ABSTRACT
Purpose
An ethyl alcohol-precipitated silk sericin/PVA scaffold that controlled the release of silk sericin was previously developed and applied for the treatment of full-thickness wounds in rats and demonstrated efficient healing. In this study, we aimed to further evaluate the clinical potential of this scaffold, hereafter called “silk sericin-releasing wound dressing”, for the treatment of split-thickness skin graft donor sites by comparison with the clinically available wound dressing known as “Bactigras®”.
Methods
In vitro characterization and in vivo evaluation for safety of the wound dressings were performed. A clinical trial of the wound dressings was conducted according to standard protocols.
Results
The sericin released from the wound dressing was not toxic to HaCat human keratinocytes. A peel test indicated that the silk sericin-releasing wound dressing was less adhesive than Bactigras®, potentially reducing trauma and the risk of repeated injury upon removal. There was no evidence of skin irritation upon treatment with either wound dressing. When tested in patients with split-thickness skin graft donor sites, the wounds treated with the silk sericin-releasing wound dressing exhibited complete healing at 12 ± 5.0 days, whereas those treated with Bactigras® were completely healed at 14 ± 5.2 days (p = 1.99 × 10−4). In addition, treatment with the silk sericin-releasing wound dressing significantly reduced pain compared with Bactigras® particularly during the first 4 postoperative days (p = 2.70 × 10−5 on day 1).
Conclusion
We introduce this novel silk sericin-releasing wound dressing as an alternative treatment for split-thickness skin graft donor sites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
A wound dressing is generally applied in direct contact with the wound to promote healing and prevent further injury. The wound dressing should maintain an appropriate moisture and temperature over the wound to promote cell migration and early epithelialization. However, an excessively warm and moist environment favors microbial growth (1,2). The wound dressing should also provide good absorption of postoperative bleeding to avoid the need for frequent changing of the dressing, which can irritate the wound. Furthermore, the dressing must not contain allergenic or toxic agents that can irritate the wound (3). Generally, wound dressings can be classified into two groups: passive and bioactive dressings. A passive dressing is a traditional absorbent wound pad that is attached with tape and is primarily designed to absorb the fluid from the wound and to protect the wound. A bioactive dressing is designed to interact with the wound surface by providing an optimal microenvironment for the wound or by delivering bioactive molecules to accelerate wound healing (4–6).
Numerous types of bioactive dressings are available and are used clinically. For example, a hydrofiber dressing (Aquacel®) applied with adhesive polyurethane film has been shown to heal wounds after hip replacement surgery with reduced wound area reactions (blister, erythema, edema, skin injury, and hematoma) (3). Manuka honey tulle dressings (Activon®), which display antibacterial activity, successfully treated recalcitrant surgical wounds in a wound care clinic (7). The application of honey to severely infected cutaneous wounds can clear infection from the wound and improve healing. However, many types of bioactive wound dressings have disadvantages. For example, alginate dressings, which are generally used to treat wounds with heavy exudates or infected wounds, do not provide thermoregulation and may require a secondary dressing (8). Application of alginate dressings to deep wounds requires careful monitoring because overstimulation of fibroblasts can delay healing. Semi-permeable wound dressings can be applied to surgical wounds or nearly healed wounds in the epithelialization stage with very little exudate but cannot be used on infected wounds or wounds with heavy exudates (9). Therefore, a high demand remains for the development of more effective bioactive wound dressings, particularly dressings that can deliver active molecules to the wound to accelerate healing.
The silk protein sericin, which is derived from silkworm cocoons, has been recently investigated by many researchers for possible new applications in the biomedical field. We previously demonstrate that silk sericin can activate collagen production in wounds, which subsequently induced epithelialization (10–12). Furthermore, silk sericin has been reported to promote the attachment and proliferation of human skin fibroblasts and keratinocytes (13–17). These properties contributed to the excellent suitability of silk sericin as a wound dressing material. However, the potential applications of a silk sericin wound dressing have not been clinically investigated. We previously developed and tested in vitro and in vivo studies of an ethyl alcohol-precipitated silk sericin/polyvinyl alcohol (PVA) scaffold (18). We demonstrated that silk sericin could be released from this scaffold in a sustained manner. The controlled release of silk sericin from the scaffold promoted the proliferation of L929 mouse fibroblasts and efficiently healed full-thickness wounds in rats, with a greater extent of collagen formation and epithelialization than those treated with a control scaffold without silk sericin.
In this study, we aimed to further investigate the clinical potential of this ethyl alcohol-precipitated silk sericin/PVA scaffold as a novel bioactive wound dressing, hereafter called a “silk sericin-releasing wound dressing”, for the treatment of split-thickness skin graft donor sites in comparison with the commercially available wound dressing known as “Bactigras®”. In vitro studies of the wound dressings were performed, including the evaluation of oxygen permeation, microbial penetration, toxicity and wound healing model using scratch assay, in addition to peel assays. All of these properties are essential and beneficial for clinical applications. In vivo evaluation of the safety of the wound dressings was performed according to ISO 10993–6 (Biological evaluation of medical devices-Part 6: Tests for local effects after implantation). Finally, a clinical trial of the wound dressings was conducted according to standard protocols. A patch test was performed on healthy volunteers to evaluate skin irritation, whereas a split-thickness skin wound test was performed on patients who received split-thickness skin grafts to evaluate healing efficiency. Erythema and melanin levels in addition to elevated responses including edema, papules, vesicles, and bullae on the skin of healthy volunteers after receiving a patch test were assessed to determine the extent of skin irritation. Clinical wound healing efficacy was evaluated on split-thickness skin graft donor sites in terms of a pain score during treatment and the period required for complete healing.
MATERIALS AND METHODS
Materials
Fresh bivoltine white-shell cocoons of Bombyx mori that were produced in a controlled environment were kindly supplied by Chul Thai Silk Co., Ltd. (Petchaboon province, Thailand). PVA (MW 77,000-82,000) was purchased from Ajax Finechem (New South Wales, Australia). Ethyl alcohol, glycerin, and other chemicals were of analytical grade and used without further purification. Bactigras®, which is a fine mesh gauze impregnated with paraffin and chlorhexidine acetate, was purchased from Smith & Nephew, London, UK.
