Abstract
How do we inspire new ideas that could lead to potential treatments for rare or neglected diseases, and allow for serendipity that could help to catalyze them? How many potentially good ideas are lost because they are never tested? What if those ideas could have lead to new therapeutic approaches and major healthcare advances? If a clinician or anyone for that matter, has a new idea they want to test to develop a molecule or therapeutic that they could translate to the clinic, how would they do it without a laboratory or funding? These are not idle theoretical questions but addressing them could have potentially huge economic implications for nations. If we fail to capture the diversity of ideas and test them we may also lose out on the next blockbuster treatments. Many of those involved in the process of ideation may be discouraged and simply not know where to go. We try to address these questions and describe how there are options to raising funding, how even small scale investments can foster preclinical or clinical translation, and how there are several approaches to outsourcing the experiments, whether to collaborators or commercial enterprises. While these are not new or far from complete solutions, they are first steps that can be taken by virtually anyone while we work on other solutions to build a more concrete structure for the “idea—hypothesis testing—proof of concept—translation—breakthrough pathway”.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The Challenge of Ideas
As you read this perhaps you have already thought about some if not all of the following scenarios, or maybe one day you will. How do we enable ground breaking therapeutic approaches to happen? Let us imagine three interconnected scenarios.
Firstly how do we enable or enhance serendipity in the hope that we can foster innovation? It could be argued that some important advances in the biological sciences have come from serendipity, the accidental observation informing a key insight that was then taken further and explored. For example the overused case of Viagra’s use for erectile dysfunction or the many other examples of drugs whose discovery was accidental (1). In today’s research environment it seems that we have almost totally removed serendipity from modern drug discovery. We also do not allow ideas to come from outside of those in academia or industry. Accidental discoveries tend not to happen or at least scientists and clinicians are less aware of them or do not want them. Instead research is regimented and industrialized, distilled down to a pipeline with predefined stages (2). Unfortunately, this approach, while logically motivated, has been demonstrably unsuccessful (3). How can we alter the drug discovery landscape in the 21st century and perhaps ‘disrupt it’ as suggested by others (4,5). What future approaches might we take to tap back into the serendipitous insights of researchers and clinicians or even those outside the walls of academia and industry (citizen scientists)?
Secondly, you might have a brilliant idea to do drug discovery, you think you can find some new molecule that has therapeutic effect on a horrific disease or you might have noticed a common trait within a rare disease that you think can be addressed by a drug currently approved by the Food and Drug Administration (FDA) or other regulatory body. As a clinician, with no laboratory space of your own, no funding to do research of this type, or drug discovery experience how do you move this idea from a mere thought or idea to test and put it into clinical practice?
Third, what happens when as a clinician you have an idea and yet you do not have a laboratory or expertise to test your idea? You also may not have that all important source of funding to pay for someone else to test your idea either. What do you do?
Whether in a large academic institute or in a small hospital, these posited scenarios probably happen thousands of times every day to clinicians and our lack of taking them further could be hampering our ability to bring new medicines to patients. But it does not have to stop with clinicians or other researchers. Increasingly, we are seeing rare disease foundations fund research through academics or pursue research themselves (6–8). Similarly, individual rare disease families may also be in a similar situation to those described earlier, when they have an idea for a potential treatment but they have no means themselves to test it, they therefore have to find and collaborate with academics as well as raise funding (9). Families dealing with a child or family member with a rare disease may not have an idea to pursue but they may instead have the ability to raise funds which could be made available to anyone else (scientists or clinicians) with a good idea that would help them. How do these distinct individuals and groups come together in a way that is organized and synergistic?
For example, an academic clinician generally may not have access to their own lab. In order to test their ideas they need to find a colleague with a lab or pay someone to do their proposed work. This assumes their hospital is affiliated with a university or institute. If not they have to go further afield. Certainly if the individual with the idea is not a clinician or a scientist then the odds are further stacked against them even in the era of citizen scientists (10), biohacking (11) and crowdsourcing etc.
