ABSTRACT
Purpose
The present study was undertaken to gain insight into the molecular mechanism of G2/M phase cell cycle arrest resulting from treatment of DU145 cells with diallyl trisulfide (DATS), a promising cancer chemopreventive constituent of garlic.
Methods
Cell cycle distribution was determined by flow cytometry. Immunoblotting was performed to determine protein expression. Overexpression of wild-type or mutant Cdc25C was achieved by transient transfection. Nuclear and cytoplasmic localization of cyclin B1 and cyclin-dependent kinase 1 (cdk1) was studied by immunoblotting.
Results
Exposure of DU145 human prostate cancer cells to DATS resulted in concentration- and time-dependent accumulation of G2/M phase cells, which correlated with down-regulation as well as increased S216 phosphorylation of Cdc25C. Ectopic expression of wild-type or redox-insensitive mutants (C330S and C330S/C377S) or S216A mutant of Cdc25C failed to confer protection against DATS-induced G2/M phase arrest. The DATS-mediated G2/M phase cell cycle arrest was also independent of reduced complex formation between cdk1 and cyclin B1, but correlated with delayed nuclear translocation of cdk1.
Conclusion
The present study indicates that the DATS-mediated G2/M phase cell cycle arrest in DU145 cells results from differential kinetics of nuclear localization of cdk1 and cyclin B1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Epidemiological studies have suggested that dietary intake of Allium vegetables may reduce the risk of different malignancies, including cancer of the prostate (1–4). Anticancer effect of Allium vegetables (e.g., garlic) is attributed to organosulfur compounds (OSCs), which are released upon processing of these vegetables (5,6). The Allium vegetable-derived OSCs, including diallyl sulfide, diallyl disulfide, and diallyl trisulfide (DATS), have been shown to offer significant protection against chemically induced cancer in experimental animals. For example, naturally occurring OSCs inhibit benzo[a]pyrene-induced forestomach and pulmonary carcinogenesis in mice (7), N-nitrosomethylbenzylamine-induced esophageal cancer in rats (8), and azoxymethane-induced colon carcinogenesis in rats (9). The OSC-mediated prevention of chemically induced cancer correlates with inhibition of cytochrome P450-dependent monooxygenases and induction of phase 2 carcinogen inactivating enzymes (7,10–13). We have also shown recently that oral administration of DATS inhibits prostate cancer development and pulmonary metastasis multiplicity in Transgenic Adenocarcinoma of Mouse Prostate (TRAMP) mice without causing weight loss or any other adverse side effects (14).
Besides prevention of cancer in experimental animals, some naturally occurring OSCs are capable of suppressing growth of cancer cells in culture (15–18). The OSC-mediated inhibition of cancer cell proliferation correlates with cell cycle arrest and apoptosis induction (15–25). Milner and co-workers were the first to document apoptosis induction and cell cycle arrest by diallyl disulfide in human colon cancer cells (15,16). Cell cycle arrest and apoptosis induction responses were subsequently extended to other OSCs in a variety of cancer cell types (6,10,17–23). Studies from our laboratory have revealed that DATS is significantly more potent in suppressing growth of human prostate cancer cells compared with either diallyl sulfide or diallyl disulfide (18). Interestingly, a normal prostate epithelial cell line (PrEC) is significantly more resistant to growth arrest and apoptosis induction by DATS compared with prostate cancer cells (19, 25). Oral administration of DATS inhibits growth of PC-3 xenografts in male athymic mice without causing any overt toxicity (26).
Studies during the past 6 years have significantly advanced our understanding of the mechanisms underlying growth arrest and apoptosis induction in DATS-treated cancer cells (18–26). For example, the DATS-induced apoptosis in prostate cancer cells is caused by c-Jun N-terminal kinase-mediated phosphorylation (inactivation) of Bcl-2 as well as suppression of serine/threonine kinase Akt leading to activation of intrinsic caspase pathway (18,22,25). We have also shown previously that the DATS-treated prostate cancer cells are arrested in prometaphase stage due to checkpoint kinase 1-mediated inactivation of anaphase promoting complex/cyclosome (20,21), but the mechanism for G2 phase arrest remains elusive.
Results presented herein demonstrate that the DATS-mediated G2/M phase cell cycle arrest in DU145 human prostate cancer cell line is transient and caused by differential kinetics of nuclear localization of cyclin-dependent kinase 1 (cdk1) and cyclin B1.
