ABSTRACT
Purpose
To investigate the cause of the observed instability of dulanermin in 100 ml polyolefin (PO) infusion bags containing saline.
Methods
Diluted dulanermin in IV bags was collected and frozen prior to analysis by size exclusion chromatography. The UV absorption profiles of the IV bag solutions were characterized by using spectrophotometry. Inductively Coupled Plasma Mass Spectrometry (ICP-MS) measured the metal content. Leachables from IV bags were identified by LC-UV-high resolution MS/MS analysis.
Results
An elevated loss of dulanermin monomers was observed only in 100 ml PO bags. These IV bag solutions have a compound that contains zinc and has absorbance at 320 nm. This compound was identified to be 2-Mercaptobenzothiazole, and its zinc salt and was found to come from the stopper used in the 100 ml PO bags. The manufacturer has subsequently corrected this problem by using non-latex components in the 100 ml PO IV bag.
Conclusions
End-users need to be aware that IV bags made from a particular polymer by the same manufacturer may contain components or use a manufacturing process that results in a different product. Analysis of samples after freezing and thawing proved to be useful in identifying potential incompatibility of dulanermin in the IV bags.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Intravenous drug administration is commonly used for many types of therapies, especially for oncology drugs. Since there is the potential of drug instability due to dilution or interaction with plastic surfaces or compounds that are absorbed into solution from the plastic surfaces, the physical and chemical stability of drugs in IV infusion bags and infusion sets should be investigated to ensure compatibility and safety. This assessment process is complex due to the large selection of bag types in the market and the complexity of the plastic materials. In addition, parameters such as temperature and duration of storage in the bags may affect the outcome of the study. Recently, the practice of using a centralized intravenous additive service (CIVAS) in Europe has resulted in often preparing IV drugs in advance and freezing the IV bag and solution for later thawing and administration. Thus, the assessment of product compatibility should also include a freeze-thaw step.
The materials and the manufacturing process used in the construction of IV infusion bags and sets are complicated and generally regarded as proprietary by the manufacturers. The plasticizers, polymerization initiators, and stabilizing additives commonly used in IV bag production to enhance their manufacturability and/or physical properties can differ in different types or the same type of plastic materials [1–3]. Compounds that include organics, metals and volatile sulfur-containing compounds may leach from the plastic IV infusion containers into the contained solution [4–7]. The inter- and intra-batch variability of the leachables’ levels in commercially available IV solutions across the same or different manufactures can vary significantly [8]. Jenke provided an extensive review on the compatibility of plastic materials with pharmaceutical products with special attention given to the extractable and leachables related to safety [9]. Although leachables may not be vastly harmful to humans, they may alter the pH of the diluents or interact with the drug and impact product safety and efficacy. As an example, zinc-based organic compounds are often used to initiate polymerization, and wide-ranging trace levels of zinc are found in IV bag diluents [10]. The zinc can contribute to particulate or precipitate formation as shown for Protonix IV, Zosyn® (original formulation), and aminophylline in IV solutions [4,11]. Another example is di-2-ethylhexyl-phthalate (DEHP), a known carcinogen found in many IV diluents stored in PVC bags. A further complication is that the level of DEHP that leaches into the IV solution depends on the characteristics of the drug, formulation and concentration, as well as the temperature, duration and total area of contact with the interior plastic surface [8,12–14].
Losses of drug from the solution by adsorption onto the walls of the bags or infusion sets are also a concern. This could result in under-dose, and a greater amount of drug to compensate for the adsorption loss may be required. Although the significance of the inner surface morphologies of IV bags is not certain, different brands of IV bags can differ very significantly [15]. Loss of Factor VIII (FVIII) activity in IV infusion systems was reported to be caused by FVIII binding to plastic surfaces [16,17]; the two reports differ in that the former reported initial drop in recovery, while the latter reported a continuous loss of activity. The differences in the findings may reflect the difference in the types and sizes of the containers.
The most common commercially available IV infusion bags and infusion sets in the US are polyvinyl chloride (PVC) and polyolefin (PO). There are numerous reports on the leachables, such as metals, DEHP, cyclohexanone, and other organic and acidic compounds, found in the solution of PVC bags [15,18–22]. The leachables have the potential to interact with and alter the drug to be administered [23,24]. As mentioned earlier, DEHP is a carcinogen, but PVC infusion bags and sets having DEHP as the plasticizer are still widely used in the US despite the FDA’s recommendation that health-care practitioners and patients take action to reduce DEHP exposures.
