ABSTRACT
Purpose
To investigate the cause of unexpected and erratic increase in aggregation during long-term storage of an IgG2 monoclonal antibody in a trehalose formulation at −20°C.
Methods
Frozen matrix was sampled, stored frozen at various temperatures and analyzed by SEC over time.
Results
Aggregation increased with time at −20°C but not at −40°C or −10°C. The cause of the instability was the crystallization of freeze-concentrated trehalose from the frozen solute when the storage temperature exceeds the glass transition temperature of the matrix (−29°C). Crystallization at −20°C deprives the protein of the cryoprotectant, leading to a slow increase in aggregation. Storage at −10°C also leads to crystallization of trehalose but no increase in aggregation. It is hypothesized that significantly higher mobility in the matrix at −10°C allows protein molecules that are unfolded at the ice interface on freezing to refold back before significant aggregation can occur. In contrast, lack of mobility at −40°C prevents crystallization, refolding, and aggregation.
Conclusions
Aggregation in the frozen state when stored above the glass transition temperature is a consequence of balance between rate of crystallization leading to loss of cryoprotectant, rate of aggregation of the unfolded protein molecules, and rate of refolding that prevents aggregation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Frozen storage of bulk protein solutions increases the flexibility for manufacturing of protein therapeutics (1). Freezing the protein bulk slows degradation rates, reduces risk of microbial contamination, and simplifies transportation. Despite its relative technical simplicity, a number of complex fundamental phenomena underlie the formulation and process and determine the ultimate outcome of this unit operation.
The phenomena that occur as a consequence of the freezing process subject the protein to a variety of destabilizing stresses. During freezing, water is removed from solution as ice, while the excluded solutes including the protein are subject to cryoconcentration. Formation of ice thus desiccates the protein, exposing the molecules to osmotic stress. The protein molecule, in the cryoconcentrated solute matrix, also experiences a crowded molecular environment containing both high concentrations of protein as well as significantly increased levels of other solutes. The solutes including protein occupy the region between the growing ice crystals and become increasingly concentrated, accompanied by a significant increase in viscosity. The nature of the solute determines its behavior in this cryo-concentrating matrix. Non-crystallizing solutes will eventually form a glass as the viscosity rises. For non-crystallizing solutes, such as disaccharides, the concentration of the solute in the maximally freeze-concentrated matrix can reach approximately 80% w/w (defined by the state diagram). Crystallizing solutes such as NaCl will go through a eutectic and crystallize, although the extent of crystallization will be dependent on the rate of cooling, other solutes present, etc. Apart from molecular crowding, cryoconcentration subjects protein to high ionic concentrations and potential pH changes both from the simple impact of temperature on pKa as well as pH drift due to precipitation/eutectic crystallization of buffer salts. Proteins have also been shown to adsorb and unfold/denature at the ice surface. Depending on the freezing process, the nature and extent of ice-solute interface varies and, therefore, also the fraction of protein impacted by this interface. Proteins also have a critical temperature at which they may undergo cold denaturation. All of these phenomena, potentially acting in concert, can make freezing and frozen storage an operation that is not simple to execute successfully, especially at large-scales, as discussed later. Aggregation is the most likely consequence of an unsuccessful operation, while the rates of chemical degradation reactions, such as deamidation, hydrolysis or oxidation, are generally significantly reduced.
Detailed reviews on these fundamental aspects of proteins in the frozen state are available, although many questions remain (2–5). Stabilization of proteins in solution by trehalose and other osmolytes through the preferential exclusion mechanism is well established and understood (6). This mechanism is also invoked for the cryoprotection relying on the notion that the basic thermodynamic principles governing protein stability in the frozen state should not differ from those in the aqueous state. The effect is maintenance of the hydration shell and thereby the structure of the protein under conditions of lowered water activity, irrespective of the temperature (7,8). An assumption that dilute solution state concepts also apply in the freeze-concentrated state is required, and the significance of the viscosity and mobility changes is neglected (2). The second mechanism that may enable protective solutes to function in the frozen state is kinetic stabilization by the glassy state-vitrification mechanism (2). Kinetic stabilization implies that the cryoprotectant forms a glass at the end of the freezing process, reduces molecular mobility and thus slows down the rate of all degradation pathways including unfolding and aggregation. The two mechanisms described above are not mutually exclusive, and both are likely to be involved in cryoprotection. For example, sucrose has been shown to decrease cold denaturation temperature (thermodynamic stabilization through preferential exclusion) as well as increase unfolding time (kinetic stabilization) (9). However, the implications of the presence of these two mechanisms are important. During freeze-thaw cycling studies, inhibition of denaturation and consequent aggregation by addition of sugars or polyols implies that preferential exclusion (thermodynamic stabilization) against cold-induced denaturation is at work. By implication, kinetics of processes leading to aggregation are too slow within the time-frame of these experiments. In this situation, material that is subject to freeze-thaw cycling would be found to be unchanged when examined after thaw. If this mechanism were the sole destabilization pathway in the frozen state, the formulation could then potentially be stored for a length of time at any frozen state temperature. On the other hand, if storage in the frozen state results in denaturation or aggregation, the implication is that despite the thermodynamic stabilization, the kinetics of aggregation have not been slowed down. The latter scenario would require that the dynamics of the frozen state be slowed further, normally by reducing the storage temperature to preferably below the Tg’. Note that the above thermodynamic stabilization mechanism ignores molecules which may have undergone interface-induced denaturation. Kinetic stabilization would apply to all molecules, although the molecules denatured at the interface are the most susceptible to aggregation if this stabilization mechanism is not functional.
