ABSTRACT
Purpose
To gain a better understanding of the physical state and the unusual thermal behavior of milled griseofulvin.
Methods
Griseofulvin crystals and amorphous melt quench samples were milled in a vibrating ball mill for different times and then analyzed using differential scanning calorimetry (DSC) and powder X-ray diffraction (PXRD). Modulated DSC (mDSC) and annealing studies were done for the milled amorphous samples to further probe the effects of milling.
Results
Milling of griseofulvin crystals results in decrease in crystallinity and amorphization of the compound. A double peak is seen for crystallization in the DSC, which is also seen for the milled melt quench sample. Both enthalpy and temperature of crystallization decrease for the milled melt quenched sample. Tg is visible under the first peak with the mDSC, and annealing shows that increasing milling time results in faster crystallization upon storage.
Conclusion
Milling of griseofulvin results in the formation of an amorphous form and not a mesophase. It increases the amount of surface created and the overall energy of the amorphous griseofulvin, which leads to a decreased temperature of crystallization. The two exotherms in the DSC are due to some particles having nuclei on the surface.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
The amorphous state of compounds has been under intense investigation mainly for the advantageous solubility and dissolution rates it offers over crystalline states. Depending upon the nature of a given material, various methods may be used to produce its amorphous state, such as melt-quenching, freeze-drying, spray-drying, milling and dehydration (1,2). When amorphous samples are prepared using different methods, it is revealed that the method of preparation influences the physical stability in terms of enthalpic relaxation and crystallization behavior (3).
Among the various methods that can produce the amorphous form, milling is a high energy process mainly used for size reduction of particles which, depending on the compound, can result in a product being of a smaller size as well as of reduced crystallinity (4). Depending on the crystal structure, milling can either yield highly strained crystals with small particles sizes or the crystals can lose their structure completely and form the amorphous state as seen in indomethacin (5). The formation of reactive amorphous sites due to milling was found to have an effect on the Maillard reaction between metoclopramide HCl and lactose (6), and it has been observed that most of the amorphous regions produced during milling tend to concentrate more on the surface than the bulk (7).
The two main mechanisms by which a compound is said to undergo amorphization during milling are the gradual formation of crystal defects which accumulate to lose all long-range order and the possibility of quench melting of the compound due to the high local temperatures produced during milling (8,9). For the amorphous form to occur, it is necessary for the temperature of operation to be below the glass transition temperature (Tg) of the amorphous state, since above Tg the transiently formed amorphous state transforms to the stable crystal phase (10).
Recently, it has been reported that certain compounds, when cryomilled, form amorphous systems, while others, such as griseofulvin (Fig. 1), have been said to form a different high energy physical state which only resembles the amorphous state when analyzed by X-ray powder diffraction (5,11). This higher energy state has been defined as being a new “defective” state having a different thermal behavior as compared to the truly amorphous phase and has recently even been said to exist in a “mesophase” (12). This belief was caused by the observation of a significantly different amorphous profile for their quenched melt showing only one maximum of diffuse scattering instead of the usual two. Our extensive efforts to reproduce that melt-quenched amorphous profile for griseofulvin were not successful, as the X-ray profiles for the cryomilled and melt-quenched samples appeared invariably the same, showing the usual two maxima of diffusive scattering, leading us to believe that this does not correspond to a mesophase. On the other hand, our thermal analyses on milled samples showed the characteristic splitting up of the exotherm of crystallization, similar to what we described, and we therefore wanted to take a closer look at the interplay of milling and the amorphous state of griseofulvin. In this paper, we investigate and show how the presence of crystal nuclei may play a role in this intriguing behavior.
MATERIALS AND METHODS
Materials
Griseofulvin (GSF) was obtained from Sigma (St. Louis, MO) in the crystalline form at 95% purity. It exhibits a melting temperature of around 218°C and has a very low solubility of 30.56 μg/ml in water at 25°C (13).
