of tumorigenic potential inherent in implanted cells.
Exosomes have been shown to modulate lipid metabolism, adipogenesis, insulin resistance, and secretion, all processes are heavily correlated with the morbidity of obesity and metabolic syndrome (3,111). Due to this regulatory property, there is great potential for using or targeting these exosomes as a treatment for these diseases and others involving dysregulated metabolic processes. Exosomal production is permanently increased during obesity and is correlated with the onset of obesity-related diseases (75). In an adipocyte specific Sirt1 knockout mouse model that resulted in obesity, treatment with the exosome production inhibitor, GW4869, resulted in reduced body weight and improved insulin sensitivity, suggesting that ablation of adipocyte exosome release may be a possible treatment for obesity (112). The delivery of miRNA sponge-containing exosomes that target miRNAs responsible for disease progression may also be a viable method that avoids possible off-target effects of reducing total exosome production. Exosomes from cancer cells have been demonstrated to significantly increase the metabolic activity of adipocytes, mostly by increasing lipolysis (103). While this may be highly detrimental during cachexia, it may be possible to use exosomes with similar effects as a method for inducing browning and fat mass loss in obesity treatment. A potentially useful cargo for this purpose would be a new category of secreted molecules deemed “exerkines”, these are released by various tissues during exercise and are demonstrated to be highly beneficial for regulating metabolic homeostasis (113). Exerkines are speculated to be a crucial aspect of organ crosstalk and responsible for many of the health benefits associated with exercise (114). Adiponectin, an exerkine primarily released by adipocytes, was demonstrated to alter exosome production when associated with T-cadherin, suggesting that exerkine release may play a role in regulating exosome production (66). In addition, there is growing evidence that exosomes and other extracellular vesicles are a major vehicle for these factors (115). Apelin, an exerkine, was demonstrated to be able to prevent obesity and even increased AT browning (116). Irisin, another myokine that is released during exercise is also capable of inducing a similar effect in adipocytes and is negatively correlated with obesity-associated NAFLD and insulin resistance (114). There are countless other exerkines with similarly beneficial properties, thus, creation of an efficient method for producing exosomes containing these factors may be an effective method for combating much of the adipocyte metabolic dysregulation associated with obesity.
Although exosomes have great therapeutic potentials, several key issues still must be addressed before further utilization. Optimizing cells for therapeutic exosomal production is an undeveloped area of research. The exosomal secretion profile of cells is still highly dynamic and the underlying molecular regulation is poorly understood (117). For example, the process of exactly how cells select cargo for packing into these extracellular vesicles is largely unknown. Several groups have tried to culture cells optimized for therapeutic exosomal production by altering growth conditions or increasing cytoplasmic concentration of desired therapeutics, yet the effect on therapeutic viability remains to be seen (118,119,120). Despite there being certain post-translational modifications and nucleotide sequences that appear to increase exosomal incorporation, utilizing these in therapeutic cargo changes their inherent properties and may also affect the efficiency of production. Further research into this area would greatly improve their utility in a clinical setting, allowing for optimized production and avoidance of undesired cargo being incorporated. Another issue is the general lack of specificity in exosomal targeting. Exosomes do not appear to exhibit target cell selectivity, raising a concern of off-target effects and the necessity for much higher dosages to ensure that target cells will receive an effective dosage. In order to address these issues, incorporation of targeting molecules or peptides into exosomal membranes may be a viable strategy as these tend to preferentially target desired cells (121). Therefore, elucidating exosomal targeting mechanisms and/or discovering effective targeting modifications would greatly increase potency.
Summary and Future Directions
AT is a multifaceted organ that has critical roles in metabolic homeostasis through its energy storage and endocrine functions. The secretome of AT is complex and consists of countless components with exosomes being one of the most diverse. These exosomes are capable of targeting a wide variety of tissues and cells throughout the body to regulate countless numbers of processes through many mechanisms. As with many other AT functions, exosomal secretion is one that is dependent upon physiological context and is heavily altered during periods of adipose dysfunction such as obesity and metabolic syndrome. This altered exosomal secretion may be responsible for many of the associated maladies that accompany obesity, thus representing a possible opportunity for research into therapeutic avenues to alleviate consequences of the prevalent obesity pandemic. In the context of cancer, exosomal communication between adipose and tumor tissue may also be a critical component of disease progression and initiation, again opening a wide area of research. However, there are still critical aspects of exosomal secretion that must be investigated further in order to facilitate subsequent applications. The mechanisms by which cells regulate exosomal production is largely unknown, further research into this area would greatly enhance the ability to viably produce exosomal therapeutics and provide avenues of treatment for exosome related pathologies. In addition, the cell type or culture strategy optimal for consistent and efficient exosome production has still not yet been identified, leading to an increased possibility of variable results for exosomal research. Lastly, methods for targeted delivery of exosomes to specific cell types are urgently needed. In conclusion, exosomes are an aspect of extracellular communication that have great potential for further study and manipulation to improve both our understanding and treatment of AT-related diseases such as obesity, cancer, and type 2 diabetes.
