ABSTRACT
Since ancient times, natural products, herbs and spices have been used for preventing several diseases, including cancer. The term chemoprevention was coined in the late 1970s and referred to the prevention of cancer by selective use of phytochemicals or their analogs. The field utilizes experimental carcinogenesis models to examine the efficacy of chemopreventive agents in a stage-specific manner. The concept of using naturally derived chemicals as potential chemopreventive agents has advanced the field dramatically. Throughout the years, a vast number of chemopreventive agents present in natural products have been evaluated using various experimental models. A number of them have progressed to early clinical trials. More recently, the focus has been directed towards molecular targeting of chemopreventive agents to identify mechanism(s) of action of these newly discovered bioactive compounds. Moreover, it has been recognized that single agents may not always be sufficient to provide chemopreventive efficacy, and, therefore, the new concept of combination chemoprevention by multiple agents or by the consumption of “whole foods” has become an increasingly attractive area of study. Novel technologies, such as nanotechnology, along with a better understanding of cancer stem cells, are certain to continue the advancement of the field of cancer chemoprevention in years to come.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
DIETARY PREVENTION OF CANCER
Since early in the history of medicine, an association between diet and disease has persisted. Hippocrates, the father of modern medicine said two-and-a-half thousand years ago, “Let food be thy medicine and medicine be thy food.” Galen of Pegamon (129–199 A.D.), a Greek physician and follower of Hippocrates’ teachings was said to have prescribed various foods, including peeled barley, milk, and various vegetables for the treatment of cancer (1). Despite the vast interest in diet as a mode to prevent and cure cancer, it was not until the late twentieth century that the mechanism of action of diet-derived chemoprevention began to unravel. Approximately 35 years ago, the National Cancer Institute initiated a Diet and Cancer Program to provide researchers with the resources needed to better elucidate the role of food and its nutrients in cancer prevention. Some of the initial studies in the field of diet and cancer were ecologic, examining correlations between cancer rates and dietary exposures based on national food consumption data and corresponding incidence or mortality rates. These studies were among the first to show that consumption of fruits, vegetables, and whole grains are associated with a decreased risk of many types of cancers (2). Subsequently, numerous case-control studies were conducted which yielded several potential dietary constituents as possible chemopreventive agents.
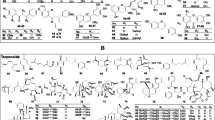
The beneficial effects of fruits and vegetables have been attributed to, among other things, the high content of bioactive compounds that are non-nutrient constituents commonly present in food (2). Studies conducted in the last few decades have shown that these bioactive compounds have important roles in the prevention of chronic diseases, including cancer, diabetes and hypercholesteremia (3). Noteworthy examples of diet-derived substances that have been shown to reduce experimental carcinogenesis are indole-3-carbinol (I3C) from cruciferous vegetables (4,5), curcumin from the root of curcuma (6), and epigallocatechin gallate (EGCG) from tea (7). Vegetables and fruits contain fiber, vitamins, minerals and a variety of bioactive compounds, such as carotenoids, flavonoids, indoles and sterols, all of which could account for this protective effect (Fig. 1).
Some of the herbs widely consumed in USA and reported to have chemopreventive efficacy in the literature. The squares indicate the name of the herbs and active phytochemicals present in them. The circles indicate reported mechanism(s) of action for these agents. Numbers in the parentheses (*) represent the estimated US population consuming these herbs.
One concern often raised has been that while animal models of carcinogenesis have been instrumental in demonstrating the efficacy of a number of diet-derived chemopreventive agents, the protective effect of these agents to cancer risk in humans has not been established conclusively. These inconsistencies are likely to reflect the complex nature of food and of the biological systems (i.e., humans) being investigated. The complexity of defining the role of diet is underscored by numerous diverse essential and non-essential components that may alter one or more phases of the cancer process. Among the many reasons for the lack of clear and consistent associations between diet and cancer are difficulties with assessments of dietary intake in free living populations that often change their consumption patterns over time, as well as the complexity of the food, (e.g., varied amount of mineral in vegetables grown in different parts of the world). Yet another possibility may be related to the absorption, metabolism, distribution, or excretion of the bioactive compounds. Several years ago, we examined the metabolism of resveratrol using both in vitro (human and rat hepatocytes) and in vivo (oral or intraperitoneal administration of resveratrol to rats and mice) studies to better elucidate the fate of resveratrol in the body. From these experiments, we were able to conclude that the half life of resveratrol is very short, with only a trace amount found even within fifteen minutes of administration (8). On the other hand, an abundant amount of trans-resveratrol-3-O-glucuronide and trans-resveratrol-3-sulfate were identified in rat urine, mouse serum, and incubations with rat and human hepatocytes (8). Consequently, the chemopreventive efficacy of trans-resveratrol-3-O-glucuronide and trans-resveratrol-3-sulfate has also been evaluated. In a similar fashion, I3C has shown great promise as a chemopreventive agent for several types of cancer, yet enthusiasm for this compound has been somewhat diminished due to its unstable characteristics upon exposure to acids in the stomach. This observation led to the synthesis of diindolylmethane (DIM), an acid-stable dimmer, that has been reported to be detectable in biological tissues and found to be several-fold more biologically active than I3C (9). In summary, the absorption, metabolism, distribution, and excretion profile of bioactive compounds is essential to assess the full potential of promising chemopreventive agents and may help guide in the design of novel synthetic analogs. In the last decades, the role of polymorphic genetic variance has also been found to contribute to the different responses observed in humans. For example, several studies have shown that individuals or groups with different polymorphic variants exhibit very different susceptibility to bioactive components (e.g., cruciferous vegetables) as compared to the general population (4). In some cases, polymorphic forms are associated with differential risk of carcinogenesis as well as responsiveness to chemopreventive agents (10). A better understanding of how the genetic traits may affect cancer risk could be used to develop better cancer prevention strategies.
