ABSTRACT
Purpose
Develop a preclinical in vitro algorithm enabling de novo design of semisolid vaginal drug delivery gels, by using biomechanical modeling of gel spreading in the vaginal canal and empirically relating gel composition to mechanical properties and predicted performance.
Methods
Gel performance was defined through a multivariate objective function constructed from gels’ mechanical properties and selected performance criteria for gel spreading within the vaginal canal. Mixture design of experiment was used to establish a semi-empirical relationship linking composition-property and property-performance relationships for gels with varying concentrations of hydroxyethylcellulose and Carbopol 974P. This permits definition of a local optimum for gel composition and volume of administration, within a defined gel composition space.
Results
Rheological behavior and, consequently, the value of the objective function varied broadly with composition. The algorithm indicated a 3.0 wt% HEC gel as the near optimal composition for a 3.5 mL applied volume for gels designed to spread throughout the vagina.
Conclusions
The algorithm introduced herein is a novel tool that facilitates an understanding of the composition-property-performance relationship for vaginal semisolid drug delivery gels. This approach has promise as a scientific methodology for evaluation and optimization of vaginal gels prior to in vivo investigations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
To date, most vaginal semisolid gels have been designed empirically, with mechanical properties mimicking those of other commercial semisolid products. For drug delivery products, this approach may have originated, in part, due to inadequate recognition of the role of delivery vehicles in the overall effectivenesses and acceptabilities of the products (1–4). Given the nature of the historical approach, we have limited appreciation for the extent to which these existing vaginal products achieve their designated functions. We therefore posit that there is a need for methodologies to help formulation scientists to objectively and intelligently select gel compositions for target applications. Performance criteria will nonetheless vary based on the use of the vaginal product and the drug(s) it contains. Therefore, it is inevitable that pharmaceutical scientists will need to vary the properties of gel formulations to satisfy desired performance criteria. For this reason, it is important to understand the underlying composition-property-performance relationships (CPPR) of the gels, so that their compositions can be rationally varied to achieve given sets of properties and performance standards.
Vaginal gels are a primary dosage form for microbicides, which are topically applied products for the delivery of antiviral agents, intended to provide protection against the sexual transmission of HIV-1 and other sexually transmitted pathogens (5–7). The success of 1.0 wt% tenofovir (TFV) gel in the CAPRISA 004 microbicide trial is a historic milestone in the field of microbicides and reinforces the hope for preventive strategies in the fight against HIV (8). The performance of a vaginal microbicide gel is both biological (i.e. drug delivery) and behavioral (i.e. user acceptability). Performance can be measured with respect to a number of functions, including the gel’s ability to spread along the vaginal canal and coat the vaginal epithelium. This function governs delivery of active ingredient(s) to the target tissue; it also can lead to leakage outside the vagina, which adversely influences the acceptability of the product (1,9,10). Gel performance in relation to the above criteria is strongly influenced by the administered volume, an often neglected parameter in gel design. Careful selection of the gel volume is crucial, since it has a causal relationship to the dynamics of gel coating (11) and the potential for leakage. That is, an ideal microbicide gel must provide desired coating with minimal leakage for a given volume of administration. To address this trade-off between coating and leakage, an objective, quantitative method relating gel composition to both of those performance attributes is needed. An initial set of biomechanical flow models has been created that relate gel rheological properties, gel volume, vaginal forces acting on a gel, and vaginal geometry to measures of gel spreading along the vaginal canal (12–14). These fluid flow models enable us to predict the coating and leakage of a gel for a given volume and set of properties. However, such information, alone, is of limited value to pharmaceutical scientists: the performance of a gel and the properties that give rise to it are multivariate; they depend on the composition-property relationships of the polymers and excipients within the formulation. Given the complexity of the relationships amongst composition, properties and performance, it is unsurprising that most vaginal gel compositions were selected empirically with little effort devoted to quantitatively relate composition to properties and performance (4).
To address this critical gap in the field of vaginal drug delivery from gels, a set of tools was developed to quantitatively explore composition-property-performance relationships in semisolid gels. A multivariate objective function constructed from gel properties, computations of flow behavior of the gel, and performance criteria for that flow behavior, is described herein. Analogous multivariate optimization approaches have previously been used in many fields, including computational biology, bioinformatics, drug discovery and molecular optimization for ligand binding (15–17). Mixture Design of Experiments (MDOE) methodology (18–20) was used to establish a semi-empirical, quantitative relationship between the composition-property and property-performance relationships; this approach enabled us to obtain a local optimum within a given composition space (Fig. 1). In the past few years, considerable work has been done on the development of hydrogels composed of polyacrylic acid (PAA), cellulose, chitosan and hyaluronic acid for ocular, buccal, vaginal and intestinal delivery (21). Among these, cellulose and PAA-based polymers have received increasing interest for cervical and vaginal drug delivery (9). The major attributes of the above-mentioned polymeric carriers that make them attractive candidates for vaginal drug delivery are the GRAS status (9), low cost and bioadhesion (21). On the basis of the extensive literature available on the use of these polymers for vaginal drug delivery, we chose Carbopol 974P and hydroxyethylcellulose (HEC) as the two polymeric carriers to be evaluated in our design (1,4,9,10,21,22); however, the general methodology we present below can be used in any semisolid design space. The antiretroviral drugs used in development of the models were the HIV-1 reverse transcriptase inhibitors UC781 (23–25) and TFV (26–28).