Abbreviations
- ADSC:
-
Adipose-derived stem cells
- AKT:
-
Protein kinase B
- AM:
-
Adrenomedullin
- AMPK:
-
AMP-activated protein kinase
- APAF:
-
Apoptotic protease activating factor
- AT:
-
Adipose tissue
- AT-exos:
-
Adipose tissue derived exosomes
- ARG:
-
Arginase
- BAT:
-
Brown adipose tissue
- BCAA:
-
Branched-chain amino acid transaminase, FABP, fatty acid binding protein
- CCL:
-
Chemokine (C-C motif) ligand
- CEBP:
-
CCAAT/ enhancer-binding protein
- DLL:
-
Delta like canonical Notch ligand
- ERK:
-
Extracellular signal regulated kinase
- EVs:
-
Extracellular vesicles
- FBXW7:
-
F-Box and WD repeat domain containing
- FGF:
-
Fibroblast growth factor
- FNDC:
-
Fibronectin type III domain-containing protein
- GDM:
-
Gestational diabetes mellitus, aP, adipocyte protein
- GLUT:
-
Glucose transporter
- HSL:
-
Hormone sensitive lipase
- HUVEC:
-
Human umbilical vein endothelial cells
- IGFR:
-
Insulin-like growth factor receptor
- IL:
-
Interleukin
- IRS:
-
Insulin receptor substrate
- KLF:
-
Kruppel-like factor
- MALAT:
-
Metastasis associated lung adenocarcinoma transcript
- MAPK:
-
Mitogen-activated protein kinase
- MDM:
-
Mouse double minute homolog
- MMP:
-
Matrix metalloproteinase
- mTOR:
-
Mammalian target of rapamycin
- NAFLD:
-
Nonalcoholic fatty liver disease, Bcl, B cell lymphoma
- ob:
-
Leptin
- PI3K:
-
Phosphoinositide 3-kinase
- POMC:
-
Pro-opiomelanocortin
- PPAR:
-
Peroxisome proliferator-activated receptor
- PRDM:
-
PR domain containing
- Ptch:
-
Patched-1 protein
- PUM:
-
Pumilio RNA binding family member
- RNCR3:
-
Retinal noncoding RNA3
- SDF:
-
Stromal cell derived factor
- SHH:
-
Sonic hedgehog
- SIRT:
-
Sirtuin
- STAT:
-
Signal transducer and activator of transcription
- TGF:
-
Transforming growth factor
- TIMP:
-
Tissue inhibitor of metalloproteinases
- TLR:
-
Toll-like receptor
- TNF:
-
Tumor necrosis factor
- UCP:
-
Uncoupling protein
- VEGF:
-
Vascular endothelial growth factor
- WAT:
-
White adipose tissue
- WISP:
-
WNT1 inducible signaling pathway protein, HIF, hypoxia induced factor
References
Patrikoski M, Mannerström B, Miettinen S. Perspectives for clinical translation of adipose stromal/stem cells. Stem Cells Int. 2019;2019:1–21.
Fasshauer M, Blüher M. Adipokines in health and disease. Trends Pharmacol Sci. 2015. p. 461–70.
Zhang Y, Yu M, Tian W. Physiological and pathological impact of exosomes of adipose tissue. Cell Prolif. 2016;49:3–13.
Choi CHJ, Cohen P. Adipose crosstalk with other cell types in health and disease. Exp. Cell Res. 2017. p. 6–11.
Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–9.