There are significant technical difficulties involved in studying proteins in the frozen state, which has limited fundamental studies in this area. Pharmaceutical scientists have, however, developed protocols to identify formulations that stabilize proteins for storage in this state. The protocols are focused towards addressing the concentration and interface-induced stresses on the protein by identifying cryoprotectants and surfactants. This is primarily done through freeze-thaw (FT) cycling studies in which formulations are frozen and thawed between certain specified temperatures for a number of cycles to generate stress on the system. These FT cycling studies are usually carried out at very small scales which, while suitable for rapid formulation definition, may neglect the impact of kinetics of freezing and thawing processes on the FT behavior, as discussed below. Furthermore, FT cycling experiments do not capture the long-term consequences of storage in the frozen state. The latter are primarily determined by diffusion kinetics, and these do not come into play in the relatively short time-scale of a freeze-thaw cycling study. Stability studies are then conducted to assess the long-term frozen state viability of the identified formulations.
Practical freezing processes in large-scale systems have heat- and mass-transfer dimensions much larger than those normally used during formulation identification. Large-scale systems take much longer to process, with rates of ice formation or melting varying significantly through the duration of the process. For example, based upon the authors’ experience, a 300-L tank takes approximately 10–12 h to process down to −20°C, while most formulation studies are done at the 1–5 mL scale and will freeze in a matter of a few minutes (3,4). It is generally accepted that the transition period between solution and solid is when the protein is most likely to suffer the greatest damage from the stresses of the freezing or thawing process (2). This would be especially true in formulations where significant pH or ionic strength changes occur as a consequence of the transition. FT cycling experiments are therefore supplemented with studies that mimic the process scale and/or time where possible. The Cryowedge™ used in the work reported here is an example of such a system where the heat and mass transfer dimensions are equivalent to that of a 300-L vessel and where the process time is closely approximated to that of the full-scale system by controlling the temperature profile of the heat-transfer fluid. However, gravity-driven convective processes cannot be completely replicated in the cryowedge (10,11).
For proteins being stored in the liquid state, long-term stability can be assessed through “accelerated studies” performed by raising the temperature to accelerate the degradation reactions. For example, for a protein intended to be stored at 2–8°C, studies can be performed at 25°C to obtain a reasonable estimate of shelf-life in a shorter amount of time, for most degradation pathways. However, such “accelerated” stability studies are not useful to assess the corresponding long-term behavior of the protein in the frozen state. Arrhenius kinetics do not apply in the frozen state. Diffusion and other molecular relaxation phenomena take on a more complicated dependence on temperature, such as those given by the Williams-Landel-Ferry (WLF) equation ((12); Conrad and de Pablo (13) showed the applicability of the WLF equation to analysis of their trehalose diffusivity data in high concentrated trehalose solutions). The phase behavior of complex solutions in the frozen state can vary significantly with temperature. Thus, short-term behavior at, say, −20°C will not necessarily have any correlation to long-term behavior at −40°C.
We report here on our investigation into some unexpected and erratic aggregation data on long-term frozen storage of an IgG2 monoclonal antibody (mAb) at large-scale (300-L). The investigation was conducted by taking frozen core samples from a small-scale model of the large-scale storage vessel and storing them at −10°C, −20°C and −40°C. The results show that the frozen state aggregation behavior is likely determined by a combination of the slow crystallization of trehalose excipient leading to loss of cryoprotection, which promotes aggregation, and mobility afforded to the protein molecules allowing them to refold, thus avoiding aggregation. Storage temperature, in this case, is the key to the long-term outcome.
MATERIALS AND METHODS
The protein was an in-house IgG2 monoclonal antibody (mAb) in solution at 20 mg/mL in a 20 mM Histidine HCl buffer, pH 5.5 with 0.2 mg/mL polysorbate 80, 0.1 mg/mL disodium edetate dihydrate, and 84 mg/mL trehalose dihydrate. Various batches of the mAb were studied and are identified in the data tables.
Long-Term Storage Stability of mAb at −20°C in Mini-Tanks and Cryovessels
Protein solution was stored in 30 mL aliquots in 60-mL nominal volume cylindrical stainless steel (SS 316L) mini-tanks of an in-house design (5 cm inner diameter). These mini-tanks were frozen by simply placing them in a walk-in −20°C freezer room, and were stored there for the long-term. The mini-tanks were placed on shelves, slightly separated from each other, in one layer. These tanks were thawed by simply keeping them at room temperature for 6–8 h before gently mixing the contents and placing them at 2–8°C until analysis. Long-term storage study was performed at −20°C with four batches of the mAb. A new mini-tank was thawed and analyzed at each stability time point. Soluble aggregate levels were measured by SEC. No change in protein concentration as measured by UV280 was observed.
Protein solution was also stored in large 300-L Cryovessels (Sartorious Stedim Biotech S.A., Aubagne Cedex, France; www.sartorius-stedim.com), with fill volumes of the order of 250–300 L. These cryovessels consist of jacketed cylindrical stainless steel tank with an internal radial finned-heat exchanger that effectively divides the tank into 8 longitudinal sections. The cryovessels are processed by a cooling unit that circulates a heat transfer fluid at −50°C until the last point to freeze has reached a temperature of −20°C. The last point to freeze in these vessels is determined from installation and performance qualification studies and lies along an axial line in each of the longitudinal sections that is furthest from all heat transfer surfaces. This is most easily visualized as a point along a vertical line passing through the region of highest concentration in the cryowedge map in Fig. 1 (11). Freezing of bulk in a cryovessel generally takes about 10–12 h depending on the fill volume. After freezing, these vessels were subsequently stored in a walk-in −20°C cold-storage facility. Thawing was performed via the same processing unit that circulates heat transfer fluid at 25°C, while gently rocking the vessel to ensure mixing while thawing. Thawing was taken to be complete when the bulk temperature reaches 15°C and would take approximately 10 h (11). Long-term storage study was performed at −20°C with the same four batches of the mAb as used in the mini-tank studies described above. At designated time intervals, the cryovessels were thawed, sampled for analysis, refrozen and returned to storage. Soluble aggregate levels were measured by SEC. No change in protein concentration (by UV280) was observed.