Preparation of Amorphous Form
Amorphous griseofulvin was prepared by melting pure crystalline griseofulvin over a hot plate to a few degrees above its melting temperature under nitrogen flow, keeping it long enough to ensure complete melting and then immersing in liquid nitrogen to quench it. These amorphous solids were immediately placed in a desiccator over P2O5 to prevent water sorption.
Preparation of Cryomilled Form
Crystalline griseofulvin was cryo-milled using a SPEX centriprep 6750 cryogenic impact mill. The milling was performed for 60 min using cycles of 2 min milling followed by a 2 min cooling phase. The intensity of milling was set to 20 impacts per second. The sample was then allowed to come to room temperature in a desiccator containing P2O5.
Room Temperature Ball Milling
Melt-quenched griseofulvin was placed in a small stainless steel vial with one steel ball bearing and then fixed to the wig-L-bug crescent amalgamator (Dentsply International, Surrey, UK) and milled for 15, 30 and 60 s in 5 s intervals to ensure that the powder did not become too hot. Milling for increased time was not done to avoid crystallization of griseofulvin. Milling was also done for the crystalline form using milling times of 5, 10 and 15 min.
Thermal Analysis
The samples were analyzed using a DSC 2920 (TA instruments, New Castle, DE) in both the standard and modulated mode. The standard mode was run at a rate of 10°C per minute, and the modulated scan was performed at an underlying rate of 2°C per minute with an amplitude of 1°C and a modulation frequency of 60 s. The instrument was calibrated for baseline and cell constant using Indium as a standard material at both heating rates. Nitrogen was used as a purge gas at 40 ml/min. Samples for the standard mode were around 3 mg while for the modulated scan the samples were 8 mg to get better signal. The samples were sealed in hermetic aluminum pans and run from 20°C to 250°C. The results were analyzed using Universal analysis software version 4.1D (TA Instruments).
X-ray Powder Diffraction (XRPD)
The samples were analyzed using a Siemens D-5000 diffractometer using Cu Kα radiation with a wavelength of 1.54 Å. Scans were taken from 4 to 40° 2θ at a step size of 0.02° and a scan rate of 1 s per step. The X-ray generator voltage and amperage were set to 40 kV and 40 mA, respectively. The divergence and scattering slits were 1.0 mm wide, and the receiving slit was 0.1 mm wide. The samples were mounted in a silicone sample holder with a well diameter of 1.3 cm and well depth of 1 mm.
RESULTS AND DISCUSSION
Difference Between Milled and Melt-Quenched Griseofulvin
Griseofulvin when cryomilled has been shown to progressively reduce in crystallinity as milling time is increased (11). It exhibited an unusual thermal behavior where the milled sample had two distinct exotherms followed by the endotherm of melting. It was concluded that the exotherm occurring below the Tg must have been the rearrangement of the defective regions to a configuration closer to the original crystal. It was also suggested that the state obtained after cryomilling GSF is different from the melt quench amorphous form, and this was further corroborated in a recent paper where the X-ray profiles of the melt quench and the cryomilled sample were compared (12). It was pointed out in the paper that the X-ray profiles of the milled samples had two diffuse intensity regions in the amorphous profile, while the melt quench had only one intensity region. In our experiments, when the melt-quenched GSF was analyzed by XRPD, the profile consistently showed two regions in the amorphous profile (Fig. 2). Many attempts were made to observe the single region profile by varying the conditions of quenching the melt. A literature survey shows that other authors also report that melt-quench amorphous GSF results in an amorphous profile with two intensity regions (14). For our current investigation, we thus accept that the X-ray profiles of the melt-quenched and the cryomilled samples are more or less the same, which is consistent with other compounds, such as indomethacin, piroxicam and trehalose (15–17). We agree that the observation of two significantly different X-ray amorphous profiles would have suggested two different states of order, but our present results cannot confirm that for griseofulvin. We thus conclude that there is no major structural difference in our studies between the amorphous materials obtained from milling and quenching the melt.