MATERIALS AND METHODS
Materials
Micronized UC781 and TFV were obtained from the CONRAD Program (Arlington, VA, USA). Carbopol 974P NF was purchased from Lubrizol (Wickliffe, OH, USA). HEC 250 HX PHARM was purchased from Hercules (Wilmington, DE, USA). Glycerin NF was purchased from J.T.Baker (Phillipsburg, NJ, USA). Methylparaben and propylparaben were purchased from Fluka (Milwaukee, WI, USA). The commercial gels Replens® (lot # RD01015), K-Y Jelly® (lot # 0385C), Advantage-S™ (lot # A05101), Conceptrol® (lot # 8MM6346), and RepHresh® (lot # F09142) were purchased from a local pharmacy store. Methyl cellulose placebo gel (lot # 100306) and Carraguard (lot # 23175) were obtained from the Population Council (New York, NY, USA). The HEC clinical placebo gel (GMID #70018699), 6% Cellulose Sulfate microbicide gel (lot # C1564A001), TFV 1% microbicide gel from the CAPRISA trial (GMID #70018698) and UC781 microbicide gel (lot # 1061) were obtained from the CONRAD Program (Arlington, VA).
Experimental Design
This work was part of our development of semisolid vaginal microbicide gels for the delivery of two highly potent antiretroviral drugs—TFV (26) and UC781 (24)—and these drugs were included in all formulations. To elucidate the relationship between composition-property and property-performance, a statistical tool—Mixture Design of Experiments (MDOE)—was used. The fractions of the two polymers, X 1 (Carbopol 974P) and X 2 (HEC), were selected as two independent variables, which were varied simultaneously. Selection of model compositions and the optimization were performed using Design Expert® 7.1 (Stat-Ease, Inc., Minneapolis, MN) software.
In order to determine maximum concentrations of gelling agents, viscosity measurements were obtained on a series of gels with varying concentrations of Carbopol 974P and HEC. For initial comparison of these gels based on viscosity, a shear rate of 50 s−1 representing an intermediate physiologically relevant (1–100 s−1) value was chosen (29). Upper limits on the concentrations of the gelling agents were chosen such that each polymer at its highest concentration would create a gel with viscosity comparable to that for commercially available gels at 50 s−1. Based on these preliminary investigations, 2.5 wt% and 3.0 wt% were chosen as the maximum concentrations to be tested for Carbopol 974P and HEC, respectively. Fixed gel components consisted of 5 wt% glycerin, 0.1 wt% UC781, 1.0 wt% TFV, 0.15 wt% methylparaben and 0.05 wt% propylparaben (preservatives), totaling 6.3 wt% of the gel. DI water was used to make up the weight of the gel to 100 wt% (Table I).
Design space for an ‘n’ component mixture is represented by a surface in ‘n−1’ dimensions. Thus, an equilateral triangle in two-dimensional space represents the mixture design space for a three-component system. In this work, not all factors varied over the same range; the design space was represented by a fraction of the equilateral triangle, as shown in Fig. 2. A design matrix comprising 19 model compositions was constructed (Table II). This included three vertices of the triangular space (X 1 , X 2 , X 3 ) to determine the effect of the pure components, three binary blends (X 1 X 2 , X 2 X 3 , X 3 X 1 ) to determine the effect of combinations of gelling agents, and one centroid point (X 1 X 2 X 3 ) which represents a center point with each ingredient at one third the maximum concentration. The order in which the gels were made and tested was randomized to counteract any differences due to aging or time-related effects (Table II).
Twelve-point mixture design for a three-component mixture. The constraints on the model are Carbopol 974P (0 ≤ X 1 ≤ 2.5 wt%); HEC (0 ≤ X 2 ≤ 3.0 wt%) and Water (78.2 ≤ X 3 ≤ 83.7 wt%). Red points in the design space indicate the 19 model compositions. The numbers next to the point indicate number of repeats of each composition.
Model Selection
The cubic model was chosen to model the responses because it provides a reasonable approximation of actual response surface for a three-component mixture design. The Scheffé polynomial was used to eliminate redundant coefficients introduced by the constraints imposed by the mixture design (30). In addition, to obtain a good response surface, extra points were augmented in the model to evaluate goodness of fit and pure error. Equation 1 gives the polynomial for the non-linear cubic model (20).
Here, y is the response; X i , X j , X k represent the three components of the mixture; β i , β j , β k represent the linear coefficients; β ij , β jk , β ik represent the non-linear blending model coefficients; and β ijk, δ ij represent the model coefficient for higher order non-linear blending.
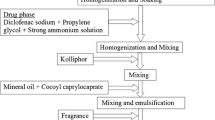
Preparation of the Carbopol 974P and HEC Gels
UC781 (0.1 wt%) was dispersed in glycerin (3.5 wt%) to form a homogenous paste which was then added to 1 wt% TFV solution in water. Required amounts of Carbopol 974P were added to the above solution and stirred with a paddle mixer at 350 rpm for 45 min to ensure complete hydration of the polymer. Stainless steel paddles (passivated 316 L) and USP grade water should be used to avoid introduction of trace amounts of metal into the gel during preparation. Methylparaben (0.15 wt%) and propylparben (0.05 wt%) were weighed and mixed with pre-heated glycerin (1.5 wt%) at 65°C until all solids dissolved. Required amount of HEC was added to the glycerin paste after cooling to room temperature. The glycerin paste was then added to the Carbopol 974P dispersion with constant stirring. The pH of the gel was adjusted to 5.2 ± 0.2 with 10 wt% NaOH. Finally, the overall weight of the gel was adjusted to 100 g with 18 mΩ DI water.
pH and Osmolality Measurements
The pH of the gels was recorded at room temperature using a 423 microprobe electrode (Mettler Toledo Instruments, Germany) prior to rheological measurements. Osmolality of gels was measured using a Vapro 5220 osmometer (Wescor Inc., UT, USA), with the goal of constraining hyperosmolality. Ten μL of gel were loaded on solvent-free disc and placed in the pre-calibrated osmometer for osmolality measurements. Averages of five measurements were taken. The pH of the gels was 5.2 ± 0.2, and all osmolality values were <1,000 mOsm/kg.