Jayabalan N, Lai A, Ormazabal V, Adam S, Guanzon D, Palma C, et al. Adipose tissue Exosomal proteomic profile reveals a role on placenta glucose metabolism in gestational diabetes mellitus. J Clin Endocrinol Metab. 2019;104:1735–52.
Garcia NA, González-King H, Grueso E, Sánchez R, Martinez-Romero A, Jávega B, et al. Circulating exosomes deliver free fatty acids from the bloodstream to cardiac cells: possible role of CD36. PLoS One. 2019;14:e0217546.
Deng ZB, Poliakov A, Hardy RW, Clements R, Liu C, Liu Y, et al. Adipose tissue exosome-like vesicles mediate activation of macrophage-induced insulin resistance. Diabetes. 2009;58:2498–505.
Liang X, Zhang L, Wang S, Han Q, Zhao RC. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J Cell Sci. 2016;129:2182–9.
Gao X, Salomon C, Freeman DJ. Extracellular vesicles from adipose tissue-A potential role in obesity and type 2 diabetes? Front. Endocrinol. (Lausanne). 2017.
Lorente-Cebrián S, González-Muniesa P, Milagro FI, Alfredo MJ. MicroRNAs and other non-coding RNAs in adipose tissue and obesity: emerging roles as biomarkers and therapeutic targets. Clin Sci. 2019;133:23–40.
Bruun K, Schermer E, Sivendra A, Valaik E, Wise RB, Said R, et al. Therapeutic applications of adipose-derived stem cells in cardiovascular disease. Am J Stem Cells. 2018;7:94–103.
Kowal J, Tkach M, Théry C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014. p. 116–25.
Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97:329–39.
Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33:967–78.
Escola JM, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem. 1998;273:20121–7.
Bebelman MP, Smit MJ, Pegtel DM, Baglio SR. Biogenesis and function of extracellular vesicles in cancer. Pharmacol Ther. 2018. p. 1–11.
Gruenberg J, Van Der Goot FG. Mechanisms of pathogen entry through the endosomal compartments. Nat Rev Mol Cell Biol. 2006;7:495–504.
Salih M, Fenton RA, Knipscheer J, Janssen JW, Vredenbregt-Van Den Berg MS, Jenster G, et al. An immunoassay for urinary extracellular vesicles. Am J Physiol Ren Physiol 2016;310:F796–F801.
Tang YT, Huang YY, Zheng L, Qin SH, Xu XP, An TX, et al. Comparison of isolation methods of exosomes and exosomal RNA from cell culture medium and serum. Int J Mol Med. 2017;40:834–44.
Lin F, Zeng Z, Song Y, Li L, Wu Z, Zhang X, et al. YBX-1 mediated sorting of miR-133 into derived exosomes to increase fibroblast angiogenesis and MEndoT. 2019;1–13.
Cao Q, Guo Z, Yan Y, Wu J, Song C. Exosomal long noncoding RNAs in aging and age-related diseases 2019;1–11.
Zhang H, Deng T, Ge S, Liu Y, Bai M, Zhu K, et al. Exosome circRNA secreted from adipocytes promotes the growth of hepatocellular carcinoma by targeting deubiquitination-related USP7. Oncogene. 2019;38:2844–59.
Zhang H, Zhu L, Bai M, Liu Y, Zhan Y, Deng T, et al. Exosomal circRNA derived from gastric tumor promotes white adipose browning by targeting the miR-133/PRDM16 pathway. Int J Cancer. 2019;144:2501–15.
Pace KR, Dutt R, Galileo DS. Exosomal L1CAM stimulates glioblastoma cell motility, proliferation, and invasiveness. Int J Mol Sci. 2019;20.
Beloribi S, Ristorcelli E, Breuzard G, Silvy F, Bertrand-Michel J, Beraud E, et al. Exosomal lipids impact notch signaling and induce death of human pancreatic tumoral SOJ-6 Cells. Hoheisel JD, editor. PLoS One. 2012;7:e47480.
Mori MA, Ludwig RG, Garcia-Martin R, Brandão BB, Kahn CR. Extracellular miRNAs: from biomarkers to mediators of physiology and disease. Cell Metab. 2019. p. 656–73.
Zomer A, Steenbeek SC, Maynard C, Van Rheenen J. Studying extracellular vesicle transfer by a Cre-loxP method. Nat Protoc. 2016;11:87–101.