MATERIALS AND METHODS
Reagents
DATS (purity >98%) was purchased from LKT Laboratories (St Paul, MN). Cell culture media, non-essential amino acids, sodium pyruvate, penicillin/streptomycin antibiotic mixture, and fetal bovine serum were from GIBCO (Grand Island, NY), and RNaseA was from Promega (Madison, WI). The antibodies against cyclin B1, cdk1, Cdc25C, and S216 phosphorylated Cdc25C were purchased from Santa Cruz Biotechnology (Santa Cruz, CA); anti-actin and anti-α-tubulin antibodies were from Sigma (St Louis, MO); and the antibody against poly-(ADP-ribose)-polymerase (PARP) was from Biomol (Plymouth Meeting, PA).
Cell Culture
DU145 cell line was purchased from American Type Culture Collection (ATCC) and cultured as described by us previously (24). Stock solution of DATS was prepared in dimethyl sulfoxide (DMSO), and an equal volume of DMSO (final concentration <0.2%) was added to the control.
Analysis of Cell Cycle Distribution
The effect of DATS treatment on cell cycle distribution was determined by flow cytometry following staining with propidium iodide as described by us previously (20,21). Briefly, DU145 cells were plated in 100-mm culture dishes and allowed to attach by overnight incubation. The medium was replaced with fresh complete medium containing desired concentrations of DATS or DMSO (control). After incubation at 37°C for desired time point, floating and adherent cells were collected, washed with phosphate-buffered saline (PBS), and fixed with 70% ethanol. The cells were then treated with RNaseA and propidium iodide, and cell cycle distribution was determined by flow cytometry as described by us previously (19,20).
Immunoblotting
The DU145 cells were treated with the desired concentrations of DATS for specified time periods. Cells were lysed as described by us previously (27). The cell lysate was cleared by centrifugation at 14,000 rpm for 30 min. The cytosolic and nuclear fractions from control and DATS-treated cells were prepared using a kit from Pierce (Rockford, IL) according to the manufacturer’s recommendations. Proteins were resolved by sodium-dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto PVDF membrane. The membrane was incubated with a solution containing Tris-buffered saline, 0.05% Tween-20, and 5% (w/v) non-fat dry milk. The membrane was then treated with the desired primary antibody for 1 h at room temperature or overnight at 4°C. Following treatment with appropriate secondary antibody, the immunoreactive bands were visualized using enhanced chemiluminescence method. Change in protein level was determined by densitometric scanning and corrected for loading control.
Transient Transfection
The DU145 cells were transiently transfected with empty vector (pcDNA3.1 or pRC/CMV) or vector encoding for wild-type Cdc25C, redox-insensitive mutants of Cdc25C (C330S or C330S/C377S double mutant) or S216A mutant of Cdc25C. Plasmids for C330S and C330S/C377S (hereafter abbreviated as C2) mutants of Cdc25C were kindly provided by Dr. T. Finkel, National Institutes of Health, Bethesda, MD (28). Plasmids for wild-type Cdc25C and S216A mutant of Cdc25C were generously provided by Dr. Helen Piwnica-Worms, Howard Hughes Medical Institute, Washington University School of Medicine, St. Louis, MO (29). Briefly, cells were plated in 6-well plates and transfected with 1–2 μg plasmid DNA using Lipofectamine2000 or Fugene6 transfection reagent. Twenty-four hours after transfection, the cells were treated with DMSO (control) or DATS for specified time period and used for immunoblotting or analysis of cell cycle distribution.
Analysis of cdk1/Cyclin B1 Complex Formation
The DU145 cells (1 × 105) were treated with DMSO (control) or 40 µM DATS for 8 h. The cells were washed twice with ice-cold PBS and lysed as described above. Aliquot containing 500 μg of lysate protein was incubated overnight at 4°C with anti-cdk1 antibody. Protein A-agarose was added, and the incubation was continued for an additional 3 h at 4°C with gentle shaking. The immunoprecipitates were subjected to sodium-dodecyl sulfate polyacrylamide gel electrophoresis followed by immunoblotting using anti-cyclin B1 antibody.