Fabrication of the Silk Sericin-Releasing Wound Dressing
A silk sericin solution was prepared according to a previously reported method (19). The silkworm cocoons were cut into small pieces, and the silk sericin was extracted using a high temperature and pressure degumming technique. The final concentration of the silk sericin solution was approximately 7 wt%. The ethyl alcohol-precipitated sericin/PVA scaffold was fabricated according to previously published techniques (18). Briefly, a silk sericin solution (3 wt%), PVA solution (2 wt%), and glycerin solution (1 wt%) were mixed at room temperature for 30 min. The mixture was poured into a Teflon mold and frozen at −20°C, followed by lyophilization (Heto LL 3000 lyophilizer, Allerod, Denmark) for 72 h. The scaffold was then precipitated by soaking in 70 vol% ethyl alcohol for 5 min, followed by soaking in a 20 wt% glycerin solution for an additional 20 min. The ethyl alcohol-precipitated silk sericin/PVA scaffold, hereafter called the “silk sericin-releasing wound dressing”, was thus obtained. The wound dressing was sterilized by gamma irradiation (20).
Oxygen Penetration
Oxygen penetration through the wound dressings was determined by gluing each dressing to the top of a flask filled with 300 ml deionized water (21). A flask sealed with an airtight cap served as the negative control, whereas an open flask that allowed oxygen to enter the flask and dissolve in the water served as the positive control. Test flasks were placed in an open environment with constant agitation for 7 and 14 days. The collected water samples were then analyzed for dissolved oxygen content according to the Winkler method (n = 3). The test procedure was adopted from standard methods for the examination of water and wastewater (22).
Microbial Penetration
The ability of the wound dressings to prevent microbial penetration was determined by attaching the dressings to the top of glass test tubes containing 20 ml of standard nutrient broth (21). The dressings were sterilized by overnight incubation in 70 vol% ethyl alcohol supplemented with 0.1 vol% penicillin/streptomycin, followed by exposure to UV light for 30 min. Nutrient broth and glass test tubes were sterilized in an autoclave for 20 min at 121°C. Sterile nutrient broth in a glass test tube sealed with an airtight cap served as the negative control, whereas sterile nutrient broth in a test tube that was open to air served as the positive control. The turbidity of the nutrient broth in the test tubes after 7, 14, and 30 days of incubation in an ambient environment was determined to assess microbial contamination. Spectrophotometric measurements were obtained at 600 nm in a microplate spectrophotometer (n = 3).
Cytotoxicity Evaluation
HaCat human keratinocyte cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, high glucose–glutamine) supplemented with 10 vol% fetal bovine serum and 10 U/ml penicillin/streptomycin at 37°C under a humidified atmosphere of 5 vol% CO2/95 vol% air. At approximately 80–90% confluence, the cells were trypsinized for passaging. The cells were seeded in each well of 24-well plates at an initial seeding density of 3 × 104 cells/well. After 24 h of seeding, the sterilized wound dressings (2 × 2 cm2) were placed in the medium. After 24 and 48 h of culture, the number of cells was quantified using the conventional 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (n = 3). Cells cultured in a well without any wound dressing served as a control.
The spreading and migration capabilities of L929 mouse fibroblast cells were assessed using a scratch assay (injury to the cell monolayer) that measured the expansion of a cell population on the surface. The cells (5 × 104 cells/ml) in DMEM containing 10% FBS were seeded in a 6-well plate. Once the confluent monolayer was formed, a linear scratch was generated in the monolayer with a sterile pipette tip. Any cellular debris was removed by washing with phosphate buffer saline (PBS) and replaced with 2 ml of DMEM containing silk sericin-releasing wound dressing and DMEM without sample served as a control. Photographs were taken at a 10× magnification using a microphotograph (Olympus CK2, Japan) on day 0 then plates were incubated at 37°C with 5% CO2 and photographs were taken again after 24, 48 and 72 h. The images acquired for each sample were further analyzed quantitatively by using computer software (Image J 1.42q/Java 1.6.0.10). By comparing the images from day 0 to 1, the distance of each scratch closure was determined and the percentage migration rate was calculated. In each well, two scratches were made (left and right) and six points per scratch were considered. Average of left scratch and right scratch were taken separately. Percentage of migration was calculated from left scratch and then right scratch using following equation:
Samples were in quadruplicates. Percentage of migration obtained from all four wells were averaged and recorded.
Cell Cycle Analysis
HaCat human keratinocyte cells were cultured on the wound dressings for 24 and 48 h as previously described. Subsequently, the cells were collected by trypsinization and fixed with 70 vol% cold ethyl alcohol for 24 h. Cells were incubated with 1 mg/ml RNase A (Sigma–Aldrich, St. Louis, MO, USA) for 30 min, followed by staining with 50 mg/ml propidium iodide (Sigma–Aldrich, St. Louis, MO, USA) at 37°C for an additional 30 min. DNA fluorescence was measured using a flow cytometer. The percentages of cells in the subG0, G0/G1, S, and G2/M phases of the cell cycle were determined using FlowJo software.
Peel Test with Porcine Skin
Porcine skin was used within 2 h after sacrifice. A full-thickness wound was generated from a 1-cm-deep skin incision. The silk sericin-releasing wound dressing and Bactigras® were randomly attached to the wounds. After 12 h, the dressings were removed, and the number of cells attached on the dressings was analyzed by fluorometric quantification of cellular DNA according to the method reported by Takahashi et al. (23). The adhesive force applied to peel the dressings from the wounds was also determined by a modified fixed peeling angle peel test (24). Briefly, the porcine skin with the attached dressings (150 mm in length and 25 mm in width) was placed on a liner translation stage of a universal testing machine (Instron, No. 5567) at a fixed peeling angle of 135°. The sample holder was fixed at the upper side of the dressings, and the peeling force was applied at a constant tensile rate of 5 mm/min. The adhesive force, which was defined as the steady-state peeling force used to peel the dressings from the wounds, was determined from a load–displacement curve (n = 5).