Dilution of Gels with Vaginal Fluid Simulant (VFS)
To study the effect of dilution of gels with vaginal fluid, we performed a complete rheological characterization on gels mechanically mixed with VFS at a biologically relevant ratio (1:4 dilution; VFS: gel) (29). The VFS was prepared as previously reported (31). One gram of the VFS was mechanically mixed with 4 g of gel. Diluted gels were allowed to equilibrate for 24 hrs before rheological measurements.
Determination of Rheological Properties
Viscosities of gel formulations (undiluted, diluted) were measured using a stress-controlled AR 550 rheometer (TA Instruments, DE, USA) equipped with a 20 mm 4° steel cone geometry. One-hundred-fifty μL of the gel were loaded using a positive displacement pipette (Gilson, WI, USA) and allowed to equilibrate for 2 min on the Peltier plate at 37°C, after which a steady-state shear was applied. Shear rates experienced in vivo range from <0.1 s−1 during passive seeping between epithelial surfaces to the order of ~100 s−1 during coitus (29). Thus, viscosity was determined over the range of 10−4–1,000 s−1 to simulate physiological conditions (29).
Stress Growth and Relaxation Measurements
The yield and residual stresses in a gel influence its initial flow and also its sustained retention within the vaginal canal. We performed stress growth and relaxation studies to measure residual stresses, on both undiluted and diluted gels. For most gels, we used a Brookfield model 5HBDV-III Ultra rheometer equipped with a CPE40 cone at a shear rate of 10 s−1. Gels (7,11,14) had low viscosities and failed to produce measurable torque on this instrument; therefore, a Brookfield model LVDV-III rheometer equipped with a CP40 cone at a shear rate of 300 s−1 was used for those gels.
Fitting Rheological Data to Constitutive Models
Data obtained from the steady-state rheological studies and the stress growth and relaxation studies were fitted to the Power law and Herschel-Bulkley models (Table III). The latter was used when a non-zero residual stress was measured, and the residual stress value was used to approximate the yield stress. Logarithmic values of shear stress or viscosity as a function of shear rate (over the range of 10−4 to 100 s−1) were averaged over the replicate experiments, and fitted to the models using a Matlab function to minimize the residuals.
Squeezing Flow Model
Estimates of the vaginal area coated by the gel (A) were made using a biomechanical fluid flow model of the coating process (12,13). This model utilizes the concept of constant-force squeezing of fluid between two plates, simulating the elastic forces from the posterior and anterior walls of the vagina. Inputs to the squeezing flow model were the rheological parameters from the constitutive model, initial height of the bolus (0.2 cm), volume of the gel, vaginal dimensions, and forces exerted by the vaginal muscles on the gel (1–10 N) (12). The output from the flow model is the area coated as a function of time. We focused on that area at 120 s after the onset of gel flow, which serves as a measure of the deployment of the viscoelastic gel (12,13).
Confirmation of the Squeezing Flow Model
The results of the squeezing flow model were confirmed through calculation of area coated and in vivo MRI measurements of gel coating on four commercial over-the counter-products—K-Y Jelly®, Replens®, Conceptrol® and Advantage-S™ (32,33).
Development of the Objective Function
An objective function was designed to serve as a DOE response factor, which was constructed as a piecewise continuous function of A: it has different analytical forms for A ≤ A max and for A > A max , which give equal results at A = A max . The domain of the function is defined from zero to unity such that when its value, termed the score (S), equals 1, the gel fully meets the performance requirements (Fig. 3).
If we assume that the amount of drug delivered by a gel is derived from drug diffusion within the gel, and that the process is quasi-steady, then drug flux is inversely proportional to gel thickness, (h).
where is diffusion flux, Ф is concentration and x is length or thickness. Volume of the gel (V) can be expressed as
For constant value of V, h is inversely proportional to A.
Since the amount of drug delivered (Q) depends on the flux and the surface area, Q is proportional to A 2.
We chose a simple form of the objective function based upon this result, and the criterion that S = 1 when A = A max .
This expression is evaluated at a fixed time, 120 s, and does not, of course, include the time course of the increase in area coated with time. As such, it represents an upper bound on drug delivery over this time interval.
When the area coated exceeds A max, the gel is predicted to leak from the vagina. Thus, its merit would decrease in relation to the amount of predicted leakage. Letting V L be the volume of gel that has leaked, Eq. 10 relates V L to A *, which is the value of A at which the score becomes zero. A simple linear function was used to model the decline of the score from unity to zero, as area coated increases above A max (Fig. 3). Our expression inputs a maximum value of V L at which the value of the objective function has declined to zero. Subsequently, we also tested alternative non-linear functions—quadratic and exponential (cf. sensitivity analysis in the Supplementary Material), to model the decline of score to zero as leakage increased.
Separate computations were performed for undiluted (S undiluted ) and diluted (S diluted ) gels. These scores served as responses to create response surface models. In this algorithm, there are four key inputs: V, A max and V L and the mathematical form of the objective function. V was chosen to be 3.5 mL for the initial set of studies because this value is a common dose volume of microbicide gels. Maximum volume of the gel leaked after application was taken as V L = 1.5 mL, at which the score declined to zero. This volume was based upon inputs from clinical investigators evaluating acceptability of gels in microbicide trials. Choosing the value A max , which is a specified target area for optimal coating, requires information about the relationship between the squeezing model predictions of area coated and actual values of areas coated in women (obtained from imaging studies). This is complex, in part because gels likely become non-uniformly diluted as they spread over vaginal surfaces. Consequently, their rheological properties vary with respect to location and time. In the present study, we used values of area coated computed for the undiluted methylcellulose (MC) placebo gel from the Carraguard® trials as a reference; this gel was found to have good vaginal coating (34).