Polyolefin (PO), a relatively new material used in IV infusion bags in the US and the most popular alternative to PVC bags, is produced from olefin or alkene polymers. The PO bags and IV sets can be polypropylene (PP), polyethylene (PE), or ethylene and propylene copolymer. Manufacturers usually claim that their PO bags and infusion sets are essentially free of plasticizers, especially DEHP; however, other leachables, such as organic acids, Irganox, and anti-oxidants have been reported [6,7,9,25,26].
Many drugs diluted in PO containers have been reported to be more stable than the drug in PVC bags [6–8,13] for a variety of reasons. It has also been shown that that drug loss by adsorption onto PO surfaces is less compared to PVC surfaces [14,27]. One exception is insulin where adsorption in PO containers is substantial [14].
This report discusses the evaluation of 100 ml and 250 ml PVC and PO IV infusion bags and sets, which are most commonly used in the US, by using recombinant dulanermin, an investigational anti-cancer agent, as the test drug.
MATERIALS AND METHODS
Materials
Commercially available polyvinyl chloride (PVC) infusion bags containing 0.9% Sodium Chloride Injection, USP were purchased from a major US PVC bag manufacturer A. The bags from the same and different lots were evaluated before their expiration date. Both of the 100 ml and 250 ml PVC bags are very flexible and packaged with a secondary wrapper.
Commercially available polyolefin (PO) infusion bags containing 0.9% Sodium Chloride Injection, USP were purchased from a major US PO bag manufacturer B. The manufacturer claimed that these IV bags are made from 100% ethylene and propylene copolymer and free of plasticizers and additives. The 100 ml bags are rigid and sold without a secondary packaging, whereas the 250 ml bags are flexible, multiple-layered, and sold with a secondary packaging wrapper. A 13 mm rubber stopper, at the med-add port contacting the IV solution, is present only in the 100 ml bags. The 100 ml PO bags produced prior to July 2007 with lot numbers before J7H956 contain natural rubber stoppers, whereas the lots produced after July 2007 contain 13 mm latex-free chlorobutyl stoppers. Bags from the same and different lots were evaluated before their expiration date.
Two types of latex-free IV infusion sets were purchased from Alaris Medical Systems, San Diego, CA and Baxter Healthcare Corp., Deerfield, IL. The former (REF 2260–0500) has no inline filter, while the later (2H6480) has an inline filter.
Dulanermin is an apoptosis-inducing ligand belonging to the tumor necrosis factor (TNF) gene super family [28]. It consists of amino acid residues 114 to 281 of the natural ligand and is a non-covalent, zinc-bound 60-kDa homotrimer. X–ray crystallography studies reveal that a zinc ion is located at the trimer interface, coordinated by the single cysteine residue of each monomer [29,30]. The zinc ion is required for maintaining the native structure and the stability and, hence, the biological activity of dulanermin [29,30]. The trimeric form binds with two known death receptors and induces apoptosis in vitro in a wide variety of human cancer cells [30,31]. The recombinant-derived dulanermin molecule was produced at Genentech, Inc., South San Francisco, CA as a single-use lyophilized formulation and contains no preservatives. After reconstitution with Sterile Water for Injection, the resulting solution contains 20 mg/ml dulanermin in 0.5 M arginine succinate, 20 mM TRIS HCl, 0.02% polysorbate 20, pH 7.2 [32,33].
TSK G2000SWXL 7.8 mm x 30 cm HPLC columns were purchased from TosoHaas Bioscience LLC, Montgomeryville, PA. 2-Mercaptobenzothiazole zinc salt, 97% was purchased from AK Scientific, Inc., Mountain View, CA, while 2-Mercaptobenzothiazole was purchased from Spectrum Chemical Mfg. Corp., Gardena, CA and Fluka, Sigma-Aldrich GmbH, Steinheim, Germany.
Methods
Dilution of Dulanermin into IV Bags Containing 0.9% Sodium Chloride Solution
Dulanermin was diluted into 100 ml or 250 ml IV bags (PVC and PO) by the addition of dulanermin after the removal of an equal volume of saline from the IV bags. The final nominal dulanermin concentrations were 0.08, 0.2, and 18 mg/ml. An aliquot (10 ml) was aseptically removed from each bag immediately after the addition of dulanermin and used as the initial time point (< 5 min) sample. The remaining liquid in the IV bags was incubated at 30°C overnight, and another 10 ml sample was removed from each bag the next morning (∼ 16 h) and used as the 16 h time point sample.