In Vivo Evaluation of the Safety of the Wound Dressings (ISO 10993–6 Standard)
The in vivo evaluation was approved by the Ethics Committee of the Faculty of Medicine, Chulalongkorn University (No. 09/52). The animal experiments were performed according to the Chulalongkorn University Animal Care and Use Committee (CU-ACUC) under standard sterile conditions. The silk sericin-releasing wound dressing and Bactigras® (2 × 2 cm2) were implanted into the subcutaneous tissue of female Wistar rats (8 weeks old, 200–300 g). Briefly, the rats were anesthetized, the hair was shaved, and the skin was disinfected with 70 vol% ethyl alcohol. A 1-cm skin incision was made to form pockets in the subcutaneous tissue, and the wound dressings were inserted into each pocket. The wound was closed with a 6–0 prolene suture and disinfected with Betadine® (povidone-iodine topical antiseptics) solution. After 3, 7, 14, and 28 days of implantation, the rats were sacrificed by overdose with thiopental sodium. The samples and surrounding tissue were retrieved, fixed with 10 vol% formalin solution, and embedded in paraffin. The paraffin-embedded samples were sectioned and stained with hematoxylin and eosin (H&E) for histological evaluation. Both macro- and microscopic assessments were performed. For histological assessment, the H&E slides were semi-quantitatively scored following ISO 10993–6 (25). Inflammatory cell types, neovascularization, fibrosis, and fatty infiltrate were scored by one pathologist at two different times. The intensity of inflammatory cells, neovascularization, fibrosis, and fatty infiltrate was recorded using 0–4 scales (0=not observed, 1=rare, 2=minimal, 3=heavy infiltrate, and 4=packed infiltrate). The final score was calculated according to Eq. 1 and classified as follows: 0.0–2.9 (the sample is a nonirritant), 3.0–8.9 (the sample is a slight irritant), 9.0–15 (the sample is a moderate irritant), and >15 (the sample is a severe irritant). The level of irritation was compared with that of the control (Bactigras®).
where I i is the total number of polymorphonuclear cells, lymphocytes, plasma cells, macrophages, giant cells, and necrosis of sample i (i=test sample (t) and control (c)); and N i is the total number of neovascularization, fibrosis, and fatty infiltrate of sample i (i=test sample (t) and control (c)).
Clinical Trial
Patch Test to Evaluate the Safety of the Wound Dressings on Healthy Volunteers
A prospective, randomized, controlled matched-pair study was conducted from April 2011 to January 2012 at the Department of Pharmacy Practice, Pharmaceutical Sciences, Chulalongkorn University and Police General Hospital in Bangkok, Thailand, to determine the safety profile of the silk sericin-releasing wound dressing in comparison to the commercial wound dressing “Bactigras®”, a standard wound dressing used to treat split-thickness skin graft donor sites at Chulalongkorn Hospital. A total of 110 healthy Thai volunteers, male and female, between the ages of 20 and 57 years and who fulfilled the criteria were recruited in this study. The volunteers were required to be dependable, to be able to read, understand, and follow instructions, and not exhibit any physical or dermatological condition that would preclude the application of the test materials (26). The study protocol was reviewed and approved by the institutional review board of the Police General Hospital (Approval number 30/2554). Adequate explanation of the objectives, methods, and potential risks of the study was provided to each individual, and written informed consent was obtained. After signing the consent forms, demographic data were recorded from all volunteers. The back of the volunteers was chosen to provide a sufficient area for the simultaneous application of both the silk sericin-releasing wound dressing and Bactigras®. The left and right areas on the back were randomly allocated to attach the silk sericin-releasing wound dressing or Bactigras®.
On the first visit, the silk sericin-releasing wound dressing or Bactigras® (4 × 4 cm2) were applied to the assigned back areas. Both dressings were then covered with gauze and retention tape and left for 3 days. This process was performed in two cycles during the induction phase. Seven to ten days after the application of the last induction patch, the dressings were repeatedly applied on identical areas and left for an additional 3 days. This latter process was called the challenge phase. At each visit, both skin redness (erythema) and skin darkness (melanin) were measured using a Mexameter MX 18 (Courage+Khazaka electronic GmbH, Germany), and photos of the skin were taken from both areas within 30 min after the dressings were removed. The volunteers were also interviewed by the investigator for details of any perceived skin irritation. All photos of the skin were evaluated for any visual skin irritation using the human repeated insult patch test (HRIPT) scored by three clinical dermatologists who were unaware of the origin of the samples. At the end of this phase, all study information from the participants was carefully gathered for analysis and interpretation (27).
Evaluation of the Efficacy of the Wound Dressings on Patients who Received Split-Thickness Skin Grafts
The prospective, randomized, controlled matched-pair study was conducted from March to December 2012 at the Division of Plastic and Reconstructive Surgery, Department of Surgery, King Chulalongkorn Memorial Hospital in Bangkok, Thailand, to determine the efficacy and safety of the silk sericin-releasing wound dressing for the treatment of split-thickness skin graft donor sites in comparison with Bactigras®, a standard treatment. A total of 30 patients both male and female between the ages of 18 and 65 years with split-thickness skin graft donor sites and who fulfilled the criteria were recruited for this clinical trial. The donor site (a minimum of 6 × 10 cm2) at the proximal thigh area was selected. Patients were excluded if the donor site was anywhere other than the thigh area or if they did not comply with the study protocol. All patients were given both verbal and written information on the purpose and design of the study and provided written consent before any study-related procedure. This study complied with the latest revision of the Declaration of Helsinki and was approved by the Institutional Review Board, Research Affairs, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand (IRB No.4/55; Clinical Trial Registration Number NCT01539980 titled “Clinical study on silk sericin wound dressing for split-thickness skin graft donor sites treatment”).