RESULTS
Rheological Measurements
Viscosity values vs. shear rate for different combinations of HEC and Carbopol 974P are presented in Fig. 4. Shear rates are shown from 1–100 s−1 for undiluted gels (Fig. 4A) and 1–1,000 s−1 for gels diluted with VFS (Fig. 4B). Viscosity varied between 1-50 Pa s and 0.5–20 Pa s at 50 s−1 for undiluted and diluted gels, respectively. Combinations of Carbopol 974P and HEC were investigated in relation to any synergistic effect between the two polymers on gel viscosity. All combination gels exhibited significant increases in viscosity at low shear rates. Similar observations for combinations of Carbopol 974P and HPMC have previously been reported (35). At low shear rates (~1 s−1) the viscosities of gels with both gelling agents were approximately an order of magnitude higher than the sums of the viscosities for gels with the individual polymers. However, at higher shear rates (~100 s−1) such differences became marginal. This result indicates that the shear rate-dependent viscosity profile is critical and that simply comparing viscosities at specific shear rates is insufficient in understanding and comparing rheological behavior of gels. When gels were diluted with VFS, those consisting of both HEC and Carbopol 974P exhibited >50% loss in viscosity vs. undiluted values. The maximum impact of dilution was observed with gels consisting of 2.5 wt% Carbopol 974P and 3.0 wt% HEC (Fig. 4B).
Results of the steady-state flow experiments on undiluted gels (A) and gels after 20% dilution with VFS (B). All gels exhibited shear thinning behavior, with viscosity decreasing by approximately one order of magnitude as shear rate increased from 1 s−1 to 100 s−1. Dilution with VFS had a greater impact on the viscosity vs. shear rate curves for Carbopol 974P gels than for pure HEC gels. Overall, the effect of dilution with VFS on gel viscosity and shear thinning behavior was strongly influenced by the composition of the gel.
Fitting Rheological Data to Constitutive Models
The Power law model, and its counterpart Herschel-Bulkley model when a yield stress exists, fitted the rheological data for the gels both undiluted and diluted. Table IV summarizes the composition-property relationship in the gels. In general, the consistency index decreased on dilution with VFS, whereas the shear thinning coefficient increased, i.e. the degree of shear thinning decreased.
Stress Growth and Relaxation Measurements
As expected, gels with HEC alone did not demonstrate measurable residual stresses (12). However, gels consisting of Carbopol 974P alone or in combination with HEC exhibited measurable residual stress. In addition, Carbopol 974P gels demonstrated a concentration-dependent increase in measured residual stress. For all gels diluted with VFS (Fig. 5B), there was a significant decrease in the residual stress. Carbopol 974P gels, in the absence of HEC, showed ~50% loss in residual stress on dilution; in contrast, gels containing both Carbopol 974P and HEC showed significant but less dramatic losses in residual stress compared to pure Carbopol 974P gels. Gels with Carbopol 974P at its highest concentration, in the presence of HEC, demonstrated the highest residual stress (~150–200 Pa) of all compositions tested, and were the least affected by dilution (Fig. 5).
Comparisons of the stress growth and relaxation curves for gels from the MDOE. Plots A and B show the stress growth and the relaxation curve for the undiluted and diluted gels as a function of time, respectively. Gels with HEC alone did not demonstrate measurable residual stresses. Gels consisting of Carbopol 974P alone or a combination of HEC and Carbopol 974P demonstrated concentration-dependent increase in measured residual stress. On dilution with VFS, gels containing both Carbopol 974P and HEC showed less dramatic loss in residual stress, when compared to pure Carbopol 974P gels.
Squeezing Flow Model
The MC placebo gel from the Carraguard® clinical trial (34,36) has been previously reported to provide near complete coating of the vaginal epithelium in vivo, immediately after application with insignificant leakage post application (2). For this gel we obtained an area coated of 117 cm2 at 120 s for a 3.5 mL volume. This value is close to the actual value of human vaginal surface area (12,37). To compensate for the overestimations of area coated by the flow model, we renormalized all data with respect to the MC placebo. Thus, A max for both undiluted and diluted gels was set at 117 cm2, for a volume of administration of 3.5 mL.
In Vivo Confirmation of the Squeezing Flow Model
To validate the predictions of area coated obtained from the squeezing flow model, model data were compared to the percentage A obtained from in vivo imaging studies for four over-the-counter (OTC) gels—K-Y Jelly®, Replens®, Conceptrol® and Advantage-S™. The values of A predicted by the squeezing flow model showed good agreement (<25% difference) with the results from imaging studies reported in literature, with a consistent but small overestimation of the in vivo values (Table V) (32). Please refer to the Supplementary Material for the rheological data on the OTC gels.
Area Coated from Squeezing Flow Model
Figure 6 summarizes the results of the squeezing flow computations on the gels. Upon dilution with VFS, gels with highest concentration of HEC and/or Carbopol 974P displayed 15–20% increase in the predicted area coated, whereas, at lower concentrations, there was a more pronounced effect of dilution (30–50%). At fixed concentrations of one gelling agent and increasing concentrations of the other agent, spreading of gels decreased significantly. These results are consistent with the trends observed in the viscosity vs. shear rate curves (Fig. 4).
Predicted vaginal surface area covered after 120 s of constant-volume squeezing, using the squeezing flow model for a gel volume = 3.5 mL. A Undiluted gels and B gels diluted 20% with VFS. Increasing size of the bubble and color gradations from black to gray represents increasing values of area coated. 1.5 wt% HEC gel gave the highest area coated followed by 3.0 wt% HEC gel.
