ABSTRACT
Purpose
To investigate interactions between protein and silicone oil so that we can provide some mechanistic understanding of protein aggregation in silicone oil lubricated syringes and its prevention by formulation additives such as Polysorbate 80 and Poloxamer 188.
Methods
Interfacial tension values of silicone oil/water interface of abatacept solutions with and without formulation additives were obtained under equilibrium conditions using Attension Theta optical tensiometer. Their adsorption and desorption profiles were measured using Quartz Crystal Microbalancing with Dissipation monitoring (QCM-D). The degree of aggregation of abatacept was assessed based on size exclusion measurement.
Results
Adsorption of abatacept at the oil/water interface was shown. Polysorbat 80 was more effective than Poloxamer 188 in preventing abatacept adsorption. Moreover, it was noted that some of the adsorbed abatacept molecules were not desorbed readily upon buffer rinse. Finally, no homogeneous aggregation was observed at room temperature and a slight increase of aggregation was only observed for samples measured at 40°C which can be prevented using Polysorbate 80.
Conclusions
Interfacial adsorption of proteins is the key step and maybe responsible for the phenomenon of soluble-protein loss when contacting silicone oil and the irreversible adsorption of protein may be associated with protein denaturation/aggregation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
As proteins become important therapeutic agents in treating diseases, the delivery of these drugs faces ever increasing challenges. Prefilled glass syringes (PFS) have become the container of choice for storing and administering therapeutic protein products to patients (1). Proteins tend to interact with surfaces of such delivery device due to their amphiphilic nature, leading to aggregate formation. Particularly, their interaction with a syringe lubricant (silicone oil) has been shown to adversely affect formulations (2,3). The effect of silicone oil on the stability of proteins has been investigated (3–5). It was found that silicone oil can induce soluble protein loss, and the loss of protein is likely attributed to the adsorption of proteins at the oil/water interface followed by coalescence of oil droplets since there is lack of evidence of homogeneous aggregation of proteins (4). The addition of formulation additive such as a non-ionic surfactant, Polysorbate 20 can decrease silicone oil-induced loss of soluble proteins. It is hypothesized that Polysorbate 20 can be preferentially adsorbed at the oil/water interface (4). However, there is still lack of mechanistic understanding of how proteins interact with silicone oil at the interface, and how surfactants participate in this process. In this study, our objective is to provide some mechanistic understanding of protein-silicone oil interactions. A silicone oil/water interface, mimicking protein formulations in lubricated glass syringes, was selected as a model system for investigating interactions between protein and silicone oil at the oil/water interface. Abatacept, a therapeutic protein for treating rheumatoid arthritis (6), was used as a model compound. Typical non-ionic surfactants used in protein formulations such as Polysorbate 80 and Poloxamer 188 were chosen to study their interactions with silicone oil at the interface in protein solutions (7). The interfacial tensions values of protein solutions with and without formulation additives were measured using a method based on drop shape analysis (8), and the adsorption kinetics of abatacept at the oil/water interface was measured using Quartz Crystal Microbalance with dissipation monitoring (QCM-D) (9). Information obtained from this study provides a better understanding of how formulation additives impact product stability.
MATERIALS AND METHODS
Materials and Protein Solution Preparation
Abatacept was obtained from Bristol-Myers Squibb Company (New Brunswick, NJ 08903). Silicone oil (Dow Corning Corporation 200®fluid, viscosity 5 cSt (25°C)) and polysorbate 80 (Tween 80) as well as acetone were purchased from Sigma-Aldrich chemical company (St. Louis, Missouri 63103), and Poloxamer 188 was obtained from BASF Corporation (Florham Park, NJ 07932). The surface tension of the silicone oil (200®fluid) is about 20 mN/m and the physical-chemical properties of abatacept and surfactants are listed in Table I. Sodium phosphate monobasic and sodium chloride were obtained from EMD (Gibbstown, NJ 08027) and Alfa Aesar (Ward Hill, MA 01835) respectively.