Determination of cdk1/Cyclin B1 Kinase Activity
The kinase activity of cdk1/cyclin B1 complex in control and DATS-treated DU145 cells was measured using a kit from Upstate Biotechnology-Millipore (Billerica, MA) according to the manufacturer’s recommendations. The DU145 cells were treated with 40 μM DATS for specified time period. Total cell extract was prepared in 50 mM Tris (pH 7.5) containing 1% Triton X-100, 150 mM NaCl, 0.5 mM EDTA, and protease and phosphatase inhibitors. The lysate was cleared by centrifugation at 14,000 rpm for 15 min and pre-cleared with protein A-agarose (50 μl, Santa Cruz Biotechnology) for 30 min at 4°C. Aliquot containing 0.5–1 mg lysate protein was incubated with anti-cdk1 antibody overnight at 4°C. Protein A-agarose (50 μl) was then added to each sample, and incubation was continued for an additional 3 h at 4°C with gentle shaking. Kinase activity was then determined as recommended by the manufacturer.
RESULTS
DATS Treatment Resulted in G2/M Phase Cell Cycle Arrest in DU145 Cells
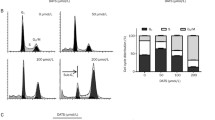
We have shown previously that DATS treatment causes G2/M phase as well as prometaphase arrest in human prostate cancer cells regardless of androgen-responsiveness or p53 status (19–21). However, the mechanism behind DATS-mediated G2/M phase cell cycle arrest was not fully understood. In the present study, we used an androgen-independent human prostate cancer cell line (DU145) to first characterize dose- and time-course kinetic response of DATS-mediated G2/M phase cell cycle arrest. Fig. 1a depicts cell cycle distribution in DU145 cultures after treatment with different concentrations of DATS for 8 h. The DATS treatment resulted in enrichment of G2/M fraction in a concentration-dependent manner (Fig. 1b). For example, the percentage of G2/M fraction in DU145 cultures treated for 8 h with 20 and 40 µM DATS was increased by about 1.6- and 1.9-fold, respectively, compared with DMSO-treated control (Fig. 1b). In a time-course experiment using 40 µM DATS, the G2/M phase cell cycle arrest was evident as early as 4 h after treatment (Fig. 1c). These results indicated that the DATS treatment caused dose- and time-dependent accumulation of G2/M fraction in DU145 cells.
DATS treatment caused G2/M phase cell cycle arrest in DU145 cells in a concentration- and time-dependent manner. a Representative flow histograms depicting cell cycle distribution in DU145 cultures following 8 h treatment with the indicated concentrations of DATS. b Percentage of G2/M fraction in DU145 cultures after 8 h treatment with the indicated concentrations of DATS. c Percentage of G2/M fraction in DU145 cultures after treatment with 40 μM DATS for the indicated time periods. Results shown are mean ± SE (n = 3). *, P < 0.05, significantly different compared with control by one-way ANOVA followed by Dunnett’s test.
Effect of Ectopic Expression of Cdc25C on DATS-Induced G2/M Arrest
Previous studies from our laboratory have shown that the G2/M phase cell cycle arrest resulting from DATS exposure in prostate cancer cells correlates with decline in protein level of Cdc25C (19,24), which is involved in regulation of G2/M transition (30,31). We also found that the DATS-mediated decline in Cdc25C protein level is attenuated in the presence of antioxidants, implicating an oxidation component in this cellular response (19,24), which is consistent with redox regulation of Cdc25C stability due to oxidation of cysteine residues at positions 330 and 377 (28). However, the functional significance of DATS-mediated down-regulation of Cdc25C in cell cycle arrest was not clear. As a start, we validated our previous observations by examining the effect of DATS treatment on protein level of Cdc25C by immunoblotting. As can be seen in Fig. 2a, the level of Cdc25C protein was reduced by >85% by 8 h treatment of DU145 cells with 20 or 40 μM DATS. In a time-course experiment using 40 μM DATS, down-regulation of Cdc25C protein level was clearly evident at 8–24 h time points (Fig. 2b). We reasoned that DATS-induced cell cycle arrest would be attenuated by overexpression of Cdc25C and/or C330S or C2 mutants if redox-sensitive down-regulation of this protein contributed to the cell cycle arrest in our model. We explored this possibility by transient overexpression of wild-type Cdc25C as well as its redox-insensitive mutants. As shown in Fig. 2c, the level of Cdc25C protein was increased markedly in DU145 cells transiently transfected with vector encoding for wild-type as well as mutated versions of Cdc25C in comparison with empty-vector transfected control cells. Of note, the faint band with faster electrophoretic mobility in cells transfected with C330S, C2, and wild-type Cdc25C plasmids represents endogenous form of the Cdc25C protein, while the higher intensity band represents ectopically expressed protein (Fig. 2c). Similar to the results shown in Fig. 2a, the endogenous Cdc25C protein level was decreased upon treatment with DATS in cells transfected with C330S and wild-type Cdc25C (Fig. 2c), but not in the cells with ectopic expression of C2 mutant (Fig. 2c). In addition, the electrophoretic mobility of the ectopically expressed C2 mutant was different from that of C330S mutant or the wild-type Cdc25C (Fig. 2c) for reasons not clear. Nonetheless, the G2/M phase cell cycle arrest resulting from 8 h treatment with 40 μM DATS was observed not only in cells transfected with the empty vector but also in cells with ectopic expression of wild-type Cdc25C as well as its redox-insensitive mutants C330S and C2 (Fig. 2d).