Skin grafts were harvested from all patients using an identical standardized procedure. After the patients were administered general anesthesia, the donor site skin was prepared for split-thickness skin grafting by scrubbing with 10 vol% povidone iodine followed by 5 vol% chlorhexidine gluconate solution. A split-thickness skin graft was harvested from the thigh area (0.15–0.45 mm in thickness) using a powerized Zimmer® dermatome (Zimmer, Inc., Warsaw, IN, USA) and used to cover the defects of the patients. After harvesting the graft, the wound at the donor site was covered with adrenaline-soaked gauze for a few minutes to stop bleeding. Each site on the patient was treated with both dressings, i.e., the silk sericin-releasing wound dressing and Bactigras®, in a study-dependent manner. After equally dividing the wound into two parts, the cephalad half and caudal half were designated A and B, respectively. The A side was randomized for treatment with the silk sericin-releasing wound dressing using simple randomization. Randomization was performed using a random number table; odd numbers represented A, and even numbers represented B. The other side was then automatically treated with Bactigras®. Photos of the donor site were also taken before and after the application of the dressing in the presence of a ruler to measure the wound area. Secondary absorbent gauze and elastic bandages were then tightly applied to the wound to protect it from external shear force. Two days later, the top absorbent layers and bandage were carefully removed; the primary dressing was left in place to prevent wound damage. The dressing was not changed, and the donor sites were left undisturbed as much as possible unless there was excessive fluid leakage or any sign of infection. On five consecutive postoperative days, all patients were asked to assess the severity of pain at each part of the donor site by marking a visual analog scale (VAS). The VAS was composed of a 10-cm line equal to 10 points in which a score of “0” represented “no pain” and a score of “10” represented “the worst intolerable pain”.
On the day that the donor site was completely healed, surgeons observed the donor site and other local adverse effects. The time required for complete healing of the donor site was determined as the period from the operative day to the day that the dressing material spontaneously peeled off without causing pain and the wound underneath exhibited complete epithelialization without fluid leakage (28). The medical records of the patients were thoroughly reviewed by the investigators to record relevant clinical data. Physical examination, vital signs, and medications of the patients during the study procedure were also recorded. All study information was gathered for analysis and interpretation.
Statistical Analysis
All quantitative data represent the mean±standard deviation. For in vitro data, the statistical significance was determined by paired and unpaired Student’s t-tests and ANOVA. For the clinical study, the Statistical Package for the Social Sciences (SPSS) for Windows (SPSS Inc., Chicago, IL), version 13.0, was used for statistical analysis. A value of p < 0.05 was considered significant.
RESULTS
Oxygen Penetration Through the Wound Dressings
The extent of oxygen penetration through the wound dressing was assessed due to a significant role of oxygen in wound healing by providing the additional energy source for the repair process. The exchange of oxygen through the dressing provides an optimal environment for wound healing. Figure 1 shows the concentration of oxygen dissolved in water after penetration through the silk sericin-releasing wound dressing and Bactigras®. The concentrations of oxygen that penetrated each wound dressing did not differ and were nearly equal to that of the positive control (open flask). Conversely, the negative control (the flask sealed with an airtight cap) demonstrated the lowest concentration of penetrated oxygen. The concentration of penetrated oxygen was not dependent on the incubation period.
Oxygen penetration through the wound dressings measured as the concentration of oxygen dissolved in DI water at 7 ( ) and 14 days (■). A flask sealed with an airtight cap served as the negative control, whereas an open flask that allowed oxygen to enter the flask and dissolve in the water served as the positive control.
) and 14 days (■). A flask sealed with an airtight cap served as the negative control, whereas an open flask that allowed oxygen to enter the flask and dissolve in the water served as the positive control.
Microbial Penetration through the Wound Dressings
The antimicrobial nature of the wound dressing is important to prevent wound infection. The extent of microbial penetration through the wound dressings is shown in Fig. 2. In the test tubes containing nutrient broth that were capped with the silk sericin-releasing wound dressing, the optical density of the microbial contamination was as low as that of the negative control (nutrient broth in a glass test tube sealed with an airtight cap) after 30 days of incubation. Conversely, the optical density of the nutrient broth in the glass test tube capped with Bactigras® was as high as that of the positive control (nutrient broth in a test tube open to air). The optical density of the nutrient broth also increased with increasing incubation time. At corresponding time points, the optical densities of the nutrient broth in the glass test tube capped with Bactigras® and the positive control were significantly higher than those of the negative control (p < 0.05).
Microbial penetration through the wound dressings measured based on the optical density of nutrient broths covered with the wound dressings for 7 ( ), 14 (
), 14 ( ), and 30 days (■). Sterile nutrient broth in a glass test tube sealed with an airtight cap served as the negative control, whereas sterile nutrient broth in a test tube open to air served as the positive control. * p < 0.05 when compared to the negative control at the corresponding time was considered significant.
), and 30 days (■). Sterile nutrient broth in a glass test tube sealed with an airtight cap served as the negative control, whereas sterile nutrient broth in a test tube open to air served as the positive control. * p < 0.05 when compared to the negative control at the corresponding time was considered significant.
Viability, Cell Cycle Distribution of Cells Cultured and Scratch Assay in the Presence of the Wound Dressings
A standard cytotoxicity test was performed to evaluate the in vitro safety of the wound dressings. The viability of HaCat human keratinocytes cultured on a polystyrene plate in the presence of the wound dressings is presented in Fig. 3a. Cells cultured in the presence of the silk sericin-releasing wound dressing exhibited 92–94% viability, whereas approximately 76% and 23% of cells cultured in the presence of Bactigras® remained viable after incubation for 24 and 48 h, respectively. Figure 3b presents the flow cytometric profiles of cells cultured on a polystyrene plate in the presence of the wound dressings. The profile of cells cultured in the presence of the silk sericin-releasing wound dressing was similar to that of the control (cells cultured in the absence of a wound dressing), whereas cells cultured in the presence of Bactigras® displayed a different profile. The cell cycle distribution (the percentage of cells in the subG0, G0/G1, S, and G2/M phases), which was analyzed by flow cytometric analysis of the DNA content, is shown in Fig. 3c. After 24 h of culture, the presence of both wound dressings decreased the percentage of cells in the G0/G1 and G2/M phases, whereas the percentage of cells in the S phase increased compared with control cells cultured without any wound dressing. After 48 h of culture, all cells cultured in the presence of Bactigras® were in the subG0 phase (100%) and did not proceed further to the G0/G1, S, and G2/M phases. The cells cultured in the presence of the silk sericin-releasing wound dressing demonstrated a nearly similar percentage of cells in each phase as did the control cells.