Fitting Responses to Model
S undiluted and S diluted computed for the model gels were simultaneously fitted to linear, quadratic, cubic and higher order special cubic models. The cubic model provided the best estimation of coefficients for both responses. Comparative values of model fits are summarized in Table VI along with the regression equation generated for each response. The fits indicated that a significant model did exist (p < 0.05), with insignificant lack of fit (p > 0.10) and an adequate signal-to-noise ratio (precision >4). Using the backward elimination function in the program, only statistically significant (p < 0.05) coefficients were included in the regression equations.
In general, positive values of model coefficients (β i , β j , β k ; Eq. 1) indicate that the selected effect favors optimization of the response, whereas negative values suggest an inverse relationship. From the linear coefficients for the pure component, it is evident that Carbopol 974P (X 1 ) with a negative β i has an adverse effect on S undiluted . This can be interpreted as being due to the yield stress acting to diminish spreading. The linear coefficient for HEC (X 2 ) is positive and indicates that HEC favors S undiluted . The effect of variation in X 3 was ~3 orders of magnitude lower on both the responses, which can be considered negligible. The coefficients in the regression equations (Table VI) are large, which is due to the L-pseudo transformation that converts the compositions on a scale of 0 to 1. Thus, to compare the effect of each component on the score optimization, we must multiply the two-component terms (β ij, β jk, β ki ) by one-fourth (1/2 × 1/2) and the three-component term (β ijk ) by 1/27 (1/3 × 1/3 × 1/3). Unlike the case of S undiluted , both X 1 and X 2 favor the optimization of the S diluted , and the two-component non-linear blending coefficients show an antagonistic effect on S diluted . The surface for the response S diluted is more complex, due to the constant interplay between the linear and the non-linear blending coefficients, as shown in the regression equation. In summary, based on the regression equations, it is evident that the factors X 1 and X 2 play an important role in the optimization process. HEC (X 2 ) has a favorable effect on both the responses, whereas Carbopol (X 1 ) favors only the optimization of S diluted .
Validation of the Cubic Model and RSM
To evaluate the ability of the model and RSM to predict the score, we chose four checkpoints within the design space. Rheological characterization of these gels was performed in the same manner as on all other gels in the study. Table VII shows the composition of the checkpoints, their predicted and experimental values of the score, and the percentage error in predictions. The low magnitude of error (<15%) in the model confirms the high prognostic ability of the RSM.
Response Surface Methodology (RSM)
Response surfaces provide visual depiction of the relationship between composition and scores. The score is suggestive of the relative performance of the gels. Figure 7 shows the surface plots for the score of the gels under undiluted and diluted conditions. All the responses exhibited non-linear relationships with composition. Figure 7D and E show the 2D contour plots, while Figure 7A and B are 3D surface plots depicting effects of change in composition on gel score. S undiluted increases with increasing concentrations of HEC as shown in Figure 7A. The response surface peaks at the highest concentration of HEC in the absence of Carbopol 974P, whereas the surface that fits S diluted is more complex. This surface exhibits curvature at the intermediate concentrations of HEC (2.25–3.0 wt%) and Carbopol 974P (1.25 wt%). However, further increase in Carbopol 974P concentration results in a dip in the surface as shown in Figure 7B. (cf. statistical analysis in the Supplementary material).
3D and 2D response surface plot showing the effect of concentration of Carbopol 974P (X 1 ) and HEC (X 2 ) on—(A) 3D response surface plot of S undiluted ; B 3D response surface plot of S diluted ; and C 3D response surface plot of desirability for the optimization of gels based on maximizing two responses—S undiluted and S diluted . D and E are the 2D response plots for S undiluted and S diluted , respectively and (F) 2D response surface plot of desirability. S undiluted increased with increasing concentrations of HEC and the response surface peaked at the highest concentration of HEC. The response surface for S diluted exhibited a curvature at the intermediate concentrations of HEC (2.25–3.0 wt%) and Carbopol 974P (1.25 wt%) and further increase in Carbopol 974P concentration resulted in a dip in the surface. Based on the desirability plot, 0.0 wt% Carbopol 974P and 3.0 wt% HEC was identified as the optimum composition.
Vehicle Optimization Using RSM
Individual response surfaces for the undiluted and diluted gels at 3.5 mL volume were generated. A multiple response optimization methodology was used to maximize S undiluted and S diluted simultaneously, with the constraints of Carbopol 974P (0–2.5 wt%), HEC (0–3.0 wt%) and score (0–1). Responses S undiluted and S diluted varied in the range of 0–0.876 and 0–0.830, respectively. For multiple response optimizations, the software utilizes a function that converts multiple responses into a univariate function—desirability, which ranges from 0–1 (least to most desirable). From the desirability plot, 0.0 wt% Carbopol 974P and 3.0 wt% HEC was identified as the optimum composition for 3.5 mL volume and A max of 117 cm2 (Fig. 7C and F).
DISCUSSION
The prophylactic capabilities of a vaginal microbicide depends on the potency of the Active Pharmaceutical Ingredients (API) and the capability of the vehicle to deliver it appropriately to the vaginal lumen (4). For microbicide gels, the latter derives directly from the time course of gel deployment within the lumen. In general, an effective microbicide gel should achieve maximum coating of the vaginal epithelium, a coating which is retained for the desired duration of action (10,38). Thus, the deployment potential of microbicide gels should be a key component in product design. Only when this potential is understood, and can be related to gel composition during development, can rational design and optimization of vaginal gel carriers be realized.
Rheological behavior governs the ability of a gel to initially flow and coat the vaginal epithelial surfaces after insertion, as well as to resist further flow, facilitating retention (2,10,38). The most commonly used parameter to characterize the rheological behavior of vaginal gels is viscosity (10,38). However, because a majority of these gels are non-Newtonian, there is no single value of viscosity that characterizes spreading behavior. In general, it is the shear rate dependence of viscosity (which requires specification of at least two parameters), and also the value of the yield stress (an additional parameter, should a yield stress exist) governing the gel spreading process. The presence of Carbopol 974P in gels (but not HEC alone) gave rise to a non-zero yield stress. We evaluated the dependence of gel rheological behavior across jointly varying concentrations of Carbopol 974P and HEC. Consistent with results of other studies, viscosities of these gels increased as functions of concentration of the gelling agent (35). All gels exhibited shear thinning behavior, with viscosity decreasing by approximately one order of magnitude as shear rate increased from 1 s−1 to 100 s−1.