Chen IH, Xue L, Hsu CC, Paez JSP, Panb L, Andaluz H, et al. Phosphoproteins in extracellular vesicles as candidate markers for breast cancer. Proc Natl Acad Sci U S A. 2017;114:3175–80.
Griffiths SG, Cormier MT, Clayton A, Doucette AA. Differential proteome analysis of extracellular vesicles from breast cancer cell lines by chaperone affinity enrichment. Proteomes. 2017;5:1–16.
Ghaben AL, Scherer PE. Adipogenesis and metabolic health. Nat Rev Mol Cell Biol. 2019. p. 242–58.
Dai M, Yu M, Zhang Y, Tian W. Exosome-like vesicles derived from adipose tissue provide biochemical cues for adipose tissue regeneration. Tissue Eng - Part A. 2017;23:1221–30.
Zhang Y, Yu M, Dai M, Chen C, Tang Q, Jing W, et al. miR-450a-5p within rat adipose tissue exosome-like vesicles promotes adipogenic differentiation by targeting WISP2. J Cell Sci. 2017;130:1158–68.
Gentile P, Garcovich S. Concise review: adipose-derived stem cells (ASCs) and adipocyte-secreted Exosomal microRNA (A-SE-miR) modulate Cancer growth and proMote wound repair. J Clin Med. 2019;8:855.
Liu K, Chen C, Zhang H, Chen Y, Zhou S. ASC -derived exosomes in combination with hyaluronic acid accelerate wound healing through enhancing re-epithelialization and vascularization. Br J Dermatol. 2019:15–6.
An Y, Zhao J, Nie F, Qin Z, Xue H, Wang G, et al. Exosomes from adipose-derived stem cells (ADSCs) overexpressing miR-21 promote vascularization of endothelial cells. Sci Rep. 2019;9:1–10.
Monfared H, Jahangard Y, Nikkhah M, Mirnajafi-Zadeh J, Mowla SJ. Potential therapeutic effects of Exosomes packed with a miR-21-sponge construct in a rat model of Glioblastoma. Front Oncol. 2019;9:782.
Madonna R, Angelucci S, Di Giuseppe F, Doria V, Giricz Z, Görbe A, et al. Proteomic analysis of the secretome of adipose tissue-derived murine mesenchymal cells overexpressing telomerase and myocardin. J Mol Cell Cardiol. 2019;131:171–86.
Wang X, Wang H, Cao J, Ye C. Exosomes from adipose-derived stem cells promotes VEGF-C-dependent Lymphangiogenesis by regulating miRNA-132/TGF-β pathway. Cell Physiol Biochem. 2018;49:160–71.
Reddy P, Lent-Schochet D, Ramakrishnan N, McLaughlin M, Jialal I. Metabolic syndrome is an inflammatory disorder: A conspiracy between adipose tissue and phagocytes. Clin Chim Acta. 2019. p. 35–44.
Pan Y, Hui X, Chong Hoo RL, Ye D, Cheung Chan CY, Feng T, et al. Adipocyte-secreted exosomal microRNA-34a inhibits M2 macrophage polarization to promote obesity-induced adipose inflammation. J Clin Invest. 2019;129:834–49.
Batista-Gonzalez A, Vidal R, Criollo A, Carreño LJ. New insights on the role of lipid metabolism in the metabolic reprogramming of macrophages. Front Immunol. 2020;10:2993.
Flaherty SE, Grijalva A, Xu X, Ables E, Nomani A, Ferrante AW. A lipase-independent pathway of lipid release and immune modulation by adipocytes. Science (80- ). 2019;363:989–93.
Domenis R, Cifù A, Quaglia S, Pistis C, Moretti M, Vicario A, et al. Pro inflammatory stimuli enhance the immunosuppressive functions of adipose mesenchymal stem cells-derived exosomes. Sci Rep. 2018;8:13325.
Jiang M, Wang H, Jin M, Yang X, Ji H, Jiang Y, et al. Exosomes from MiR-30d-5p-ADSCs reverse acute ischemic stroke-induced, autophagy-mediated brain injury by promoting M2 microglial/macrophage polarization. Cell Physiol Biochem. 2018;47:864–78.
Zhao H, Shang Q, Pan Z, Bai Y, Li Z, Zhang H, et al. Exosomes from adipose-derived stem cells attenuate adipose inflammation and obesity through polarizing M2 macrophages and beiging in white adipose tissue. Diabetes. 2018;67:235–47.