THE BIRTH OF CHEMOPREVENTION
It has generally been accepted that prevention of any disease is preferable over treatment. Healthy diets along with exercise have been found to be associated with the prevention of multiple diseases, including cancer. Although, until recently, the focus has been diverted towards the use of extracts or compounds from plant-derived natural products for the prevention of disease. More recently, the use of phytochemicals (e.g., genistein) has become an increasingly popular approach to prevent or delay disease occurrence. At this point, it is important to note that, in fact, the practical use of these plants and the medicinal properties of plant components have been in existence for many centuries. Ayurveda, the “Science of Life,” which originated in India centuries ago, is still being practiced effectively in many parts of the world. It is based on the concept of using natural products to prevent and cure disease. Ayurvedic literature called Charaka and Sushruta clearly describes the use of natural products in the management of “minor neoplasms” (Granthi) and “major neoplasms” (Arbuda), or benign and malignant tumors, respectively (11). In a similar fashion, Traditional Chinese Medicine (TCM) represents another ancient culture which uses natural products and exercise to maintain health and prevent disease (12). As a last example, the Mayan civilization, known for their knowledge of astronomy and advanced calendar system, also developed an elaborated study of medicinal plants. Medicine among the ancient Mayans was a blend of religion and science. It was practiced by priests who inherited their positions and received extensive education from childhood. The area inhabited by the ancient Mayans as well as their present day descendents has been identified as having a complex ecosystem and is considered one of the richest source for vascular plant species in the world. As such, it is not surprising that the Mayan medicine men, ah-men, used up to 1,500 different plants for their medicinal properties (13). In the last two decades, there has been an increased awareness of the potential use of the plants used by the Mayans. Recently, the cytotoxic activity of nine plants used in Mayan traditional medicine has been demonstrated using various cancer cell lines (14). Thus, the application of natural products for the management of disease is not a novel concept. In a similar fashion, the literature shows that all three ancient civilizations included guidelines for the proper use of herbs or natural products to avoid unwarranted toxicity of the therapeutic agents. Today, the issue of toxicity continues to be a subject of great concern. For example, the use of chemotherapeutic agents among cancer patients is often accompanied by side effects due to the toxic nature of the drugs. Yet, for the most part, these side effects are acceptable provided that benefits outweigh the risk. The use of pharmacological doses of chemopreventive agents among individuals at high risk for developing cancer may also be considered acceptable provided that the benefits associated with the use of the drugs outweighs the risk of toxic side effects. On the other hand, chemopreventive agents intended for the general population (i.e., healthy individuals) must have no toxicity associated with their use in order for them to be acceptable. Since it is relatively safe to assume that the food we consume exhibits no toxicity, there has been a great emphasis on studying diet-derived agents as potential chemopreventive agents.
The modern day concept of chemoprevention and the term were first proposed by Sporn in the early 1970s (15). He defined the term chemoprevention as the use of natural or synthetic compounds to inhibit, suppress or reverse the development and progression of cancer. The work that immediately followed the introduction of the term chemoprevention, utilized synthetic analogs of vitamin A (termed Retinoids) to demonstrate the chemopreventive potential of vitamin A. These studies concluded that the natural product, retinol (vitamin A), was not as effective as all-trans retinoic acid, and the later had teratogenic properties and toxicity associated with it (15). This led to the synthesis of analogs of retinoic acid with the intention of developing a less toxic and more efficacious chemopreventive agents. Soon after the initial series of publications on retinoids demonstrating the modulating effects on models of chemically induced carcinogenesis, the concept of selective inhibition of carcinogenesis during either initiation, promotion or progression phases was articulated by Wattenberg (16). Since then, the field of cancer chemoprevention has exploded.
There is overwhelming literature describing the chemopreventive efficacy of numerous phytochemicals and their synthetic analogs for several classes of chemopreventive agents. These studies have largely been carried out using experimental carcinogenesis models of mammary gland, colon, prostate, lung, skin, pancreas and esophagus. We recently reviewed chemopreventive agents which have been evaluated using these various models of experimental carcinogenesis as well as those in clinical trials and therefore will not discuss this topic in depth here (17). Based on reviewing the numerous chemoprevention studies, one can conclude that there is considerable evidence pointing to a target-organ specificity for the majority of the chemopreventive agents. For example, most of the retinoids are efficacious against breast, prostate or urinary bladder carcinogenesis but ineffective against colon or esophageal cancers. Similarly, agents such as piroxicam or folate have been shown to be effective against several colon cancer models but not against mammary carcinogenesis. This target organ selectivity demonstrates the need to conduct the evaluation of chemopreventive agents and phytochemicals in a variety of carcinogenesis models in order to define the true chemopreventive potential of novel drugs.
THE ROLE OF PHYTOCHEMICALS IN CANCER PREVENTION AND THERAPY
In general, natural products and phytochemicals can serve both as chemopreventive as well as chemotherapeutic agents. Until relatively recent times, the discovery and use of natural products was focused on the development of chemotherapeutic agents. For example, the plant-derived anticancer agents commonly classified into one of four major classes: 1) vinca alkeloids (vinblastin, vincristine and vindesine), 2) epipodophyllotoxins (etoposide and teniposides), 3) texanes (paclitaxel and docetaxel), and 4) camtothecins (camptothecin and irinotecan) were developed to be used as chemotherapeutic agents and, as a result, had some accepted toxicity associated with their use. In contrast, chemopreventive agents can be delivered in foods or as dietary supplements with little or no toxicity. Taking into consideration the sequence of events in the process of carcinogenesis (i.e., initiation, promotion and progression), it has become increasingly unclear as to when the role of a preventive agent ends during the progression of cancer and when the role of a therapeutic agent begins. There appears to be an undefined grey line, which provides a venue for utilizing chemopreventive agents as an adjunct to therapeutic agents. The overall goal would be to reduce the toxicity induced by chemotherapeutic agents. An argument can be made that chemopreventive agents clearly have a chemotherapeutic role since, in fact, most of the chemopreventive agents are effective in reducing cell proliferation or inducing apoptosis in tissue culture of human cancer cells. In light of the fact that the mechanism of action of the majority of phytochemicals and their synthetic analogs have historically been studied in cancer cells, one could easily extrapolate the chemotherapeutic value of the phytochemicals in a cancer setting.