Thermal Behavior of Milled Crystalline Griseofulvin
For crystalline GSF samples, mechanical activation occurring due to dry mixing was found to begin at the particle surface and gradually form a surface layer with higher energy (18). The thickness of this layer was suggested to be around 0.1–1 μm corresponding to a depth of 100–1000 molecules (19). Thus, for these samples it seemed that some amount of mechanical stress resulted in disordered/amorphous surface with a crystalline core. These changes occurred after many hours of processing. Since, in our case, larger amounts of force are utilized, the same changes can be said to occur at a faster rate with additional reduction in particle size when the crystals are milled.
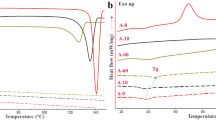
While the X-ray profiles do not show a significant difference between the melt-quenched and the cryomilled samples, a major difference is, on the other hand, seen in the thermal behavior (Fig. 3) as determined by DSC. The DSC experiments show an exotherm of crystallization as a single peak with an onset of about 131°C for the amorphous material from the quenched melt. The cryomilled sample shows a much broader temperature range of (re)crystallization, starting as low as about 65°C. The profile of this exotherm looks unusual as it clearly has multiple maxima. This indicates a non-uniform distribution of energy in the sample.
After about 1 h of cryomilling, the sample appears nearly completely amorphous. It is possible that the milled sample still contains some seed crystals and that the exotherm is the crystallization of the bulk occurring due to these seed crystals. It is known that when an amorphous sample is in the glassy region, the molecular motion is very low and reduces to negligible as the temperature is reduced even further. Even then, at temperatures not too far below the Tg, there is some residual mobility in the molecules—enough to cause them to relax towards the equilibrium state and thus crystallize (20). When in the metastable equilibrium stage, the sample does not relax, as it is already in equilibrium, but crystallization can occur in this region upon the introduction or formation of crystal nuclei which acts as a catalyst. It is possible that the presence of small amounts of crystalline material in the GSF sample after 60 min of cryomilling affected the crystallization behavior of the remaining amorphous phase by acting as seeds for nucleation. However, this does not explain the formation of two distinct exothermic peaks.
Comparing the DSC scan for the triturated melt-quenched amorphous and the cryomilled sample (Fig. 3), the first exotherm in the cryomilled sample appears in a region between 65°C to 100°C which overlaps with the glass transition of the unmilled amorphous sample. It is not fully understood why the crystallization appears to occur in two sequential stages. Yu explains that the rate of crystallization of the surface of amorphous material is higher than that of the bulk (21). A difference in rate can be explained by a difference in molecular mobility between the surface and the bulk, a difference in the activation energy needed to nucleate and/or grow, or a combination thereof. Molecules at the surface are in a less confined arrangement than in the bulk, and the surface energy implies that they intrinsically must have a higher state of energy than in the bulk. Thus, according to Yu’s explanation, the first exotherm would represent the crystallization related to the surface of the amorphous particles, and the second exotherm would then correspond to 3D nucleation and growth of the bulk of the particles. The close proximity to Tg suggests that the thermal modes that become accessible above that temperature play a role in the kinetics.
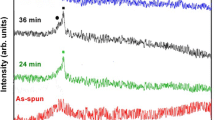
To see if milling at non-cryogenic temperatures gives the same results, the crystalline GSF powder was milled without temperature control and analyzed by X-ray (Fig. 4). The X-ray profile looked very similar to that of the cryomilled material, and the method was actually found to be more efficient, since far less time was required to produce the same change in XRPD profile at non-cryogenic conditions than in a cryogenic environment. The time progression shows a gradual decrease of intensity of the crystalline phase accompanied by the appearance of an amorphous region, without appreciable broadening of the peaks. Thus, it seems that low temperature is not a requirement for amorphization of GSF.
DSC scans of these briefly milled samples (Fig. 5) again show two exotherms. With an increase of milling time, the peak maxima of the exotherms creep to lower temperatures, and their total enthalpy increases with the first exotherm increasing relative to the higher temperature exotherm.