Chen H-H, Lin K-C, Wallace CG, Chen Y-T, Yang C-C, Leu S, et al. Additional benefit of combined therapy with melatonin and apoptotic adipose-derived mesenchymal stem cell against sepsis-induced kidney injury. J Pineal Res. 2014;57:16–32.
Song M, Han L, Chen FF, Wang D, Wang F, Zhang L, et al. Adipocyte-derived exosomes carrying sonic hedgehog mediate M1 macrophage polarization-induced insulin resistance via Ptch and PI3K pathways. Cell Physiol Biochem. 2018;48:1416–32.
Mahmoudi M, Taghavi-Farahabadi M, Rezaei N, Hashemi SM. Comparison of the effects of adipose tissue mesenchymal stromal cell-derived exosomes with conditioned media on neutrophil function and apoptosis. Int Immunopharmacol. 2019;74:105689.
Liu T, Sun YC, Cheng P, Shao HG. Adipose tissue macrophage-derived exosomal miR-29a regulates obesity-associated insulin resistance. Biochem Biophys Res Commun. 2019;515:352–8.
Shi J, Duan J, Gong H, Pang Y, Wang L, Yan Y. Exosomes from miR-20b-3p-overexpressing stromal cells ameliorate calcium oxalate deposition in rat kidney. J Cell Mol Med. 2019;23:7268–78.
Baranova A, Maltseva D, Tonevitsky A. Adipose may actively delay progression of NAFLD by releasing tumor-suppressing, anti-fibrotic miR-122 into circulation. Obes Rev. 2019;20:108–18.
Qu Y, Zhang Q, Cai X, Li F, Ma Z, Xu M, et al. Exosomes derived from miR-181-5p-modified adipose-derived mesenchymal stem cells prevent liver fibrosis via autophagy activation. J Cell Mol Med. 2017;21:2491–502.
Rong B, Feng R, Liu C, Wu Q, Sun C. Reduced delivery of epididymal adipocyte-derived exosomal resistin is essential for melatonin ameliorating hepatic steatosis in mice. J Pineal Res. 2019;66:1–20.
Ix JH, Sharma K. Mechanisms linking obesity, chronic kidney disease, and fatty liver disease: The roles of fetuin-A, adiponectin, and AMPK [Internet]. J Am Soc Nephrol. 2010. p. 406–12.
Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, et al. Identification and importance of Brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–17.
Thomou T, Mori MA, Dreyfuss JM, Konishi M, Sakaguchi M, Wolfrum C, et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature. 2017;542:450–5.
Chen Y, Pfeifer A. Brown fat-derived exosomes: Small vesicles with big impact [Internet]. Cell Metab. 2017. p. 759–60.
Zhou X, Li Z, Qi M, Zhao P, Duan Y, Yang G, et al. Brown adipose tissue-derived exosomes mitigate the metabolic syndrome in high fat diet mice. Theranostics. 2020;10:8197–210.
Ali Khan A, Hansson J, Weber P, Foehr S, Krijgsveld J, Herzig S, et al. Comparative secretome analyses of primary murine white and brown adipocytes reveal novel adipokines. Mol Cell Proteomics. 2018;17:2358–70.
Yang J, Zhang X, Chen X, Wang L, Yang G. Exosome mediated delivery of miR-124 promotes neurogenesis after ischemia. Mol Ther - Nucleic Acids. 2017;7:278–87.
Bonafede, Brandi, Manfredi, Scambi, Schiaffino, Merigo, et al. The Anti-Apoptotic Effect of ASC-Exosomes in an In Vitro ALS Model and Their Proteomic Analysis. Cells. 2019;8:1087.
Gao J, Li X, Wang Y, Cao Y, Yao D, Sun L, et al. Adipocyte-derived extracellular vesicles modulate appetite and weight through mTOR signalling in the hypothalamus. Acta Physiol. 2019:1–16.
Ching RC, Wiberg M, Kingham PJ. Schwann cell-like differentiated adipose stem cells promote neurite outgrowth via secreted exosomes and RNA transfer 06 biological sciences 0601 biochemistry and cell biology. Stem Cell Res Ther. 2018;9:266.