If a scientist in academia has an idea they can go to their laboratory and possibly do the experiment or work with a collaborator. If the idea is more fundamental in scope and perhaps something larger they could write a grant and submit to the National Institutes of Health (NIH), Department of Defense, National Science Foundation or other funding body for peer review. They might not get the funding but there is at least a pretty well defined process in place. This also ultimately limits anyone to test an idea that fits in a predefined funding bodies grant budget or within their desired project areas which they will fund. Bigger ideas likely do not fit and neither do those that swim in the opposite direction to the prevailing paradigm for a disease or means of treatment. Over decades, volumes have been written on fostering and funding scientific creativity and innovation in general and this will not add to them, but instead we briefly deal with the practical component of going from ideation to validation of the idea. What we now call the ‘proof of concept’. For the purposes of this article this is a broad term but it could cover in vitro, in vivo or even clinical research. It could be defined as providing some degree of validation or confidence that the idea has merit, or that the hypothesis is proven.
Solutions to Funding and Testing Ideas
Nowadays, there are other options to funding and testing ideas than what would have been possible even 5 years ago. Perhaps the individual with the hypothesis to test could outsource the proposed experiment to specialized clinical research organizations (CROs) via companies like Scientist (previously called Assay Depot) (12) or Science Exchange (13) or even groups that will perform experiments as you design them like Emerald Cloud Lab (14). There are likely many more groups like this that will emerge as early drug discovery continues to fragment and shift from pharmaceutical companies to academia and beyond (15).
Some universities and biomedical research institutes offer seed funding for their scientists to pursue projects but these may themselves have unrealistic expectations that basically exclude early stage preclinical exploratory research. Recent alternatives for funding early stage research could include funding biomedical research through online efforts to obtain small donations from large numbers of donors or ‘crowdfunding’ which has become popular as government funding for science has stagnated or decreased (16). Crowdfunding provides small amounts of funding suitable for generating preliminary data and several useful descriptions of resources and experiences of various crowdfunding sites are available (17–20) . The downside of this approach is that it can be time consuming, requires the individual to actively market their science and they must possess an extensive social network in order to maximize their chance of obtaining funding. Several examples of successful scientific crowdfunding efforts have raised over $25,000 (21,22) and there are European examples which have raised over 44,000 Euros (23). Even undergraduate students in the US have been able to raise over $5000 to fund their undergraduate project (24). Established scientists at non-profit institutes have raised similar sums to pay for experiments (25). In all cases they have made use of commercial crowdfunding sites or alternatively their own internal university crowdfunding infrastructure. Crowdfunding provides a role similar to small charitable foundations (which can generally be found though searching the internet) without the time consuming need for a proposal and peer review. However, pursuing these foundations may be a good investment of time as some very small family or disease foundations are willing to provide anywhere from $10,000 to $30,000 to researchers or clinicians to help get their ideas off the ground, if they align with the wishes of the bequest or the foundation. Some time needs to be spent sourcing the foundations, building connections with them and writing the application. In adition there may be opportunities for continued funding if the initial research is successful.
Protecting Intellectual Property
Imagine that you have successfully been able to fund your research (through any of the above examples) and have managed to found a group, collaborator or CRO to test your idea, then what? Once you have done the proof of concept work and it is successful you might want to file a provisional patent which also costs money. In an institute there is nearly always the need for internal champions for research and support of filing patents, or some other submission process to be followed (whether documented or not) without which you are unlikely to progress and obtain the support of the technology transfer group. So this will be another hurdle to jump unless again you can raise more funds and are able to pursue patenting outside any institute which you are affiliated with. While filing a provisional patent is relatively cheap and gives you coverage for a year to do further experiments, filing a patent a year later is costly and this presents a challenge for academia (26). From our diverse experiences working with different collaborators in academia, few scientists or clinicians realize when they have anything that is potentially patentable, and the possibility for early or accidental disclosure is high (26). One could argue, by focusing on continued publication of their findings without filing a provisional patent, universities and institutes are losing out on potential revenue. Of course the downside of pursuing patents might be the ownership of IP and the potential for conflict this could create. It should be noted that ownership of IP is an important prerequisite for some of the NIH grants to be described next. We have not in our experiences found it a disadvantage to pursue IP and then publish or share ideas.