DATS-mediated G2/M phase cell cycle arrest was not reversed by ectopic expression of wild-type Cdc25C or its redox-insensitive mutants (C330S and double mutant C2). a Immunoblotting for Cdc25C using lysates from DU145 cells treated for 8 h with the indicated concentrations of DATS. b Immunoblotting for Cdc25C using lysates from DU145 cells treated with 40 μM DATS for the indicated time periods. In panels A and B, the blots were stripped and reprobed with anti-actin antibody to ensure equal protein loading, and the numbers on top of bands represent densitometric quantitation relative to control. c Immunoblotting for Cdc25C using lysates from DU145 cells transfected with empty-vector (pcDNA3.1) or vector encoding for wild-type or mutant Cdc25C (C330S single mutant or C330S/C377S double mutant C2) and treated for 8 h with DMSO (control) or 40 μM DATS. The blot was stripped and reprobed with anti-actin antibody to ensure equal protein loading. d Percentage of G2/M fraction in DU145 cells transfected with empty-vector (pcDNA3.1) or vector encoding for wild-type or mutant Cdc25C and treated for 8 h with DMSO (control) or 40 μM DATS. Results shown are mean ± SE (n = 3). *, P < 0.05, significantly different compared with respective control by t-test.
We have shown previously that DATS treatment increases S216 phosphorylation of Cdc25C in human prostate cancer cells (19), but functional significance of this observation was not clear. The S216 phosphorylation of Cdc25C affects its nuclear localization (29,32). Initially, we validated our previous observations on DATS-mediated S216 hyperphosphorylation of Cdc25C in DU145 cells. As can be seen in Fig. 3a, exposure of DU145 cells to 40 μM DATS resulted in increased S216 phosphorylation of Cdc25C in comparison with control, which was evident as early as 4 h after treatment. To test functional significance of increased S216 phosphorylation of Cdc25C, we determined the effect of overexpression of S216A mutant of Cdc25C on DATS-induced G2/M arrest. Cells were also transfected with empty pRC/CMV vector or pRC/CMV vector encoding for wild-type Cdc25C as controls. Transient transfection of DU145 cells with vector encoding for wild-type Cdc25C or its S216A mutant resulted in overexpression of the respective protein compared with empty-vector transfected control cells (Fig. 3b). However, the G2/M phase cell cycle arrest resulting from DATS treatment was observed not only in the empty-vector transfected cells but also in DU145 cells with forced expression of wild-type Cdc25C and its S216A mutant (Fig. 3c). The DATS-mediated enrichment of G2/M fraction was relatively less pronounced in pRC/CMV-vector transfected control cells as well as in the cells transiently transfected with vector encoding for wild-type Cdc25C or S216A mutant (Fig. 3c) when compared with un-transfected cells (Fig. 1b). This discrepancy may be attributable to transfection and/or difference in treatment time (8 h in Fig. 1B versus 16 h in Fig. 3c). Nonetheless, these results indicated that even though DATS treatment caused a decrease in Cdc25C protein level and increased its phosphorylation at S216, these cellular changes were not responsible for the cell cycle arrest in our model.