Abatacept solutions at 10 mg/mL and 1 mg/mL were prepared by diluting abatacept stock solution with 25 mM sodium phosphate, 50 mM sodium chloride at pH 7.5. The abatacept solutions with surfactants were prepared by adding surfactants into the protein solutions to final concentration of 0.05% (w/w) for Polysorbate 80 (Tween 80) or 4 mg/mL for Poloxamer 188 followed by gentle mixing for about 30 min.
Interfacial Tension Measurement
Interfacial tension measurements were performed on Attension Theta optical tensiometer (Biolin Scientific Inc. MD 21090). Inverted pendant drop method was used to measure interfacial tension between silicone oil and aqueous solutions of abatacept at various concentrations, with or without surfactants. An inverted pendant drop of silicon oil at its maximum size was pushed from a syringe tip in different sample solutions. Images were taken after the drop was allowed to reach equilibrium state for 60 s. Total measurement time was about a few minutes. The shape of the drop was fitted using Young-Laplace equation in order to obtain the interfacial tension.
Adsorption/Desorption Measurement
Sensor Preparation
SiO2 coated QCM-D sensors (Q-Sense) were UV/Ozon treated for 10 min prior to spin coating. Silicon oil (200®fluid) was pre-mixed with acetone (50%, w/w). Approximately 10 μl of the sample was pippetted onto the center of the sensor and spin coating was then performed at a speed of 4000 rpm using Laurell 400 series spin coater (Laurell Technologies Corporation, PA 19454). A homogeneous film of silicon oil was observed and the sensors were left over night at room-temperature for solvent evaporation.
QCM-D Measurements
All QCM-D measurements were performed using Q-Sense (Biolin Scientific Inc. MD 21090) which allows simultaneous monitoring at four individual sensors. The silicone oil coated SiO2−sensor were allowed to equilibrate in 1 X PBS buffer (pH 7.5) at 25°C until a stable baseline of Δf (frequency) and ΔD (dissipation) was observed. The protein (abatacept) solutions, with and without surfactants, were then flown over the above sensors and changes in F and D were recorded in real time. Measurements of abatacept alone and surfactants alone (Polysorbate 80 and Poloxamer 188) were also performed as control experiments. All adsorption experiments were performed at a flow rate of 50 μl/min. 50 ul/min flow rate was chosen to mimic the storage condition of protein drug product in prefilled syringe.
Size Exclusion Measurement
For size exclusion high-performance liquid chromatography (SE-HPLC) analysis, 200 μg of abatacept protein was injected into Waters Alliance Separation Module 2695 connected to Waters Photodiode Array 996 Detector. Various size components of the protein were separated using Tosoh Biosep TSK G3000SWXL 30 cm × 7.8 mm column with 0.2 M KH2PO4, 0.9% NaCl, pH 6.8 as aqueous mobile phase. The isocratic elution profile is monitored at 280 nm at flow rate of 1 mL/min. The high molecular weight (HMW) species eluted before the monomer peak were expressed as percentage of the total protein in each sample. The increase of HMW was compared to control samples without any treatment.