(a) Percentage of viable HaCat human keratinocytes cultured on a polystyrene plate in the presence of the wound dressings for 24 ( ) and 48 h (■). * p < 0.05 when compared to the value of the control (cells cultured on the polystyrene plate without sample) was considered significant. (b) Flow cytometric cell cycle profiles of HaCat human keratinocytes cultured on a polystyrene plate in the presence of the wound dressings for 48 h. (c) The cell cycle distribution (subG0, G0/G1, S, and G2/M phases) of HaCat human keratinocytes analyzed by flow cytometric analysis of the DNA content in (
) and 48 h (■). * p < 0.05 when compared to the value of the control (cells cultured on the polystyrene plate without sample) was considered significant. (b) Flow cytometric cell cycle profiles of HaCat human keratinocytes cultured on a polystyrene plate in the presence of the wound dressings for 48 h. (c) The cell cycle distribution (subG0, G0/G1, S, and G2/M phases) of HaCat human keratinocytes analyzed by flow cytometric analysis of the DNA content in ( ) the control (cells cultured on the polystyrene plate without sample), (
) the control (cells cultured on the polystyrene plate without sample), ( ) cells cultured on a polystyrene plate in the presence of Bactigras®, and (■) cells cultured on a polystyrene plate in the presence of the silk sericin-releasing wound dressing.
) cells cultured on a polystyrene plate in the presence of Bactigras®, and (■) cells cultured on a polystyrene plate in the presence of the silk sericin-releasing wound dressing.
Furthermore, we performed an in vitro scratch assay using L929 mouse fibroblast cells to evaluate cell migration when cultured in the presence of the wound dressings (Figure S1). L929 cells cultured in the presence of the silk sericin-releasing wound dressing migrated during the culture period (24–72 h) and became confluent (100% migration) at 72 h, in contrast to the control. By contrast, L929 cells cultured in the presence of Bactigras® were round in shape and did not migrate during the incubation period.
Adhesive Property of the Wound Dressings
A nonadhesive wound dressing is required to reduce trauma and the risk of repeated injury upon removal. The adhesive property of the wound dressings was evaluated in terms of the number of cells present on the wound dressings (Fig. 4a) and the adhesive force applied to remove the wound dressings after attachment to full-thickness wounds on porcine skin (Fig. 4b). After 24 h of attachment on the wound, the number of cells on the silk sericin-releasing wound dressing was significantly lower than that on the Bactigras® dressing. The adhesive force applied to remove the silk sericin-releasing wound dressing was significantly lower than that required to remove Bactigras®.
(a) Number of cells present on the wound dressings after attachment to full-thickness wounds on porcine skin for 8 ( ) and 24 h (■). (b) Adhesive force applied to remove the wound dressings after attachment to full-thickness wounds on porcine skin for 24 h. * p < 0.05 when compared to Bactigras® was considered significant.
) and 24 h (■). (b) Adhesive force applied to remove the wound dressings after attachment to full-thickness wounds on porcine skin for 24 h. * p < 0.05 when compared to Bactigras® was considered significant.
In Vivo Safety of the Wound Dressings
The standard safety test was performed according to ISO 10993–6 to evaluate the in vivo safety of the silk sericin-releasing wound dressing in comparison to Bactigras® prior to further clinical investigation. The rats that were implanted with the two types of wound dressings were healthy throughout the implantation period, and no signs of inflammation (i.e., redness, swelling, pain, and heat) were observed. No excessive inflammatory reaction was detected around the implantation sites (unpublished data). Figure 5 shows the images of H&E-stained sections of the implanted Bactigras® and silk sericin-releasing wound dressings. Both samples remained even after 28 days of implantation (the arrow indicates the interface between the implanted sample and the surrounding tissue). The infiltration of inflammatory cells into the implanted samples is shown in Fig. 6. At each time point during the implantation period, the number of inflammatory cells that infiltrated the silk sericin-releasing wound dressing was comparable to that of Bactigras®. The intensity of inflammatory cells, necrosis, fibrosis, neovascularization, and fatty infiltrate was graded as presented in Table I. After 3 days of implantation, polymorphonuclear cells demonstrated packed infiltration in both samples; however, the intensity gradually reduced thereafter. Lymphocytes, macrophages, and neovascularization were observed throughout the implantation period. Heavy infiltration of giant cells and fibrosis was observed after 14 and 28 days of implantation. The Bactigras® implant demonstrated more intense fatty infiltration than the silk sericin-releasing wound dressing. Conversely, plasma cells and necrosis were not observed in either sample during the implantation period. Overall, the intensity of inflammatory cells, necrosis, fibrosis, and neovascularization in the silk sericin-releasing wound dressing was comparable to that of Bactigras®. The implantation of the silk sericin-releasing wound dressing was determined to be non-irritating throughout the implantation period in comparison to Bactigras® as a control.
Clinical Safety of the Wound Dressings
A patch test was performed on healthy volunteers to evaluate the clinical safety of the wound dressing. The descriptive statistics of the healthy volunteers who were included in the patch test are summarized in Table II. The sample size was based on a review of critical factors in the conduct and interpretation of the human patch test of McNamee et al. (29), who recommended that the sample size for testing any transdermal delivery device or dressing should be at least 100 healthy volunteers who participate in a complete study. This sample size had a probability of 0.99 to determine any adverse skin effects that likely had an incidence equal to 5% in the target population. The drop-out rate was determined; therefore, the sample size should be at least 110 healthy volunteers. After application of the wound dressings on healthy volunteers, the safety of the wound dressings was evaluated in terms of the levels of erythema and melanin (Fig. 7) and the percentage of elevated responses on the skin (Table III). The levels of erythema and melanin before and after the application of both wound dressings did not differ (approximately 250 units for erythema and approximately 220 units for melanin). Regarding the elevated responses on the skin, the majority of the area did not exhibit edema (77.3–88.8% for Bactigras® and 78.5–85.5% for the silk sericin-releasing wound dressing) or papules (93.0–96.4% for Bactigras® and 92.7–97.9% for the silk sericin-releasing wound dressing). No evidence of vesicles or bullae was observed on the skin of any volunteer.