The remaining dulanermin solution in the IV bags was then passed through an IV solution set at ambient temperature. The PVC bags from manufacturer A were coupled with Baxter Solution Sets and delivered without the aid of an infusion pump. The PO bags from manufacturer B were coupled with Alaris Solution Sets with or without using a Gemini pump. The flow rate was approximately 100 ml per hour. Samples were collected, frozen immediately, and stored at –70°C for greater than 16 h prior to analysis. Multiple freeze (at –70°C) and thaw (at ambient temperature) cycles of the diluted dulanermin collected from the IV bags were also performed to test the compatibility of dulanermin with the IV solutions under the freeze-thaw stress condition.
pH Determination
The pH was measured using a Radiometer Copenhagen PHM82 pH meter at room temperature. Standard solutions of pH 4.00, 7.00 and 10.00 (EM Science) were used for calibration of the pH meter.
Protein Concentration Determination
The concentration of dulanermin was determined with a diode array spectrophotometer (Model 8453, Hewlett Packard, Mountain View, CA) using absorptivity at 277 nm of 1.53 (mg/ml)−1 cm−1. The absorptivity was determined for dulanermin using a modification of the method of Bewley [34].
Spectrophotometric Scanning
The absorption profile of various saline solutions was characterized by spectral scanning of solutions from 240 to 500 nm using a diode array spectrophotometer (Model 8453, Hewlett Packard, Mountain View, CA).
High Performance Size Exclusion Chromatography
The molecular size distribution of dulanermin monomer and the amount of disulfide-linked dimer was assessed by sodium dodecyl sulfate (SDS) non-native size exclusion chromatography at 0.1% SDS using a TSK G2000SWXL column on an Agilent 1100 HPLC System. Prior to analysis, free sulphydryls were blocked by reaction with iodoacetamide in the presence of 0.5% SDS at 50°C and pH 9. This is to prevent the formation of disulphides between monomers upon the dissociation of dulanermin homotrimer by SDS.
Zinc Analysis
The total zinc content was determined by Inductively Coupled Plasma Mass Spectrometry (ICP-MS). All samples were diluted to a final concentration of 5% nitric acid prior to analysis. Measurements were made with a Thermo Scientific X series II ICP-MS instrument. A small volume of each sample was dissociated into its elemental components using an argon plasma torch, which ionizes the elements so they can be subsequently identified by mass using conventional mass spectroscopy. The detection range of the ICP-MS method used is 1–100 ppb x a sample dilution factor.
Identification of Leachables
Reverse phase HPLC-UV-high resolution MS/MS analysis was performed to identify the leachables from the 100 ml polyolefin bag and the stopper in its Med-Add port. UV-absorption was monitored at 310 nm, and the eluent was connected to an electrospray ionization source. A high resolution Orbitrap mass analyzer (Thermo Fisher Scientific, San Jose, CA) was used for measuring both intact mass and fragmentation spectrum. Mass accuracy of 5 ppm allowed predicting possible elemental compositions for the species with absorption at 310 nm. Product ion spectrum (ms/ms) was used to match the spectrum with those species predicted from elemental composition.
RESULTS AND DISCUSSION
Surface-to-Liquid Ratio of IV Infusion Bags
To determine the surface-to-liquid ratio of the PVC and PO infusion bags, the polymer surface areas were measured. Nominal liquid volumes in the IV bags were obtained from the product data sheets provided by the manufacturers. The ratios of the polymer surface areas to the nominal liquid volume were then calculated. The results indicated that the IV bags containing 100 ml saline have surface-to-volume ratios 70% (PVC) and 130 % (PO) higher than that of the 250 ml bags (Table I). These results suggest that amount of drug that potentially interacts or adsorbs to the polymer surface and the amount of leachates from these surfaces may be expected to be greater for the 100 ml bags.
Dulanermin is Stable in 100 ml PVC Bags, but Not Compatible with 100 ml Polyolefin Bags
To assess the compatibility of dulanermin with the IV infusion bags, the protein was diluted into 100 ml IV bags through their Med-Add ports to final concentrations of 0.08, 0.2, and 18 mg/ml after the removal of an equal volume of saline from the IV bags. The stability of dulanermin was examined immediately (< 5 min) and after 16 h residence time in the bags.
Results revealed that the measured and expected dulanermin concentrations are comparable from the respective bags (Table II), suggesting no detectable loss of dulanermin in the PVC and PO bags by surface adsorption.
The stability of dulanermin was analyzed by size exclusion chromatography under denaturing (SDS-SEC) conditions. Dulanermin is stable regardless of the protein concentration and duration of exposure in the PVC bags (Table II). Furthermore, the sample is stable upon freezing and thawing, as the monomer content of the protein remained unchanged (Fig. 1). Results from the 100 ml PO bags revealed that dulanermin is most stable in the IV bags at the highest concentration (18 mg/ml) and least stable at the lowest concentration (0.08 mg/ml) where the dulanermin monomer decreased by as much as 5% (Table II and Fig. 1) as compared to the original solution. This decrease in monomer content, concurrent with the increase of disulfide-linked dimer, was seen immediately (< 5 min) upon the dilution of dulanermin into the 100 ml PO bags. After 16 h in the IV bags, only slight additional loss of monomer content was seen.