RESULTS AND DISCUSSION
Interfacial Tension Reduction by Abatacept and Surfactants
Tables II, III and IV shows the interfacial tension values of solutions of abatacept with and without surfactants as well as the interfacial tension values of the buffer alone and surfactants alone. In addition, the calculated surface pressure, Π = γ0-γ, is also shown in Tables II, III and IV. As seen from Table II, the interface between silicone oil and the buffer yielded the interfacial tension of ∼34 mN/m, and the presence of abatacept in the solutions (1 mg/mL) lowered the interfacial tension to ∼21 mN/m (a reduction of ∼13 mN/m (surface pressure)), indicating that protein molecules were likely to be adsorbed at the interface. Furthermore, the interfacial tension was further reduced to ∼17 mN/m when the protein concentration increased to 10 mg/mL, indicating that more of protein molecules were adsorbed at the interface. This is in line with the prediction of Eqs. 1–4 (Appendix), in which the surface pressure (interfacial tension reduction) increases with the concentration of proteins in the interfacial layer as well as the bulk concentration, due to the proportionality between the interfacial layer and bulk given that other parameters remain unchanged. When a surfactant, either Polysorbate 80 or Poloxamer 188, was added into solutions of abatacept, the interfacial tension was further reduced as shown in Tables III and IV. This is also expected based on the prediction from Eqs. 1–4 (Appendix). In this case, the presence of surfactant would compete with protein for adsorption at the oil/water interface. As more surfactant molecules were adsorbed at the oil/water interface (Γ2 increases) and the interfacial tension decreased and the surface pressure increased. The same phenomenon was observed for other proteins such as bovine serum albumin (BSA) (data not shown here). When the effect of Polysorbate 80 was compared with Poloxamer 188, the former was found to be more effective in reducing the interfacial tension. As indicated in Tables III and IV, the presence of Poloysorbate 80 in solutions reduced the interfacial tension more than Poloxamer 188. In the case of 10 mg/mL abatacept solution, the presence of 0.05% Tween 80 reduced the surface tension of the solution to10.93 mN/m. In general, adsorption of surfactant at interfaces is affected by a few factors including bulk concentration (c) partition coefficient (b), and molar surface area (ω) (10). Given that both surfactants have similar bulk molar concentrations in solution, and Polysorbate 80 is above it critical micelle concentration (CMC) and Poloxamer 188 is below CMC (see Table V for calculated values) (11), the main contributing factors for the interfacial adsorption of these two surfactants appear to be the partition coefficient b and surface molar area ω. As shown in Table I, Polysorbate 80 has a much lower CMC values compared with Poloxamer 188, indicating that Polysorbate 80 molecules is much more hydrophobic tending to move away from water and self-associate. Therefore, Polysorbate 80 molecules likely prefer to be adsorbed at the hydrophobic interface such as the one investigated here. This is also reflected in their hydrophilic and lipophilic balance (HLB) values. Polysorbate 80 has a HLB value of 15 which is much less than that of Poloxamer 188 (HLB = 29). Based on their HLB values, Polysorbate 80 is more hydrophobic than Poloxamer 188. For abatacept solutions with surfactants, as seen from Tables II, III and IV, their interfacial tension values are lower than both of protein solution alone and surfactant solution alone, suggesting that adsorption of both protein and surfactant molecules occurred at the oil/water interface. In addition, the interfacial tension decreased with increasing protein concentration for both abatacept solutions with Polysorbate 80 and Poloxamer 188. This indicated that more protein molecules were adsorbed at the interface as the protein concentration increased. As seen from Eqs. 1–4 (Appendix) qualitatively, the surface pressure (interfacial tension reduction) is related to the adsorption of molecules. With increasing the bulk concentration of abatacept in the presence of surfactant, the protein concentration in the interfacial layer increases, which results in a reduction of interfacial tension and increase of the surface pressure. In the following sections, the adsorption of abatacept and abatacept/surfactants solutions will be investigated.
Time-Resolved Analyses of Abatacept Adsorption/Desorption