Commercializing or Translating the Idea
Once you have some preliminary work that builds on your original idea then perhaps you now have enough which can enable you to write a bigger grant that might fit the NIH grant criteria. If you can start a company or find a collaborator with a small company you could also write a Small Business Technology Transfer (STTR) or Small Business Innovation Research (SBIR) (27) grant to fund more experiments or put the therapeutic in animal models before going to the clinic as further proof of concept studies. At this point a clinical collaborator would be needed who could design and conduct studies under the stringent regulatory and ethical conditions.
But the challenges do not end here as outsourcing preclinical animal models in the USA is not cheap. Outsourcing anything here in the USA either ends up in Europe or China and that is a problem because NIH grants will not generally fund this work performed abroad without justification and permission. From our own experience the same mouse pharmacokinetic study performed in the USA costs many times what it would in China. So again if you do not have the facilities to do the work, e.g. a laboratory of your own, how do you get the work done? Perhaps this is one of the many reasons why the innovators in biopharma will continue to struggle in the USA unless they are surrounded with the drug discovery facilities and expertise which they can leverage. This is a solvable problem by ensuring competitive clinical research organizations dealing with animal models in the USA or providing incentives to create or use such companies. Universities or institutes that have set up drug discovery centers may be better resourced and have access to the necessary facilities to perform small animal pharmacokinetics studies at reasonable cost (28).
So what else may be needed? As an individual or a small company trying to translate some of the therapeutic ideas you come up with alongside collaborators, it may seem a constant uphill battle to just go beyond the idea. When you make significant progress you then need the help of someone else beyond the person doing the experimental testing, for example a patent lawyer or technology transfer group, which adds further to the complexity and costs in a university or institute. Few scientists know or can take on all these skillsets which generally take decades to learn and master. Scientists need support along the process to make discoveries, to cure diseases, to invent and to patent.
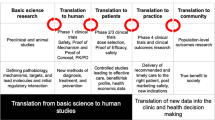
Imagine if you are outside of this framework as a non-scientist with a hypothesis to test, it would be virtually impossible. Increasingly this may be the reality though as rare disease parent and foundations join forces with scientists and clinicians to discover and advance treatments (9,29,30). There will be challenges ahead as they move from step to step in the Idea to breakthrough pathway (Fig. 1). There needs to be some mechanism developed to help them. Therefore to improve the situation for those coming up with ideas in academia or outside of it we certainly need more accessible walk-up or flexible lab space where scientists or clinicians (or for that matter any member of the public) can do experiments (assisted by professionals to ensure safety and correct interpretation of the data) that could serve as a foundation for moving their idea along to something bigger, namely the next experiment. Beyond that there needs to be new sources of funds for breakout ideas from non-traditional sources, be they clinicians, rare disease parents or patients or others that are inspired by an idea. There also needs to be accessible, low cost help for patenting and generally translating ideas to the clinic that can be eventually commercialized by companies.
Testing Ideas Cheaply
We should not be holding back the ideas from clinicians, researchers, non-scientists or anyone with unique insights into a disease or ground breaking approaches to treating a disease. We should be testing as many of these ideas as feasibly possible by doing the experiment/s and making sure if they work they reach the patient quickly for clinical trials after testing for efficacy and safety etc. Perhaps we also need mechanisms for capturing and testing ideas for therapeutics quickly that would need to be secure and private (or open if desired, though this might interfere with potential intellectual property) but could be developed in the same way that we store much of our data on the cloud from our chemistry or biology experiments (31). If there are too many ideas then we need to investigate computational methods and algorithms that could be used to evaluate the ideas in a cost effective manner to triage them. Perhaps we could also use machine learning or deep learning approaches that could identify what are potentially viable or good ideas in the same way that such software can recognize images or be used to make decisions (32,33). For example, increasingly over the past decade we have seen the development of cell (34), organ (35) and disease models (36–42). If these methods could be made more accessible as well, then perhaps it would be possible for those with ideas to refine them further before testing, or at least provide the person coming up with the ideas with a free computational hypothesis testing pathway.