Stability of dulanermin in 100 ml PVC, (solid symbols) or PO (open symbols) IV infusion bags as determined by the effect of freezing and thawing. Dulanermin was diluted into 100 ml IV bags to a final concentration of 0.08 mg/ml and then removed for analysis immediately (● and ○) or after 16 h (▲ and △) at 30°C. The solution was then stressed by multiple cycles of freezing and thawing. Linear regression lines are shown for clarity of visualizing the data trend.
Overall, these results indicate that dulanermin is stable in and compatible with the 100 ml PVC bags but is not stable in the 100 ml PO bags. These results suggest that either the saline or the surfaces of the PVC bags and PO bags are different.
UV Absorption Spectroscopy of Saline from IV Bags
To determine whether the observed stability differences between PVC and PO bags may be caused by differences between the saline solutions stored in the PVC and PO bags, the saline contained in each type of bag was analyzed by scanning UV/Vis absorption spectroscopy from 240 to 500 nm (Fig. 2). As expected, freshly prepared saline that was never stored in an IV bag did not have any absorbance over the 240 to 500 nm range. However, saline stored in either 100 ml PVC or PO bags exhibited higher UV absorption over the range of 240 to ∼320 nm as compared to saline that was never exposed to IV bag interior contact surfaces. There are clear differences between the PVC and PO bags in this absorption range. Even more noticeable is the appearance of a strong absorption peak at ∼320 nm, which was only observed in the saline from the 100 ml PO bags. Moreover, the intensity of this peak varied between the 100 ml PO bags from the same manufacturing lot. Saline from the 100 ml and 250 ml PVC and 250 ml PO IV bags as well as saline that was never stored in the bags did not exhibit this absorption peak at 320 nm. What was surprising was that saline in PO bags of different size made by the same manufacturer showed huge differences in their UV absorption, particularly at 320 nm.
To further determine whether the 320 nm absorption peak was contributed by leachables from the 100 ml PO bags, the IV bags were incubated at 60°C and monitored. The intensity of the 320 nm peak increased proportionally with incubation time (Fig. 3). These results suggest that the 320 nm peak came from a compound leached from the 100 ml PO bags and that the compound could be leached at a faster rate at elevated temperature.
Zn Content of Saline in IV Bags
The UV spectral analysis revealed a difference between saline from PVC vs. PO bags. Since zinc-containing compounds are commonly used in the manufacturing of polymers, an analysis of zinc content by ICP-MS was undertaken to ascertain if different amounts of zinc were leached from the different IV bags. Results revealed that the zinc content was higher for the saline solution from 100 ml PO bags (Table III) as compared to the other saline solutions. In addition, the zinc levels varied between the 100 ml PO bags of the same manufacturing lot and could be correlated with the intensity of the 320 nm absorption peak.
These results suggest that a zinc-containing compound having an absorbance at 320 nm was leached from the 100 ml PO bags and that this leachate is not present in the saline from 100 ml PVC and 250 ml PO bags. In addition, the assay shows that there are likely variable amounts of this compound between bags, suggesting there may be lot-to-lot variation.
Freeze-and-Thaw Stress Accelerated Dulanermin Degradation in PO Bags
As previously mentioned, the practice of preparation of the IV bag dosing solution that includes a freeze-thaw step is becoming more frequent. Thus, to examine the effect of such a stress condition on the stability of the diluted dulanermin from IV bags, the samples having 0.08 mg/ml dulanermin were taken, placed into polypropylene tubes, and subjected to freeze-and-thaw cycles and then analyzed. Results revealed that dulanermin from 100 PVC bags was stable upon freezing and thawing, whereas the samples from 100 ml PO bags were not stable; significant monomer loss proportional to the number of freeze-and-thaw cycles (Fig. 1) was observed.
Although solutions in IV bags are not always subjected to freezing and thawing under standard condition for use, it is useful to assess stability under stressed conditions. This is often done in comparability protocols since, for example, a protein made using different processes is expected to respond in a similar fashion to a stressed condition. Thus, assessment under a stressed condition can be used to identify potential incompatibility of the protein drug with the IV bags.
Comparing the Components of PVC and PO IV Infusion Bags
In an effort to locate the source of the leachables contributing to the elevated zinc and the 320 nm absorption, 100 ml and 250 ml IV bags were disassembled by taking away all of the removable parts and examined (Fig. 4). Regardless of the types and the manufacturers of the IV bags, all bags contained small rubber components besides PVC and PO polymers. Upon close examination of the parts and structures, all of the small rubber components were not in direct contact with saline except for that of the 100 ml PO bags. The 100 ml PO infusion bag contained a 13 mm rubber stopper in its Med-Add port that was in direct contact with the stored saline. This observation suggested that the leachables in the 100 ml PO bag could be from the 13 mm rubber stopper.