It appeared from interfacial tension measurement that both protein molecules and surfactant molecules were adsorbed at the oil/water interface (12). However, the adsorption kinetics is still unknown since the interfacial tension values were measured at equilibrium or close to equilibrium conditions. As indicated by Eqs. 1–4 (Appendix), interfacial tension reduction is related to the concentration of adsorbed molecules in the interfacial layers, and therefore the adsorption of either protein molecules or surfactant molecules, or both plays a critical role in the reduction of interfacial tension. QCM-D measures the change in oscillation frequency (Δf) and change in dissiptaion (ΔD), associated with the mass of adsorbed molecules and the viscoelastic property of the interfacial layer, respectively (13,14). For a rigid film formed on the sensor, f (Hz) decreases with increasing mass based on the Sauerbrey relation \( (\Delta m \propto - \Delta {f_n}) \) (15) and D, the dissipation parameter, measures the ratio of dissipated (Edissipated) and stored energy (Estored) according to the following relationship: \( D = \frac{{{E_{{dissipated}}}}}{{2\pi {E_{{stored}}}}} \) (16,17). In addition, the solution concentration in the bulk can also cause changes in f and its effect can be eliminated by rinsing the sensor with PBS buffer in which the bulk concentration of protein is reduced close to zero. Figure 1 shows the effect of protein concentration on the adsorption kinetics of abatacept at the oil/water interface. The solution of abatacept at 10 mg/mL exhibited a larger final frequency (f) decrease (Fig. 1a) as compared to the solution of 1 mg/mL, indicating that there were more abatacept molecules adsorbed at the oil/water interface at 10 mg/mL. In this case, the frequency change was truly due to the effect of the mass adsorbed since the difference still existed even after PBS buffer rinsing. Dynamically, it is expected that a protein solution of high bulk concentration had more adsorption at the interface as shown by Eqs. 5 and 6 (Appendix). Figure 1b shows that dissipation increased significantly as the protein concentration increased from 1 mg/mL to 10 mg/mL even though both returned to approximately the same level after PBS rinsing. Figure 2 displays the adsorption kinetics of Polysorbate 80 and Poloxamer 188 solutions alone at the oil/water interface. The solution of Polysorbate 80 exhibited a fast adsorption (60 s) with a smaller frequency decrease (Fig. 2a) while the solution of Poloxamer 188 showed a slow adsorption (over 300 s) with a larger frequency change. This is because Poloxamer 188, a larger molecule than Polysorbate 80, has a smaller diffusion coefficient besides that the concentration of Poloxamer 188 is below its CMC and the concentration of Polysorbate 80 is above its CMC and thereby a slower adsorption is expected (see Eqs. 5 and 6). Furthermore, the higher mass from adsorption of a larger molecule caused larger frequency change. It is also observed that Poloxamer 188 solution had a significant increase in dissipation parameter. This is due to the fact that Poloxamer 188 is relatively hydrophilic compared with Polysorbate 80 (see Table I for their HLB number), and it interacts with water strongly, which increases the hydrodynamic thickness of interfacial layer, and therefore increase the energy of dissipation.
To further understand the impact of surfactants on the adsorption of abatacept at the oil/water interface, the adsorption kinetics of the solutions of abatacept with either Polysorbate 80 or Poloxamer 188 at both 1 mg/mL and 10 mg/mL were measured.
Low Concentration of Abatacept
Figure 3 displays the adsorption kinetics of the solutions of Abatacept at concentration of 1 mg/mL with either polysorbate 80 or poloxamer 188. As shown in Fig. 3 the presence of Polysorbate 80 with the 1 mg/ml abatacept solution resulted in a frequency increase (Fig. 3a) and dissipation decrease (Fig. 3b) (in comparison with Fig. 1), indicating a reduction of the overall adsorbed mass and the formation of more compacted interfacial layer. A likely explanation for the observed behavior can be that Polysorbate 80 molecules competed with abatacept molecules for adsorption at the oil/water interface and some protein molecules were replaced by Polysorbate 80. Since Polysorbate 80 molecules are smaller in size compared with abatacept (Table I), replacement of some of the abatacept molecules with Polysorbate 80 reduced the total protein mass adsorbed and formed a more compacted interfacial layer. The same phenomena was observed for BSA (data not shown here)
For the protein solution containing Poloxamer 188 (Fig. 3), the presence of the surfactant actually further decreased the frequency (Fig. 3a) and increased energy dissipation (Fig. 3b) (compared with Fig. 1). This can be explained by the fact that Poloxamer 188 is a relatively larger molecule in comparison to Polysorbate 80, although it is smaller than abatacept, and that the adsorption of poloxamer 188 at the interface increased the overall mass and the thickness of hydrodynamic layer which caused more energy dissipation (18). However, the frequency reduction of the abatacept solution with 4 mg/mL Poloxamer 188 (1 mg/mL) is almost same as the sum of frequency reduction of abatacept and Poloxamer 188 alone, suggesting that both surfactant and protein were adsorbed at the interface independently. Further investigation is needed to verify these results.