Getting to the Next Breakthrough
It may be unrealistic to expect this short perspective to provide a route to cure all the ills in biomedical research. However, it does at least lay out a potential pathway for clinicians or others with ideas but currently no outlet to test them (Fig. 2). We have described some of the many challenges which we ourselves have experienced first-hand and have also provided some possible solutions. There may of course be other steps we have neglected and we welcome suggestions. For some with ideas that could one day become therapeutics it may be simply they do not know how to get to the next stage in the “idea—hypothesis testing—proof of concept—translation—breakthrough” pathway (Fig. 1). While the early stages in idea testing may be held up by lack of funding, the amounts needed to test the hypothesis or to show proof of concept may be very small (perhaps $10,000–$20,000), in fact much smaller than many of the coveted NIH grants that are required by institutes to ensure tenure. As a society we may need to shift our focus to ensure that we promote and fund those that have good ideas which can be brought to fruition, rather than solely those that can obtain RO1 and other grants which ensure career longevity currently. It is hoped that this will encourage those with new ideas (whether obtained via serendipity or elsewhere) to take the next step and pursue their ideas further (Table I). Our source of future healthcare breakthroughs may very much depend on them doing it.
References
Sneader W. Drug discovery a history. Cheppenham: Wiley; 2005.
Nicolaou KC. The chemistry-biology-medicine continuum and the drug discovery and development process in academia. Chem Biol. 2014;21(9):1039–45.
Paul SM, Mytelka DS, Dunwiddie CT, Persinger CC, Munos BH, Lindborg SR, et al. How to improve R&D productivity: the pharmaceutical industry’s grand challenge. Nat Rev Drug Discov. 2010;9(3):203–14.
Munos BH, Orloff JJ. Disruptive innovation and transformation of the drug discovery and development enterprise. Natl Acad Med. 2016.
Baxter K, Horn E, Gal-Edd N, Zonno K, O’Leary J, Terry PF, et al. An end to the myth: there is no drug development pipeline. Sci Transl Med. 2013;5(171):171cm171.
Pariser AR, Gahl WA. Important role of translational science in rare disease innovation, discovery, and drug development. J Gen Intern Med. 2014;29 Suppl 3:S804–7.
Kerkovich D, Drew A. Designing a plan for drug discovery in rare pediatric neurodegenerative disease. Cerebrum. 2011;2011:11.
Litterman NK, Rhee M, Swinney DC, Ekins S. Collaboration for rare disease drug discovery research. F1000Res. 2014;3:261.
Wood J, Sames L, Moore A, Ekins S. Multifaceted roles of ultra-rare and rare disease patients/parents in drug discovery. Drug Discov Today. 2013;18:1043–51.
Council SE, Horvath JE. Tools for citizen-science recruitment and student engagement in your research and in your classroom. J Microbiol Biol Educ. 2016;17(1):38–40.
Wohlsen M. Biopunk: DIY scientists hack the software of life current hardcover. 2011.
Anon. scientist. Available from: http://www.scientist.com/.
Anon. Science exchange. Available from: https://www.scienceexchange.com/.
Anon. Emerald Cloud Lab. Available from: http://www.emeraldcloudlab.com/.
Ekins S, Waller CL, Bradley MP, Clark AM, Williams AJ. Four disruptive strategies for removing drug discovery bottlenecks. Drug Discov Today. 2013;18(5–6):265–71.
Siva N. Crowdfunding for medical research picks up pace. Lancet. 2014;384(9948):1085–6.
Dahlhausen K, Krebs BL, Watters JV, Ganz HH. Crowdfunding campaigns help researchers launch projects and generate outreach. J Microbiol Biol Educ. 2016;17(1):32–7.
Larkin M. How to use crowdfunding to support your research. Available from: https://www.elsevier.com/connect/how-to-use-crowdfunding-to-support-your-research.
Vachelard J, Gambarra-Soares T, Augustini G, Riul P, Maracaja-Coutinho V. A guide to scientific crowdfunding. PLoS Biol. 2016;14(2):e1002373.
Perlstein EO. Anatomy of the Crowd4Discovery crowdfunding campaign. Springerplus. 2013;2:560.
Pollastri MP. Finding new collaboration models for enabling neglected tropical disease drug discovery. PLoS Negl Trop Dis. 2014;8(7):e2866.
Pollastri MP. Improving collaborations for neglected tropical diseases. Available from: https://experiment.com/projects/improving-collaborations-for-neglected-tropical-diseases.