UV Absorption and Zinc Analysis of Leachates from the 13 mm Stoppers from the 100 ml PO IV Bags
To determine if the elevated zinc levels and 320 nm absorption peak were from the leachates from the 13 mm rubber stopper, these stoppers were removed from 100 ml PO bags and used as stoppers for 3 ml glass vials filled with control solutions of freshly prepared saline. The vials were then inverted to ensure contact of the saline with the stopper and incubated for 7 days at 30°C. Spectral UV/Vis analysis of the saline from these vials showed a 320 nm absorption peak, whereas the control solutions did not exhibit the 320 nm peak (Fig. 5). Furthermore, an elevated zinc level was obtained from the inverted vials (Table IV). These results confirmed that the elevated zinc and 320 nm absorption peak of the saline were due to material that leached from the rubber stopper used in the 100 ml PO bags. These results also explain the differences between the 250 and 100 ml PO bags. Interestingly, the UV/Vis absorption of the saline contained in 250 ml PO bags is much lower than that observed for the PVC bags (Fig. 2), which is consistent with the claims that the PO polymers are essentially free of many potential leachates as compared to PVC. A comparison of 100 ml PVC bags with 250 ml bags also shows a greater UV absorption, which is consistent with the larger surface-area-to-volume ratio for the 100 ml bags (Fig. 2).
Identification of the Leachate by LC-UV-High Resolution MS/MS
The saline solution from the 100 ml PO bags was subjected to analysis using LC-UV-high resolution MS/MS in order to identify the chemical composition of the leachates from the 13 mm rubber stopper. Absorption at 310 nm was monitored, and chromatograms of saline from the 100 ml PO bags showed the presence of a peak at 14.63 min, which was absent in the control saline solution. Electrospray mass spectrum at the corresponding retention time showed a strong peak with an m/z 167.9993 with a measurement accuracy of +/- 5 ppm. This accuracy of 5 ppm allowed prediction of possible elemental compositions of the species involved. There were only two possibilities: C7H6N1S2 (−2.15 ppm) and C6H2O5N3 (+3.02 ppm), and the first one corresponds to 2-Mercaptobenzothiazole. Further analysis using high resolution MS/MS revealed that the fragmentation pattern and their m/z values only matched with that predicted for 2-Mercaptobenzothiazole (Fig. 6).
LC-UV-high resolution mass spectrum of the species with 320 nm absorption from the leachables of 13 nm stopper showed a strong peak at m/z 167.9993 (+/- 5 ppm). Two elemental compositions could be predicted from this value. High resolution MS/MS of m/z 167.99 species matched with one of those predicted as 2-mercaptobenzothiazole.
UV Spectral Analysis of 2-Mercaptobenzothiazole and its Zinc Salt in Saline
2-Mercaptobenzothiazole and its salt (Zinc-2-Mercaptobenzothiazole) at 5 and 10 µM in saline were analyzed by spectral scanning from 240 to 500 nm. These saline solutions have a 320 nm absorption peak similar to that observed for saline from the 100 ml PO bags (Fig. 7a and b). Moreover, the intensity of the absorbance peaks was proportional to the concentration of the 2-Mercaptobenzothiazole and zinc-2-Mercaptobenzothiazole. Based on the peak heights determined for saline from the two 100 ml PO bags, the amount of 2-Mercaptobenzothiazole or its zinc salt leached from these 100 ml PO bags was estimated to be at 5 or 10 µM, respectively.
Stability of Dulanermin After Incubation with 2-Mercaptobenzothiazole and its Zinc Salt
Dulanermin stability at 0.08 mg/ml was assessed after incubating 16 h at 30°C in solutions containing freshly prepared saline and 2-Mercaptobenzothiazole or its zinc salt at 5 or 10 µM. The results revealed that after freeze-thaw, 2-Mercaptobenzothiazole and its salt destabilized dulanermin similarly to what was observed for saline contained in the 100 ml PO IV bags (Fig. 8a and b).
Stability of dulanermin in saline solutions containing 0 ( ), 5 (
), 5 ( ), or 10 (
), or 10 ( ) µM of 2-Mercaptobenzothiazole (a) and its zinc salt (b) and the effect of freezing and thawing. Dulanermin was at 0.08 mg/ml in freshly prepared saline with varying concentrations of 2-Mercaptobenzothiazole or its zinc salt. The solution was stressed by multiple cycles of freezing and thawing. Linear regression lines are shown for clarity of visualizing the data trend.