Portions of the mAb batches were also stored in a second set of cryovessels at −20°C. These vessels were thawed at one designated long-term time point (approximately 20 months after freezing), sampled and analyzed as above.
Cryoconcentration Map in Cryowedge and Long-Term Storage Stability of mAb in Core Samples at −40°C, −20°C or −10°C
The cryoconcentration behavior and its impact was investigated via a Cryowedge34, which represents a scaled-down model of the 300-L cryovessels. The lateral heat and mass transfer surfaces/distances in the cryowedge are identical to those in the cryovessel of corresponding size. Details of the study and analysis conducted with the cryowedge have been published elsewhere (11,14). Briefly, 4-L of mAb solution was frozen in the cryowedge and cylindrical cores (1.5 cm diameter, approximately 2.5 cm long) drilled out from various positions in the frozen block using a hand-held electric drill with a custom-made hollow cylindrical steel drill bit. Some of the cores were used to obtain a detailed solute and protein distribution map of the frozen mass in the cryowedge. This profile represents the macro-cryoconcentration in the frozen block since the results are determined by the size of the core. The true or micro-cryoconcentration in the inter-dendritic spaces at all points in the frozen matrix corresponds to the maximal freeze-concentrated level at the glass transition point, identified in the state diagram (11). The results of the mapping, however, clearly show that significant diffusive and convective transport occurs in front of the moving ice boundary. The highest concentrations are recorded in the region near the last point to freeze in the cryowedge, being farthest from the heat transfer surfaces. Furthermore, convective flows are created by temperature- and concentration-induced density gradients during cooling. These lead to cryoconcentration differences in the vertical direction also, with the lower half of the cryowedge showing a significantly higher macro-cryoconcentration. The detailed map is shown in Fig. 1.
After completion of the above mapping, a number of blocks of the mAb solution were frozen under identical conditions, removed and stored at −40°C until needed for further processing. Based upon the previously obtained solute and protein map, cores (dimensions as defined above) from a number of pre-defined positions were removed from both top and bottom half of the frozen blocks, rapidly transferred into glass vials without thawing, and immediately placed at −40°C until completion of processing. These coring operations were carried out in a −20°C freezer room. The targeted protein concentrations in the cores (after thawing) were approx. 10–60 mg/mL. After completion of coring, the vials containing the frozen cores were distributed for long-term storage at three temperatures (−40°C, −20°C, or −10°C). Vials were removed, thawed, gently mixed, and analyzed at pre-determined time points for soluble aggregates (by SEC) and concentration (by UV280). Osmolality was measured on a vapor pressure osmometer (Model 3320 Osmometer, Advanced Instruments Inc., Norwood, MA, USA). A new core sample (vial) from each position was analyzed at each time point.
Note that due to the intensive processing required to produce the cores and the consequent risk for extraneous contamination, subvisible particulates were not measured on these samples. Results are therefore limited to examination of the soluble aggregate levels measured by SEC.
Size Exclusion Chromatography (SEC)
The column used for this analysis was a TSK3000SWXL (300 × 7.8 mm; Tosoh Bioscience LLC, King of Prussia, PA, USA) in a 50 mM Sodium phosphate pH 7.0 with 600 mM Sodium chloride running buffer at 1.0 mL/min over 20 min. Detection was by UV absorbance at 214 nm. In-house reference standard was injected before (3X), after every 10 samples (1X), and at the end (1X) of each chromatography batch run. Peak area for the reference standard runs was monitored, as well as tracked over time, to confirm system suitability and eliminate any impact on performance due to instrument or column drift. Results are reported as total% aggregates and incorporate primarily dimeric and some higher order species. Results within 0.2% of each other are not considered significantly different.
Biophysical Characterization
Solutions containing aggregates were directly assessed by various biophysical techniques. Far-UV circular dichroism (CD) spectra from 190 to 260 nm were collected using a ChiraScan™(Applied Photophysics, Leatherhead, Surrey, UK). IgG2 samples were diluted to 0.2 mg/mL and analyzed at room temperature in a quartz cuvette of 0.2 cm path-length. Spectra were collected at a scan rate of 50 nm/min with a resolution of 0.5 nm using a response time of 0.5 s. Two accumulations of spectra were collected and averaged. Spectra for protein were corrected for background with histidine buffer that had been diluted similarly.
The intrinsic fluorescence spectra of the IgG2 samples was measured on a Cary Eclipse Fluorescence Spectrophotometer (Varian Instruments, Walnut Creek, CA, USA) at an excitation wavelength of 295 nm and recorded from 305 to 450 nm. All samples were diluted to 0.2 mg/mL and analyzed at room temperature in a quartz cuvette.
To enhance the impact of aggregates (15), samples were diluted and mixed with 4,4′-bis-1-anilinonaphthalene-8-sulfonate (bis-ANS) stock solution (300 μM in 10 mM Histidine buffer) before fluorescence measurement (Cary Eclipse Fluorescence Spectrophotometer, Varian Instruments, Walnut Creek, CA, USA). The final concentration of IgG2 was 0.1 mg/mL, and bis-ANS concentration was at 10 μM. The fluorescence spectra were recorded between 405 to 580 nm at an excitation of 385 nm.