When a gel is inserted into the vagina using an applicator, it is delivered as a bolus near the fornix. In situ, the bolus experiences a continuous squeezing force from the vaginal musculature, initiating spreading (12,13). Therefore, in addition to the measurement of rheological properties of gels and its incorporation in constitutive models, in vitro methodologies have been developed to simulate gel flow and changes in gel properties in vivo (29,39). Once the gel begins to spread, it undergoes dilution with the vaginal secretions, likely further enhancing the spreading (29). The rate and extent of dilution of the formulation in the vagina may not be homogenous. This behavior may be due to several factors, including a) the highly heterogeneous vaginal epithelium (5,29), b) variability between women (37), c) cyclic changes in vaginal epithelium with changes in menstrual cycle (5,37,40), and d) variability in the local dilution of the gels with vaginal secretions (29). In order to study the effect of dilution of gels with vaginal fluid, we performed rheological characterization on gels diluted with VFS (1:4 VFS:gel) (29). This single ratio and the one time mechanical mixing do not mimic the kinetics of dilution in vivo, but they do provide an extreme case of dilution, which is relevant in understanding the potential effect of dilution on the deployment of gels. The degree of shear thinning decreased (i.e. n increased), and the consistency index (m) decreased after dilution with VFS. That is, the gels became less non-Newtonian. In addition, yield stress decreased on dilution. Consequently, there was a significant increase in the predicted area coated for the diluted gels as compared to the undiluted gels (Fig. 6). Details of the response to dilution with VFS differed amongst gels with varying composition, with a greater sensitivity to dilution when Carbopol 974P was present. Overall, computation of coated area and consequent computation of the score derived from interactions amongst the multiple rheological properties of the gels, whole and diluted. This methodology points to a 3.0 wt% HEC gel as the optimal candidate for a vaginal microbicide gel capable of quickly spreading throughout the vagina across the HEC-Carbopol 974P mixture space analyzed in this study. However, TFV, one of the two active ingredients incorporated in the gel is a sodium salt of ({[(2R)-1-(6-amino-9H-purin-9-yl)propan-2-yl]oxy}methyl)phosphonic acid. Since Carbopol is an ionic polymer; viscosity of Carbopol 974P gels was significantly affected by the presence of TFV. Therefore, the gels optimized in the work herein are specific to the delivery of 1 wt% TFV and will need to be re-optimized for other drugs or gelling agents.
To study the impact of gel volume on the score, we calculated scores for the 3.0 wt% HEC gel at varying volumes. S undiluted increased as volume increased (up to 4.0 mL), whereas S diluted exhibited a maximum at 3.5 mL. S combined was calculated from the arithmetic average of S undiluted and S diluted . The use of the arithmetic average of Sundiluted and Sdiluted is a first approximation, accounting for the complex process of progressive gel dilution during spreading in vivo. The 4:1 gel dilution with vaginal fluid (gel:vaginal fluid) is an upper bound on what is believed to be the extent of gel dilution with vaginal fluid in vivo. The arithmetic average for S equally weights the spreading of undiluted and fully diluted gel, and effectively assumes that net gel performance (i.e. the value of S) can be represented as the simple average of the lower (undiluted) and upper (diluted) bounds to the extent of gel spreading. S combined showed a maximum score at 3.5 mL (Fig. 8). This supports our selection of 3.0 wt% HEC as the optimum spreading gel at an administered volume of 3.5 mL. Since the optimized gel lies towards the extreme corner of the tested design space, additional studies were performed to ensure optimization was not compromised or limited by the design space. In order to evaluate the effect of increasing concentrations of HEC, we prepared gels with increasing HEC concentrations over the range 2.5–5.0 wt%. As shown in Fig. 8, the maximum for S undiluted was around 3.0 wt% HEC, and for S diluted it was around 4.0 wt% HEC. Since we co-optimized the scores for both undiluted and diluted gels—using the S combined —the 3.0 wt% HEC gel proved to be the optimum gel followed by the 4.0 wt% HEC gel.
The squeezing flow model has been found to predict spreading reasonably well, compared to data from in vivo imaging studies (Table V), for four polyacrylic acid or cellulose-based gels. Further improvements to the model are underway (14) to improve its accuracy and extend its applicability across different gels. Of course, all such modeling should be compared and validated against imaging studies whenever sufficient in vivo data are available. In addition to the above analysis, we compared the values of the score (undiluted, diluted) for the 3.0 wt% HEC gel created here with values for other microbicide gels and placebos, as well as for commercial over-the-counter vaginal gels used for lubrication and moisturization. Table VIII summarizes these results. In general, all undiluted gels exhibited spreading that did not exceed the target A max = 117 cm2. Upon dilution, spreading increased for all gels: for some, A still did not exceed A max , and thus S increased. However, for others, A exceeded A max ; for all gels evaluated here, this resulted in a lower score after dilution. We note that the HEC Universal Placebo gel is a 2.7 wt% HEC gel (22), and it has score values (0.77, 0.90; 0.84 undiluted, diluted; combined) very close to those of the optimal HEC gel created here (0.73, 0.94; 0.84). Comparisons with the other gels vary. TFV 1% microbicide gel from the CAPRISA 004 trial (0.92, 0.80; 0.86) and Carraguard (0.88, 0.51; 0.70) have good scores. In contrast, the 6% cellulose sulfate (0.58, 0.03; 0.31) and UC781 clinical gel (0.44, 0.00; 0.22) have low scores when diluted, because their significant reductions in viscosity cause them to be prone to leakage. The contrasts across these results exemplify the uncertainty about gel coating performance inherent to traditional, empirical vaginal gel design. The three commercial gels exhibit contrasting behavior. Two have higher scores after dilution: RepHresh® (0.24, 0.85; 0.55) and K-Y Jelly® (0.59, 0.82; 0.71). The other gel, Replens®, exhibits the opposite trend (0.81, 0.56; 0.69).