) µM of 2-Mercaptobenzothiazole (a) and its zinc salt (b) and the effect of freezing and thawing. Dulanermin was at 0.08 mg/ml in freshly prepared saline with varying concentrations of 2-Mercaptobenzothiazole or its zinc salt. The solution was stressed by multiple cycles of freezing and thawing. Linear regression lines are shown for clarity of visualizing the data trend.
Results of Manufacturing Changes
During the course of this study, the PO manufacturer changed the IV bag production process. All of the 100 ml PO bags manufactured before July 2007 used natural rubber stoppers (latex containing) in the Med-Add port, while the 100 ml PO bags manufactured after July 2007 used latex-free synthetic chlorobutyl rubber. The saline solutions from the bags produced before and after July 2007 were examined and compared. The saline solution from 100 ml PO bags with latex-free 13 mm rubber stoppers does not exhibit the absorption peak at 320 nm (Fig. 9), and zinc is below the detection limit of 200 ppb by ICP-MS analysis, which is significantly lower than what was determined in the 100 mL PO bag with the natural rubber stopper and highest absorbance value at 320 nm (Table V). Furthermore, dulanermin is stable in and compatible with the latex-free 100 ml PO bags and has stability comparable to duanermin in a 100 mL PVC IV bag (Fig. 10).
Stability as a result of freezing and thawing of dulanermin in latex-free 100 ml PO IV infusion bags with a chlorobutyl stopper compared to dulanerin in a 100 mL PVC IV infusion bag. Dulanermin was diluted into 100 ml PO IV bags to final concentrations at 0.08 ( ), 0.2 (
), 0.2 ( ) and 16 (
) and 16 ( ) mg/mL and into a 100 mL PVC IV bag to a final concentration of 0.1 mg/mL(
) mg/mL and into a 100 mL PVC IV bag to a final concentration of 0.1 mg/mL( ). Solutions were then removed for analysis after 16 h at 30°C and stressed by multiple cycles of freezing and thawing. Linear regression lines are shown for clarity of visualizing the data trend.
). Solutions were then removed for analysis after 16 h at 30°C and stressed by multiple cycles of freezing and thawing. Linear regression lines are shown for clarity of visualizing the data trend.
CONCLUSIONS
The studies presented here have shown that a freezing-and-thawing stress condition of diluted dulanermin from IV bags enabled the detection of a potential incompatibility between the diluted drug and 100 ml PO IV bags. Although the current practice for preparing dulanermin IV administration does not involve freezing and thawing, utilization of a centralized intravenous additive service (CIVAS) where the IV solution for small molecule drugs is stored frozen is quite common and gaining popularity [35–37]. Essentially, CIVAS would prepare the IV solutions in advance, store the solution in a freezer, and thaw the frozen solutions prior to administration. Therefore, incorporating freeze and thawing to study drug stability and compatibility in IV bags not only can provide a quick and early detection of potential incompatibility between a therapeutic agent and the IV bag solution but also can be useful in assessing this practice in CIVAS settings.
We thought, initially, that the substances that were absorbing at ∼320 nm may be leachates that migrated from plastic polymers into the IV solution, since zinc-based organic compounds have been commonly used as polymerization initiators in the manufacturing of plastics [1, 4, 5]. The studies reported here clearly demonstrate that a particular zinc compound, Zinc-2-Mercaptobenzothiazole, used in manufacturing of rubber stoppers had migrated into the stored saline in the 100 ml PO IV bag. These studies have provided an explanation for the differences observed between different size PO bags from the same manufacturer. Most importantly, the end-user needs to be aware that IV bags made from a particular polymer by the same manufacturer may contain components or methods of manufacture that result in a different product that may have an impact on the quality of the pharmaceutical being prepared for IV administration.
Zinc-2-Mercaptobenzothiazole, commonly used in natural rubber formulation, is a rubber accelerator in the production of sulfur-cured latex. The manufacturer of the PO bags changed the rubber stopper in the original 100 ml PO bags from natural rubber to synthetic chlorobutyl rubber in July 2007, in the very late stages of this investigation. “Latex-free” was added to the label of the new 100 ml PO bags. The saline solution from the new latex-free 100 ml PO bags now has undetectable levels of zinc and does not show a strong 320-nm absorbance. In particular, the dulanermin is stable in and compatible with the new latex-free 100 ml PO bags.