For thermal melt with ANS, IgG2 samples were diluted and mixed with bis-ANS stock solution (300 μM in 10 mM Histidine buffer) before fluorescence measurements on the Cary Eclipse Fluorescence Spectrophotometer (Varian Instruments, Walnut Creek, CA, USA), equipped with a Peltier element-based temperature-controlled cuvette holder. The final concentration of protein was 0.1 mg/mL with 10 μM bis-ANS. Fluorescence spectra from 405 to 580 nm (excited at 385 nm) were taken every 2°C over a range from 25 to 90°C with 300 s of thermal equilibration time at each temperature. Peak maxima were chosen based on the first derivative from a polynomial fit of the spectra, analyzed using a Microcal Origin 7.0.
RESULTS
Long-Term Storage of mAb at −20°C in Mini-Tanks
SEC results from long-term storage of four batches of the IgG2 mAb solution at −20°C in mini-tanks are provided in Table I. Data are shown as change in total aggregation level from the level at time zero. In all cases, the initial levels were in the range of 0.4–0.7%. An apparently random increase in aggregation levels is observed in a number of batches of the mAb. Note that each time point represents an independent mini-tank. The corresponding storage at 2–8°C (in vials for over 2 years, in mini-tanks for 6 months) resulted in absolutely no change in aggregation levels (data not shown).
As part of the formal investigation into the cause of aggregation, the manufacturing records of these (and other) batches were examined. No differences in the manufacturing process or anomalies in the extensive analytical characterization were found between these batches (results not shown). No specific reason for lack of aggregation in samples from Batch #3 could be pinpointed, although many possible causes were examined, including 1) more “stable” temperature in the storage location, 2) differences in surface smoothness of mini-tanks, and 3) differences in quality and/or quantity of excipients used.
Long-Term Storage of mAb at −20°C in Cryovessels
As part of the investigation into the above phenomena, a study was set up to evaluate the same batches of the mAb in cryovessels at −20°C. These cryovessels were thawed, sampled for analysis and refrozen at specific time points. A second set of observations was made by analyzing materials from cryovessels which were thawed for drug product manufacture, after about 20 months of storage at −20°C. Data from these latter two experiments are provided in Table II. The results present an interesting contrast. Contrary to expectation, absolutely no increase in aggregation was observed in the study involving repeated freeze/thaw of material in cryovessels with storage. On the other hand, varying levels of increased aggregation were seen in the material which had been stored for >20 months at −20°C without any intermediate thaw. However, the increase was much smaller than observed in the mini-tanks (compare data in Tables II to Table I).
Long-Term Storage of mAb in Cores from Cryowedge at −40°C, −20°C or −10°C
Results from SEC analysis of the cores stored at various temperatures for up to 15 months are shown in Fig. 2(a, b, c) presented as % soluble aggregates plotted against protein concentration in the thawed core. The initial soluble aggregate levels in all samples were between 0.4% to 0.7%, suggesting that the aggregate levels were uniform in the frozen block of cryowedge irrespective of position or protein concentration. At the 3 month time point, the aggregate levels remained below 1.2% for the −10°C and −40°C storage temperatures. On the other hand, aggregation in cores stored at −20°C increased with values ranging from 1.6% to 2.5%. No correlation between protein concentration and soluble aggregate levels was observed at any of the temperature conditions. The above trend was confirmed by the data at 6, 12 and 15 month time points. While cores maintained at −10°C and −40°C did not show any significant change in soluble aggregates, the corresponding cores at −20°C showed aggregate levels ranging between 3% to 4%, again with no dependence on position or protein concentration in core after thawing.
SEC chromatograms from the study are shown in Fig. 3(a, b, c). These overlays clearly show the development of the aggregates in the samples stored at −20°C. The main species of aggregates are dimers. Higher order species are seen at −20°C with increasing storage times.
Representative SEC chromatograms from analysis of core samples from cryowedge as a function of time and temperature of storage condition (−10°C, −20°C, −40°C) over 15 months, measured after thawing the core samples. Chromatograms are for core samples taken from approximately similar physical locations in the frozen blocks with thawed protein concentrations between 30–50 mg/mL.
Characterization of Solutions Containing Aggregates
All other quality parameters for the IgG2 mAb stability samples from mini-tanks and cryovessels (Tables I and II) remained effectively unchanged over the study period. The turbidity ranged between 3 and 5 FNU (per Ph. Eur. 2.2.1) for all samples/batches with no trends. Clarity was consistently reported as ≤ Ref. II (per Ph. Eur. 2.2.1). Reduced capillary gel electrophoresis analysis results ranged between 99% and 100% monomers for all samples/batches with no trends over time, indicating no fragmentation and reducible/non-covalent aggregates. The IgG2 molecule does not have any free cysteines, and no covalent aggregates were detected in non-reduced capillary gel electrophoresis, which consistently reported about 2–3% non-monomer species. Subvisible particulate matter analysis was not conducted on these samples.
Due to the relatively small amount of aggregates, isolation of the aggregates was not possible. Furthermore, no visible particles were seen, and the solutions remained clear and colorless. Test solutions were diluted from 20 mg/mL to 15, 10, 5 and 1 mg/mL in normal saline and analyzed by SEC either immediately or after holding for 24 h at either RT or 2–8°C. No change in levels of aggregates was seen, implying that the aggregates were not reversible (Fig. 4a). FT cycling with an aggregate-containing solution showed no decrease in these levels, indicating that this stress did not lead to a physical breakdown of the aggregates (Fig. 4b). The lack of increase in aggregates due to FT was not unexpected, since the formulation had been optimized to enable such a treatment. This was clearly shown by performing FT on a sample of the mAb in histidine buffer alone (Fig. 4c) or in a buffer with NaCl and PS80 and no trehalose (Fig. 4d) where increases in aggregates was seen. Lack of a cryoprotectant such as trehalose leads to aggregate formation on FT, which the presence of PS80 alone is not able to modulate, since levels of aggregation due to FT are quite similar in these two formulations (Fig. 4c and d).