DATS-induced G2/M phase cell cycle arrest in DU145 cells was independent of S216 phosphorylation of Cdc25C. a Immunoblotting for S216 phosphorylated Cdc25C using lysates from DU145 cells treated with 40 μM DATS for the indicated time periods. b Immunoblotting for Cdc25C using lysates from DU145 cells transfected with empty-vector (pRC/CMV) or vector encoding for wild-type or S216A mutant of Cdc25C and treated for 16 h with DMSO (control) or 40 μM DATS. The blot was stripped and reprobed with anti-actin antibody to ensure equal protein loading. c Percentage of G2/M fraction in DU145 cells transfected with empty-vector or vector encoding for wild-type or S216A mutant of Cdc25C and treated for 16 h with DMSO (control) or 40 μM DATS. Experiment was repeated twice in duplicate. Representative data from a single experiment are shown. Error bars are included to indicate range of values. d Western blotting for cyclin B1 using equal amounts of cdk1 immunoprecipitates from cytosolic and nuclear proteins from DU145 cells treated for 8 h with DMSO (control) or 40 μM DATS.
Effect of DATS Treatment on Complex Formation Between cdk1 and Cyclin B1
Because formation of cdk1 and cyclin B1 complex is necessary for the activation of the kinase (30), we tested the possibility whether DATS-mediated G2/M phase cell cycle arrest resulted from inhibition of cdk1/cyclin B1 complex formation. We tested this possibility by immunoprecipitation of cdk1 using lysates from control and DATS-treated (40 μM; 8 h) DU145 cells followed by immunoblotting using anti-cyclin B1 antibody. As shown in Fig. 3d, the cdk1/cyclin B1 complex was detectable only in the nuclear fraction of both control and DATS-treated cells (the upper band). Of note, the immunoreactive band in the cytosolic fractions of control and DATS-treated cells represents IgG. Interestingly, DATS treatment caused an increase, not reduction, in the level of cdk1/cyclin B1 complex compared with control (Fig. 3d). These results indicated that the DATS-mediated G2/M phase cell cycle arrest was not due to a decrease in complex formation between cdk1 and cyclin B1.
Effect of DATS Treatment on Localization of cdk1 and Cyclin B1
Fig. 4 shows the effect of DATS treatment (40 μM) on cytosolic and nuclear localization of cdk1 and cyclin B1. In control cells (first lane from left), cyclin B1 expression was predominant in the cytosolic fraction. However, DATS treatment caused an increase in nuclear level of cyclin B1 as early as 1 h post-exposure. The blots were stripped and re-probed with anti-α-tubulin and anti-PARP antibodies to rule out cross-contaminations of the nuclear and cytosolic fractions. Similar to cyclin B1, cdk1 was primarily present in the cytosol in control cells. Nuclear cdk1 in DATS-treated cells was not detectable until 2–4 h after treatment. Based on these results, we conclude that the delay in nuclear translocation of cdk1 is possibly responsible for the G2/M phase cell cycle arrest in DATS-treated cells.
Effect of DATS Treatment on Kinase Activity of cdk1/cyclin B1 Complex
We proceeded to determine kinetic effect of DATS treatment on kinase activity of cdk1/cyclin B1 complex. The kinase activity of cdk1/cyclin B1 complex was decreased by >80% after 2 h and 4 h treatment of DU145 cells with 40 μM DATS in comparison with control (Fig. 5a). Surprisingly, the DATS-mediated inhibition of cdk1/cyclin B1 kinase activity was completely abolished at the 8 h time point. Because DATS-mediated inhibition of cdk1/cyclin B1 kinase activity was reversible, we raised the question of whether the cells arrested in G2/M phase were able to escape upon extended culture beyond 8 h time point. To address this question, we determined cell cycle distribution in DU145 cultures after 24 h treatment with DMSO (control) or DATS (20 and 40 μM). As shown in Fig. 5b, the DATS-mediated enrichment of G2/M fraction at the 24 h time point was much less pronounced compared with that observed at the 8 h time point (Fig. 1b). For example, the percentage of G2/M fraction in DU145 cultures treated for 8 h with DMSO (control), 20 μM DATS, and 40 μM DATS was about 32.5%, 53.1% (1.6-fold enrichment compared with DMSO-treated control), and 61.2% (1.9-fold enrichment compared with DMSO-treated control), respectively (Fig. 1b). Enrichment of G2/M fraction following 24 h treatment with 20 and 40 μM DATS was characterized by 1.26-fold (27.6% G2/M phase cells) and 1.34-fold (29.5% G2/M phase cells) increase, respectively, compared with DMSO-treated control cells (21.9% G2/M phase cells) (Fig. 5b). Collectively, these results suggested that a fraction of cells arrested in G2/M phase upon treatment with DATS were able to escape cell cycle arrest.