Abbreviations
- CIVAS:
-
centralized intravenous additive service
- DEHP:
-
di-2-ethylhexyl-phthalate
- FVIII:
-
Factor VIII
- ICP:
-
Inductively Coupled Plasma
- IV:
-
intravenous
- LC:
-
Liquid Chromatography
- MS:
-
Mass Spectrometry
- PE:
-
polyethylene
- PO:
-
polyolefin
- PP:
-
polypropylene
- PVC:
-
polyvinyl chloride
- SDS:
-
sodium dodecyl sulfate
- UV:
-
ultraviolet
REFERENCES
Best practice for ONIDP pharmaceutical development programs leachables and extractables. II. ONIDP container closure systems. Leachables and extractables working group, Product Quality Research Institute PQRI training course, Chicago (2007).
Quackenbos HM. Plasticizers in vinyl chloride resins. Ind Engng Chem. 1954;46:1335.
Till DE, Reid RC, Schwartz PS, Sidman KR, Valentine JR, Whelan RH. Plasticizer migration from polyvinyl chloride film to solvents and foods. Food Chem Toxicol. 1982;20:95–104.
Gallelli JF, Groves MJ. USP perspectives on particle contamination of injectable products. J Parenter Sci Technol. 1993;47:289–92.
Jenke D. Extractable/leachable substances from plastic materials used as pharmaceutical product containers/devices. PDA J Pharm Sci Technol. 2002;56:332–71.
Jenke DR. Linking extractables and leachables in container/closure applications. PDA J Pharm Sci Technol. 2005;59:265–81.
Jenke DR, Jene JM, Poss M, Story J, Tsilipetros T, Odufu A, et al. Accumulation of extractables in buffer solutions from a polyolefin plastic container. Int J Pharm. 2005;297:120–33.
Nuijen B, Bouma M, Manada C, Jimeno JM, Lazaro LL, Bult A, et al. Compatibility and stability of the investigational polypeptide marine anticancer agent kahalalide F in infusion devices. Invest New Drugs. 2001;19:273–81.
Jenke D. Evaluation of the chemical compatibility of plastic contact materials and pharmaceutical products; safety considerations related to extractables and leachables. J Pharm Sci. 2007;96:2566–81.
Desai N, Shah SM, Koczone J, Vencl-Joncic M, Sisto C, Ludwig SA. Zinc content of commercial diluents widely used in drug admixtures prepared for intravenous infusion. Inter J Pharm Compd. 2007;11:426–32.
Ambados F. Incompatibility between aminophylline and element zinc injections. Aust J Hosp Pharm. 1996;26:370–1.
Boyle DA, Goldspiel BR. A review of paclitaxel (Taxol) administration, stability, and compatibility issues. Clin J Oncol Nurs. 1998;2:141–5.
Thiesen J, Kramer I. Physico-chemical stability of docetaxel premix solution and docetaxel infusion solutions in PVC bags and polyolefine containers. Pharm World Sci. 1999;21:137–41.
Waugh WN, Trissel LA, Stella VJ. Stability, compatibility, and plasticizer extraction of taxol (NSC-125973) injection diluted in infusion solutions and stored in various containers. Am J Hosp Pharm. 1991;48:1520–4.
Chawla AS, Hinberg I. Leaching of plasticizers from and surface characterization of PVC blood platelet bags. Biomater Artif Cells Immobil Biotechnol. 1991;19:761–83.
DiMichele DM, Lasak ME, Miller CH. In vitro factor VIII recovery during the delivery of ultra-pure factor III concentrate by continuous infusion. Am J Hematol. 1996;51:99–103.
McLeod AG, Walker IR, Zheng S, Hayward CPM. Loss of factor VIII activity during storage in PVC containers due to adsorption. Haemophilia. 2000;6:89–92.
Arbin A, Jacobsson S, Hanniene K, Hagman A, Ostelius J. Studies on contamination of intravenous solution from PVC bags with dynamic headspace GC-MS and LC-diode array techniques. Inter J Pharm. 1986;28:211–8.
Cheung AP, Hallock YF, Vishnuvajjala BR, Nguyenle T, Wang E. Compatibility and stability of bryostatin 1 in infusion devices. Invest New Drugs. 1998;16:227–36.
Demore B, Vigneron J, Perrin A, Hoffman MA, Hoffman M. Leaching of diethylhexyl phthalate from polyvinyl chloride bags into intravenous etoposide solution. J Clin Pharm Ther. 2002;27:139–42.
Pearson SD, Trissel LA. Leaching of diethylhexyl phthalate from polyvinyl chloride containers by selected drugs and formulation components. Am J Hosp Pharm. 1993;50:1405–9.
Ulsaker GA, Korsnes RM. Determination of cyclohexanone in intravenous solutions stored in PVC bags by gas chromatography. Analyst. 1977;102:882–3.