Aggregate levels by SEC in mAb solutions as a function of FT cycles illustrating the aggregation behavior of the mAb. (a) Batch #1/3 diluted to various levels in normal saline to assess reversibility of aggregates formed by frozen storage. (b) A high aggregation level batch #9 subject to FT cycles between −20°C/−40°C/−70°C and room temperature, showing lack of decrease or increase in aggregation. (c) IgG2 mAb solution in 20 mM histidine buffer only, subject to FT cycles between −40°C/−70°C and room temperature showing increase in aggregation due to lack of cryoprotectant and surfactant. (d) IgG2 mAb solution in 20 mM sodium acetate buffer pH 5.5 with 140 mM NaCl and 0.2 mg/mL PS80, subject to FT cycles between −40°C/−70°C and room temperature showing increase in aggregation despite presence of surfactant.
The small amounts of aggregates also limited the information available from biophysical characterization. Intrinsic fluorescence spectra on control, FT-treated and frozen-storage samples showed no differences (Fig. 5a). Adding bis-ANS enhanced the peak intensity slightly (Fig. 5b), indicating the presence of hydrophobic material (15). Fluorescence melting with bis-ANS confirmed lack of difference in the thermal behavior of these solutions (Fig. 5c). Far-UV CD spectra could not detect any impact on secondary structure due to (the small amounts of) aggregates (Fig. 5d).
Biophysical characteristics of the mAb solutions containing various levels of aggregates. Control (Batch #3) sample had 0.5% aggregates; 10X FT-treated sample had 0.9%, while the 20X FT sample had 2.0% aggregates. Batch #6 sample had 3.6%, while the Batch #4 sample had 3.9% aggregates. (a) Intrinsic fluorescence spectrum. (b) bis-ANS fluorescence spectrum. (c) Fluorescence melting curve using bis-ANS as a hydrophobic probe (d) Far-UV CD spectrum.
DISCUSSION
The analytical and biophysical characterization of the solutions containing aggregates and the aggregation behavior of the IgG2 mAb showed that the aggregates generated during long-term frozen storage were non-covalent and hydrophobic in nature. These aggregates were not reversible on dilution or through FT processing (Fig. 4a and b). FT cycling in the optimized formulation containing trehalose and surfactant did not lead to any significant increase in aggregation, irrespective of the initial level of aggregates present, i.e. the aggregates did not function as seed or nuclei for further aggregate formation. However, presence of cryoprotectant (trehalose) was important to prevent formation of aggregates during FT cycling, while presence or absence of surfactant did not have any impact at least in the FT cycling studies (Fig. 4c and d). These results help in understanding the anomalous behavior of the mAb on long-term storage. It is evident that the apparently random nature of the aggregation data in Table I is not a consequence of reversibility or de-aggregation due to the thawing process. The lack of aggregation in the large-scale cryovessels that underwent storage with FT cycling (Table II; Set A) is also consistent with these observations. Finally, these studies illustrate the key role of trehalose as a cryoprotectant in this system and form the foundation of the explanation below.
A number of factors play a role in the anomalous aggregation behavior described above (Tables I and II). However, they are all based on the molecular mobility in the system as a function of storage temperature. The Tg’ of the formulation and the cores have been measured by us previously and been found to be approximately −29°C, with little variation across the frozen block. The Tg’ is driven primarily by the trehalose component of the formulation. It is well known that the viscosity of the cryo-concentrated solute phase changes significantly in the temperature range around Tg’, and the system can have significantly higher mobility at temperatures a few degrees above Tg’ compared to that at Tg’ and below (see, e.g., Schenz (16)). Estimates of viscosity changes of concentrated solutions above their glass transition temperature (up to melting) can be made by the WLF equation using universal constants (17,18). Based on these constants, a 10° change in temperature above Tg’ (i.e. at approximately −20°C) gives a viscosity decrease by a factor of ~700 compared to that at Tg’. However, a further 10° change (i.e. at approximately −10°C) decreases viscosity by a factor of ~75000 compared to that at Tg’, i.e. a decrease by a factor of 100 over that at −20°C. An estimate of the viscosity of a 3% sucrose solution on freezing provided by Bhatnagar et al. shows viscosity decreasing by a factor of 100 when going from −30°C (~106 cP) to −20°C (~104 cP) and by another factor of 100 from −20°C to −10°C (~102 cP) (2). This lower viscosity on storage at −20°C and −10°C therefore allows molecular relaxation motions to occur, while this mobility is not present at −40°C. Macroscopically, the samples at −10°C are visibly softer and pliable compared to the samples at −40°C or even at −20°C. Molecular mobility, or lack thereof, as a function of storage temperature, thus determines the behavior of protein and excipients in the frozen matrix.