High Concentration of Abatacept
Figure 4 displays the adsorption kinetics of abatacept solutions at a concentration of 10 mg/mL with either Polysorbate 80 or Poloxamer 188. In comparison with the adsorption kinetics of abatacept (10 mg/mL) without surfactants (Fig. 1), the presence of polysorbate 80 significantly reduced both the frequency (Fig. 4a) and dissipation changes (ca 10 Hz decrease) (Fig. 4b). This is again could be most likely due to the fact that abatacept molecules were replaced by Polysorbate 80 molecules at the interface (please note Figs. 1 and 2: the adsorption kinetics of Polysorbate 80 alone is faster than the adsorption kinetics of abatacept alone).
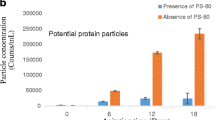
The presence of Poloxymer 188 in the abatacept solution slightly increased the frequency and significantly increased dissipation. A possible explanation for this is at a concentration of 10 mg/ml, the adsorption of protein was significantly enhanced as predicted by Eqs. 5 and 6 (Appendix), and therefore, the inhibiting effect of Poloxamer 188. The significant increase in dissipation, however, can indeed reflect a structural change of the adsorbed layer in the presence of Poloxymer 188, such as for example protein unfolding. It is known that interface may cause protein denaturation and desorption of proteins from the interface can provide some critical information on the irreversibility of adsorption caused by protein denaturation. As seen from Figs. 1, 3 and 4, there is significant degree of irreversibility for abatacept adsorption at the oil/water interface since both frequency and dissipation change did not reverse back to the original values after rinsing with PBS buffer. In addition, both surfactants showed adsorption irreversibility (results are not shown in this paper). However, in this paper, we are mainly interested in the adsorption irreversibility of protein. In some cases, the irreversibility of abatacept adsorption can reach 50% or more based on the calculation using the following formula; the reversibility is calculated as (16): \( Reversibility{\left( \% \right)} = \frac{{\Delta f_{{reversible}} }} {{\Delta f_{{adsorption}} }} \times 100 \). This indicates that a significant amount of protein remained with silicone oil after adsorption. This led to the some speculation that some of abatacept molecules may go through a conformational change upon adsorption at the interface as a typical adsorption process involves (a) diffusion, (b) overcoming the energy barrier between the subsurface and the interface, and (c) conformational change after adsorbing at the interface. The conformational change of abatacept may be the first step toward aggregation. Figure 5 shows the measured degree of aggregation in abatacept solutions by size exclusion chromatograph for abatacept samples rinsed with PBS after adsorption measurement at 40°C. As shown in Fig. 5, the rinsed solutions from adsorption experiments for the samples with Polysorbate 80 exhibit some level of aggregation in comparison with the control, which has the same level of aggregation as that of solution without contacting silicone oil. This indicated that there was some degree of homogeneous aggregation at 40°C. However, the rinsed solutions from the sample contacted silicone oil (without surfactant) have more aggregates as indicated in Fig. 5. In addition, there is no increase of aggregation observed for the rinsed solutions of all samples measured at room temperature in comparison with the control (data no shown here), indicating that at room temperature there is no detectable homogeneous aggregation of abatacept even after contacting silicone oil. It may also indicate that aggregated protein molecules still remain at the oil/water interface after adsorption, which is consistent with the literature report that the loss of soluble-protein after contact with silicone oil emulsion droplets is due to adsorption of protein molecules at the silicone oil interface. This phenomenon need to be further investigated since it is related to the product stability of biologics.
Implications to Formulation Development
This study aims to provide general mechanistic understanding of interactions between proteins and silicone oil, and abatacept was used as a model compound. The system selected is simplified for the purpose of providing mechanistic understanding, which should not directly compare with the actual product formulation containing many other components. Nevertheless, the results show that protein abatacept does not undergo homogeneous aggregation at room temperature even after contacting silicone oil and the same conclusion was drawn by Ludwig et al. for other proteins (4). As shown in the “Results” section, adsorption of proteins at silicone oil/water interface is the key step for protein-silicone oil interactions. It appears that surfactants such as Polysorbate 80 and Poloxamer 188 can reduce protein adsorption at the silicone oil/water interface through competing adsorption mechanism although mutual adsorption is not excluded at lower protein concentration. This supports the conclusions from literature that surfactants can reduce soluble-protein loss (4). In addition, if silicone oil is not migrated into bulk solution from the syringe surface, the adsorbed protein is negligible compared with bulk solution due to limited interface available.