Suryanarayanan et al. distinguish the one-state and two-state models when evaluating the concept of partial crystallinity (22). The two-state model assumes that material is either 100% amorphous or 100% crystalline and that partial crystallinity is exhibited as a combination of those two. The one-state model, on the other hand, sees the amorphous and crystalline content as being present in a continuously changing scale of “lattice order.” The one-state model implies that an event in a DSC, for instance, shows as a single peak that may shift temperature depending upon the crystalline/amorphous ratio. X-ray peaks are expected to gradually broaden from the sharp crystalline peaks into an amorphous halo. The two-state model is essentially expected to produce a linear combination of signals. Our X-ray studies show that the diffraction peaks of the crystalline griseofulvin broaden only slightly at the beginning and then decrease in intensity as the amorphous halo intensifies with increasing milling time without showing any drastic changes in peak width. The DSC profile of the crystals after milling for 1 min shows only one broad exotherm which on further milling results in two sharper exotherms. Thus, it appears that the crystals initially become defective and then undergo amorphization instead of gradual development of the defects. Our X-ray and DSC analyses after the initial defect formation thus show behavior that is consistent with the two-state model. Even though no metastable polymorphs have been detected yet for griseofulvin, there is always a possibility that the first exotherm could be due to formation of a higher energy crystal form. Our FTIR experiments (data not shown) on the milled melt-quenched sample after heating beyond the first exothermic peak in the DSC show that we are dealing with only one crystal form. This result was similar to etravarine, which, when cryomilled, had a DSC profile showing more than one recrystallization peak with the X-ray showing that the solid was amorphous (23). The ATR-FTIR experiments in that case also confirmed that the amorphous sample was transforming to only one polymorph during both the crystallization exotherms.
Milling of Amorphous Griseofulvin
To investigate the presence of the crystalline phase and thereby the role of seeding on the recrystallization in the milled samples as well as the effect of surface creation, we prepared amorphous material by quenching griseofulvin from the melt. Subsequently, this material was milled briefly using the Wig-L-Bug for 15, 30 and 60 s.
For these three samples X-rays taken directly after milling showed no evidence of the presence of crystalline material. As opposed to the single exotherm produced by unmilled melt-quench, the milled samples show a second exotherm at lower temperature (Fig. 6). The enthalpy of crystallization reduced from 82 J/g for the pure melt-quenched to 62 J/g for both peaks in the sample milled for 15 s. There are various reasons that can explain this reduction in enthalpy. One of the reasons is that amorphous samples do not recrystallize to the same extent when the crystallization occurs at a lower temperature. This was seen by the fact that the melting enthalpy after recrystallization reduced from around 112 J/g for the melt-quenched GSF to around 102 J/g for the milled sample. This reduction in crystallization and melting enthalpy was also seen in milled trehalose (17). Another reason is the fact that at lower temperatures the heat capacity (Cp) of the solid is also lower. Thus, the lower the temperature at which the recrystallization takes place, the smaller the recrystallization enthalpy. This is more pronounced in this case since part of the first exotherm overlaps the Tg where there is a drastic reduction of the Cp. Finally, it must be mentioned that though the X-ray profile of the milled sample does not show any crystalline peaks, that does not mean there is no crystalline material. Small amounts of crystalline material (i.e. nuclei) would not be observed in an X-ray profile.
The lower temperature exotherm starts to appear at around the same temperature as the Tg, and eventually the Tg is no longer visible. Increasing the milling time results in more surface created and also increases the area of the first exotherm and reduces the second exotherm, while the overall enthalpy under the two exotherms tends to reduce only slightly as milling time is increased (Fig. 7). The enthalpies of recrystallization were 62.16 (2.8) J/g for the 15 s sample, 58.4 (1.0) J/g for the 30 s and 56.7 (1.4) for the 60 s samples. This reduction could be due to the fact that the exotherms move to lower temperatures to areas where the solid has much lower Cp. Earlier explanations that this peak corresponds to the curing of crystalline defects cannot hold true, since the peak is also seen for material that had no crystalline content to begin with. Also, the first exotherm moved more to the left upon further milling (Fig. 7), indicating the possibility of the Tg reducing upon milling due to the fact that the molecules in the ground sample relax faster than those in the melt-quenched sample. A similar effect was seen when indomethacin was ground from the melt quench (24).