It has been shown (for a number of proteins) that as freezing proceeds and ice forms, the protein at the ice surface can undergo a marked increase in flexibility due to a loosening of the native fold and loss of secondary and tertiary structure (19–21). However, once the temperature drops further (below approximately −30°C), the mobility is lost due to the increased viscosity of the matrix. Although these experiments were done with protein in buffer only, a similar behavior is likely in our system with the temperatures shifted lower due to the colligative effect of the excipients. Thus, on completion of freezing at −40°C, a fraction of the protein molecules exist in an unfolded state. When the cores are moved to −20°C or −10°C, some degree of mobility becomes possible. On extended storage at these conditions, the protein molecules can either relax/refold back to native state or interact with neighboring (unfolded) molecules to form aggregates. We hypothesize that between these two competing processes, storage at −10°C allows the refolding process to proceed at a faster rate than aggregation, while the opposite holds at −20°C. The significantly lower viscosity of the matrix at −10°C (as discussed above) implies that the protein molecules effectively exist in a “solution” state, albeit highly concentrated. On the other hand, the low molecular mobility at −40°C basically traps the protein in whatever conformation state they find themselves in at the end of the freezing. Lack of mobility does not allow for refolding to native state or interaction with neighboring molecules to form aggregates. Another possibility that reduces the aggregation rate at −10°C could be that the protein concentration is lower at −10°C than at −20°C or −40°C due to ice melt. This is shown on the trehalose-water state diagram in Fig. 6, on which the movement of the matrix compositions over time for various storage temperatures has been outlined. The authors’ note that the IgG2 mAb in this study is not significantly susceptible to aggregation in the liquid state even when concentrated up to 100 mg/mL, which would represent a five-fold higher concentration. However, the study at 100 mg/mL did not incorporate a corresponding five-fold increase in trehalose concentration (data not shown). In the frozen state under equilibrium freezing, the concentration solutes (and of the protein) would increase by a factor of about 7 at −10°C, from 8.4% in the liquid to approximately 56% (see Fig. 6).
Schematic state diagram of a non-crystallizing solute. The solubility curves for trehalose (and sucrose) are shown. Cores when first removed from frozen block correspond to position A. On placement at −20°C or −10°C, they equilibrate rapidly to positions B and C, respectively. Due to enhanced mobility at these temperatures and the large difference in eutectic solubility compared to the actual concentration of trehalose in the glassy matrix, trehalose dihydrate begins to crystallize out on long-term storage. This crystallization is negligible from position A due to the lack of mobility. Sucrose has a smaller driving force due to its higher eutectic solubility. [Note that this plot is for pure disaccharide. Addition of protein raises Tg’, thus reducing the drive for crystallization, and additionally serves as a steric hinder to crystallization. Extent and rate of crystallization will depend on protein content and its distribution within the trehalose matrix.]
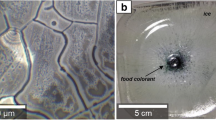
Trehalose is a key formulation component added to the mAb solution as a stabilizer and cryoprotectant. The equilibrium phase diagram for the trehalose-water system postulates the eutectic crystallization at temperatures slightly below 0°C at a composition limit of about 31% w/w (22,23). However, in practical systems, eutectic crystallization is generally hindered due to the increased viscosity and relatively fast non-equilibrium cooling rates, resulting in the formation of a glass with a maximally freeze-concentrated matrix of nearly 80% w/w (Fig. 6). Annealing by storage above the Tg’ can, however, facilitate the crystallization, thus relieving the stress in the system. Sundaramurthi and Suryanarayanan (24) have recently reported on the crystallization of trehalose dihydrate from a frozen matrix when annealed at −18°C for about 3 days. (In an analogous earlier report, sorbitol has also been shown to crystallize slowly in a frozen matrix when held at −30°C (above its Tg’ of −44°C), although not at −20°C(25).) Although the protein present in our matrix could potentially hinder this, the relatively high concentration of trehalose in our system (84 mg/mL in solution) makes crystallization likely over time. This possibility is supported by the map of the frozen block published by Kolhe et al. (11,14) showing the high levels of macroscopic cryoconcentration in parts of the matrix, and the fact that the micro-cryococentration will be closer to the maximally-freeze-concentrated matrix (Fig. 1). (Osmolality values greater than 1,000 mOsm/Kg were measured in some of the cores, implying regions with a macroscopic trehalose concentration of greater than 350 mg/mL; the microscopic-cryoconcentration levels are expected to be close to 80% w/w.) Again, storage at −40°C completely hinders the crystallization, while storage at −20°C and at −10°C facilitates it. Fig. 7 shows photographs of crystallization in small amounts of placebo (buffer + trehalose), mAb formulation, and (water + trehalose) solutions placed on petri dishes and stored at various temperatures. These photographs confirm the crystallization of trehalose in these matrices. The only solute that can crystallize from the (water + trehalose) solutions is clearly trehalose, and trehalose is also the only solute present in amounts significant enough to be able to crystallize in the other solutions. Although our experiments did not include PXRD measurements, data presented by Sundaramurthi and Suryanarayanan (24) in an analogous system provide strong supporting evidence for our conclusions to be correct. T. Patapoff also reported crystallization of trehalose and consequent aggregation in similar systems at −20°C (presentation at 2010 National Biotechnology Conference, San Francisco, CA; no reprint available). We also observed that the presence of protein hinders the crystallization of trehalose, as would be expected for any mixed-solute system, especially one containing a crystallizing and a non-crystallizing component (see, e.g., (26,27)), and also reported by Piedmonte et al. (25) for crystallization of sorbitol.
Photographs illustrating crystallization of trehalose from (buffer + trehalose), mAb, and (water + trehalose) solutions stored at various temperatures. No changes are seen at −40°C, while growth of crystals is apparent at −20°C and −10°C in all samples, indicating that it is the trehalose that crystallizes. All samples were frozen at −40°C for 2 days before moving to the indicated temperatures. [A red dye was added to a set of (water + trehalose) solutions to simply aid in visualization of the crystals. Lack of PS80 in these samples causes the solution to bead-up in the petri dish.]