Clinical Efficacy of the Wound Dressings
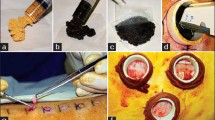
The split-thickness skin wound test was performed on patients with split-thickness skin grafts to evaluate the efficacy of the wound dressing for wound healing. The descriptive statistics of the patients who were included in the split-thickness skin wound test are summarized in Table II. The sample size was calculated according to the data obtained from a study by Angspatt et al. (30) that compared the time required for the complete healing of split-thickness skin grafts treated with carboxymethyl chitosan and Bactigras®. Defining a type I error of 5% and a power of 90% for the study results in at least 30 required sites. The gross images (Fig. 8), period for complete healing (Table IV), and pain score (Table V) were determined to demonstrate the clinical efficacy of the silk sericin-releasing wound dressing in comparison with Bactigras®. The gross image presented in Fig. 8d indicates a completely healed wound such that the dressings could be spontaneously removed without causing pain, and the wound underneath exhibited complete epithelialization without fluid leakage. After complete healing, the Bactigras®-treated area exhibited slightly darker skin than the area treated with the silk sericin-releasing wound dressing. The time required for the complete healing of the split-thickness skin wound treated with the silk sericin-releasing wound dressing (12 days) was significantly shorter than that required for wounds treated with Bactigras® (14 days) (Table IV). The determined pain scores indicated that wounds treated with the silk sericin-releasing wound dressing demonstrated a significantly lower pain score than those treated with Bactigras®, particularly during the initial three postoperative days (Table V).
Gross images of a split-thickness skin wound before and after treatment with Bactigras® and the silk sericin-releasing wound dressing. (a) Skin prior to surgery; (b) split-thickness skin wound following operation; (c) split-thickness skin wound treated with Bactigras® and the silk sericin-releasing wound dressing; and (d) healed split-thickness skin wound.
DISCUSSION
We previously developed an ethyl alcohol-precipitated silk sericin/PVA scaffold for use as a bioactive wound dressing (18). The promotion of L929 mouse fibroblast proliferation and efficient healing of full-thickness wounds in rats through the controlled release of silk sericin from this scaffold was reported. In the present study, we aimed to further evaluate the clinical potential of this scaffold, hereafter called the “silk sericin-releasing wound dressing”, to heal split-thickness skin graft donor sites in comparison with the commercially available wound dressing “Bactigras®”. Herein, the in vitro characteristics of both the silk sericin-releasing wound dressing and Bactigras® were evaluated to confirm their suitability as wound dressings. Oxygen plays a significant role in wound healing by providing an additional energy source for the repair process. In addition, the exchange of oxygen and other gases through the wound dressing provides the optimal environment for wound healing, including nutritional state, immune function, blood flow, blood volume, temperature, and hormonal mediators (31). In this study, neither wound dressing impeded oxygen permeability (Fig. 1), due to their porous structures. The porous structure of the silk sericin-releasing wound dressing was demonstrated previously (18). Bactigras® possesses a mesh gauze structure that also facilitates oxygen penetration. The silk sericin-releasing wound dressing effectively prevented microbial penetration (Fig. 2), possibly due to the antimicrobial activities of the remaining ethyl alcohol and silk sericin components (32,33). This property of the silk sericin-releasing wound dressing is important to prevent wound infection. Conversely, in vitro, a mesh gauze with a loose open-weave structure, as in Bactigras®, may allow microbial penetration even though it contains chlorhexidine acetate, which is an antimicrobial agent. However, when applied to the wound, it is presumed that the antimicrobial agent in Bactigras® mixes with wound exudates to occlude the loose open-weave structure, resulting in a higher efficiency of microbial penetration prevention.
The cytotoxicity evaluation verified that the sericin released from the wound dressing was not toxic to HaCat human keratinocytes (Fig. 3a). By contrast, the cells exhibited low viability when cultured in the presence of Bactigras®. This finding may be due to the paraffin component of Bactigras®, which is toxic to cells. The cytotoxicity of paraffin toward cells such as macrophages has been reported previously (34,35). The result of the cell viability assay was further confirmed by the determination of the cell cycle distribution (Fig. 3b and c). All cells cultured in the presence of Bactigras® for 48 h were in the subG0 phase (a resting phase in which the cells have left the cell cycle and stopped dividing). By contrast, the cells cultured in the presence of the silk sericin-releasing wound dressing for 48 h were in the G0/G1 (cells increase in size and ensure that DNA synthesis can proceed), S (DNA replication), and G2/M phases (cells continue to grow and divide into two distinct cells) at percentages similar to those of the control cells. This finding indicated normal cellular activity of cells cultured in the presence of the silk sericin-releasing wound dressing. The results indicate the noncytotoxic nature of our wound dressing and that sericin released from the wound dressing would be safe for further study in animals and in the clinic. The in vitro scratch assay also indicated that L929 cells cultured in the presence of the silk sericin-releasing wound dressing migrated during the culture period and became confluent (100% migration) at 72 h while L929 cells cultured in the presence of Bactigras® were round in shape and did not migrate during the incubation period, corresponding to the cytotoxicity evaluation. The results from the scratch test confirmed the in vitro efficacy of the silk sericin-releasing wound dressing.
Nonadhesive wound dressings would reduce trauma and the risk of repeated injury upon removal. In this study, we determined that the silk sericin-releasing wound dressing was less adhesive than Bactigras® (Fig. 4), possibly due to the glycerin component in the silk sericin-releasing wound dressing. Thin layer mesh gauze such as that used in Bactigras® typically adheres tightly to the wound surface, which may injure the new epithelial cells upon removal.