DSC profiles of quenched melt griseofulvin which have been milled in the Wig-L-Bug for 15, 30 and 60 s along with the X-ray profiles (Inset) after milling show that the exotherms move to lower temperatures, while the first exotherm increases in size upon further milling. The corresponding X-ray profiles show that the crystalline phase cannot be detected by X-ray upon milling for such short times.
Modulated DSC (mDSC) of Milled Amorphous and Crystalline Griseofulvin
The melt-quenched sample milled for 30 s was analyzed by mDSC to see if any Tg was visible. This time, the first exotherm was even more pronounced, possibly due to the slower heating rate, while the second exotherm was small. The reversible signal showed only the beginning of a Tg below the first endotherm and was immediately followed by some transitions due to the crystallization of the sample.
To ensure that the Tg can be clearly observed, the melt-quenched sample was ground for about 2–3 s with a mortar and pestle and then analyzed by modulated DSC. This time, the first exotherm was quite small followed by a larger second exotherm, as expected (Fig. 8). In the normal heat flow profile, the Tg is again not visible, but upon going to the reversible signal, the Tg appears very clearly with a ΔCp of around 0.2306 J/g/°C. The fact that a clear exotherm is visible at the Tg just upon mere triturating of the melt-quenched solid should put to rest the hypothesis that this could be due to defective crystals.
If the milled crystalline GSF is amorphous and not defective, then it should exhibit a glass transition which should be visible in the reversible signal, since defective crystals would only show a very broad and indistinct exotherm due to annealing of the defects but no Tg. To see if a Tg is visible in the milled crystalline samples, the sample milled for 5 min was analyzed by mDSC, the slow heating rate of which resulted in the two exotherms merging into one broad exotherm. The glass transition was, however, picked up by the reversible signal under the crystallization exotherm (Fig. 9). The glass transition occurred at a significantly lower temperature (around 60°C) than that observed for a fresh melt-quenched solid (around 85°C). The transition was also more spread-out with a ΔCp of around 0.1 J/g/°C before it was interrupted by the crystallization event. The presence of the Tg clearly indicates that the exotherms in the DSC are due to the crystallization of amorphous regions in the milled samples.
Effect of Mechanical Stress and Surface Nuclei Formation
When crystalline, cryomilled, and melt-quenched amorphous griseofulvin samples were analyzed using inverse gas chromatography (IGC), the milled samples showed much higher surface energy values compared to the others (25). Besides the fact that milling reduces the particle size and thus increases the surface-to-bulk ratio, the surface which is created upon milling is also expected to be more energetic. This elevation of surface energy can be envisioned to have many contributions, such as unfavorable non-equilibrium conformations of molecules, dislocation of molecules from their crystallographic positions and more exposure of high energy functional groups.
For glassy materials, it is known that the preparation method governs the thermal history of the amorphous sample and therefore has a drastic effect on its behavior. For example, tri-O-methyl-β-cyclodextrin, when melt-quenched and milled, showed different enthalpic relaxation with the milled sample having an unusual temperature dependence (26). There is also the phenomenon of crystallization induced by mechanical stress. A recent publication reports this effect on indomethacin (24). Melt-quenched samples with absolutely no mechanical stress did not show a recrystallization peak using DSC even at the slowest scan rate of 1°C per minute, while those which were ground did show a clear recrystallization exotherm. The greater the applied stress, lower the temperature at which recrystallization was observed. This behavior is consistent with our current observations for melt-quenched griseofulvin for which the recrystallization temperature was also lowered upon mild grinding.