CONCLUSIONS
Proteins such as abatacept were adsorbed at the silicone oil/water interface and the adsorption increased with protein bulk concentration. Formulation additives such as Polysorbate 80 and Poloxamer 188 can reduce protein adsorption through competitive adsorption although it is possible that at 1 mg/mL abatacept concentration mutual adsorption became dominant. Polysorbate 80 has rapid adsorption kinetics and more reduction of interfacial tension due to its hydrophobic nature. The fast adsorption kinetics of Polysorbate 80 allows surfactant molecules to reach the silicon oil/water interface quicker than abatacept molecules. On the other hand, Poloxamer 188 reached the interface slower. However, it did yield an increased dissipation because it is a larger molecule and the hydrodynamic layer thickness is larger owing to its interaction with water, which may provide steric forces against flocculation. In addition, it appears that a significant amount of the adsorbed protein molecules bind in an irreversible manner. This is probably because adsorption of proteins may result in conformational change or possibly even aggregation, which needs to be further investigated. The irreversibility of protein binding at the oil/water interface may be responsible for the loss of soluble-proteins observed by other researchers. However, the amount of proteins adsorbed at the interface is minute compared to amount of proteins in the bulk since there is a limited interfacial area available in the syringes. SEC results confirmed that there is no homogeneous protein aggregation at room temperature. However, there is a slight increase of aggregation at 40°C after abatacept contacts the silicone oil, which can be reduced by formulation additive (surfactant). In the future, a detailed study will be conducted for the adsorption of abatacept and other proteins at various formulation conditions. The effect of surfactant structure on adsorption of proteins at silicon oil/water interface will be also explored in future studies.
REFERENCES
Thompson I. New-generation auto-injectors: completing the scale of convenience for self-injection. Drug Delivery Report. 2005; Autum/winter, 47–49.
Jones LS, Kaufmann A, Middaugh CR. Silicone oil induced aggregation of proteins. J Pharm Sci. 2005;94:918–27.
Thirumangalathu R, Krishnan S, Ricci MS, Brems DN, Randolph TW, Carpenter JF. Silicone oil-and agitation-induced aggregation of a monoclonal antibody in aqueous solution. J Pharm Sci. 2009;98:3167–81.
Ludwig DB, Carpenter JF, Hamel JB, Randolph TW. Protein adsorption and excipient effect on kinetic stability of silicone oil emulsions. J Pharm Sci. 2010;99:1721–33.
Ludwig DB, Trotter JT, Gabrielson JP, Carpenter JF, Randolph TW. Flow cytometry: a promising technique for the study of silicone oil-induced particulate formation in protein formulations. Anal Biochem. 2011;410:191–9.
2009. Orencia [product monograph]. Canada: Bristol-Myers Squibb Company.
Rowe RC, Sheskey PJ, Weller PJ. Handbook of pharmaceutical excipients. London: Pharmaceutical; 2003.
Chen P, Prokop RM, Susnar SS, Neumann AW. Interfacial tensions of protein solutions using axisymmetric drop shape analysis. In: Möbius D, Miller R, editors. Proteins at liquid linterfaces. Amsterdam: Elsevier; 1998. p. 303–40.
Messina GML, Satriano C, Marletta G. A multitechnique study of preferential protein adsorption on hydrophobic and hydrophilic plasma-modified polymer surfaces. Colloids Surf B. 2009;70:76–83.
Miller R, Makievski AV, Fainerman VB. Dynamics of adsorption from solutions. In: Fainerman VB, Mobius D, Miller R, editors. Surfactants: chemistry, interfacial properties, applications. Amsterdam: Elsevier, 2001. p. 287–399.
Alexandridis P, Holzwarth JF, Hatton TA. Micellization of poly (ethylene-oxide)-poly (propylene-oxide)-poly (ethylene-oxide) triblock copolymers in aqueous solutions: thermodynamics of copolymer association. Macromolecules. 1994;27:2414–25.