Cui X, He Z, Liang Z, Chen Z, Wang H, Zhang J. Exosomes from adipose-derived Mesenchymal stem cells protect the myocardium against ischemia/reperfusion injury through Wnt/b-catenin signaling pathway. J Cardiovasc Pharmacol. 2017;70:225–31.
Obata Y, Kita S, Koyama Y, Fukuda S, Takeda H, Takahashi M, et al. Adiponectin/T-cadherin system enhances exosome biogenesis and decreases cellular ceramides by exosomal release. JCI insight. 2018;3.
Tsuchiya A, Takeuchi S, Watanabe T, Yoshida T, Nojiri S, Ogawa M, et al. Mesenchymal stem cell therapies for liver cirrhosis: MSCs as “conducting cells” for improvement of liver fibrosis and regeneration. Inflamm Regen. 2019;39:18.
Shen T, Zheng QQ, Shen J, Li QS, Song XH, Luo HB, et al. Effects of adipose-derived mesenchymal stem cell exosomes on corneal stromal fibroblast viability and extracellular matrix synthesis. Chin Med J (Engl). 2018;131:704–12.
Cooper DR, Wang C, Patel R, Trujillo A, Patel NA, Prather J, et al. Human adipose-derived stem cell conditioned media and Exosomes containing MALAT1 promote human dermal fibroblast migration and ischemic wound healing. Adv Wound Care. 2018;7:299–308.
Zhang W, Bai X, Zhao B, Li Y, Zhang Y, Li Z, et al. Cell-free therapy based on adipose tissue stem cell-derived exosomes promotes wound healing via the PI3K/Akt signaling pathway. Exp Cell Res. 2018;370:333–42.
Liu X, Wang S, Wu S, Hao Q, Li Y, Guo Z, et al. Exosomes secreted by adipose-derived mesenchymal stem cells regulate type i collagen metabolism in fibroblasts from women with stress urinary incontinence. Stem Cell Res Ther. 2018;9:1–10.
Zhang X, Chen L, Xiao B, Liu H, Su Y. Circ_0075932 in adipocyte-derived exosomes induces inflammation and apoptosis in human dermal keratinocytes by directly binding with PUM2 and promoting PUM2-mediated activation of AuroraA/NF-κB pathway. Biochem Biophys Res Commun. 2019;511:551–8.
Arnold M, Pandeya N, Byrnes G, Renehan AG, Stevens GA, Ezzati M, et al. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol. 2015;16:36–46.
Castaño C, Kalko S, Novials A, Párrizas M. Obesity-associated exosomal miRNAs modulate glucose and lipid metabolism in mice. Proc Natl Acad Sci U S A. 2018;115:12158–63.
Pardo F, Villalobos-Labra R, Sobrevia B, Toledo F, Sobrevia L. Extracellular vesicles in obesity and diabetes mellitus. Mol. Aspects Med. 2018.
Stolarczyk E. Adipose tissue inflammation in obesity: a metabolic or immune response? Curr Opin Pharmacol. 2017;37:35–40.
Klöting N, Blüher M. Adipocyte dysfunction, inflammation and metabolic syndrome. Rev Endocr Metab Disord. 2014. p. 277–87.
Li C, Qu L, Farragher C, Vella A, Zhou B. MicroRNA regulated macrophage activation in obesity. J Transl Intern Med. 2019;7:46–52.
Sharif O, Brunner JS, Vogel A, Schabbauer G. Macrophage rewiring by nutrient associated PI3K dependent pathways. Front Immunol. 2019.
Deiuliis JA, Syed R, Duggineni D, Rutsky J, Rengasamy P, Zhang J, et al. Visceral adipose MicroRNA 223 is upregulated in human and murine obesity and modulates the inflammatory phenotype of macrophages. PLoS One. 2016;11:1–15.
Du W, Su L, Zhang N, Wang H. Exosomes derived from preadipocytes improve osteogenic differentiation, potentially via reduced miR-223 expression. Mol Med Rep. 2019;19:951–8.
Zhang Y, Mei H, Chang X, Chen F, Zhu Y, Han X. Adipocyte-derived microvesicles from obese mice induce M1 macrophage phenotype through secreted miR-155. J Mol Cell Biol. 2016;8:505–17.
Bae Y, Kim Y, Lee H, Kim H, Jeon JS, Noh H, et al. Bariatric surgery alters microRNA content of circulating Exosomes in patients with obesity. Obesity. 2019;27:264–71.