Prior to the clinical trial, the safety of the silk sericin-releasing wound dressing was evaluated in vivo according to ISO 10993–6 in comparison to Bactigras®. The implantation of the silk sericin-releasing wound dressing was determined to be non-irritating in comparison to the Bactigras® implantation. The intensities of inflammatory cells, necrosis, fibrosis, and neovascularization in the silk sericin-releasing wound dressing were comparable to those in Bactigras® throughout the implantation period. Therefore, our wound dressing and the sericin released from the wound dressing would be safe for clinical trials. We previously reported the in vivo efficacy of the silk sericin-releasing wound dressing for healing full-thickness wounds (18). The controlled release of silk sericin from the wound dressing potentially healed the wound with a significant reduction in the wound size and a high degree of type III collagen formation and epithelialization.
Clinically, the safety of the silk sericin-releasing wound dressing was verified in a sufficient number of healthy volunteers (n = 110). There was no evidence of skin irritation, including skin color changes or the presence of vesicles and bullae (Fig. 7, Table III). Only a small percentage of the volunteers (approximately 2–7%) exhibited papules on the treated skin. In addition, the degree of skin irritation from treatment with the silk sericin-releasing wound dressing was comparable to that of Bactigras®. This result confirmed the safety of the silk sericin-releasing wound dressing for clinical application. Finally, the clinical efficacy of the silk sericin-releasing wound dressing for the treatment of split-thickness skin graft donor sites was demonstrated. We demonstrated that split-thickness skin graft donor sites that were treated with the silk sericin-releasing wound dressing healed significantly faster than those treated with Bactigras® (Table IV). This finding may be explained by the acceleration of wound healing by the silk sericin released from the wound dressing, which promotes collagen formation and epithelialization, as previously reported (10,17,18). Treatment with the silk sericin-releasing wound dressing also reduced pain from the wound (Table V), possibly due to its reduced adhesive properties and generated moist wound environment compared to Bactigras® (Fig. 4). Furthermore, the superior in vitro characteristics of the silk sericin-releasing wound dressing relative to Bactigras®, such as the effective prevention of microbial penetration (Fig. 2), also support wound healing with a low risk of infection. The slightly darker skin color after complete healing of Bactigras®-treated wounds may indicate greater inflammation than in wounds treated with the silk sericin-releasing wound dressing. This darkening of the skin that results from inflammation, which is termed post-inflammatory hyperpigmentation, was reported by Epstein (36).
In summary, our silk sericin-releasing wound dressing possessed appropriate properties and could be applied as a bioactive wound dressing for the treatment of split-thickness skin graft donor sites with safety and high efficacy compared with the clinically available Bactigras® wound dressing. This is the first clinical report of a wound dressing made of silk sericin for accelerated wound healing. This novel silk sericin-releasing wound dressing could be an alternative treatment for split-thickness skin graft donor sites to provide accelerated healing and reduced pain.
CONCLUSION
The silk sericin-releasing wound dressing (the previously developed ethyl alcohol-precipitated silk sericin/PVA scaffold) demonstrated appropriate in vitro characteristics, including oxygen permeation, prevention of microbial penetration, and noncytotoxicity to human keratinocytes. The silk sericin-releasing wound dressing was also less adhesive than the clinically available Bactigras® wound dressing. A safety evaluation of our silk sericin-releasing wound dressing according to ISO 10993–6 and a patch test on healthy volunteers yielded no evidence of skin irritation. The evaluation of the clinical efficacy of the silk sericin-releasing wound dressing in patients with split-thickness skin graft donor sites demonstrated accelerated healing and reduced pain compared with wounds treated with Bactigras®. Therefore, we suggest the use of this novel silk sericin-releasing wound dressing as an alternative treatment for split-thickness skin graft donor sites.
REFERENCES
Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–70.
Winter GD. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature. 1962;193:293–4.
Harle S, Korhonen A, Kettunen JA, Seitsalo S. A randomised clinical trial of two different wound dressing materials for hip replacement patients. J Orthopaed Nurs. 2005;9:205–10.
Ramakrishnan KM, Jayaraman V. Management of partial-thickness burn wounds by amniotic membrane: a cost-effective treatment in developing countries. Burns. 1997;23:S33–6.
Gore MA, Akolekar D. Evaluation of banana leaf dressing for partial thickness burn wounds. Burns. 2003;29:487–92.
Middelkoop E, van den Bogaerdt AJ, Lamme EN, Hoekstra MJ, Brandsma K, Ulrich MMW. Porcine wound models for skin substitution and burn treatment. Biomaterials. 2004;25:1559–67.
Visavadia BG, Honeysett J, Danford MH. Manuka honey dressing: an effective treatment for chronic wound infections. Brit J Oral Max Surg. 2008;46:55–6.
van der Weyden EA. Treatment of a venous leg ulcer with a honey alginate dressing. Br J Community Nurs. 2005;10:S21, S24, S26-S27.
Skorkowska-Telichowska K, Czemplik M, Kulma A, Szopa J. The local treatment and available dressings designed for chronic wounds. J Am Acad Dermatol. 2013;68:e117–26.
Aramwit P, Sangcakul A. The effects of sericin cream on wound healing in rats. Biosci Biotechnol Biochem. 2007;71:2473–7.
Aramwit P, Kanokpanont S, De-Eknamkul W, Kamei K, Srichana T. The effect of sericin with variable amino-acid content from different silk strains on the production of collagen and nitric oxide. J Biomater Sci Polym Ed. 2009;20:1295–306.
Aramwit P, Kanokpanont S, Nakpheng T, Srichana T. The effect of sericin from various extraction methods on cell viability and collagen production. Int J Mol Sci. 2010;11:2200–11.
Terada S, Nishimura T, Sasaki M, Yamada H, Miki M. Sericin, a protein derived from silkworms, accelerates the proliferation of several mammalian cell lines including a hybridoma. Cytotechnology. 2002;40:3–12.
Sasaki M, Kato Y, Yamada H, Terada S. Development of a novel serum-free freezing medium for mammalian cells using the silk protein sericin. Biotechnol Appl Biochem. 2005;42:183–8.
Tsubouchi K, Igarashi Y, Takasu Y, Yamada H. Sericin enhances attachment of cultured human skin fibroblasts. Biosci Biotechnol Biochem. 2005;69:403–5.