The above discussion provides an explanation for the aggregation data in Tables I and II and Fig. 2. The system is immobile at −40°C, preventing both crystallization of trehalose and aggregation of protein. Extended storage at −20°C allows trehalose to crystallize, depriving the protein of the protective effects of the cryoprotectant, even as the protein at the ice interfaces remains in an unfolded state. This protein lacks the mobility to refold but, having lost the cryoprotectant, is able to interact with neighbors and form aggregates. The resultant effect is the increase in aggregation observed over time. At −10°C, although trehalose crystallization still occurs, the much greater overall mobility allows the protein to also effectively refold. The protein molecules at this temperature are essentially in a “solution,” and the lack of cryoprotectant therefore does not result in aggregation. This behavior is illustrated schematically in Fig. 8.
Schematic rate diagram illustrating the various processes occurring in the frozen system as a function of temperature. Aggregation is a result of competition between trehalose crystallization and protein refolding processes. Crystallization leads to loss of cryoprotection for the protein and increases potential for aggregation. The unfolded protein at the ice interface can interact with nearest (unfolded) neighbors and aggregate. However, given sufficient mobility at increasing temperatures, the protein can also completely refold and thus avoid aggregation even though the cryoprotectant has also crystallized. A maximum is therefore obtained in the aggregation rate at an intermediate temperature.
The apparently random nature of aggregation in the mini-tanks in Table I is a consequence of the probabilistic nature of the nucleation/crystallization phenomena. Likelihood of trehalose crystallization is dependent upon the pattern of ice/solute development in the individual mini-tanks and the temperature variations and other disturbances experienced by it. In the stability study in cryovessels where the vessels were thawed, sampled and frozen repeatedly, the act of thawing “nulls” the process and “resets” the material. The time in frozen state between the thaws (~6–8 months) is not long enough to allow significant crystallization and aggregation to develop. However, when stored continuously at −20°C for 20 months or more, a measurable increase in aggregation is seen. The formulation has been optimized for freeze/thaw so the impact of such repeat processing itself is negligible, as shown in Fig. 4b.
The significantly higher level of aggregation in the mini-tanks compared to the large cryovesssels suggests that a larger fraction of the trehalose is able to crystallize in the mini-tanks. One probable cause could be that the mini-tanks are likely to be more sensitive to temperature fluctuations due to their smaller (thermal) mass. Another contributing factor could be that a larger fraction of protein is in contact with ice interfaces due to the relatively faster cooling in a mini-tank compared to the cryovessel. Slow formation of ice allows longer time for solutes to diffuse ahead of the ice front as well as larger crystals (4). Dong et al. (21) also showed that greater fraction of protein and trehalose was occluded in the ice phase when rapid freezing occurred after a high degree of supercooling. Such freezing would result in a large number of nuclei and therefore a larger number of smaller ice crystals.
Addition of protein raises the Tg’ of the solution slightly (from −30°C reported for a pure aqueous trehalose solution (13,22), −31°C measured by us in histidine buffer with PS80, to −29°C in the IgG2 mAb solution of this study). This reduces the driver for crystallization at −20°C and even −10°C, but crystallization does still occur in the long-term. However, the protein also serves as a steric hindrance to the crystallization of trehalose, and it is possible that higher protein concentrations (in the range of 150–200 mg/mL) could suppress crystallization for practically viable periods of time. The trehalose content in our system was chosen to provide an isotonic solution. It is likely that increasing the protein-to-trehalose ratio would also increase the resistance to crystallization. The impact of (lack of) PS80 in the formulation on its long-term storage stability was not studied. From the data presented in Fig. 4c and d, it appears that the IgG2 mAb in this study is primarily protected from aggregation by the cryoprotectant and that the presence of PS80 does not have a significant protective impact, at least under FT cycling; the levels of aggregation in Fig. 4c and d are similar. The expectation is, however, that PS80 will have a greater role in stabilization for long-term frozen storage since its presence would reduce the amount of protein adsorbed/unfolded at the ice interface. Thus, frozen storage without PS80 would probably lead to even greater (rates and levels of) aggregation.
CONCLUSIONS
Long-term outcome of frozen state storage of an IgG2 mAb in trehalose formulation was observed to be dependent on the storage temperature vis a vis the Tg’, due to an interplay of various phenomena that are triggered by the mobility of the system. Storage below Tg’ (= −29°C) at −40°C effectively immobilizes the constituent molecules. This prevents crystallization of trehalose that protects as well as prevents the protein molecules from aggregation, irrespective of their degree of unfolding. Storage (at −10°C) significantly above Tg’ allows the protein molecules to refold and effectively places them in a “solution” environment where, even though deprived of the full protective effect of cryoprotectant (through crystallization), aggregation does not occur. Reduction of the protein concentration at −10°C due to ice melt could also contribute to this lack of aggregation. Storage above Tg’ at −20°C seems to present a situation where there is enough mobility for loss of cryoprotectant (through crystallization) but not enough mobility for the refolding of protein, thus resulting in increase in aggregation over a period of time.
The precautionary conclusion from this investigation is that bulk drug substance should be stored at temperatures preferably 10°C lower than the Tg’. Relevance of small-scale models to estimate stability of large-scale storage must be carefully assessed. However, the above observations also suggest some interesting alternative possibilities for frozen-state storage from the point of view of preventing aggregation (although impact on other quality parameters would have to be assessed on an individual protein basis):
-
1)
Periodically thawing the frozen material can effectively “reset” the material prior to re-freezing, if the protein in the formulation is stable to the FT process itself.
-
2)
Long-term storage at −10°C could be a viable, significantly simpler, and less energy-intensive alternative to −40°C storage.
-
3)
Sucrose might be used as an alternative cryoprotectant since its eutectic solubility limit (~64% w/w) is much higher than that for trehalose. The main technical limitation for sucrose would be the greater risk (in the liquid state) for its hydrolysis, generation of glucose and subsequent glycation of the protein, especially at low pHs (28,29).