Cui X, You L, Zhu L, Wang X, Zhou Y, Li Y, et al. Change in circulating microRNA profile of obese children indicates future risk of adult diabetes. Metabolism. 2018;78:95–105.
Kim NH, Ahn J, Choi YM, Son HJ, Choi WH, Cho HJ, et al. Differential circulating and visceral fat microRNA expression of non-obese and obese subjects. Clin Nutr. 2020;39:910–6.
Yu Y, Du H, Wei S, Feng L, Li J, Yao F, et al. Adipocyte-derived exosomal MiR-27a induces insulin resistance in skeletal muscle through repression of PPARγ. Theranostics. 2018;8:2171–88.
Ying W, Riopel M, Bandyopadhyay G, Dong Y, Birmingham A, Seo JB, et al. Adipose tissue macrophage-derived exosomal mirnas can modulate in vivo and in vitro insulin sensitivity. Cell. 2017;171:372–384.e12.
Thompson MD, Cismowski MJ, Serpico M, Pusateri A, Brigstock DR. Elevation of circulating microRNA levels in obese children compared to healthy controls. Clin Obes. 2017;7:216–21.
Dang SY, Leng Y, Wang ZX, Xiao X, Zhang X, Wen T, et al. Exosomal transfer of obesity adipose tissue for decreased miR-141-3p mediate insulin resistance of hepatocytes. Int J Biol Sci. 2019;15:351–68.
Lee JE, Moon PG, Lee IK, Baek MC. Proteomic analysis of extracellular vesicles released by adipocytes of Otsuka long-Evans Tokushima fatty (OLETF) rats. Protein J. 2015;34:220–35.
Blüher M. Adipose tissue dysfunction contributes to obesity related metabolic diseases. Best Pract Res Clin Endocrinol Metab. 2013;27:163–77.
Zaki MB, Abulsoud AI, Elsisi AM, Doghish AS, Mansour OAE, Amin AI, et al. Potential role of circulating microRNAs (486-5p, 497, 509-5p and 605) in metabolic syndrome Egyptian male patients. Diabetes, Metab Syndr Obes Targets Ther. 2019;12:601–11.
El-Dawy khalifa, Ali H, Abdo S. Characterization of circulating exosomal micrornas and its role as biomarkers for metabolic syndrome. Zagazig Vet J. 2019;47:78–90.
Pek SLT, Sum CF, Lin MX, Cheng AKS, Wong MTK, Lim SC, et al. Circulating and visceral adipose miR-100 is down-regulated in patients with obesity and type 2 diabetes. Mol Cell Endocrinol. 2016;427:112–23.
Parr EB, Camera DM, Burke LM, Phillips SM, Coffey VG, Hawley JA. Circulating microrna responses between “high” and “low” responders to a 16-Wk diet and exercise weight loss intervention. PLoS One. 2016;11:1–14.
Iacomino G, Russo P, Marena P, Lauria F, Venezia A, Ahrens W, et al. Circulating microRNAs are associated with early childhood obesity: Results of the I.Family Study. Genes Nutr. 2019;14:2.
Witczak JK, Min T, Prior SL, Stephens JW, James PE, Rees A. Bariatric surgery is accompanied by changes in extracellular vesicle-associated and plasma fatty acid binding protein 4. Obes Surg. 2018;28:767–74.
Santamaria-Martos F, Benitez ID, Latorre J, Lluch A, Moreno-Navarrete JM, Sabater M, et al. Comparative and functional analysis of plasma membrane-derived extracellular vesicles from obese vs. nonobese women. Clin Nutr. 2020;39:1067–76.
Sapp RM, Shill DD, Dash C, Hicks JC, Adams-Campbell LL, Hagberg JM. Circulating microRNAs and endothelial cell migration rate are associated with metabolic syndrome and fitness level in postmenopausal African American women. Physiol Rep. 2019;7:e14173.
Rubio DS, Pastor L, Lazaro J, Marin JM, Aragón IIS. Effect of CPAP on circulating exosomal MicroRNAs in patients with morbid obesity and obstructive sleep apnea ( OSA ). 174:5603.
Donghui T, Shuang B, Xulong L, Meng Y, Yujing G, Yujie H, et al. Improvement of microvascular endothelial dysfunction induced by exercise and diet is associated with microRNA-126 in obese adolescents. Microvasc Res. 2019;123:86–91.