Dash R, Mandal M, Ghosh SK, Kundu SC. Silk sericin protein of tropical tasar silkworm inhibits UVB—induced apoptosis in human skin keratinocytes. Mol Cell Biochem. 2008;311:111–9.
Aramwit P, Siritienthong T, Ratanavaraporn J. Accelerated healing of full-thickness wounds by genipin-crosslinked silk sericin/PVA scaffolds. Cells Tissues Organs. 2013;197:224–38.
Siritienthong T, Ratanavaraporn J, Aramwit P. Development of ethyl alcohol-precipitated silk sericin/polyvinyl alcohol scaffolds for accelerated healing of full-thickness wounds. Int J Pharm. 2012;439:175–86.
Lee K, Kweon H, Yeo JH, Woo SO, Lee YW, Cho CS, et al. Effect of methyl alcohol on the morphology and conformational characteristics of silk sericin. Int J Biol Macromol. 2003;33:75–80.
Siritientong T, Srichana T, Aramwit P. The effect of sterilization methods on the physical properties of silk sericin scaffolds. AAPS Pharm Sci Tech. 2011;12:771–81.
Wittaya-areekul S, Prahsarn C. Development and in vitro evaluation of chitosanpolysaccharides composite wound dressings. Int J Pharm. 2006;313:123–8.
Standard Methods for the Examination of Water and Wastewater, Copyright by American Public Health Association, American Water Works Association, Water Environment Federation, 1999. Available from URL: http://www.mwa.co.th/download/file_upload/SMWW_1000-3000.pdf.
Takahashi Y, Yamamoto M, Tabata Y. Osteogenic differentiation of mesenchymal stem cells in biodegradable sponges composed of gelatin and β-tricalcium phosphate. Biomaterials. 2005;26:3587–96.
Zhang Y, Hazelton DW, Knoll AR, Duval JM, Brownsey P, Repnoy S, et al. Adhesion strength study of IBAD–MOCVD-based 2G HTS wire using a peel test. Physica C. 2012;473:41–7.
ISO10993-6. Biological evaluation of medical devices—part 6: tests for local effects after implantation.
Politano VT, Api AM. The research institute for fragrance materials’ human repeated insult patch test protocol. Regul Toxicol Pharm. 2008;52:35–8.
Akhtar N, Yazan Y. Formulation and in-vivo evaluation of a cosmetic multiple emulsion containing vitamin C and wheat protein. Pak J Pharm Sci. 2008;21:45–50.
Rakel BA, Bermel MA, Abbott LI, Baumler SK, Burger MR, Dawson CJ, et al. Split-thickness skin graft donor site care: a quantitative synthesis of the research. Appl Nurs Res. 1998;11:174–82.
McNamee P, Api A, Basketter D, Gerberick G, Gilpin D, Hall B, et al. A review of critical factors in the conduct and interpretation of the human repeat insult patch test. Regul Toxicol Pharmacol. 2008;52:24–34.
Angspatt A, Taweerattanasil B, Janvikul W, Chokrungvaranont P, Wimon S. Carboxymethylchitosan, alginate and tulle gauze wound dressings: a comparative study in the treatment of partial-thickness wounds. Asian Biomed. 2011;5:413–6.
Hunt TK, Zederfeldt B, Goldstick TK. Oxygen and healing. Am J Surg. 1969;1:521–5.
Fraise AP. Choosing disinfectants. J Hosp Infect. 1999;43:255–64.
Rajendran R, Balakumar C, Sivakumar R, Amruta T, Devaki N. Extraction and application of natural silk protein sericin from Bombyx mori as antimicrobial finish for cotton fabrics. J Text Inst. 2012;103:458–62.
Hoglen NC, Regan SP, Hensel JL, Younis HS, Sauer JM, Steup DR, et al. Alteration of Kupffer cell function and morphology by low melt point paraffin wax in female Fischer-344 but not Sprague–Dawley rats. Toxicol Sci. 1998;46:176–84.
Flescher E, Gonen P, Keisari Y. Oxidative burst-dependent tumoricidal and tumorostatic activities of paraffin oil-elicited mouse macrophages. J Natl Cancer Inst. 1984;72:1341–7.
Epstein JH. Postinflammatory hyperpigmentation. Clin Dermatol. 1989;7:55–65.
ACKNOWLEDGMENTS AND DISCLOSURES
We gratefully acknowledge the financial support from the Thailand Research Fund through the Royal Golden Jubilee Ph.D. Program (Grant No. PHD/0115/2551) to Tippawan Siritientong and Pornanong Aramwit and also the support from the National Research Council of Thailand.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Figure S1
(A) Percentage of migrated L929 mouse fibroblast cells after the monolayer was scratched and cultured in the presence of the wound dressings for  and 72 h (■). * p < 0.05 when compared to the control (cells cultured on a polystyrene plate without sample) was considered significant. (B) Phase-contrast microscopic images of L929 cells after the monolayer were scratched (t0) and cultured in the presence of the wound dressings for 24, 48, and 72 h, respectively. (JPEG 83.6 kb)
and 72 h (■). * p < 0.05 when compared to the control (cells cultured on a polystyrene plate without sample) was considered significant. (B) Phase-contrast microscopic images of L929 cells after the monolayer were scratched (t0) and cultured in the presence of the wound dressings for 24, 48, and 72 h, respectively. (JPEG 83.6 kb)
Rights and permissions
About this article
Cite this article
Siritientong, T., Angspatt, A., Ratanavaraporn, J. et al. Clinical Potential of a Silk Sericin-Releasing Bioactive Wound Dressing for the Treatment of Split-Thickness Skin Graft Donor Sites. Pharm Res 31, 104–116 (2014). https://doi.org/10.1007/s11095-013-1136-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-013-1136-y











 ) Before the patch test, (
) Before the patch test, ( ) after the induction phase of the patch test, and (■) after the challenge phase of the patch test.
) after the induction phase of the patch test, and (■) after the challenge phase of the patch test.