The onset of crystallization for amorphous compounds generally begins at the surface, and this has been illustrated using studies of the onset of crystallization of a melt-quenched sample kept between two cover slips where the exposed amorphous sample at the edges starts to crystallize first and then moves inward into the bulk (21). We performed a similar experiment using griseofulvin of which a melt-quenched sample was held between a cover slip and a glass slide. The exposed amorphous part at the edge of the cover slip was mildly scratched before the sample was heated in a hot stage under a microscope. It was seen that at around 115°C the regions which were scratched started crystallizing sooner than those areas that were undisturbed (Fig. 10). From this observation, we may deduce that the creation of a large amount of surface by milling as well as mechanical stress can easily reduce the crystallization time and temperature.
As indicated above, the XRPD is not sensitive enough to detect crystalline nuclei. Melt-quenched (not ground) samples were kept at room temperature for about a month to partially crystallize the solid. After a month, it was seen that only the parts of the surface had crystallized, and the bulk was more or less still amorphous. A DSC scan was obtained at 10°C/min and compared with the fresh melt quench. The re-crystallization enthalpy for the nucleated sample was 74 J/g as compared to 82 J/g for the fresh melt quench. The temperature at which the recrystallization occurred had not changed; thus, it appears that presence of nuclei on the surface of the amorphous solid does not influence the temperature at which crystallization of the bulk happens. The Tg also looked normal but upon closer inspection showed a very interesting behavior where the Tg was not smooth but actually appeared to have a small bump (Fig. 11). Griseofulvin was then quench-cooled in such a way that some crystals were present within the bulk of the material. DSC scans of melt quench with bulk crystal nuclei did not show such a pattern in the Tg, indicating that this is a surface-specific phenomenon.
Thermal behavior at the glass transition temperature for a fresh melt quench and b melt quench with partial surface crystallization. A small bump is seen when the surface is partially crystallized. Inset in a shows that the fresh melt quench is transparent, while the insets in b show that in top view the surface is opaque due to crystallization, while the side view reveals that the bulk is still transparent.
Triturating the fresh melt-quench and heating in the DSC at 10°C/min reduced the temperature of recrystallization to a lower temperature and also reduced the enthalpy to around 65 J/g. In this case, however, an exotherm at the Tg is not visible. Triturating the partially surface-crystallized melt-quench solid resulted in a DSC profile in which even at 10°C/min a small exotherm is seen at the Tg (Fig. 12).
From these results, it appears that disruption of the melt quench by grinding results in creation of a lot of surface with high mobility which can subsequently lead to the creation of nuclei on the surface. When these particles are heated, the surfaces of the particles gain high mobility around the Tg, and those particles that have the nuclei on their surface will crystallize completely on the surface, resulting in the first exotherm. The second exotherm will consist of crystallization of the bulk regions of those particles as well as the crystallization of particles without the nuclei. Thus, for the first peak to occur significantly, the two conditions of high surface area and presence of nuclei seem necessary. The second exotherm occurs at lower temperature relative to the fresh melt quench due to the higher mobility from the excess surface and also the energy imparted by mechanical stress. Attempts were made to visualize the mechanics of the crystallization using the hot stage microscope under cross polarizers to see how the two stages of crystallization are related. Unfortunately, however, the resolution of the hot stage microscope was insufficient to clearly discern individual amorphous particles, let alone to distinguish the bulk from the surface in these particles.
Annealing of Milled Amorphous Samples
To investigate how the crystallization changes with time after briefly milling melt-quenched samples, the following studies were performed. Samples were milled for either 15 or 60 s and then annealed at 60°C for 30 and 45 min. The samples were analyzed for crystallinity by X-ray analysis immediately after milling and after annealing. Fig. 13 shows that the sample milled for 15 s is still mostly amorphous after 30 min, but some Bragg reflections are starting to show just above the noise level, which indicates the presence of a significant amount of crystalline material. At 45 min, these peaks have clearly developed further even though the sample is still mostly amorphous. In contrast, the sample milled for 60 s had clearly completely crystallized after 30 min. This difference clearly shows that milling induces crystallization of the amorphous griseofulvin. DSC was performed to further analyze the energetic effects of the annealing.