Neidhardt E, Koval R, Burke E, Warne N. In vitro evaluation of B-domain deleted recombinant factor VIII (ReFacto) stability during simulated continuous infusion administration. Haemophilia. 2005;11:319–25.
Rock GA, Farrah G, Rozon G, Smiley RK, Cole R, Vileneuve D, et al. Analysis of contaminants in factor VIII preparations administered to patients with hemophilia. Can Med Ass J. 1983;128:403–8.
Labow RS, Tocchi M, Rock G. A leachable materia from polyolefin bags (abstr.) Presented at 20th International Society Blood Transfusion, Munich (1984 ).
Labow RS, Tocchi M, Rock G. Contamination of platelet storage bags by phthalate esters. J Toxicol Environ Health. 1986;19:591–8.
Beitz C, Bertsch T, Hannak D, Schrammel W, Einberger C, Wehling M. Compatibility of plastics with cytotoxic drug solutions-comparison of polyethylene with other container materials. Int J Pharm. 1999;185:113–21.
Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501.
Bodmer JL, Meier P, Tschopp J, Schneider P. Cysteine 230 is essential for the structure and activity of the cytotoxic ligand TRAIL. J Biol Chem. 2000;275:20632–7.
Hymowitz SG, O’Connell MP, Ultsch MH, Hurst A, Totpal K, Ashkenazi A, et al. A unique zinc-binding site revealed by a high-resolution X-ray structure of homotrimeric Apo2L/TRAIL. Biochemistry. 2000;39:633–40.
Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–8.
Flores H, Lin TP, Matthews TC, Pai R, Shahrokh Z. Apo2L ligand/trail formulations. United State Patent Application 20050020498 (2005).
Lin TP, Shahrokh Z, Flores H, Pai R. Apo2L ligand/trail formulations. United State Patent Application 20050080006 (2005).
Bewley TA. A novel procedure for determining protein concentrations from absorption spectra of enzyme digests. Anal Biochem. 1982;123:55–65.
Boitquin LP, Hecq JD, Vanbeckbergen DF, Jamart J, Galanti LM. Stability of sufentanil citrate with levobupivacaine HCl in NaCl 0.9% infusion after microwave freeze-thaw treatment. Ann Pharmacother. 2004;38:1836–9.
Hecq JD. Ten years of European hospital pharmacy history: centralized intravenous additives services. Eu J Hosp Pharm Sci. 2004;10:47.
Lebitasy M, Hecq JD, Athanassopoulos A, Vanbeckbergen D, Jamart J, Galanti L. Effect of freeze-thawing on the long-term stability of calcium levofolinate in 5% dextrose stored on polyolefin infusion bags. J Clin Pharm Ther. 2009;34:423–8.
ACKNOWLEDGMENTS
The authors wish to thank Heather Flores, Bruce Kabakoff and Sherry Martin-Moe for their support of this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chang, J.Y., Xiao, N.J., Zhu, M. et al. Leachables from Saline-Containing IV Bags Can Alter Therapeutic Protein Properties. Pharm Res 27, 2402–2413 (2010). https://doi.org/10.1007/s11095-010-0193-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-010-0193-8



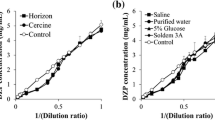



 ) and bag #2 (
) and bag #2 ( ); 100 mL PVC (
); 100 mL PVC ( ); 250 mL PO (
); 250 mL PO ( ); 250 mL PVC (
); 250 mL PVC ( ); Saline, not exposed to IV bag (
); Saline, not exposed to IV bag ( ).
).
 ) 4 (
) 4 ( ), 7 (
), 7 ( ) and 18 (
) and 18 ( ) days .
) days .

 ) and without (
) and without ( ) exposure to the med-add stopper from the 100 ml PO bag. A control saline solution was filled into a 3 cc glass vial, stoppered with a stopper removed from 100 ml PO bag, and stored inverted for 7 days at 30°C.
) exposure to the med-add stopper from the 100 ml PO bag. A control saline solution was filled into a 3 cc glass vial, stoppered with a stopper removed from 100 ml PO bag, and stored inverted for 7 days at 30°C.

 ), 5 (
), 5 ( ), or 10 (
), or 10 ( ) µM 2-Mercaptobenzothiazole (a) and its Zinc salt (b).
) µM 2-Mercaptobenzothiazole (a) and its Zinc salt (b).

 ) and after (
) and after ( ) July 2007. A natural rubber stopper was used in the med-add port of the 100 ml PO bags manufactured prior to July 2007, while a chlorobutyl stopper was used in bags after July 2007.
) July 2007. A natural rubber stopper was used in the med-add port of the 100 ml PO bags manufactured prior to July 2007, while a chlorobutyl stopper was used in bags after July 2007.