Li X, Ballantyne LL, Yu Y, Funk CD. Perivascular adipose tissue–derived extracellular vesicle miR-221-3p mediates vascular remodeling. FASEB J. 2019;33:12704–22.
Chitti SV, Fonseka P, Mathivanan S. Emerging role of extracellular vesicles in mediating cancer cachexia. Biochem Soc Trans. 2018;46:1129–36.
Sun S, Wu Q, Li J, Li Z, Sun S, Zhu S, et al. Exosomes from the tumour-adipocyte interplay stimulate beige/brown differentiation and reprogram metabolism in stromal adipocytes to promote tumour progression. J Exp Clin Cancer Res. 2019;38:1–20.
Wu Q, Li B, Li Z, Li J, Sun S, Sun S. Cancer-associated adipocytes: key players in breast cancer progression. Oncol: J. Hematol; 2019.
Au Yeung CL, Co NN, Tsuruga T, Yeung TL, Kwan SY, Leung CS, et al. Exosomal transfer of stroma-derived miR21 confers paclitaxel resistance in ovarian cancer cells through targeting APAF1. Nat Commun. 2016;7:1–14.
De Lope LR, Alcíbar OL, López AA, Hergueta-Redondo M, Peinado H. Tumour–adipose tissue crosstalk: fuelling tumour metastasis by extracellular vesicles. Philos Trans R Soc B Biol Sci. 2018;373.
Sagar G, Sah RP, Javeed N, Dutta SK, Smyrk TC, Lau JS, et al. Pathogenesis of pancreatic cancer exosome-induced lipolysis in adipose tissue. Gut. 2016;65:1165–74.
Casadei L, Calore F, Braggio DA, Zewdu A, Deshmukh AA, Fadda P, et al. MDM2 derived from dedifferentiated liposarcoma extracellular vesicles induces MMP2 production from preadipocytes. Cancer Res. 2019;79:4911–22.
Liu C, Su C. Design strategies and application progress of therapeutic exosomes. Theranostics. 2019. p. 1015–28.
Sun Y, Shi H, Yin S, Ji C, Zhang X, Zhang B, et al. Human mesenchymal stem cell derived exosomes alleviate type 2 diabetes mellitus by reversing peripheral insulin resistance and relieving β-cell destruction. ACS Nano. 2018;12:7613–28.
Li F, Li H, Jin X, Zhang Y, Kang X, Zhang Z, et al. Adipose-specific knockdown of Sirt1 results in obesity and insulin resistance by promoting exosomes release. Cell Cycle. 2019;18:2067–82.
Safdar A, Saleem A, Tarnopolsky MA. The potential of endurance exercise-derived exosomes to treat metabolic diseases. Nat. Rev. Endocrinol. 2016. p. 504–17.
Yu M, Tsai SF, Kuo YM. The therapeutic potential of anti-inflammatory exerkines in the treatment of atherosclerosis. Int J Mol Sci. 2017;18.
Safdar A, Tarnopolsky MA. Exosomes as mediators of the systemic adaptations to endurance exercise. Cold Spring Harb Perspect Med. 2018;8.
Son JS, Zhao L, Chen Y, Chen K, Chae SA, De Avila JM, et al. Maternal exercise via exerkine apelin enhances brown adipogenesis and prevents metabolic dysfunction in offspring mice. Sci Adv. 2020;6:eaaz0359.
Yu X, Harris SL, Levine AJ. The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res. 2006;66:4795–801.
King HW, Michael MZ, Gleadle JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;12:421.
Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A, et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem. 2009;284:34211–22.
Pascucci L, Coccè V, Bonomi A, Ami D, Ceccarelli P, Ciusani E, et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. J Control Release. 2014;192:262–70.
Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35:2383–90.
ACKNOWLEDGMENTS AND DISCLOSURES
This work was partially supported by grants from the National Cancer Institute of US National Institutes of Health (R01CA212609), and Purdue University Center for Cancer Research (P30CA023168).
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: Meng Deng and Shihuan Kuang
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Quan, M., Kuang, S. Exosomal Secretion of Adipose Tissue during Various Physiological States. Pharm Res 37, 221 (2020). https://doi.org/10.1007/s11095-020-02941-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-020-02941-6