Fainerman VB, Lucassen-Reynders EH, Miller R. Adsorption of surfactants and proteins at fluid interfaces. Colloids and Surf A. 1998;143:141–65.
Albet-Torres N, Gunnarsson A, Persson M, Balaz M, Hook F, Mansson A. Molecular motors on lipid bilayers and silicon dioxide: different driving forces for adsorption. Soft Mater. 2010;6:3211–9.
Berglin M, Pinori E, Sellborn A, Andersson M, Hulander M, Elwing H. Fibrinogen adsorption and conformational change on model polymers: novel aspects of mutual molecular rearrangement. Langmuir. 2009;25:5602–8.
Sauerbrey G. The use of quartz oscillators for weighing thin layers and for microweighing. Zeitschrift fuer Physik. 1959;155:206–22.
Vogt BD, Lin EK, Wu W-L, White CC. Effect of film thickness on the validity of the sauerbrey equation for hydrated polyelectrolyte films. J Phys Chem B. 2004;108:12685–90.
Jordan JL, Fernandez EJ. QCM-D sensitivity to protein adsorption reversibility. Biotechnol Bioeng. 2008;101:837–42.
Tadros TF. Applied surfactants: principles and applications. Weinheim: Wiley-VCH; 2008. p. 100.
Lucassen-Reynders EH. Competitive adsorption of emulsifiers 1. theory for adsorption of small and large molecules. Colloids Surf A. 1994;91:79–88.
Fainerman VB, Lucassen-Reynders EH, Miller R. Description of the adsorption behavior of proteins at water/fluid interfaces in the framework of a two-dimensional solution model. Adv Colloid Interface Sci. 2003;106:237–59.
Fainerman VB, Miller R. Thermodynamics of adsorption of surfactants at the fluid interfaces. In: Fainerman VB, Möbius D, Miller R, editors. Surfactants: chemistry, interfacial properties, applications. Amsterdam: Elsevier; 2001. p. 99–188.
AcknowledgmentS & DISCLOSURES
Authors would like to thank the support of Drug Product Science and Technology Management of Bristol-Myers Squibb Company and Dr. M. Hussain for critical review.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Some Theoretical Considerations
In this study, it is assumed that the oil/water (buffer) has a sharp interface (19,20) and its interfacial tension value is γ0 which can be measured, and the adsorption of protein molecules or surfactant molecules or both at the oil/water interface can reduce the interfacial tension to γ. Therefore, the surface pressure is expressed as: Π = γ0-γ, and Π can be modeled as the concentration (Г) in the interfacial layer, which is also a function of protein bulk concentration c, and the interfacial molar area (ω). For a surfactant (component 2) in a protein solution (component 1), the following equation can be derived based on the equation of state assuming the protein is in the state with minimal molar area and the surfactant is in a single adsorption state:
while the expressions for the adsorption isotherm of protein and surfactant are
with
The average molar area of adsorbed component 1 and 2 is
Here c1, c2, ω1, ω2, b1, b2 are the concentrations, molar interfacial area, and bulk/interface distribution coefficients of protein and surfactant, and Γ1 and Γ2 are the concentration of protein and surfactant in the interfacial layer. ael is a parameter related to the electrostatic interaction in the solution depending on the dielectric constant of the protein solution, the total concentration of electrolytes, the number of non-bound unit charges in the protein molecules, etc (20,21). Dynamically, the time-dependent adsorption, Γ(t), depends on the diffusion coefficient of the molecules, bulk concentration and time, and adsorption kinetic model (10) is shown as the following:
where D is the diffusion coefficient and c0 is the bulk concentration, t is the time. In the following text, Eqs. 1–5 will be used as qualitative guidance for discussion. For a simplied system, the above equation can be reduced to
Rights and permissions
About this article
Cite this article
Li, J., Pinnamaneni, S., Quan, Y. et al. Mechanistic Understanding of Protein-Silicone Oil Interactions. Pharm Res 29, 1689–1697 (2012). https://doi.org/10.1007/s11095-012-0696-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-012-0696-6