In recent years, the effects of phytochemicals on cell transformation and suppression of transformed cells during the different phases of carcinogenesis have been a topic of interest to many laboratories. As such, it has become increasingly clear that exposure to phytochemicals, both via diet or at pharmacological doses, yields alterations in key genomic responses, which may stop or delay the process of carcinogenesis. As shown in Fig. 2, the chemopreventive process can occur during both initiation and promotion of carcinogenesis. Clinically, chemoprevention is applicable to healthy individuals in whom a diet rich in phytochemicals can provide the protective effects. The pharmacological agents which are classified as chemopreventive agents and have been purified as individual phytochemical(s) (or their analogs) may be used for individuals at high risk of developing cancer, such as patients whose colon polyps have been removed or individuals who may be at a higher risk of developing cancer due to family history. On the other hand, for cancer patients in whom the goal is to kill the cancer cells, chemotherapeutic agents are routinely used. It is important to point out that in the case of the cancer patient, chemopreventive agents may be used in combination with chemotherapeutic agents in hopes of providing additive or synergistic effects. Moreover, dietary recommendations which include a variety of fruits, vegetables and whole grains may provide an added protection and may be a necessary component in the post-therapy of cancer patients, yet chemoprevention in these terms has not received much attention. Since recurrence of the disease after chemotherapy is an undesired consequence, the goal would be to prevent local relapses, delay the metastasis, and prevent second primary tumors. This process of protection that allows for the silencing or reduction of the growth rate of the cancer can be termed chemoquiescence. Thus, while active disease requires chemotherapeutic intervention in order to kill the cancerous cells, both dietary and pharmacological doses of chemopreventive agents may also prove beneficial to prevent the recurrence and aggressive progression of the disease. This progression of cancer from initiation to an advanced metastatic disease is governed by a complex interaction of genes which results in altered protein expression as well as changes in signaling transduction processes within the cell necessary to acquire transformed phenotype or to reacquire the normal cellular function (18). As a result, there is a need for revisiting the definition of chemoprevention to include chemoprevention at a molecular level. Thus, chemoprevention can be redefined as the use of chemicals or the combination of chemicals, which can suppress or reverse gene and/or protein expression of molecular targets responsible for transforming normal cells or the proliferation and progression of the transformed cells. We must bear in mind that although the molecular targets for drug discovery are extremely relevant, they cannot replace the conventional assays previously established during the past two decades (15,16).
A schematic diagram to show selective responsiveness of healthy population as well as cancer patients to chemopreventive agents. Accordingly, chemopreventive agents (CPA) can be useful to all populations; for cancer patients, it is feasible that CPA can be used in combination with chemotherapeutic agents. For post-therapy patients, dietary modification along with pharmacological intervention should be considered for suppressing or inhibiting the recurrence.
Today, genetically altered mice offer a valuable tool to evaluate the efficacy of chemopreventive agents and to help in determining the agents’ molecular mechanism of action. The generation of transgenic models, which involves introduction of oncogene function, loss of suppressor gene function, targeted organ-specific gene targeting knockout, or gene silencing by knocking out the gene function or Tetracyclin (Tet)-inducible functions, has become an area of intense research, which has generated several important models. For example, the TRAMP (transgenic adenocarcinoma mouse prostate) model, in which the mice develop high-grade PIN and/or prostate cancer within 12 weeks of age and cancer by 30 weeks, has been commonly used to test the efficacy of chemopreventive agents which have previously demonstrated growth-inhibitory actions on prostate cancer cell lines. Several reviews, including Singh et al., have summarized the advances in chemoprevention with the use of the TRAMP model (19,20). In summary, major efforts continue to be directed towards generating suitable transgenic models which can serve to better elucidate the mechanism of action of novel agents while at the same time providing a reliable approach to evaluate the efficacy of new chemopreventive agents. Modern chemoprevention has developed into a multi-disciplinary field, which incorporates basic traditional approaches (e.g., models of chemically-induced carcinogenesis, in vitro apoptosis studies) and state-of-the-art molecular biology-based methods (e.g., genetically engineered in vitro and in vivo models) (21).
IN SEARCH OF BIOACTIVE COMPONENTS AND SYNTHETIC ANALOGS
In the last two decades, the area of drug discovery has expanded to include natural products as a source for novel chemopreventive agents. However, with the presence of hundreds or perhaps even thousands of chemicals, it is necessary to develop various approaches to catalog the different classes of agents to better study these complex mixtures found in natural plants (22). For example, a system to classify the various bioactive compounds found in natural products can be used. These compounds can be classified into several major groups including polyphenols and flavonoids, vitamins, carotenoids, alkaloids, selenium compounds, organosulfurs, fatty acids and other miscellaneous agents, such as chlorophyll, as shown in Fig. 3.