Our work is the first to relate gel composition—comprised of two commonly used gelling agents—to gel performance as predicted using a deterministic vaginal squeezing flow model. The methodology described herein offers a quick, empirically-derived, mathematical method that can be used to select composition of vaginal semisolid gels based on performance predictions. Furthermore, use of the objective function establishes an analytical and biologically relevant method for gel evaluation that can easily be modified to incorporate a range of desired performance parameters. Application of this model could enable expedited characterization and prediction of performances of the next generation of microbicide gels. This approach is flexible, and by knowing the volume of the gel and the predicted area coated, one can create objective predictions about gel performance in vivo prior to human trials. Our methodology here includes only the pre-dilution and post-dilution rheological properties and deployment predictions. However, this DOE approach can be diversified to account for additional critical parameters of the formulation such as other gelling agents and excipients, drug release rate, drug stability, bioadhesion, toxicity, and dilution with other ambient vaginal fluids, such as semen. Indeed, this approach could potentially be applied to a diverse range of vaginal delivery products used for artificial insemination, antifungal delivery, vaginal moisturization and hormone delivery. Furthermore, use of such an approach circumvents the problems associated with traditional methods of gel optimization and composition selection, which contain many subjective elements, and can lead to the reassessment of gel compositions to meet specific clinical applications, beyond microbicides.
CONCLUSION
This study is the first to describe the composition-property-performance relationship of vaginal semisolid drug delivery gels consisting of HEC and Carbopol 974P. The multivariate optimization approach introduced here, used in conjunction with rheological characterizations, fluid flow models and statistical tools, offers an integrated multi-tier approach for the optimization and selection of vaginal microbicide gels prior to in vivo investigations.
Abbreviations
- A:
-
vaginal area coated by the gel
- A* :
-
value of A at which the score becomes zero
- Amax :
-
area of the vaginal canal
- API:
-
active Pharmaceutical Ingredient
- CPPR:
-
composition-property-performance relationship
- HEC:
-
Hydroxyethylcellulose
- MDOE:
-
mixture design of experiment
- RSM:
-
response surface modeling
- S:
-
score
- Scombined :
-
average of Sundiluted and Sdiluted
- Sdiluted :
-
score for gels diluted 1:4 with VFS
- Sundiluted :
-
score for undiluted gels
- TFV:
-
Tenofovir
- V:
-
volume of the gel
- VFS:
-
vaginal fluid simulant
- VL :
-
volume of the gel leaked
REFERENCES
das Neves J, Bahia MF. Gels as vaginal drug delivery systems. Int J Pharm. 2006;318:1–14.
Barnhart K, Kulp JL, Rosen M, Shera DM. A randomized trial to determine the distribution of four topical gel formulations in the human vagina. Contraception. 2009;79:297–303.
Barnhart KT, Pretorius ES, Shera DM, Shabbout M, Shaunik A. The optimal analysis of MRI data to quantify the distribution of a microbicide. Contraception. 2006;73:82–7.
das Neves J, da Silva MV, Goncalves MP, Amaral MH, Bahia MF. Rheological properties of vaginal hydrophilic polymer gels. Curr Drug Deliv. 2009;6:83–92.
Hendrix CW, Cao YJ, Fuchs EJ. Topical microbicides to prevent HIV: clinical drug development challenges. Annu Rev Pharmacol Toxicol. 2009;49:349–75.
Shattock RJ, Moore JP. Inhibiting sexual transmission of HIV-1 infection. Nat Rev Microbiol. 2003;1:25–34.
Grant RM, Hamer D, Hope T, Johnston R, Lange J, Lederman MM, et al. Whither or wither microbicides? Science. 2008;321:532–4.
Cohen J. HIV/AIDS. At last, vaginal gel scores victory against HIV. Science 329:374–5.
Justin-Temu M, Damian F, Kinget R, Van Den Mooter G. Intravaginal gels as drug delivery systems. J Women’s Health. 2004;13:834–44.
Owen DH, Peters JJ, Kieweg SL, Geonnotti AR, Schnaare RL, Katz DF. Biophysical analysis of prototype microbicidal gels. J Pharm Sci. 2007;96:661–9.
Barnhart KT, Pretorius ES, Shaunik A, Timbers K, Nasution M, Mauck C. Vaginal distribution of two volumes of the novel microbicide gel cellulose sulfate (2.5 and 3.5 mL). Contraception. 2005;72:65.
Kieweg Sarah L, Katz David F. Squeezing flows of vaginal gel formulations relevant to microbicide drug delivery. J Biomech Eng. 2006;128:540–53.
Kieweg SL, Katz DF. Interpreting properties of microbicide drug delivery gels: analyzing deployment kinetics due to squeezing. J Pharm Sci. 2007;96:835–50.
Szeri AJ, Park SC, Verguet S, Weiss A, Katz DF. A model of transluminal flow of an anti-HIV microbicide vehicle: combined elastic squeezing and gravitational sliding. Phys Fluids. 2008;20:83101.
Nicolaou CA, Brown N, Pattichis CS. Molecular optimization using computational multi-objective methods. Curr Opin Drug Discov Devel. 2007;10:316–24.