The DSC results in Fig. 14 show how the samples milled for 15 s develop with annealing time at 60°C. The annealing causes the combined exotherms to decrease in heat flow from 59 to 42 J/g. At the same time, the peak maxima have shifted to lower temperatures by as much as 3°C after 45 min of annealing. Clearly, the lower value of the heat flow corresponds to the observation that a portion of the material has already crystallized during the annealing as is indicated by the X-ray scans. An interesting observation is made on the relative contributions to the heat flow of crystallization. The first exotherm is relatively constant at about 10 J/g, whereas the second exotherm reduces in size to account for the total loss of heat flow upon annealing.
This result can be explained by the fact that the region most likely to crystallize first will be the nucleated surface due to it high mobility. While this is expected to result in a decrease in the first exotherm, annealing also causes new nuclei to be formed on the surfaces of particles which did not previously have nuclei. Thus, after annealing, a DSC run would still show two exothermic peaks due to the crystallization of the surface where new nuclei have been formed followed by the crystallization of the bulk and remaining non-nucleated surface. However, for very small particles, as obtained after milling, the reactive surface that crystallizes can be thick enough to result in crystallization of the entire particle. Therefore, for very small particles, the two exotherms can represent crystallizations of entire particles having surfaces with (first exothermic peak) and without (second exothermic peak) nuclei.
CONCLUSION
Although in principle the amorphous phase represents a random ensemble of molecular orientations, conformations and molecular interactions, in practice it is not completely random. Annealing or aging typically cause the material to relax its higher energy content to lower energy modifications, causing the average enthalpy to reduce gradually. Milling causes a dramatic disruption of the local arrangement of the molecules. This may include the formation of new surface, cracks, defects, higher energy molecular conformations, nuclei and so on. In this study, we have shown that for melt-quenched samples these disruptions, especially creation of the surface, cause crystal nuclei to be formed which ultimately play a decisive role in the crystallization behavior of the remaining amorphous content. The occurrence of the two exotherms in the DSC run was found to be due to the crystallization of the nucleated surface resulting in the first exotherm with the crystallization of the bulk and non-nucleated surface producing the second exotherm.
REFERENCES
Patterson JE, James MB, Forster AH, Lancaster RW, Butler JM, Rades T. Preparation of glass solutions of three poorly water soluble drugs by spray drying, melt extrusion and ball milling. Int J Pharm. 2007;336(1):22–34.
Sussich F, Cesaro A. Trehalose amorphization and recrystallization. Carbohydr Res. 2008;343(15):2667–74.
Surana R, Pyne A, Suryanarayanan R. Effect of preparation method on physical properties of amorphous trehalose. Pharm Res. 2004;21(7):1167–76.
Chikhalia V, Forbes RT, Storey RA, Ticehurst M. The effect of crystal morphology and mill type on milling induced crystal disorder. Eur J Pharm Sci. 2006;27(1):19–26.
Crowley KJ, Zografi G. Cryogenic grinding of indomethacin polymorphs and solvates: assessment of amorphous phase formation and amorphous phase physical stability. J Pharm Sci. 2002;91(2):492–507.
Qiu ZH, Stowell JG, Cao WJ, Morris KR, Byrn SR, Carvajal MT. Effect of milling and compression on the solid-state Maillard reaction. J Pharm Sci. 2005;94(11):2568–80.
Ward GH, Schultz RK. Process-induced crystallinity changes in albuterol sulfate and its effect on powder physical stability. Pharm Res. 1995;12(5):773–9.
Fecht HJ. Defect induced melting and solid-state amorphization. Nature 1992;356(6365):133–5.
Willart JF, Descamps M. Solid state amorphization of pharmaceuticals. Mol Pharmacol. 2008;5(6):905–20.