There are several efficient programs throughout the world in which active chemopreventive agents are discovered from edible plants or plant parts. In one such program led by Pezzuto, we identified several novel chemopreventive agents, some of which have progressed from the bench to clinical trials (23). With this approach, using all the resources available, more than 3,000 rationally selected plants from all over the world were collected. The plants were taxonomically identified and, when necessary, cultivated under well-controlled conditions without pesticides. The extracts from the plants were evaluated for activity using a variety of in vitro bioassays. The bioassays were based on mechanism of action that would have apoptotic, differentiating, anti-aromatase, anti-estrogenic or anti-oxidant activities. Extracts considered as active in any one of these assays were further evaluated in a secondary screen using pre-cancerous lesions as an end-point of choice (i.e., mouse mammary gland organ culture or colon aberrant crypt assay). Then, if the extract showed efficacy in either model, the extract was fractionated into various parts, and the active fraction was then selected for identification of chemicals present in that chosen fraction. In this form, novel agents were identified and their chemopreventive efficacy established both in primary and secondary bioassays (22). Subsequently, this novel agent would become a lead compound, and in vivo carcinogenesis studies were then conducted. Given the large quantity of agent needed to complete the in vivo studies, analogs often were designed and synthesized based on active pharmacophores. In order to identify molecular targets, several molecular bioassays were incorporated to better elucidate the mechanism of action and signal transduction pathways being regulated by the chemopreventive agent. This sequence of events as a process for identification of new compounds is not perfect but has shown great success for the discovery of new lead agents. This method resulted in discovery of several chemopreventive agents, including deguelin, abyssinone, sulforamate, brassinin, and resveratrol. To this end, analogs of all newly discovered chemopreventive agents have been synthesized and evaluated for their chemopreventive activity using various models (17,21). Moreover, toxicity studies for many of the compounds were completed using both rodents and, in some instances, non-human primates (NHPs). Using this concept, Sporn and colleagues identified N-4-hydroxyphenylretinamide (4-HPR, Fenretinide) and 13-cis retinoic acid as chemopreventive agents for mammary, prostate, skin and oral carcinogenesis (24).
It is out of scope to list all major chemopreventive agents that are currently being evaluated in a variety of in vitro and in vivo assays. As an example, considerable effort has been diverted towards studying selenium, organosulfurs, catachins, triterpanoids, curcumin and many others (24). This has been a valuable ongoing process, and numerous laboratories are actively pursuing the quest of discovering novel chemopreventive agents. In our laboratory, we have focused on investigating the role of vitamin D in various cancers. Evidence for the role of vitamin D in cancer prevention has been expanding in recent years; as such, vitamin D analogs have gained considerable attention. Today, there are over one-thousand analogs of vitamin D synthesized, yet only a handful of <10 analogs, including the one we synthesized and developed (1α-hydroxyvitamin D5), have shown to be more efficacious at non-toxic concentrations (25). Ongoing studies in our laboratory continue to investigate the mechanism of action by which vitamin D compounds can serve as promising chemopreventive agents for various types of cancer.
POTENTIAL SYNERGY BETWEEN EVALUATION OF WHOLE FOODS AND ISOLATED CONSTITUENTS
Fruits, vegetables, and whole grains have been estimated to have somewhere between 5,000 and 25,000 individual phytochemicals, of which only a small fraction have been identified (26). Moreover, interactions between the different components within the food or in combination with other foods may explain why the isolated dietary components do not always prove efficacious for cancer prevention in models of experimental carcinogenesis and/or clinical trials. In recent years, there has been an increased interest in the concept of whole-food synergy. It is hypothesized that a combination of foods and/or multiple dietary agents may offer increased chemoprotection against cancer as compared to isolated compounds. Until now, only a few studies have undertaken the “wholefood-based” approach to cancer dietary chemoprevention. Liu et al. (27) examined the anticancer activity of apple extracts in a 7,12-dimethylbenz(a)anthracene (DMBA)-induced rat mammary cancer model. Results showed that intragastric treatment of 0–20 g/kg apple extract inhibited mammary tumor multiplicity in a dose-dependent manner. Moreover, the expression of proliferating cell nuclear antigen (PCNA), cyclin D1, and Bcl-2 decreased, while Bax expression and apoptosis were found to increase with escalating doses of apple extract. In a similar fashion, a few other investigators have evaluated various foods using mouse models for mammary, colon and prostate cancer. Using a tumor implant model with Dunning R3327-H-prostate tumors, the combination of freeze-dried tomato and broccoli as compared to either food alone was found to significantly enhance the anti-tumor activity as evident by decreased tumor weight (28). Others have studied the chemopreventive properties of various other foods, including dry beans [Phaseolus vulgaris, L.], soy and garbanzo flour (29,30). The importance of conducting these types of studies is highlighted in a recent publication by Stoner (31) in which a step-wise scheme for evaluating the chemopreventive potential of berry powder is described. These types of study designs will be essential to better elucidate the role of whole foods. Ultimately, it will be necessary to have complementary studies on both the whole food as well as the isolated dietary agents to achieve the greatest understanding of the efficacy and mechanism(s) by which the dietary-derived agents modulate the carcinogenesis process. Furthermore, these types of designs provide a means to better define any interaction which can result in greater efficacy or in some cases decreased potency by the combination.
MOLECULAR TARGETS AND SIGNALING PATHWAYS CRITICAL TO CHEMOPREVENTION
The process of carcinogenesis, in which normal cells become malignant, is quite complex. In a simplified manner, the process is accompanied by changes in structure and function of genetic information coded in the DNA. Moreover, multiple signaling pathways become deregulated during the development of cancer. Bioactive compounds have been found to affect one or more of the deregulated pathways. The pathways which have received considerable attention as they relate to the process of carcinogenesis include carcinogen metabolism, DNA repair, cell proliferation, apoptosis, cell cycle, angiogenesis, and metastasis. Carcinogenesis from initiation to metastasis involves numerous molecular dysfunctions summarized in Fig. 4.
The process of carcinogenesis is defined as initiation, promotion and progression. Progression is shown here to include the growth of malignant tumors, invasion and metastasis. In this diagram for each of the stages, various major actions of phytochemicals involving signaling pathways are summarized.