REFERENCES
Singh SK. Storage consideration as part of the formulation development program for biologics. Am Pharm Rev. 2007;10(3):26–33.
Bhatnagar B, Bogner RH, Pikal MJ. Protein stability during freezing: separation of stresses and mechanisms of protein stabilization. Pharm Dev Technol. 2007;12:505–23.
Singh SK, Kolhe P, Wang W, Nema S. Freezing of biologics—A practitioner’s review. Part II: Practical advice. Bioprocess Int. 2009;7(10):34–42.
Singh SK, Kolhe P, Wang W, Nema S. Freezing of biologics—A practitioner’s review. Part I: Fundamental aspects. Bioprocess Int. 2009;7(9):32–44.
Singh SK, Nema S. Freezing and thawing of protein solutions. In: Jameel F, Hershenson S, editors. Formulation and process development strategies for manufacturing biopharmaceuticals. New York: Wiley; 2010. p. 625–75.
Kaushik JK, Bhat R. Why is trehalose and exceptional protein stabilizer? An analysis of the thermal stability of proteins in the presence of the compatible osmolyte trehalose. J Biol Chem. 2003;278(29):26458–65.
Carpenter JF, Crowe JH. The mechanism of cryoprotection of proteins by solutes. Cryobiology. 1988;25:244–55.
Franks F. Biophysics and biochemistry at low temperatures. Cambridge: Cambridge University Press; 1985. p. 86–9.
Tang X, Pikal MJ. The effect of stabilizers and denaturants on the cold denaturation temperatures of proteins and implications for freeze drying. Pharm Res. 2005;22(7):1167–75.
Wisniewski R, Wu V. Large-scale freezing and thawing of biopharmaceutical products. In: Avis KE, Wu VL, editors. Biotechnology and biopharmaceutical manufacturing, processing, and preservation. Buffalo Grove: Interpharm Press; 1996. p. 7–59.
Kolhe P, Holding E, Lary A, Chico S, Singh SK. Large-scale freezing of biologics—Part 2. Understanding protein and solute concentration changes in a cryovessel in the frozen state. BioPharm Int. 2010;23(7):40–9.
Williams ML, Landel RF, Ferry JD. The temperature dependence of relaation mechanisms in amorphous polymers and other glass-forming liquids. J Am Chem Soc. 1955;77:3701–7.
Conrad PB, de Pablo JJ. Computer simulation of the cryoprotectant disaccharide α, α-trehaloset in aqueous solution. J Phys Chem A. 1999;103:4049–55.
Kolhe P, Holding E, Lary A, Chico S, Singh SK. Large-scale freezing of biologics—Part 1. Understanding protein and solute concentration changes in a cryovessel during freezing. BioPharm Int. 2010;23(6):53–60.
Hawe A, Sutter M, Jiskoot W. Extrinsic fluorescent dyes as tools for protein characterization. Pharm Res. 2008;25(7):1487–99.
Schenz TW. Glass transitions and product stability—an overview. Food Hydrocolloids. 1995;9(4):307–15.
Roos Y, Karel M. Amorphous state and delayed ice formation in sucrose solutions. Int J Food Sci Technol. 1991;26:553–66.
Soesanto T, Williams MC. Volumetric interpretation of viscosity for concentrated and dilute sugar solutions. J Phys Chem. 1981;85:3338–41.
Strambini GB, Gabellieri E. Proteins in frozen solutions: evidence of ice-induced partial unfolding. Biophys J. 1996;70:971–6.
Strambini GB, Gonnelli M. Protein stability in ice. Biophys J. 2007;92:2131–8.
Dong J, Hubel A, Bischof JC, Aksan A. Freezing-induced phase separation and spatial microheterogeneity in protein solutions. J Phys Chem B. 2009;113:10081–7.
Miller DP, De Pablo JJ, Corti H. Thermophysical properties of trehalose and its concentrated aqueous solutions. Pharm Res. 1997;14:578–90.
Cesa’ro A, De Giacomo O, Sussich F. Water interplay in trehalose polymorphism. Food Chem. 2008;106:1318–28.
Sundaramurthi P, Suryanarayanan R. Trehalose crystallization during freeze-drying: implications on lyoprotection. J Phys Chem Lett. 2010;1:510–4.
Piedmonte DM, Summers C, McAuley A, Karamujic L, Ratnaswamy G. Sorbitol crystallization can lead to protein aggregation in frozen protein formulations. Pharm Res. 2007;24(1):136–46.
Johnson RE, Kirchhoff CF, Gaud HT. Mannitol-sucrose mixtures—Versatile formulations for protein lyophilization. J Pharm Sci. 2002;91(4):914–22.
Izutsu K, Yoshioka S, Kojima S. Effect of cryoprotectants on the eutectic crystallization of NaCl in frozen solutions studied by differential scanning calorimetry (DSC) and broad-line pulsed NMR. Chem Pharm Bull. 1995;43:1804–6.
Gadgil HS, Bondarenko PV, Pipes G, Rehder D, McAuley A, Perico N, et al. The LC/MS analysis of glycation of IgG molecules in sucrose containing formulations. J Pharm Sci. 2007;96(10):2607–21.
Fischer S, Hoernschemeyer J, Mahler H-C. Glycation during storage and administration of monoclonal antibody formulations. Eur J Pharm Biopharm. 2008;70:42–50.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, S.K., Kolhe, P., Mehta, A.P. et al. Frozen State Storage Instability of a Monoclonal Antibody: Aggregation as a Consequence of Trehalose Crystallization and Protein Unfolding. Pharm Res 28, 873–885 (2011). https://doi.org/10.1007/s11095-010-0343-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-010-0343-z