Handl J, Kell DB, Knowles J. Multiobjective optimization in bioinformatics and computational biology. IEEE/ACM Trans Comput Biol Bioinform. 2007;4:279.
Rajamani R, Good AC. Ranking poses in structure-based lead discovery and optimization: current trends in scoring function development. Curr Opin Drug Discov Devel. 2007;10:308–15.
Chopra S, Motwani SK, Iqbal Z, Talegaonkar S, Ahmad FJ, Khar RK. Optimisation of polyherbal gels for vaginal drug delivery by Box-Behnken statistical design. Eur J Pharm Biopharm. 2007;67:120–31.
Furlanetto S, Cirri M, Maestrelli F, Corti G, Mura P. Study of formulation variables influencing the drug release rate from matrix tablets by experimental design. Eur J Pharm Biopharm. 2006;62:77–84.
Chu JS, Amidon GL, Weiner ND, Goldberg AH. Mixture experimental design in the development of a mucoadhesive gel formulation. Pharm Res. 1991;8:1401–7.
Valenta C. The use of mucoadhesive polymers in vaginal delivery. Adv Drug Deliv Rev. 2005;57:1692–712.
Tien D, Schnaare RL, Kang F, Cohl G, McCormick TJ, Moench TR, et al. In vitro and in vivo characterization of a potential universal placebo designed for use in vaginal microbicide clinical trials. AIDS Res Hum Retroviruses. 2005;21:845–53.
Cummins Jr JE, Guarner J, Flowers L, Guenthner PC, Bartlett J, Morken T, et al. Preclinical testing of candidate topical microbicides for anti-human immunodeficiency virus type 1 activity and tissue toxicity in a human cervical explant culture. Antimicrob Agents Chemother. 2007;51:1770–9.
Patton DL, Sweeney YT, Balkus JE, Rohan LC, Moncla BJ, Parniak MA, et al. Preclinical safety assessments of UC781 anti-human immunodeficiency virus topical microbicide formulations. Antimicrob Agents Chemother. 2007;51:1608–15.
Terrazas-Aranda K, Van Herrewege Y, Lewi PJ, Van Roey J, Vanham G. In vitro pre- and post-exposure prophylaxis using HIV inhibitors as microbicides against cell-free or cell-associated HIV-1 infection. Antiviral Chem Chemother. 2007;18:141–51.
Barditch-Crovo P, Deeks SG, Collier A, Safrin S, Coakley DF, Miller M, et al. Phase i/ii trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob Agents Chemother. 2001;45:2733–9.
Bateman C. Tenofovir gel—the new HIV prevention ‘banker’? S Afr Med J. 2007;97:496–8.
Mayer KH, Maslankowski LA, Gai F, El-Sadr WM, Justman J, Kwiecien A, et al. Safety and tolerability of tenofovir vaginal gel in abstinent and sexually active HIV-infected and uninfected women. AIDS. 2006;20:543–51.
Lai Bonnie E, Xie Yao Q, Lavine Michael L, Szeri Andrew J, Owen Derek H, Katz David F. Dilution of microbicide gels with vaginal fluid and semen simulants: effect on rheological properties and coating flow. J Pharm Sci. 2008;97:1030–8.
Cornell JA. Experiments with mixtures. 3rd ed. New York: Wiley; 2002.
Owen DH, Katz DF. A vaginal fluid simulant. Contraception. 1999;59:91–5.
Henderson MH, Couchman GM, Walmer DK, Peters JJ, Owen DH, Brown MA, et al. Optical imaging and analysis of human vaginal coating by drug delivery gels. Contraception. 2007;75:142–51.
Mauck Christine K, Katz D, Sandefer Erik P, Nasution Marlina D, Henderson M, Digenis George A, et al. Vaginal distribution of Replens and K-Y Jelly using three imaging techniques. Contraception. 2008;77:195–204.
Kilmarx PH, Blanchard K, Chaikummao S, Friedland BA, Srivirojana N, Connolly C, et al. A randomized, placebo-controlled trial to assess the safety and acceptability of use of carraguard vaginal gel by heterosexual couples in Thailand. Sex Transm Dis. 2008;35:226–32.
Wang Y, Lee CH. Characterization of a female controlled drug delivery system for microbicides. Contraception. 2002;66:281–7.
Ramjee G, Morar NS, Braunstein S, Friedland B, Jones H, van de Wijgert J. Acceptability of Carraguard, a candidate microbicide and methyl cellulose placebo vaginal gels among HIV-positive women and men in Durban. South Africa AIDS Res Ther. 2007;4:20.
Barnhart Kurt T, Izquierdo A, Pretorius ES, Shera David M, Shabbout M, Shaunik A. Baseline dimensions of the human vagina. Hum Reprod. 2006;21:618–22.
Owen DH, Peters JJ, Katz DF. Rheological properties of contraceptive gels. Contraception. 2000;62:321–6.
Szeri AJPS, Tasoglu S, Katz DF. Effects of dilution on coating flow of an anti-HIV microbicide vehicle. In American Physical Society, Minneapolis, MN; 2009.
Poonia B, Walter L, Dufour J, Harrison R, Marx PA, Veazey RS. Cyclic changes in the vaginal epithelium of normal rhesus macaques. J Endocrinol. 2006;190:829–35.
ACKNOWLEDGMENTS
This work was generously supported by CONRAD under a Cooperative Agreement with USAID (HRN-A-00-98-00020-00). The views expressed by the authors do not necessarily reflect those of USAID.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material
(DOCX 295 kb)
Rights and permissions
About this article
Cite this article
Mahalingam, A., Smith, E., Fabian, J. et al. Design of a Semisolid Vaginal Microbicide Gel by Relating Composition to Properties and Performance. Pharm Res 27, 2478–2491 (2010). https://doi.org/10.1007/s11095-010-0244-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-010-0244-1