The course of cell transformation or initiation involves gene mutation, carcinogen metabolism and aberrant DNA repair. In this initial stage, environmental carcinogens (e.g., dietary, tobacco, pollution) induce one or more simple mutations, including transitions, or small deletions in genes which control the process of carcinogenesis. During this initial step, biotransformation enzymes (phase I enzymes, including the cytochrome P450 system) convert the pro-carcinogens to the active carcinogens. On the other hand, phase II enzymes (e.g., glucoronidases, sulfotransferases) play a role in the detoxification of activated carcinogens. As a result, these agents are excreted in the urine. Futhermore there is also a chance that the enzymes may change a particular chemical to a more reactive form that binds to DNA. Reactive oxygen species (ROS) are normally generated as part of the normal oxidative metabolism or may result as end-products of the breakdown of xenobiotic compounds. Extensive DNA damage resulting from ROS is associated with increasing the rate of mutation within cells and thereby promoting oncogenic transformation. Several bioactive compounds have historically been classified as anti-initiators, including genistein, aspirin, selenium, indoles, resveratrol, and allyl sulfur compounds (16).
In humans, numerous DNA repair pathways exist to prevent or overcome DNA damage. Activated carcinogens exert their effects by forming covalent adducts with individual nucleic acids of DNA or RNA. This leads to, among other things, deletions of genetic material or mistranslation of the DNA sequence. Subsequently, when the DNA replicates, an adducted base, if not repaired, could produce mutations in critical genes, such as tumor suppressors or oncogenes. Chemopreventive agents that scavenge activated oxygen species have been shown to stimulate the repair of oxidative DNA damage. Vitamin C, genistein, and compounds originating from cruciferous vegetables are among the most well-studied for their scavenger properties (21). The anti-initiation by chemopreventive agents has been summarized in a recent review (32).
The stage of promotion is characterized by deregulation of signaling pathways which normally control cell proliferation and apoptosis. Genes that control cell cycle are often mutated in human cancers. For example, p27 mutations are common in most pancreatic tumors; similarly, the majority of colon cancers exhibit p53 mutations. Mutations in the genes that control cell cycle result in the continued proliferation of the transformed cells. As a consequence, the proliferation of transformed cells exceeds that of the normal cells. During the promotion stage, a dysfunctional cell-death system is prominent. Therefore, pathways that are involved in cell death and renewal are greatly altered. A recent review by Khan et al. (33) highlights the role of bioactive compounds during the promotion phase of carcinogenesis with particular emphasis on apoptosis.
In general, apoptosis is divided into two distinct pathways: the intrinsic (mitochondrial) pathway and the extrinsic (death receptor) pathway. Common to both apoptotic pathways are the caspases, represented by a family of cysteine proteases which are pivotal in the control of the apoptotic response. Caspase-3 is a key executioner of apoptosis, which is activated by an initiator caspase such as caspase-9. The activated caspase-3 cleaves the PARP, which is one of the hallmarks of apoptosis. Bioactive compounds for the most part have been shown to regulate the intrinsic pathways (30). In mitochondria-mediated apoptosis, the collapse of the mitochondrial membrane potential and the redistribution of cytochrome c are early steps in the apoptotic cascade. Cytochrome c, a critical factor in apoptotic process, which is released from the mitochondria into the cytosol during cell apoptosis. The released cytochrome c along with Apaf-1 and caspase-9 subsequently cleave the effector caspase-3. Moreover, the Bcl-2 family members are crucial to the control of the mitochondrial-mediated apoptosis. Bcl-2 and its homologues are capable of halting mitochondrial membrane disruption and the release of cytochrome c and other pro-apoptotic factors, while Bax promotes these events. The ratio of Bcl-2/Bax, the two contrary proteins, is usually regarded as a criterion in programmed cell death. Survivin, a recently added member of the inhibitors of apoptosis, has been shown to play a key role in regulation of apoptosis in cancer cells. Survivin is abundantly expressed in fetal tissues for survival, but not in normal adult tissues. Survivin has been shown directly to block the processing and activation of effectors caspase-3 and caspase-7, which act on a common downstream of both apoptosis signaling pathways. Similarly, apoptosis may be blocked by a decrease in pro-apoptotic molecules, such as c-jun N-terminal kinase (JNK), or the activation of the anti-apoptotic NF-kB signaling pathway.
It is not possible to list all the chemopreventive agents involved in the various processes discussed above. However, it can be concluded that anti-promotional agents can target a variety of signaling pathways. These molecular targets include, but are not limited to, hormone receptors, cell cycle check-point markers, transcription factors, mitogen-activated protein kinases, rate-limiting enzymes including ornithinedecarboxylase, cyclooxygenases, cell junctions, and tumor suppressor genes (e.g., p53). The stage of promotion, unlike initiation, is reversible. As such there has been an intense interest placed in identifying agents which can stop or reverse the process of promotion. These targets are selective and specific for chemopreventive agents and their action.
Finally, the stage of progression is characterized by genetic alterations within the karyotype of the cells brought about by accumulation of mutated genes resulting in chromosomal abnormalities. That is invasion, angiogenesis, and metastatic growth all constitute the stage of progression. In recent years, there has been a significant interest in developing agents to stop the process of metastasis. Chemopreventive agents can play a significant role in delaying cancer cell progression to metastasis. This reduced rate or halting of progression by chemopreventive agents, chemoquiescence, has considerable importance. Of particular interest are agents that can serve as anti-angiogenic compounds. Angiogenesis, the development of new blood vessels from endothelial cells, is a crucial step which allows the malignant cells to gather the needed nutrients and oxygen. During angiogenesis, endothelial cells are stimulated by various growth factors, including vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF), and travel to the site where the new blood vessels, are needed. By blocking the growth of new blood vessels, the supply of nutrients and oxygen is reduced, and, as a result, the size of the tumor and metastasis may be reduced. Bioactive compounds shown to inhibit angiogenesis are polyunsaturated fatty acids, EGCG, resveratrol, curcumin, and genistein. Another chemopreventive agent, deguelin, has also been reported to exert its effects as an anti-angiogenic agent in human hepatocellular carcinoma (34).