Riccardi G. Tuberculosis a re-emergent killer. Available from: https://universitiamo.eu/en/campaigns/tubercolosi-un-killer-riemergente.
Anon. 2016. Available from: https://www.launch.umd.edu/project/54fdb91a092065401a8df9a6.
Parish T. TB: through the looking glass. Available from: https://www.rockethub.com/projects/16030-tb-through-the-looking-glass.
Jorgensen WL. Challenges for academic drug discovery. Angew Chem Int Ed Engl. 2012;51(47):11680–4.
Anon. What are SBIR and STTR programs? Available from: http://grants.nih.gov/grants/funding/sbir.htm.
Dahlin JL, Inglese J, Walters MA. Mitigating risk in academic preclinical drug discovery. Nat Rev Drug Discov. 2015;14(4):279–94.
Sames L, Moore A, Arnold R, Ekins S. Recommendations to enable drug development for inherited neuropathies: Charcot-Marie-Tooth and Giant Axonal Neuropathy. F1000Res. 2014;3:83.
Ekins S, Wood J. Incentives for starting small companies focused on rare and neglected diseases. Pharm Res. 2016;33:809–15.
Ekins S, Hohman M, Bunin BA. Pioneering use of the cloud for development of the collaborative drug discovery (cdd) database. In: Ekins S, Hupcey MAZ, Williams AJ, editors. Collaborative computational technologies for biomedical research. Hoboken: Wiley; 2011.
LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521(7553):436–44.
Kandaswamy C, Silva LM, Alexandre LA, Santos JM. High-content analysis of breast cancer using single-cell deep transfer learning. J Biomol Screen. 2016;21(3):252–9.
Dunn SJ, Nathke IS, Osborne JM. Computational models reveal a passive mechanism for cell migration in the crypt. PLoS One. 2013;8(11):e80516.
Patel B, Gauvin R, Absar S, Gupta V, Gupta N, Nahar K, et al. Computational and bioengineered lungs as alternatives to whole animal, isolated organ, and cell-based lung models. Am J Physiol Lung Cell Mol Physiol. 2012;303(9):L733–47.
Roberts PA, Gaffney EA, Luthert PJ, Foss AJ, Byrne HM. Mathematical and computational models of the retina in health, development and disease. Prog Retin Eye Res. 2016.
Pavlides A, Hogan SJ, Bogacz R. Computational models describing possible mechanisms for generation of excessive beta oscillations in Parkinson’s disease. PLoS Comput Biol. 2015;11(12):e1004609.
Burrowes KS, De Backer J, Smallwood R, Sterk PJ, Gut I, Wirix-Speetjens R, et al. Multi-scale computational models of the airways to unravel the pathophysiological mechanisms in asthma and chronic obstructive pulmonary disease (AirPROM). Interface Focus. 2013;3(2):20120057.
Smith N, Trayanova N. Computational models of heart disease. Drug Discov Today Dis Models. 2014;14:1–2.
Sawiak SJ, Wood NI, Carpenter TA, Morton AJ. Huntington’s disease mouse models online: high-resolution MRI images with stereotaxic templates for computational neuroanatomy. PLoS One. 2012;7(12):e53361.
Moustafa AA, Gluck MA. Computational cognitive models of prefrontal-striatal-hippocampal interactions in Parkinson’s disease and schizophrenia. Neural Netw. 2011;24(6):575–91.
Habtemariam T, Tameru B, Nganwa D, Beyene G, Ayanwale L, Robnett V. Epidemiologic modeling of HIV/AIDS: use of computational models to study the population dynamics of the disease to assess effective intervention strategies for decision-making. Adv Syst Sci Appl. 2008;8(1):35–9.
ACKNOWLEDGMENTS AND DISCLOSURES
SE owns stock in Scientist (Formerly Assay Depot). SE is the CEO of Collaborations Pharmaceuticals, Inc. and Phoenix Nest, Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Joseph Stahlberg Foundation grant to Dr. Aaron McMurtray.
Rights and permissions
About this article
Cite this article
Ekins, S., Diaz, N., Chung, J. et al. Enabling Anyone to Translate Clinically Relevant Ideas to Therapies. Pharm Res 34, 1–6 (2017). https://doi.org/10.1007/s11095-016-2039-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-016-2039-5