The DATS-mediated inhibition of cdk1/cyclin B1 kinase activity was reversible. a Kinase activity of cdk1/cyclin B1 complex in DU145 cells treated with 40 μM DATS for the indicated time periods. Experiment was repeated twice in duplicate. Representative data from one such experiment are shown. Error bars are included to show range of values. b Percentage of G2/M fraction in DU145 cultures treated for 24 h with the indicated concentrations of DATS. Results shown are mean ± SE (n = 3). * P < 0.05, significantly different compared with control by one-way ANOVA.
DISCUSSION
We have shown previously that the DATS-treated prostate cancer cells are arrested in G2 as well as prometaphase stage (19–21). The DATS-mediated prometaphase arrest is caused by checkpoint kinase 1-mediated inactivation of anaphase promoting complex/cyclosome characterized by accumulation of its substrates (e.g., securin) (20,21). More recently, we showed that a fraction of cells arrested in prometaphase state upon treatment with DATS are ultimately driven to apoptotic cell death (33). However, the mechanism underlying DATS-mediated G2 arrest was not clear. The present study aims to fill this void in our knowledge using DU145 cell line as a model. Eukaryotic G2/M transition is regulated by cdk1, whose activity is dependent upon its association with regulator cyclin B1 (30). A complex between cdk1 and cyclin B1 is important for entry into mitosis in most organisms (30). The activity of cdk1/cyclin B1 kinase is negatively regulated by reversible phosphorylations at Thr14 and Tyr15 of cdk1 (30). Dephosphorylation of Thr14 and Tyr15 of cdk1, and hence activation of cdk1/cyclin B1 kinase complex, is catalysed by the Cdc25 family of dual specificity phosphatases, and this reaction is believed to be a rate-limiting step for entry into mitosis (31). In the present study, we first considered the possibility that the DATS-mediated G2/M phase cell cycle arrest was due to a decline in protein level of Cdc25C. However, ectopic expression of wild-type Cdc25C or its redox-insensitive mutants failed to confer protection against DATS-induced cell cycle arrest (Fig. 2d). Investigation of redox-insensitive mutants was carried out because hydrogen peroxide treatment was shown to cause degradation of Cdc25C due to oxidation of cysteine residues at positions 330 and 377 (28). Nonetheless, the results of the present study indicate that down-regulation of Cdc25C protein per se is not responsible for the DATS-induced G2/M phase cell cycle arrest.
Next, we explored possible contribution of S216 phosphorylation of Cdc25C in DATS-mediated cell cycle arrest. The Ser216 phosphorylation of Cdc25C creates a binding site for 14–3–3 proteins, which sequester Cdc25C in the cytoplasm (29,32). Previous studies from our laboratory have shown that DATS treatment indeed causes an increase in binding between these proteins (19). However, the cell cycle arrest resulting from DATS treatment is also maintained in cells overexpressing S216A mutant of Cdc25C (Fig. 3c). Based on these observations, we conclude that S216 phosphorylation of Cdc25C is not the cause of DATS-induced G2/M arrest. Because reduced complex formation between cdk1 and cyclin B1 can lead to G2/M arrest and such a mechanism was shown in diallyl disulfide-treated colon cancer cells (16), we examined the effect of DATS treatment on complex formation between these proteins. The DATS-treated DU145 cells exhibit an increase, rather than a decrease, in the level of cdk1/cyclin B1 complex. These results suggest that the G2/M cell cycle arrest resulting from DATS exposure is not due to reduced complex formation between cdk1 and cyclin B1 (Fig. 3d).
The cdk1 and cyclin B1 shuttle between nucleus and cytoplasm. Nuclear localization of the complex is necessary for regulation of cell cycle progression and mitotic entry. We found that cyclin B1 moves to the nucleus very quickly upon treatment with DATS, whereas nuclear translocation of cdk1 is delayed until 4–8 h post-treatment as judged by immunoblotting for these proteins using isolated nuclear and cytosolic fractions. Based on these results, we conclude that differential kinetics of nuclear translocation of cdk1 and cyclin B1 possibly contributes to the G2/M phase cell cycle arrest in DATS-treated DU145 cells.