NEW PARADIGMS OF CHEMOPREVENTION
MicroRNA
MicroRNAs (miRNAs) have received considerable attention in recent years. They represent a class of small, noncoding RNAs that control gene expression by targeting mRNA and triggering translation repression or RNA degradation. Functional studies indicate that miRNAs participate in the regulation of almost every cellular process that has been investigated and that alterations in their expression are observed in many different diseases, including cancer (35). The miRNAs are initially part of immature primary transcripts (pri-miRNA) that are cleaved into 60–100 nucleotide hairpin precursor RNAs (pre-miRNA) by the RNase enzyme, Drosha. The pre-miRNA is transported to the cytoplasm by the nuclear export factor exportin-5. In the cytoplasm, the pre-miRNA is further excised by the RNAse type III Dicer and unwound by a putative helicase to yield mature miRNAs, whose lengths are ∼19–24 nucleotides. Mature miRNAs become part of the RNA-induced silencing complex (RISC) that facilitates miRNA-mediated regulation of protein translation through base-pairing between a miRNA and sequence(s) within the 3′ untranslated region of a target mRNA. Binding of the miRNA to mRNA results in a reduced translation rate and /or increased degradation of mRNA. To date, more than 500 human miRNA genes have been identified (miRBase, http://microrna.sanger.ac.uk), and it is believed that the human genome encodes about a thousand miRNAs (36). Although the specific function of most mammalian miRNAs is unknown, it has been speculated that miRNAs could regulate ∼30% of the human genome (37). Since miRNAs can contribute to cancer development and progression and are differentially expressed in normal tissues and cancers (37), they can be identified as molecular targets for cancer chemoprevention. Recently, it has been reported that mir-21 is a potential oncogene, whereas let-7 is a potential tumor suppressor (38) which represses the HMGA2 oncogene and regulates the RAS oncogenes through post-transcriptional repression. Thus, miRNAs may be useful as biomarkers of carcinogenesis and cancer prevention as well as to serve as potential molecular targets that are influenced by dietary interventions.
Throughout this review, a large emphasis has been placed on the role of natural products on the prevention of cancer. Moreover, the actions of various agents on several key signaling pathways, including apoptosis, cell-cycle control, inflammation, angiogenesis, and DNA repair, have been described. Interestingly, all these molecular events are regulated by miRNAs. Recent evidence suggests that folates, retinoids and curcumin exert chemoprotective effects through modulation of miRNA (39). For example, folate deficiency causes a pronounced global increase in miRNA expression in human lymphoblastoid cells in culture; replenishing the folate-deficient cells to complete medium has been shown to reverse the expression of the miRNA expression to that of control cells (40). In a similar manner, retinoic acid has been shown to down-regulate Ras and Bcl2 in acute promyelocytic leukemia (APL) cell lines after treatment with 100 nmol/L all-trans-retinoic acid. These actions were found to correlate with the activation of let-7a and miR-15a/miR-16-1, respectively (41). Recently, Sun et al. (42) reported that curcumin significantly altered the expression profiles of miRNA in human BxPC-3 pancreatic cancer cells. Lastly, vitamin D has been reported to regulate expression of miRNA (43). For example, miR-203 was found to be up-regulated in differentiated human keratinocytes after vitamin D treatment. Vitamin D-treated dendritic cells also showed over-expression of miR-378. In addition, miR181 was found to participate in vitamin D-induced differentiation of HL60 and U937 cells by targeting p27, and miR125b was identified to target VDR in MCF-7 cells. We evaluated effects of 25-hydroxyvitamin D3 (25(OH)D3) on MCF12F cells using a miRNA profiling array. Results showed altered expression of selective miRNAs, including miR-26, miR182 and let-7a, in the normal-like breast epithelial cell line under low serum-induced stress as compared to cells incubated under normal serum condition. The altered expression of miRNA in stressed culture conditions was reversed by 25(OH)D3 treatment (Peng and Mehta unpublished). In a recent chemically induced lung carcinogenesis study, I3C reversed vinyl carbamate (VC)-induced deregulation of miRNA levels in lung tissues of female A/J mice (44). Microarray studies revealed that miR-21, miR-31, miR-130a, miR-146b, and miR-377 were consistently up-regulated, whereas miR-1 and miR-143 were down-regulated in lung tumors relative to normal lungs. In mice treated with VC and given I3C in the diet, levels of miR-21, miR-31, miR-130a, miR-146b, and miR-377 were reduced relative to the levels in mice treated with carcinogen only. These reports collectively suggest that miRNAs are clearly significant molecular targets for carcinogenesis and possibly cancer chemoprevention.
Nanotechnology
The field of chemoprevention has evolved into a major discipline of cancer research. Despite all the advances in the field of chemoprevention, the use of numerous phytochemicals for humans has been limited, in part due to the low bioavailability of these agents to the target tissues and/or systemic toxicity. To circumvent this problem, the field of chemoprevention has expanded to include nanotechnology as a novel approach to deliver packaged chemopreventive agents in a manner which allows them to be delivered selectively to the target tissues. For example, Mukhtar and colleagues recently reported on the bioavailability of EGCG which had been packaged into nanoparticles. Results showed that EGCG delivered by nanoparticles maintained the efficacy of EGCG both as an antiangiogenic and proapoptotic agent (45). These results indicated that nano-chemoprevention can provide a new approach to avoiding systemic toxicity and increasing bioavailability. Similarly, packaging resveratrol into solid lipid nanoparticles has been reported to improve intracellular delivery of resveratrol and to reduce resveratrol toxicity (46). The application of lipid- or polymer-based nano-particles or nano-shells for improved delivery of chemopreventive or chemotherapeutic agents has facilitated the delivery of agents to selective tissues and may serve to lessen systemic toxicity by reducing the amount of agent needed and/or limiting the exposure to the body.