Descamps M, Willart JF, Dudognon E, Caron V. Transformation of pharmaceutical compounds upon milling and comilling: the role of T-g. J Pharm Sci. 2007;96(5):1398–407.
Feng T, Pinal R, Carvajal MT. Process induced disorder in crystalline materials: differentiating defective crystals from the amorphous form of griseofulvin. J Pharm Sci. 2008;97(8):3207–21.
Feng T, Bates S, Carvajal MT. Toward understanding the evolution of griseofulvin crystal structure to a mesophase after cryogenic milling. Int J Pharm. 2009;367(1–2):16–9.
Zili Z, Sfar S, Fessi H. Preparation and characterization of poly-[var epsilon]-caprolactone nanoparticles containing griseofulvin. Int J Pharm. 2005;294(1–2):261–7.
Yamamura S, Takahira R, Momose Y. Crystallization kinetics of amorphous griseofulvin by pattern fitting procedure using X-ray diffraction data. Pharm Res. 2007;24(5):880–7.
Chieng N, Rades T, Saville D. Formation and physical stability of the amorphous phase of ranitidine hydrochloride polymorphs prepared by cryo-milling. Eur J Pharm Biopharm. 2008;68(3):771–80.
Sheth AR, Bates S, Muller FX, Grant DJW. Local structure in amorphous phases of piroxicam from powder X-ray diffractometry. Cryst Growth Des. 2005;5(2):571–8.
Willart JF, De Gusseme A, Hemon S, Odou G, Danede F, Descamps M. Direct crystal to glass transformation of trehalose induced by ball milling. Solid State Commun. 2001;119(8–9):501–5.
Hockerfelt MH, Nystrom C, Alderborn G. Dry mixing transformed micro-particles of a drug from a highly crystalline to a highly amorphous state. Pharm Dev Technol. 2009;14(3):233–9.
Mosharraf M, Nystrom C. The effect of dry mixing on the apparent solubility of hydrophobic, sparingly soluble drugs. Eur J Pharm Sci. 1999;9(2):145–56.
Hancock BC, Shamblin SL, Zografi G. Molecular mobility of amorphous pharmaceutical solids below their glass-transition temperatures. Pharm Res. 1995;12(6):799–806.
Zhu L, Wong L, Yu L. Surface-enhanced crystallization of amorphous nifedipine. Mol Pharm. 2008;5(6):921–6.
Rani M, Govindarajan R, Surana R, Suryanarayanan R. Structure in dehydrated trehalose dihydrate—Evaluation of the concept of partial crystallinity. Pharm Res. 2006;23(10):2356–67.
Qi S, Weuts I, Cort SD, Stokbroekx S, Leemans R, Reading M et al. An investigation into the crystallisation behaviour of an amorphous cryomilled pharmaceutical material above and below the glass transition temperature. J Pharm Sci. 2010;99(1):196–208.
Bhugra C, Shmeis R, Pikal MJ. Role of mechanical stress in crystallization and relaxation behavior of amorphous Indomethacin. J Pharm Sci. 2008;97(10):4446–58.
Chamarthy SP, Pinal R. The nature of crystal disorder in milled pharmaceutical materials. Colloid Surface Physicochem Eng Aspect. 2008;331(1–2):68–75.
Tsukushi I, Yamamuro O, Suga H. Heat-capacities and glass transitions of ground amorphous solid and liquid-quenched glass of tri-O-methyl-beta-cyclodextrin. J Non-Cryst Solids. 1994;175(2–3):187–94.
ACKNOWLEDGEMENTS
The authors would like to acknowledge financial support from the McKeehan Graduate Assistantship. We would also like to thank Dr. Katsuhiro Kobayashi for help with cryomilling of griseofulvin.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Trasi, N.S., Boerrigter, S.X.M. & Byrn, S.R. Investigation of the Milling-Induced Thermal Behavior of Crystalline and Amorphous Griseofulvin. Pharm Res 27, 1377–1389 (2010). https://doi.org/10.1007/s11095-010-0129-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-010-0129-3