Another interesting observation of the present study is that DATS-mediated inhibition of kinase activity of cdk1/cyclin B1 complex (2–4 h after DATS treatment) is transient and reversed by 8 h after treatment. Consistent with these results, the extent of DATS-mediated G2/M phase cell cycle arrest is more pronounced at early time points (8 h) than after 24 h of treatment (Figs. 1b and 5b). These observations led us to conclude that a fraction of cells arrested in G2/M phase after treatment with DATS are able to enter cell cycle due to re-activation of cdk1/cyclin B1 kinase. In conclusion, the present study provides experimental evidence to indicate that the DATS-induced G2/M phase cell cycle arrest in DU145 cells is not permanent and caused by differential kinetics of nuclear translocation of cdk1 and cyclin B1.
REFERENCES
You WC, Blot WJ, Chang YS, et al. Allium vegetables and reduced risk of stomach cancer. J Natl Cancer Inst. 1989;81:162–4.
Fleischauer AT, Poole C, Arab L. Garlic consumption and cancer prevention: meta-analyses of colorectal and stomach cancers. Am J Clin Nutr. 2000;72:1047–52.
Hsing AW, Chokkalingam AP, Gao YT, et al. Allium vegetables and risk of prostate cancer: a population-based study. J Natl Cancer Inst. 2002;94:1648–51.
Tao MH, Xu WH, Zheng W, et al. A case-control study in Shanghai of fruit and vegetable intake and endometrial cancer. Br J Cancer. 2005;92:2059–64.
Block E. The organosulfur chemistry of the genus Allium—implications for the organic chemistry of sulfur. Angew Chem Int Ed Engl. 1992;31:1135–78.
Herman-Antosiewicz A, Singh SV. Signal transduction pathways leading to cell cycle arrest and apoptosis induction in cancer cells by Allium vegetable-derived organosulfur compounds: a review. Mutat Res. 2004;555:121–31.
Sparnins VL, Barany G, Wattenberg LW. Effects of organosulfur compounds from garlic and onions on benzo[a]pyrene-induced neoplasia and glutathione S-transferase activity in the mouse. Carcinogenesis. 1988;9:131–4.
Wargovich MJ, Woods C, Eng VWS, Stephens LC, Gray K. Chemoprevention of N-nitrosomethylbenzylamine-induced esophageal cancer in rats by the naturally occurring thioether, diallyl sulfide. Cancer Res. 1988;48:6872–5.
Reddy BS, Rao CV, Rivenson A, Kelloff G. Chemoprevention of colon carcinogenesis by organosulfur compounds. Cancer Res. 1993;53:3493–8.
Powolny AA, Singh SV. Multitargeted prevention and therapy of cancer by diallyl trisulfide and related Allium vegetable-derived organosulfur compounds. Cancer Lett. 2008;269:305–14.
Brady JF, Ishizaki H, Fukuto JM, et al. Inhibition of cytochrome P-450 2E1 by diallyl sulfide and its metabolites. Chem Res Toxicol. 1991;4:642–7.
Hu X, Benson PJ, Srivastava SK, et al. Glutathione S-transferases of female A/J mouse liver and forestomach and their differential induction by anti-carcinogenic organosulfides from garlic. Arch Biochem Biophys. 1996;336:199–214.
Singh SV, Pan SS, Srivastava SK, et al. Differential induction of NAD(P)H:quinone oxidoreductase by anti-carcinogenic organosulfides from garlic. Biochem Biophys Res Commun. 1998;244:917–20.
Singh SV, Powolny AA, Stan SD, et al. Garlic constituent diallyl trisulfide prevents development of poorly differentiated prostate cancer and pulmonary metastasis multiplicity in TRAMP mice. Cancer Res. 2008;68:9503–11.
Sundaram SG, Milner JA. Diallyl disulfide induces apoptosis of human colon tumor cells. Carcinogenesis. 1996;17:669–73.
Knowles LM, Milner JA. Diallyl disulfide inhibits p34(cdc2) kinase activity through changes in complex formation and phosphorylation. Carcinogenesis. 2000;21:1129–34.
Nakagawa H, Tsuta K, Kiuchi K, et al. Growth inhibitory effects of diallyl disulfide on human breast cancer cell lines. Carcinogenesis. 2001;22:891–7.