Genomic/Protein Profiling
Effects of phytochemicals on the selective gene(s) expression has become a major approach to evaluate the impact that they have on selected signaling pathways. For example, using a gene array approach, we recently identified the Wnt/b-catenin pathway for deguelin action in breast cancer cells (47). Similarly, we evaluated effects of resveratrol in A549 lung cancer cells using a combination of gene/protein array analyses (48). For this study, RNA samples were isolated from cells treated with resveratrol and subjected to microarray analysis, while protein isolated from these cells was subjected to western-blot-based protein-array. For the analyses, the genes that were differentially expressed by resveratrol were matched with their respective differentially expressed proteins. This approach led to the identification of the TGFβ/SMAD pathway for resveratrol action in lung cancer cells. While this technique has provided a means to better select signaling pathways relevant to the chemopreventive agents being evaluated, a shortcoming of using this concept is that not all genes can be matched with their corresponding proteins. Commercially available protein arrays today do not include all of the proteins involved in the regulatory functions. For example, in our studies, the protein array included only one thousand proteins. Thus, there is a possibility that key signaling pathways may have been missed. Consequently, newer technology which allows for greater protein/gene combination profile will be necessary to better evaluate the role of novel agents on key functions of carcinogenesis and chemoprevention.
In summary, the concept of preventing illnesses (e.g., cancer) by natural products has been in existence for centuries. However, during the past 30 years, cancer chemoprevention has emerged as a major area of research. From the traditional experimental carcinogenesis models which were used primarily to evaluate the efficacy of phytochemicals, there has been a gradual shift towards molecular chemoprevention where specific molecular targets are identified and signaling cascades for chemopreventive agents are dissected out. This approach has received much attention. With the emergence of new technologies, including nanotechnology, and an advanced understanding of stem cells biology, novel approaches to drug delivery and targeted therapy have started to expand. While new concepts are being developed to better elucidate the role of chemopreventive agents, traditional ones continue to hold value in achieving a better understanding of the use of phytochemicals for cancer prevention.
REFERENCES
Karpozilos A, Pavlidis N. The treatment of cancer in Greek antiquity. Eur J Cancer. 2004;40:2033–40.
Rafter JJ. Scientific basis of biomarkers and benefits of functional foods for reduction of disease risk: Cancer. Br J Nutr. 2002;88:S219–24.
Colic M, Pavelic K. Molecular, cellular and medical aspects of the action of nutraceuticals and small molecules therapeutics: from chemoprevention to new drug development. Drugs Exp Clin Res. 2002;28:169–75.
Murillo G, Mehta RG. Cruciferous vegetables and cancer prevention. Nutr Cancer. 2001;41:17–28.
Ambrosone CB, Tang L. Cruciferous vegetable intake and cancer prevention: role of nutrigenetics. Cancer Prev Res. 2009;2:298–300.
Reuter S, Eifes S, Dicato M, Aggarwal BB, Diederich M. Modulation of anti-apoptotic and survival pathways by curcumin as a strategy to induce apoptosis in cancer cells. Biochem Pharmacol. 2008;76:1340–51.
Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9:429–39.
Yu C, Shin YG, Chow A, Li Y, Kosmeder JW, Lee YS, et al. Human, rat, and mouse metabolism of resveratrol. Pharm Res. 2002;19:1907–14.
Garikapaty VP, Ashok BT, Mittelman TK, Tiwari RK. Synthetic dimmer of indole-3-carbinol: second generation diet derived anti-cancer agent in hormone sensitive prostate cancer. Prostate. 2006;66:453–62.
Ko KP, Park SK, Cho LY, Gwack J, Yang JJ, Shin A, et al. Soybean product intake modifies the association between interleukin-10 genetic polymorphisms and gastric cancer risk. J Nutr. 2009;139:1008–12.
Balachandran P, Govindrajan R. Cancer an Ayurvedic perspective. Pharmacol Res. 2005;51:19–30.
Xutian S, Zhang J, Louise W. New exploration and understanding of traditional Chinese medicine. Am J Chin Med. 2009;37:411–26.
Kunow MA. Maya Medicine: traditional healing in Yucatan. New Mexico: University of New Mexico Press; 2003.
Mena-Rajon G, Caamal-Fuentes E, Cantillo-Ciau Z, Cedillo-Rivera R, Flores-Guido J, Moo-Puc R. In vitro cytotoxic activity of nine plants used in Mayan Traditional medicine. J Ethnopharmacol. 2009;121:462–5.
Sporn MB, Dunlop NM, Newton DL, Smith JM. Prevention of chemical carcinogenesis by vitamin A and its synthetic analogs (retinoids). Fed Proc. 1976;35:1332–8.
Wattenberg LW. Inhibition of carcinogenesis by minor a nutrient constituents of the diet. Proc Nutr Soc. 1990;49:173–83.
Naithani R, Huma L, Moriarty R, McCormick DL, Mehta RG. Comprehensive review of cancer chemopreventive agents evaluated in experimental chemoprevention models and clinical trials. Current Med Chem. 2008;15:1044–71.
Kwon KH, Barve A, Yu S, Huang MT, Kong AN. Cancer chemoprevention by phytochemicals potential molecular targets biomarkers and animal studies. Acta Pharmacol Sin. 2007;28:1409–21.
Singh RP, Agarwal R. Prostate cancer chemoprevention by silibinin: bench to bedside. Mol Carcinog. 2006;45:436–42.
Abate-Shen C, Brown PH, Colburn NH, Gerner EW, Green JE, Lipkin M, et al. The untapped potential of genetically engineered mouse models in chemoprevention research opportunities and challenges. Cancer Prev Res. 2008;3:161–6.
Guilford JM, Pezzuto JM. Natural products as inhibitors of carcinogenesis. Expert Opin Investig Drugs. 2008;17:1341–52.