Xiao D, Choi S, Johnson DE, et al. Diallyl trisulfide-induced apoptosis in human prostate cancer cells involves c-Jun N-terminal kinase and extracellular-signal regulated kinase-mediated phosphorylation of Bcl-2. Oncogene. 2004;23:5594–606.
Xiao D, Herman-Antosiewicz A, Antosiewicz J, et al. Diallyl trisulfide-induced G2-M phase cell cycle arrest in human prostate cancer cells is caused by reactive oxygen species-dependent destruction and hyperphosphorylation of Cdc25C. Oncogene. 2005;24:6256–68.
Herman-Antosiewicz A, Singh SV. Checkpoint kinase 1 regulates diallyl trisulfide-induced mitotic arrest in human prostate cancer cells. J Biol Chem. 2005;280:28519–28.
Herman-Antosiewicz A, Stan SD, Hahm E-R, Xiao D, Singh SV. Activation of a novel ataxia-telangiectasia mutated and Rad3 related/checkpoint kinase 1-dependent prometaphase checkpoint in cancer cells by diallyl trisulfide, a promising cancer chemopreventive constituent of processed garlic. Mol Cancer Ther. 2007;6:1249–61.
Xiao D, Singh SV. Diallyl trisulfide, a constituent of processed garlic, inactivates Akt to trigger mitochondrial translocation of BAD and caspase-mediated apoptosis in human prostate cancer cells. Carcinogenesis. 2006;27:533–40.
Hosono T, Fukao T, Ogihara J, et al. Diallyl trisulfide suppresses the proliferation and induces apoptosis of human colon cancer cells through oxidative modification of β-tubulin. J Biol Chem. 2005;280:41487–93.
Antosiewicz J, Herman-Antosiewicz A, Marynowski SW, Singh SV. c-Jun NH2-terminal kinase signaling axis regulates diallyl trisulfide-induced generation of reactive oxygen species and cell cycle arrest in human prostate cancer cells. Cancer Res. 2006;66:5379–86.
Kim YA, Xiao D, Xiao H, et al. Mitochondria-mediated apoptosis by diallyl trisulfide in human prostate cancer cells is associated with generation of reactive oxygen species and regulated by Bax/Bak. Mol Cancer Ther. 2007;6:1599–609.
Xiao D, Lew KL, Kim YA, et al. Diallyl trisulfide suppresses growth of PC-3 human prostate cancer xenograft in vivo in association with Bax and Bak induction. Clin Cancer Res. 2006;12:6836–43.
Xiao D, Srivastava SK, Lew KL, et al. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis. 2003;24:891–7.
Savitsky PA, Finkel T. Redox regulation of Cdc25C. J Biol Chem. 2002;277:20535–40.
Peng CY, Graves PR, Thoma RS, Wu Z, Shaw AS, Piwnica-Worms H. Mitotic and G2 checkpoint control: regulation of 14–3–3 protein binding by phosphorylation of Cdc25C on serine-216. Science. 1997;277:1501–5.
Molinari M. Cell cycle checkpoints and their inactivation in human cancer. Cell Prolif. 2000;33:261–74.
Draetta G, Eckstein J. Cdc25 protein phosphatases in cell proliferation. Biochim Biophys Acta. 1997;1322:M53–63.
Sanchez Y, Wong C, Thoma RS, et al. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to cdk regulation through Cdc25. Science. 1997;277:1497–501.
Xiao D, Zeng Y, Singh SV. Diallyl trisulfide-induced apoptosis in human cancer cells is linked to checkpoint kinase 1-mediated mitotic arrest. Mol Carcinog. 2009;48:1018–29.
Author information
Authors and Affiliations
Corresponding author
Additional information
Grant support
This investigation was supported by the USPHS grant RO1 CA113363–05 (to S.V.S.) awarded by the National Cancer Institute.
Rights and permissions
About this article
Cite this article
Herman-Antosiewicz, A., Kim, YA., Kim, SH. et al. Diallyl Trisulfide-Induced G2/M Phase Cell Cycle Arrest in DU145 Cells Is Associated with Delayed Nuclear Translocation of Cyclin-Dependent Kinase 1. Pharm Res 27, 1072–1079 (2010). https://doi.org/10.1007/s11095-010-0060-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-010-0060-7