Kinghorn AD, Su BN, Jang DS, Chang LC, Lee D, Gu JQ, et al. Natural inhibitors of carcinogenesis. Planta Med. 2004;70:691–705.
Kosmeder 2nd JW, Pezzuto JM. Novel plant derived anticarcinogens. IARC Sci Publ Rev. 2002;156:343–7.
Lippman SM, Hawk ET. Cancer prevention: from 1727 to milestones of the past 100 years. Cancer Res. 2009;69:5269–84.
Mehta RG, Hussain EA, Mehta RR, Das Gupta TK. Chemoprevention of mammary carcinogenesis by 1α-hydroxyvitamin D5 a synthetic analog of vitamin D. Mutation Res. 2003;523–524:253–64.
Milner JA. Nutrition and cancer: essential elements for a roadmap. Cancer Lett. 2008;269:189–98.
Liu RJ, Dong HW, Chen BQ, Zhao P, Liu RH. Fresh apples suppress mammary carcinogenesis and proliferative activity and induce apoptosis in mammary tumors of the Sprague-Dawley rat. J Agric Food Chem. 2009;57:297–304.
Canene-Adams K, Lindshield BL, Wang S, Jeffery EH, Clinton SK, Erdman Jr JW. Combination of tomato and broccoli enhanced antitumor activity in Dunning R3227-H prostate adenocarcinomas. Cancer Res. 2007;67:836–43.
Thompson MD, Thompson HJ, Brick MA, McGinley JN, Jiang W, Zhu Z, et al. Mechanisms associated with dose-dependent inhibition of rat mammary carcinogenesis by dry bean (Phaseolus vulgaris, L.). J Nutr. 2008;138:2091–7.
Murillo G, Choi JK, Pan O, Hawthorne ME, Constantinou AI, Mehta RG. Garbanzo flour is as effective as soy flour at suppressing the formation of carcinogen-induced aberrant crypt foci in the colons of CF-1 mice. Anti-Cancer Res. 2004;24:3049–55.
Stoner GD. Foodstuffs for preventing cancer: the preclinical and clinical development of berries. Cancer Res. 2009;2:187–94.
Andreassen PR, Ho GP, D’Andrea AD. DNA damage responses and their many interactions with the replication fork. Carcinogenesis. 2006;27:883–92.
Khan N, Afaq F, Mukhtar H. Apoptosis by dietary factors: the suicide solution for delaying cancer growth. Carcinogenesis. 2007;28:233–9.
Lee JH, Lee HH, Lee HS, Choi JS, Kim KW, Hong SS. Deguelin inhibits human hepatocellular carcinoma by antiangiogenesis and apoptosis. Oncol Rep. 2008;20:129–34.
Li Y, VandenBoom 2nd TG, Kong D, Wang Z, Ali S, Philip PA, et al. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res. 2009;69:6704–12.
Bentwich I, Avniel A, Karov Y, Aharonov R, Gilad S, Barad O, et al. Identification of hundreds of conserved and nonconserved human microRNAs. Nat Genet. 2005;37:766–70.
Ahmed FE. Role of miRNA in carcinogenesis and biomarker selection: a methodological view. Expert Rev Mol Diagn. 2007;7:569–603.
Lee YS, Dutta A. The tumor suppressor microRNA let-7 represses the HMGA2 oncogene. Genes Dev. 2007;21:1025–30.
Davis CD, Ross SA. Evidence for dietary regulation of microRNA expression in cancer cells. Nutr Rev. 2008;66:477–82.
Marsit CJ, Eddy K, Kelsey KT. MicroRNA responses to cellular stress. Cancer Res. 2006;66:10843–8.
Garzon R, Pichiorri F, Palumbo T, Visentini M, Aqeilan R, Cimmino A, et al. MicroRNA gene expression during retinoic acid-induced differentiation of human acute promyelocytic leukemia. Oncogene. 2007;26:4148–57.
Sun M, Estrov Z, Ji Y, Coombes KR, Harris DH, Kurzrock R. Curcumin (diferuloylmethane) alters the expression profiles of microRNAs in human pancreatic cancer cells. Mol Cancer Ther. 2008;7:464–73.
Sonkoly E, Wei T, Pavez Loriè E, Suzuki H, Kato M, Törmä H, et al. Protein kinase C-dependent upregulation of miR-203 induces the differentiation of human keratinocytes. J Invest Dermatol. 2009. doi:10.1038/jid.2009.294.
Melkamu T, Zhang X, Tan J, Zeng Y, Kassie F. Alteration of microRNA expression in vinyl-carbamate-induced mouse lung tumors and modulation by the chemopreventive agent indole-3-carbinol. Carcinogenesis. 2010;31:252–8.
Siddiqui IA, Adhami VM, Bharali DJ, Hafeez BB, Asim M, Khwaja SI, et al. Introducing nanochemoprevention as a novel approach for cancer control: proof of principle with green tea polyphenol epigallocatechin-3-gallate. Cancer Res. 2009;69:1712–6.
Teskac K, Kristi J. The evidence for solid lipid nanoparticles mediated cell uptake of resveratrol Int J Pharmacol. 2009; PMID 19833178.
Murillo G, Peng X, Torres K, Mehta RG. Deguelin inhibits growth of breast cancer cells by modulating the expression of key members of the Wnt signaling pathway. Cancer Prev Res. 2009;2:942–50.
Whyte L, Huang YY, Torres K, Mehta RG. Molecular mechanisms of resveratrol action in lung cancer cells using dual protein and microarray analyses. Cancer Res. 2007;67:12007–17.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mehta, R.G., Murillo, G., Naithani, R. et al. Cancer Chemoprevention by Natural Products: How Far Have We Come?. Pharm Res 27, 950–961 (2010). https://doi.org/10.1007/s11095-010-0085-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-010-0085-y